Abstract
Corpus callosum (CC) abnormalities have been observed in several psychiatric disorders. Maltreatment has also been associated with marked differences in CC anatomy and microstructure, though rarely controlled for in psychiatric neuroimaging studies. The aim of this study was to identify type and timing of maltreatment associated with alterations in CC microstructure and to ascertain if they differ by sex. T1 and diffusion-weighted MRIs were obtained from 345 (135 M/210 F) healthy 18–25-year-olds. The Maltreatment and Abuse Chronology of Exposure scale provided retrospective data on exposure to ten types of maltreatment across each year of childhood. AI predictive analytics were used to identify the most significant type and time risk factors. The most striking maltreatment-associated alterations in males were in axial diffusivity and were most specifically associated with exposure to emotional abuse or neglect during segment-specific sensitive periods. In contrast, maltreatment was associated with marked alteration in radial diffusivity and fractional anisotropy in females and was most specifically associated with early physical neglect during one common sensitive period involving all segments except the splenium. Overall sex differences, controlling for maltreatment, brain size, and sociodemographic factors were limited to the genu with greater fractional anisotropy in males and radial diffusivity in females. These findings suggest that maltreatment may target myelinization in females and axonal development in males and that these sex differences need to be taken into account in studies seeking to delineate the contribution of CC abnormalities and interhemispheric communication to psychiatric disorders.
Subject terms: Neuroscience, Risk factors
Introduction
The corpus callosum (CC) is the largest fiber tract interconnecting right and left hemispheres and plays an important role in sensory integration, bimanual motor actions, memory, language, creativity, and intelligence [1]. Abnormalities in CC morphometry and diffusion parameters have been reported in a host of psychiatric disorders including: schizophrenia [2], bipolar disorder [3], major depression [4], attention deficit hyperactivity disorder [5], post-traumatic stress disorder [6], autism [7], and alcohol use disorder [8].
Several studies have also assessed whether the CC is sexually dimorphic. Most of the differences in CC volume reported in earlier studies can be attributed to sex-related differences in overall brain size [9, 10]. However, a few studies using the approach of matching males and females for brain size reported differences in CC volume, with a notably larger genu in females than males [11]. Sex differences have also been reported in regional measures of myelin water ratio and in fractional anisotropy [8]. Hence, this still remains an open question.
A critically important confounding factor that has never been taken into account in studies assessing sex differences in CC structure and only rarely taken into account in studies assessing the relationship between CC anatomy and psychopathology is childhood maltreatment [12–16].
Briefly, maltreatment is the most important preventable risk factor for psychopathology and is associated with significant alterations in brain morphometry. One of the earliest and most consistent findings is reduced area and integrity of the CC. For example, in a landmark study, De Bellis et al. [17] reported that CC midsagittal area was reduced in maltreated youths with post-traumatic stress disorder (PTSD) and that the differences between maltreated and controls were greater in males than females. Similar results were reported by Teicher and colleagues [18, 19]. A number of subsequent studies have reported an association between fractional anisotropy (FA) in the CC and exposure to different types of adversity [14, 20–25].
Theoretically, maltreatment could affect the CC through alterations in myelination [26] or through effects on the number, diameter, or packing density of axons as well as through effects on distribution of internal axonal structures such as microtubules and neurofilaments. The overarching aim of this study was to delineate type and timing of maltreatment associated with alterations in CC microstructure and to assess whether there were sex differences in CC parameters once the variance attributable to specific types of maltreatment were controlled for.
The key CC parameters assessed were volume and number of fiber streams within each CC segment as well as measures of diffusivity. FA is the most frequently reported diffusion metric and though highly sensitive it is a rather non-specific indicator of neuropathology and microstructure. Radial, axial, and mean diffusivity provide additional information. Radial diffusivity (λ⊥) is strongly associated with myelination and increases in the presence of demyelination or dysmyelination [27–29]. Axial diffusivity (λ//) is minimally affected by changes in myelination [29] but is influenced by axonal parameters such as axonal degeneration or damage [28]. Acute axonal injury is associated with decreased λ//. In contrast, increased λ// has been associated with chronic axonal injury and attributed to changes in cellular infiltration, gliosis, and extracellular water due to inflammation. Mean diffusivity reflects changes in both axial and radial diffusivity and in addition is influenced by inflammation and edema [30]. The CC is an ideal white matter tract to assess these parameters in, as it has few white matter crossings and has a relatively homogeneous fiber population [31].
Methods
Subject recruitment
Partners Healthcare institutional review board approved this study and written informed consent was obtained from all participants. Recruitment followed previously reported methods [32–35] and are reported in detail in the Supplement. Briefly, participants were medically healthy, right-handed, unmedicated and between 18–25 years of age who were recruited by advertising and selected based on maltreatment history to increase percentage of participants with exposure to multiple types of maltreatment to help ensure that information could be extracted on consequences of exposure to all types of maltreatment at nearly all ages. Maltreated participants were enrolled without regard to psychiatric history to constitute a representative sample. Subjects received $25 for completing the online assessment, $100 per interview and assessment session, and $100 for a 1 h MRI protocol.
Subject assessments
Mental health professionals blind to the neuroimaging results conducted the assessments and Structured Clinical Interviews for DSM-IV psychiatric disorders [36, 37]. Parental education and perceived financial sufficiency (rated from 1—much less than enough money for our needs to 5—much more than enough money for our needs) were collected as these are an important risk factor for maltreatment and may also have effects on trajectories of brain development [38]. Verbal and non-verbal IQ were assessed using the KBITS-2 [39].
Assessment of maltreatment history
The Maltreatment and Abuse Chronology of Exposure (MACE) scale provide ratings on ten types of maltreatment (i.e., parental verbal abuse, parental non-verbal emotional abuse, parental physical abuse, sexual abuse, witnessing interparental violence, witnessing violence towards siblings, emotional neglect, physical neglect, peer emotional bullying and peer physical bullying) during each year of childhood. It consists of 52 items selected using Item Response Theory [40]. Participants indicate whether they experienced a given event and check off each year of occurrence. Each MACE category fits a Rasch Model meaning that each category provides a ‘fundamental measurement’ of exposure in which items are measured on at least an interval scale with a common unit [41].
MACE has excellent overall test-retest reliability (r = 0.91) [40] with no evidence of a significant negative attribution bias in test-retest ratings [42]. More detailed information regarding the reliability of MACE ratings at specific ages are included in the Supplement.
MRI data acquisition
MRI scans were acquired using 3 T Siemens TIM Trio (Erlangen, Germany) using previously reported methods [32, 33]. Briefly, Multiple diffusion-weighted images were acquired in 72 directions. Scan sequences are provided in the Supplement.
MRI analysis
T1-weighted image was processed with FreeSurfer version 6 (http://surfer.nmr.mgh.harvard.edu/) that partitioned the CC into five equal segments in the A-P plane and calculated volumes for each segment. Diffusion tensor images were preprocessed following ENIGMA DTI protocols (http://enigma.ini.usc.edu/protocols/dti-protocols/). Briefly, diffusion-weighted images were corrected for motion and eddy current distortions using eddy correct in FSL [43]. Collected gradient field maps were used to correct for EPI-induced susceptibility artifacts using FSL’s FUGUE. Tensors were fit with FSL’s DTIFIT providing maps of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (λ⊥), and axial diffusivity (λ//). Voxel-wise statistical analyses were performed using Tract-Based Spatial Statistics (TBSS) [44] by nonlinearly registering maps of all DTI measures to the FMRIB58_FA standard-space. CC ROIs were created manually in standard space by dividing CC lengthwise into five equal segments to align with the Freesurfer segmentation. FA, MD, λ⊥, and λ// were averaged for each segment. Fiber stream numbers were estimated using deterministic tractography and the Diffusion Toolkit and TrackVis [45], with default settings, to delineate whole-brain fiber streams, followed by counting the number of streams that went through, touched, or existed within a CC segment.
Statistical analysis of sensitive exposure periods
A critical question is whether exposure to a particular type of maltreatment at a specific age is an important risk factor for CC alterations. Conventional analytic techniques are not suitable because there is substantial collinearity in the degree of exposure to specific types of maltreatment. Instead, we identified the most important cross-validated risk factors associated with diffusivity measures using random forest regression (RFR) with conditional inference trees (CIT) (cforest in R package party; http://party.r-forge.r-project.org/), a form of artificial intelligence (AI) analytics that has been reported to be resistant to collinearity, which we have used in prior sensitive period studies after verifying its utility and superiority to other AI algorithms in Monte-Carlo simulations [42, 46–52].
RFR predicts outcome by creating a forest of decision trees with each tree generated from a different subset of the data and constrained in the number of predictors it can consider at each decision point [53]. This “wisdom of the crowd” strategy is well suited to the analysis of highly collinear data sets and provides superior predictions than conventional regression techniques [53]. The tree structure can also model interactions and does not assume a linear relationship between exposure and response.
The variable importance (VI) of each regressor in the model was assessed by permuting the variable, refitting the forest, and calculating how much permutation of that variable increased the mean square error of the fit to the test set. Permuting important regressors produces a large increase in mean square error, whereas permuting unimportant regressors have a negligible effect. For these analyses, the random forest was trained using data from 63.3% of the participants and evaluated on the withheld test set (36.7%). This process was repeated 50 times with different splits between training and test sets to derive mean measures of VI for each variable. To gauge significance, the overall process was then repeated 1000 times using reshuffled diffusivity values to calculate chance mean and SD importance levels, and the significance of the difference between mean VI and random chance VI was adjusted using Bonferroni correction to control for multiple comparisons. We set a threshold so that at least 5% of the sample needed to report some degree of exposure to a type/time risk factor for it to be included as a predictor variable. Consequently, we were not able to assess the importance of physical abuse at age 1, parental verbal abuse at 1–2, peer emotional abuse at 1–3, witnessing interparental violence at 1–3 and 17–18, witnessing violence to siblings at 1–4 and 16–18, peer physical abuse at 1–5 and sexual abuse prior to age 13 in this sample. Specific methodological details are included in the Supplement.
Although RFR-CIT provides an excellent means of identifying risk factors it does not provide a direct indication of the nature of the relationship between the risk factors and outcome. Hence, we used the saved random forest to predict the outcome by adjusting the degree of exposure to the risk factor of interest while holding all other variables constant at their modal value to ascertain direction of effect as we have done in previous studies [33, 48–50].
Results
Participants
Altogether 345 (135 M/210 F) participants were enrolled and scanned with a mean age of 21.6 ± 2.5 years. Degree of exposure to each type of maltreatment on the MACE is included in Table 1. Overall, 27.2%, 26.4%, 4.8%, and 17.6% had a definite lifetime history of major depression, any anxiety disorder, PTSD, or any personality disorder, respectively. The corresponding lifetime prevalence rates for females was 32.7%, 32.2%, 5.3%, and 22.1%, respectively. Based on self-report [54] 17.9% and 18.7% of males and 29.8% and 22.1% of females had clinically significant symptoms of depression or anxiety, respectively, at the time of assessment. Demographic information is included in supplemental Table S1 along with data on time course of exposure to maltreatment (Fig. S1).
Table 1.
Prevalence of exposure to the ten types of maltreatment on the MACE.
| Males | Females | |||
|---|---|---|---|---|
| Any exposure (%) | Significant exposure (%) | Any exposure (%) | Significant exposure (%) | |
| SexA | 14.8 | 7.4 | 30.0 | 21.0 |
| PVA | 71.9 | 33.3 | 68.6 | 42.9 |
| NVEA | 82.2 | 25.2 | 82.4 | 32.4 |
| Phys | 77.8 | 23.0 | 73.8 | 22.9 |
| WIPV | 25.2 | 15.6 | 28.6 | 16.2 |
| WSIB | 23.0 | 23.0 | 20.5 | 20.5 |
| Peer_E | 86.7 | 43.7 | 82.4 | 41.0 |
| Peer_P | 49.6 | 30.4 | 40.0 | 15.2 |
| EN | 54.1 | 22.2 | 57.6 | 30.0 |
| PN | 27.4 | 10.4 | 28.1 | 13.3 |
Volume and fiber stream measure
Males
As seen in Fig. 1 and detailed in Table 1, the volume of the CC in males was decreased primarily by emotional neglect (EN) and witnessing interparental violence (WIPV). EN between ages 1–3 and 6–11 was associated with reduced volume in anterior and mid-posterior segments, respectively. WIPV at age 4 was associated with reduced volume in mid-anterior and central portions. Peer emotional abuse (Peer_E) at age 17 was also associated with decreased anterior and parental verbal abuse (PVA) at 15 with decreased posterior volume.
Fig. 1. Results of the random forest regression analysis for measures of volume and number of fiber streams in each segment of the corpus callosum in males and females.
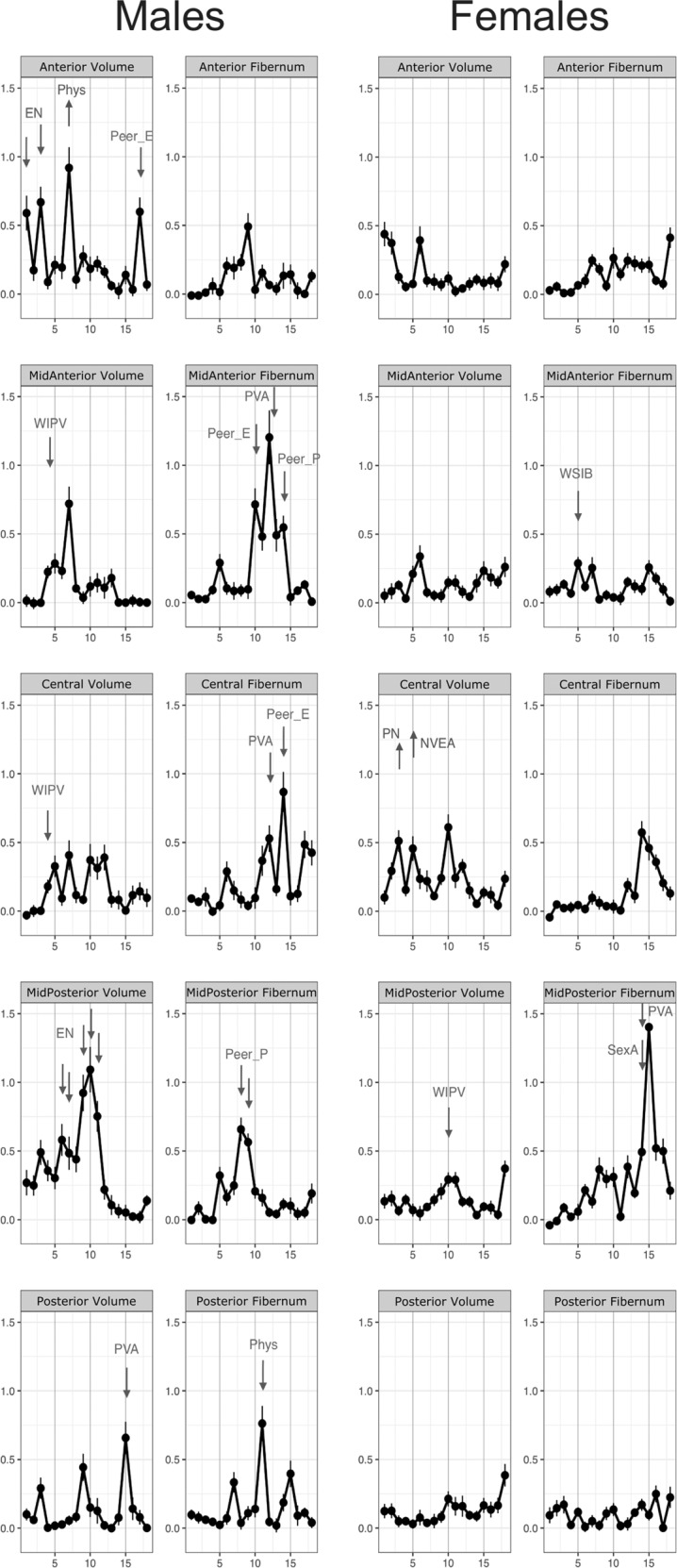
The graph indicates at each age the variable importance of exposure to the type of maltreatment having maximal importance at that age. Arrows are drawn next to ages in which a particular type of maltreatment (labeled) at that specific age emerged as a statistically significant predictor variable (or risk factors). The arrows indicate the directionality of the association, that is whether increasing exposure to that type of maltreatment at that age was associated with increasing or decreasing CC measures based on the saved random forest. Abbreviations. EN emotional neglect, NVEA non-verbal emotional abuse, Peer_E peer emotional bullying, Peer_P peer physical bullying, Phys physical abuse, PN physical neglect, PVA parental verbal abuse, SexA sexual abuse, WIPV witnessing interparental violence, WSIB witnessing violence to siblings.
Exposure to peer and parental emotional and physical abuse between ages 8–14 were associated with decreased fiber number in mid-anterior to posterior segments. Though the specific types and timing varied by segment.
Females
There were fewer discernible associations between maltreatment and volume or fiber stream numbers in females (Fig. 1, Table 1). The only significant risk factor for decreased CC volume was WIPV at age 10 in the mid-posterior segment. Interestingly, physical neglect (PN) at age 3, and non-verbal emotional abuse (NVEA) at age 5 were associated with increased volume in the central portion. Risk factors for reduced fiber stream numbers were witnessing abuse to sibling (WSIB) at age 5 in mid-anterior and sexual abuse at age 14 and parental verbal abuse (PVA) at age 15 in the mid-posterior segment.
Diffusion measures
Males
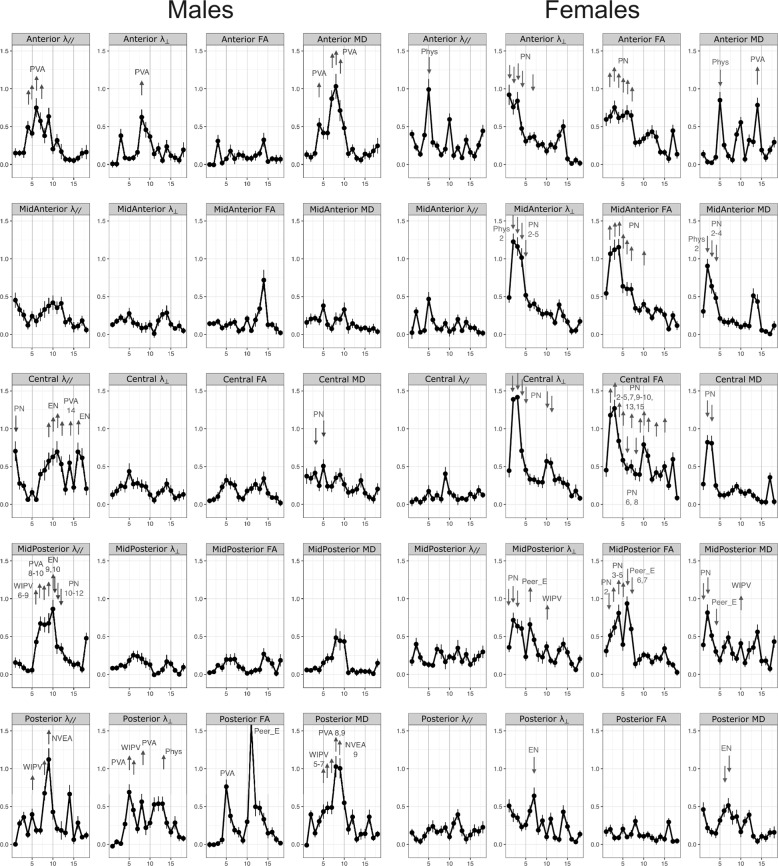
As seen in Fig. 2 and detailed in Table 2 there were marked associations between maltreatment and λ//. In particular, increased λ// was associated with PVA at 4–7 years in anterior segment and 8–10 years in mid-posterior, with WIPV from ages 6–9 for mid-posterior and posterior segments as well as with emotional neglect (EN) between ages 9–12 for central and mid-posterior segments. PN, in contrast, was associated with decreased λ//, particularly in the mid-posterior segment.
Fig. 2. Results of the random forest regression analysis for diffusion parameters of fractional anisotropy (FA), axial diffusivity (λ//), radial diffusivity (λ⊥), and mean diffusivity (MD) in each segment of the corpus callosum in males and females.
The graph indicates at each age the variable importance of exposure to the type of maltreatment having maximal importance at that age. Arrows are drawn next to ages in which a particular type of maltreatment (labeled) at that specific age emerged as a statistically significant predictor variable (or risk factors). The arrows indicate the directionality of the association, that is whether increasing exposure to that type of maltreatment at that age was associated with increasing or decreasing corpus callosum measures based on the saved random forest. See Fig. 1 for abbreviations.
Table 2.
Males: important predictors and the direction of effects on DTI measures.
| DTI measures | Predictors | CC region | Random forest regression | Direction | ||
|---|---|---|---|---|---|---|
| MAL | Age | Importance | p-value | |||
| L1 | PVA | 4 | anterior | 0.49 | 0.00005 | ↑ |
| PVA | 5 | anterior | 0.41 | 0.0083 | ↑ | |
| PVA | 6 | anterior | 0.75 | 1.14 × 10−7 | ↑ | |
| PVA | 7 | anterior | 0.57 | 0.00183 | ↑ | |
| EN | 9 | central | 0.57 | 0.00032 | ↑ | |
| EN | 10 | central | 0.63 | 0.00001 | ↑ | |
| EN | 11 | central | 0.70 | 3.46 × 10−6 | ↑ | |
| EN | 12 | central | 0.53 | 0.03680 | ↑ | |
| EN | 16 | central | 0.69 | 0.00143 | ↑ | |
| NVEA | 14 | central | 0.55 | 0.03542 | ↑ | |
| PN | 1 | central | 0.70 | 2.60 × 10−6 | ↓ | |
| EN | 9 | midposterior | 0.55 | 0.00042 | ↑ | |
| EN | 10 | midposterior | 0.64 | 6.06 × 10−6 | ↑ | |
| WIPV | 6 | midposterior | 0.43 | 2.35 × 10−6 | ↑ | |
| WIPV | 7 | midposterior | 0.67 | 2.86 × 10−18 | ↑ | |
| WIPV | 8 | midposterior | 0.48 | 8.44 × 10−6 | ↑ | |
| WIPV | 9 | midposterior | 0.21 | 0.00802 | ↑ | |
| PN | 10 | midposterior | 0.41 | 0.00775 | ↓ | |
| PN | 11 | midposterior | 0.34 | 0.02207 | ↓ | |
| PN | 12 | midposterior | 0.34 | 0.04108 | ↓ | |
| Phys | 18 | midposterior | 0.48 | 0.00004 | ↑ | |
| PVA | 8 | midposterior | 0.66 | 0.00121 | ↑ | |
| PVA | 9 | midposterior | 0.68 | 0.00054 | ↑ | |
| PVA | 10 | midposterior | 0.86 | 0.00003 | ↑ | |
| WIPV | 5 | posterior | 0.39 | 2.73 × 10−10 | ↑ | |
| WIPV | 8 | posterior | 0.68 | 3.89 × 10−12 | ↑ | |
| NVEA | 9 | posterior | 1.12 | 8.55 × 10−9 | ↑ | |
| RD | PVA | 8 | anterior | 0.62 | 0.00114 | ↑ |
| WIPV | 6 | posterior | 0.29 | 0.00011 | ↑ | |
| Phys | 13 | posterior | 0.54 | 0.00458 | ↑ | |
| PVA | 5 | posterior | 0.69 | 0.00001 | ↑ | |
| PVA | 8 | posterior | 0.56 | 0.01857 | ↑ | |
| FA | Peer_E | 11 | posterior | 1.61 | 1.16 × 10−12 | ↑ |
| PVA | 5 | posterior | 0.76 | 1.93 × 10−9 | ↓ | |
| MD | PVA | 4 | anterior | 0.52 | 5.36 × 10−7 | ↑ |
| PVA | 7 | anterior | 0.87 | 0.00013 | ↑ | |
| PVA | 8 | anterior | 1.03 | 6.04 × 10−17 | ↑ | |
| PVA | 9 | anterior | 0.71 | 0.00006 | ↑ | |
| PN | 3 | central | 0.42 | 0.00812 | ↓ | |
| PN | 5 | central | 0.51 | 0.00102 | ↓ | |
| WIPV | 5 | posterior | 0.43 | 4.46 × 10−14 | ↑ | |
| WIPV | 6 | posterior | 0.48 | 1.38 × 10−12 | ↑ | |
| WIPV | 7 | posterior | 0.49 | 8.26 × 10−6 | ↑ | |
| NVEA | 9 | posterior | 1.00 | 9.41 × 10−9 | ↑ | |
| PVA | 8 | posterior | 1.03 | 5.72 × 10−6 | ↑ | |
| PVA | 9 | posterior | 0.67 | 0.01550 | ↑ | |
| Volume | EN | 1 | anterior | 0.59 | 0.04352 | ↓ |
| EN | 3 | anterior | 0.67 | 0.00352 | ↓ | |
| Peer_E | 17 | anterior | 0.60 | 0.03250 | ↓ | |
| Phys | 7 | anterior | 0.92 | 0.00045 | ↑ | |
| WIPV | 4 | midanterior | 0.22 | 0.01753 | ↓ | |
| WIPV | 4 | central | 0.18 | 0.01110 | ↓ | |
| EN | 6 | midposterior | 0.58 | 0.00015 | ↓ | |
| EN | 7 | midposterior | 0.48 | 0.00205 | ↓ | |
| EN | 9 | midposterior | 0.92 | 3.42 × 10−12 | ↓ | |
| EN | 10 | midposterior | 1.09 | 4.16 × 10−13 | ↓ | |
| EN | 11 | midposterior | 0.75 | 7.11 × 10−8 | ↓ | |
| PVA | 15 | posterior | 0.66 | 0.01701 | ↓ | |
| Fiber numbers | Peer_P | 14 | midanterior | 0.55 | 0.00345 | ↓ |
| Peer_E | 10 | midanterior | 0.71 | 0.02899 | ↓ | |
| PVA | 12 | midanterior | 1.20 | 1.77 × 10−11 | ↓ | |
| Peer_E | 14 | central | 0.87 | 0.01109 | ↓ | |
| PVA | 12 | central | 0.53 | 0.04618 | ↓ | |
| Peer_P | 8 | midposterior | 0.66 | 0.00001 | ↓ | |
| Peer_P | 9 | midposterior | 0.56 | 3.96 × 10−6 | ↓ | |
| Phys | 11 | posterior | 0.76 | 0.00020 | ↓ | |
Increased λ⊥ was primarily observed in the posterior segment and were associated with PVA at age 5 and 8, WIPV at age 6, and physical abuse (Phys) at age 13. FA was increased with Peer_E and PVA at ages 5 and 12, respectively. Similarly, MD was also increased primarily in the posterior segment and associated with exposure to WIPV ages 5–7, PVA ages 8–9, and NVEA at 9. In contrast, PN between ages 3–5 was associated with reduced MD in the central portion.
Females
In comparison to males, there was only one significant association between maltreatment and λ// which consisted of an association between Phys at age 5 and decreased λ// in the anterior segment (Table 3 and Fig. 2). Conversely, there were numerous associations between maltreatment and measures of λ⊥, FA, and MD. In particular, PN during the first 5 years was associated with reduced λ⊥ in anterior, mid-anterior, central, and mid-posterior segments. In general, PN was also associated with increased FA in the same segments especially with exposures between ages 2–7. PN was also associated with decreased MD in mid-anterior, central, and mid-posterior segments, particularly with exposures between ages 2–4. Decreased MD was associated with exposure to EN at ages 6–7 in the posterior segment.
Table 3.
Females: important predictors and the direction of effects on DTI measures.
| DTI measures | Predictors | CC region | Random forest regression | Direction | ||
|---|---|---|---|---|---|---|
| MAL | Age | Importance | p-value | |||
| L1 | Phys | 5 | anterior | 0.99 | 4.74 × 10−9 | ↓ |
| RD | PN | 1 | anterior | 0.92 | 1.35 × 10−10 | ↓ |
| PN | 2 | anterior | 0.76 | 1.68 × 10−8 | ↓ | |
| PN | 3 | anterior | 0.84 | 2.27 × 10−10 | ↓ | |
| PN | 4 | anterior | 0.48 | 0.00005 | ↓ | |
| PN | 7 | anterior | 0.37 | 0.04520 | ↓ | |
| PN | 2 | midanterior | 1.23 | 9.08 × 10−25 | ↓ | |
| PN | 3 | midanterior | 1.16 | 9.08 × 10−21 | ↓ | |
| PN | 4 | midanterior | 1.02 | 4.14 × 10−16 | ↓ | |
| PN | 5 | midanterior | 0.52 | 0.00017 | ↓ | |
| Phys | 2 | midanterior | 0.55 | 0.00119 | ↓ | |
| PN | 2 | central | 1.39 | 1.01 × 10−18 | ↓ | |
| PN | 3 | central | 1.42 | 8.53 × 10−20 | ↓ | |
| PN | 4 | central | 0.71 | 5.65 × 10−7 | ↓ | |
| PN | 5 | central | 0.45 | 0.00021 | ↓ | |
| PN | 10 | central | 0.57 | 3.02 × 10−6 | ↓ | |
| PN | 11 | central | 0.55 | 5.25 × 10−7 | ↓ | |
| IPV | 10 | midposterior | 0.37 | 0.00002 | ↑ | |
| Peer_E | 6 | midposterior | 0.66 | 0.00040 | ↑ | |
| PN | 2 | midposterior | 0.72 | 4.87 × 10−8 | ↓ | |
| PN | 3 | midposterior | 0.64 | 1.02 × 10−6 | ↓ | |
| PN | 4 | midposterior | 0.61 | 0.00011 | ↓ | |
| EN | 7 | posterior | 0.64 | 4.29 × 10−7 | ↓ | |
| FA | PN | 2 | anterior | 0.63 | 0.00035 | ↑ |
| PN | 3 | anterior | 0.75 | 8.12 × 10−11 | ↑ | |
| PN | 4 | anterior | 0.62 | 5.00 × 10−8 | ↑ | |
| PN | 5 | anterior | 0.64 | 3.99 × 10−11 | ↑ | |
| PN | 6 | anterior | 0.69 | 3.78 × 10−10 | ↑ | |
| PN | 7 | anterior | 0.65 | 1.12 × 10−6 | ↑ | |
| PN | 2 | midanterior | 1.07 | 4.10 × 10−14 | ↑ | |
| PN | 3 | midanterior | 1.12 | 6.23 × 10−16 | ↑ | |
| PN | 4 | midanterior | 1.15 | 3.15 × 10−31 | ↑ | |
| PN | 5 | midanterior | 0.64 | 1.80 × 10−6 | ↑ | |
| PN | 6 | midanterior | 0.60 | 9.43 × 10−6 | ↑ | |
| PN | 7 | midanterior | 0.60 | 0.00007 | ↑ | |
| PN | 10 | midanterior | 0.40 | 0.00318 | ↑ | |
| PN | 2 | central | 1.18 | 1.57 × 10−25 | ↑ | |
| PN | 3 | central | 1.27 | 3.49 × 10−22 | ↑ | |
| PN | 4 | central | 0.84 | 1.51 × 10−8 | ↑ | |
| PN | 5 | central | 0.58 | 6.22 × 10−7 | ↑ | |
| PN | 6 | central | 0.47 | 0.02031 | ↓ | |
| PN | 7 | central | 0.50 | 0.00128 | ↑ | |
| PN | 8 | central | 0.40 | 0.01674 | ↓ | |
| PN | 9 | central | 0.39 | 0.01393 | ↑ | |
| PN | 10 | central | 0.79 | 4.95 × 10−17 | ↑ | |
| PN | 11 | central | 0.64 | 1.22 × 10−6 | ↑ | |
| PN | 13 | central | 0.42 | 0.00357 | ↑ | |
| PN | 15 | central | 0.50 | 0.00791 | ↑ | |
| PN | 17 | central | 0.60 | 0.01504 | ↓ | |
| Peer_E | 6 | midposterior | 0.94 | 1.30 × 10−9 | ↓ | |
| Peer_E | 7 | midposterior | 0.60 | 0.03446 | ↓ | |
| PN | 2 | midposterior | 0.51 | 0.00094 | ↓ | |
| PN | 3 | midposterior | 0.62 | 0.00009 | ↑ | |
| PN | 4 | midposterior | 0.81 | 4.27 × 10−9 | ↑ | |
| PN | 5 | midposterior | 0.39 | 0.01095 | ↑ | |
| MD | Phys | 5 | anterior | 0.85 | 0.00001 | ↓ |
| PVA | 14 | anterior | 0.78 | 0.00004 | ↑ | |
| PN | 2 | midanterior | 0.90 | 4.35 × 10−10 | ↓ | |
| PN | 3 | midanterior | 0.64 | 0.00002 | ↓ | |
| PN | 4 | midanterior | 0.48 | 0.02270 | ↓ | |
| Phys | 2 | midanterior | 0.84 | 0.00507 | ↓ | |
| PN | 2 | central | 0.82 | 0.00001 | ↓ | |
| PN | 3 | central | 0.81 | 6.39 × 10−6 | ↓ | |
| IPV | 10 | midposterior | 0.41 | 0.00001 | ↑ | |
| Peer_E | 4 | midposterior | 0.24 | 1.20 × 10−6 | ↓ | |
| PN | 2 | midposterior | 0.82 | 5.93 × 10−7 | ↓ | |
| PN | 3 | midposterior | 0.51 | 0.00105 | ↓ | |
| EN | 6 | posterior | 0.44 | 0.00196 | ↓ | |
| EN | 7 | posterior | 0.51 | 1.96 × 10−6 | ↓ | |
| Volume | NVEA | 5 | central | 0.46 | 0.00274 | ↑ |
| PN | 3 | central | 0.51 | 0.00052 | ↑ | |
| IPV | 10 | midposterior | 0.29 | 0.01685 | ↓ | |
| Fiber numbers | WSibA | 5 | midanterior | 0.29 | 0.00263 | ↓ |
| PVA | 15 | midposterior | 1.40 | 1.05 × 10−20 | ↓ | |
| SexA | 14 | midposterior | 0.49 | 1.44 × 10−8 | ↓ | |
Role of psychopathology
One question is whether maltreatment produces brain changes by increasing prevalence of psychiatric disorders. To test this hypothesis we re-ran the RFR-CIT analyses and include lifetime history of major depression, any anxiety disorder, or PTSD as risk factors as well as current symptoms of depression or anxiety on the symptom questionnaire [54]. None of these items emerged as significant risk factors for any CC measure in males or females.
Sex-related differences in volume, fiber number, and diffusivity measures
ANCOVA was used to assess sex differences in the five CC segments for the entire sample. We covaried for intracranial volume (icv), age, parental education, financial sufficiency, and verbal and non-verbal IQ scores as well as the severity of exposure to type and timing of maltreatment identified as significant risk factors for that segment. Overall, three statistically significant differences were note which were measures of FA (F1,286 = 4.76, p = 0.03), λ// (F1,286 = 4.68, p = 0.03), and λ⊥ (F1,286 = 5.60, p = 0.019) in the anterior segment, with values that were 1.2% smaller, 1.3% smaller, and 4.6% larger in females than males, respectively.
To more definitively assess whether there were gender differences we ran a mixed effects analysis using 75 pairs of male and female participants who were matched by icv (within < ±2% per pair, overall difference 0.13%, tpaired = −1.34, p = 0.18, with volumes vary slightly but not significantly larger in females than males). These analyses also included segment-specific covariates for maltreatment. Overall, two significant differences emerged. Males had greater FA values (F1,43 = 7.20, p = 0.008) and females had greater λ⊥ values (F1,43 = 5.90, p = 0.017) in the anterior segment.
Discussion
This study confirms in adults the previous finding in children that CC volume appears to be more significantly associated with maltreatment in males than the female CC [18, 55, 56]. The findings also fit with the observation that male CC volume appears to be particularly susceptible to neglect [18].
More importantly, several new findings emerged. First, there were 23 statistically significant associations between MACE scores and measure of λ// in males but only one significant association between MACE and λ// in females. Indeed, in males there were significant associations between λ// and maltreatment at 4–7 years in the anterior, 6–12 years for the midposterior, and 14–16 years for the central segments. This suggests that in males that there were segment-specific waves of susceptibility to maltreatment that were characterized by increased λ//.
This increase in λ// may seem confusing as maltreatment might be expected to reduce axon numbers and that would be associated with a decrease in λ//. A possible explanation arises from the observation of increased λ// in a neurotoxic model of selective axon injury which was associated with electron microscopy confirmed reductions of neurofilaments/microtubules in the axoplasm [57]. These elements are crucial for the growth and integrity of axons and play a key role in axonal transport. Wei et al [58] reported that early life stress markedly reduced levels of neurofilament proteins in an animal model. Reducing neurofilaments/microtubules could enhance λ// by providing less interference with free water diffusion within the axon but would likely be associated with impaired connectivity as observed in the neurotoxic model.
The second novel finding was that there were 22 significant associations between maltreatment and reduced λ⊥ in females, with evidence for a sensitive period between ages 1–5 when PN was associated with reduced λ⊥. In males there were no associations between PN and λ⊥, and the few significant associations with other types of maltreatment were with increased rather than decreased λ⊥. The λ⊥ findings in females were reflected in FA which was increased with type and timing of maltreatment associated with decreased λ⊥. The extent to which these sensitive period findings can predict CC parameters is included in the supplement.
These observations are consistent with a paper by Makita et al [21] who reported decreased λ⊥ and increased FA in the CC of youths with reactive attachment disorder, all of whom had experienced maltreatment, primarily in the form of EA, Phys and neglect. Frodl et al [14] also reported increased FA with maltreatment in individuals at ultrahigh risk for depression, but reduced FA with maltreatment in controls. Enhanced myelination would be the most likely mechanism that would lead to a decrease in λ⊥ and an increase in FA. An autopsy study by Tanti et al [59] found that childhood abuse was associated with an increased number of mature and decreased number of immature myelinating oligodendrocytes cells, and they proposed that childhood abuse may foster an adaptive acceleration in myelination.
The observation that λ⊥ was strongly associated with maltreatment in females but not males is consistent with the literature that shows prominent gender differences in key components of myelination. First, the density of CC oligodendrocytes is about 30% greater in male than female rodents [60]. Conversely, generation of new glia and apoptosis of glia, including oligodendrocytes, are approximately two times greater in the female CC indicating that the lifespan of oligodendrocytes is shorter in females than in males. Further, calpain, a protease upregulated by myelin degeneration, is dramatically increased in females [60]. Hence, myelination appears to be a more active and less stable process in females than males. In contrast, the more prominent association between maltreatment and λ// in males is consistent with observations of greater developmental alterations in axonal diameter in males than females [61, 62].
Normally, we would expect that a decrease in λ⊥ and an increase in FA would be associated with enhanced CC interhemispheric communications. We found however that there was a significant association between λ⊥ in the 5 CC segments and measures of right/left resting-state functional connectivity (rsFC) in 16 paired homotopic brain regions (e.g., right and left superior temporal gyri). In 14 of the 16 homotopic pairings that correlated significantly with λ⊥ there was a positive association between λ⊥ and rsFC indicating that a reduction in λ⊥ was associated with a reduction in interhemispheric rsFC. Hence, it is more likely that maltreatment resulted in a reduced degree of interhemispheric connectivity, despite the increase in FA.
We have proposed that alterations in brain structure and function in maltreated individuals represent phenotypic adaptations selected to enhance survival during childhood and reproductive success in adulthood [63–65]. Reduced CC connectivity may be adaptive in some situations by decreasing hemispheric integration and enabling shifts to occur in hemispheric dominance, with different emotional perceptions and memories. Such polarized hemispheric dominance could cause a person to see individuals, particularly parents and family member in an overly positive way in one state and in a rather negative way in another, and to react accordingly. This may be adaptive during childhood in coping with parents whose behavior might shift dramatically due to substance use or untreated bipolar disorder, or in reacting to other episodic threats [63]. However, this form of splitting can be maladaptive later in life. Alternatively, accelerated maturation of the CC may be another form of adaptive response, particularly in situations in which there is inadequate nurturing. We had theorized that early stress could produce precocious maturation of brain regions, leading to signs of early maturation (e.g. the “parentified child”), but may also arrest the development of this region and prevent it from reaching its full adult capacity [19, 66]. Indeed, translational studies link myelination to inhibition of neurite growth and diminished experience-dependent plasticity [67].
This study also showed that there were few significant gender differences in CC parameters. This was true using the entire sample and using a carefully match sample based on icv. The consistent differences were in FA and λ⊥ in the anterior segment.
This anterior CC segment in FreeSurfer corresponds closely to the genu in Witelson’s segmentation [68]. It is the only CC segment with a significant percentage of unmyelinated fibers and also has the greatest percentage of small myelinated axons which appear to be involved in the interhemispheric transfer of information between prefrontal association areas [69]. The slower conduction velocity of these small diameter axons may permit a greater degree of hemispheric autonomy between prefrontal association areas than other brain areas, and the apparent sex differences in FA and λ⊥ in the anterior segment may foster an even greater degree of hemispheric autonomy, on average, in females than males.
Although there were few differences between genders in morphometry there were marked differences between genders in the types of maltreatment associated with alterations in CC measures. EN, WIPV, and PVA were most frequently associated with diffusion measures in males, whereas PN was associated with the majority of diffusion abnormalities in females. The three types of MAL associated with CC abnormalities in males have in common aspects of emotional maltreatment. PN on the other hand may be more reflective of deprivation, which is the absence of exposure to expected species-typical experiences [70]. The supplement includes results of a simplified analysis of the association between measures λ⊥ and λ// with maltreatment divided into four categories: emotional abuse, emotional neglect, physical neglect, and physical-sexual abuse. The results are consistent with the ten-category analysis in highlighting the influence of physical neglect on λ⊥ in females and emotional abuse and emotional neglect on λ// in males (Figs. S2 and S3).
Key strengths of this study were the relatively large sample size of healthy right-handed, unmedicated, emerging adults who were all scanned on the same instrument. Another strength was the use of the MACE to obtain reliable data on type and timing maltreatment and our AI analytical strategy to identify the most important risk factors.
On the other hand, a key limitation is that maltreatment was assessed retrospectively, which may produce problems with recall bias and inaccurate reporting. We know that MACE results are highly reliable. However, we do not know how accurate their reports are, particularly regarding events that happened at early ages. To our knowledge no prospective study has ever been conducted that provides data on exposure to multiple types of maltreatment across each year of childhood. And such a study could not be ethically conducted without reporting certain cases to protective services and intervening on behalf of the child. Indeed, although the Adolescent Brain and Cognitive Development study assesses exposure to adversity, it omits collecting data on physical, sexual or emotional abuse, witnessing domestic violence, emotional or physical neglect, or peer emotional or physical bullying given the complexities of collecting this information in a longitudinal study. Hence, this limitation may be difficult to surmount. Nevertheless, these findings will need to be verified through longitudinal studies that collect relevant information on some of these proposed type/time risk factors.
Another limitation is that an insufficient number of males and females reported sexual abuse before age 13 for early SA to be evaluated as a risk factor. This is unfortunate and should be assessed in the future in a specifically selected sample. To partially address this problem we created a new variable for cumulative exposure to sexual abuse between ages 1–12 and re-ran the analysis using this as an additional risk factor, but this composite variable did not emerge as a significant predictor for any of the CC measures. Prior studies have reported associations between sexual abuse and CC morphometry, particularly in females. In some of these studies, participants were specifically recruited with substantial histories of sexual abuse [25, 71] or had sexual abuse and were psychiatrically hospitalized [18] or had post-traumatic stress disorder [55] or borderline personality disorder [13]. Hence, sexual abuse may emerge as a risk factor for CC alterations in participants with greater levels of exposure to sexual abuse or in those with more prominent psychiatric symptomatology. Some additional minor limitations are reported in the supplement.
Maltreatment is the most important risk factor or disease modifier for multiple psychiatric disorders such as major depression [42, 72, 73], post-traumatic stress disorder [46, 73–75], schizophrenia [76–79], bipolar disorder [80–82], attention deficit hyperactivity disorder [83–86] and alcohol use disorder [87–89] that have been associated with abnormalities in CC morphology. A key question that needs to be addressed is whether CC abnormalities are universal or specific to the maltreated subtype of these disorders [90] and if alterations in CC diffusion parameters mediate a significant portion of the risk associated with maltreatment. In any case, given the highly significant associations between maltreatment and CC diffusivity, studies assessing the contribution of CC abnormalities to psychopathology should include maltreatment as a potential confounding factor. Findings from this study suggest that data on type and timing of exposure would be more informative than overall levels of maltreatment. This study also makes clear the importance of sex differences in the potential effects of maltreatment on myelin versus axons. These sex differences in susceptibility and expression also need to be taken into account in studies on the neurobiology of psychiatric disorders.
Supplementary information
Author contributions
MHT conceived the study. MLR, CEM, EAB, KO, AK, and CMA contributed to the acquisition of the data. KO and MHT analyzed and interpreted the data. MHT and KO drafted the paper. All authors reviewed the paper and critically revised it for important intellectual content. All authors agree to be accountable for all aspects of the work.
Funding
This work was supported, in part, by the National Institute of Mental Health Award MH-091391, National Institute of Drug Abuse Award DA-017846, National Institute of Child Health and Human Development Award HD-079484 to MHT, and National Institute of Mental Health Award MH-122919 to KO. This work has not been presented.
Competing interests
MHT received royalties from Pearson until 1/1/2019. MHT developed the Maltreatment and Abuse Chronology of Exposure scale used in this study, but there is no financial conflict as he placed it into the public domain and it is available and free to use. He has no other conflicts to disclose. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01260-7.
References
- 1.Arda KN, Akay S. The relationship between corpus callosum morphometric measurements and age/gender characteristics: a comprehensive MR imaging study. J Clin Imaging Sci. 2019;9:33. doi: 10.25259/JCIS-13-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keshavan MS, Diwadkar VA, Harenski K, Rosenberg DR, Sweeney JA, Pettegrew JW. Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. J Neurol Neurosurg Psychiatry. 2002;72:757–60. doi: 10.1136/jnnp.72.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprooten E, Brumbaugh MS, Knowles EE, McKay DR, Lewis J, Barrett J, et al. Reduced white matter integrity in sibling pairs discordant for bipolar disorder. Am J Psychiatry. 2013;170:1317–25. doi: 10.1176/appi.ajp.2013.12111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, et al. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:84–8. doi: 10.1016/j.biopsych.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villarreal G, Hamilton DA, Graham DP, Driscoll I, Qualls C, Petropoulos H, et al. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. 2004;131:227–35. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, et al. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2011;21:1134–46. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu IC, Chiu CH, Chen CJ, Kuo LW, Lo YC, Tseng WY. The microstructural integrity of the corpus callosum and associated impulsivity in alcohol dependence: a tractography-based segmentation study using diffusion spectrum imaging. Psychiatry Res. 2010;184:128–34. doi: 10.1016/j.pscychresns.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neurosci Biobehav Rev. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- 10.Luders E, Toga AW, Thompson PM. Why size matters: differences in brain volume account for apparent sex differences in callosal anatomy: the sexual dimorphism of the corpus callosum. Neuroimage. 2014;84:820–4. doi: 10.1016/j.neuroimage.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiino A, Chen YW, Tanigaki K, Yamada A, Vigers P, Watanabe T, et al. Sex-related difference in human white matter volumes studied: inspection of the corpus callosum and other white matter by VBM. Sci Rep. 2017;7:39818. doi: 10.1038/srep39818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucker J, Muralidharan K, Torres IJ, Su W, Kozicky J, Silveira LE, et al. Childhood maltreatment and corpus callosum volume in recently diagnosed patients with bipolar I disorder: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM) J Psychiatr Res. 2014;48:65–72. doi: 10.1016/j.jpsychires.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Rusch N, Luders E, Lieb K, Zahn R, Ebert D, Thompson PM, et al. Corpus callosum abnormalities in women with borderline personality disorder and comorbid attention-deficit hyperactivity disorder. J Psychiatry Neurosci. 2007;32:417–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. J Psychiatry Neurosci. 2012;37:37–45. doi: 10.1503/jpn.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bellis MD, Hooper SR, Chen SD, Provenzale JM, Boyd BD, Glessner CE, et al. Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Dev Psychopathol. 2015;27:1555–76. doi: 10.1017/S0954579415000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinert S, Repple J, Nenadic I, Krug A, Jansen A, Grotegerd D, et al. Reduced fractional anisotropy in depressed patients due to childhood maltreatment rather than diagnosis. Neuropsychopharmacology. 2019;44:2065–72.. doi: 10.1038/s41386-019-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bellis MD, Keshavan MS, Clark DB, Casey Bj, Giedd JN, Boring AM. et al. A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biol Psychiatry. 1999;45:1271–84. [DOI] [PubMed]
- 18.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol psychiatry. 2004;56:80–5. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann N Y Acad Sci. 1997;821:160–75. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Gundapuneedi T, Rao U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology. 2012;37:2693–701. doi: 10.1038/npp.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makita K, Takiguchi S, Naruse H, Shimada K, Morioka S, Fujisawa TX, et al. White matter changes in children and adolescents with reactive attachment disorder: a diffusion tensor imaging study. Psychiatry Res Neuroimaging. 2020;303:111129. doi: 10.1016/j.pscychresns.2020.111129. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy-Jones S, Oestreich LKL, Lyall AE, Kikinis Z, Newell DT, Savadjiev P, et al. Childhood adversity associated with white matter alteration in the corpus callosum, corona radiata, and uncinate fasciculus of psychiatrically healthy adults. Brain Imaging Behav. 2018;12:449–58.. doi: 10.1007/s11682-017-9703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poletti S, Mazza E, Bollettini I, Locatelli C, Cavallaro R, Smeraldi E, et al. Adverse childhood experiences influence white matter microstructure in patients with schizophrenia. Psychiatry Res. 2015;234:35–43. doi: 10.1016/j.pscychresns.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, et al. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 2008;162:256–61. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinne-Albers MA, van der Werff SJ, van Hoof MJ, van Lang ND, Lamers-Winkelman F, Rombouts SA, et al. Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: a DTI study. Eur Child Adolesc Psychiatry. 2016;25:869–78. doi: 10.1007/s00787-015-0805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz PE, Tanti A, Gasecka A, Barnett-Burns S, Kim JJ, Zhou Y, et al. Association of a history of child abuse with impaired Myelination in the Anterior cingulate cortex: convergent epigenetic, transcriptional, and morphological evidence. Am J Psychiatry. 2017;174:1185–94.. doi: 10.1176/appi.ajp.2017.16111286. [DOI] [PubMed] [Google Scholar]
- 27.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 30.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi K, Anderson CM, Bolger EA, Khan A, McGreenery CE, Teicher MH. Childhood maltreatment is associated with alteration in global network fiber-tract architecture independent of history of depression and anxiety. NeuroImage. 2017;150:50–59. doi: 10.1016/j.neuroimage.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohashi K, Anderson CM, Bolger EA, Khan A, McGreenery CE, Teicher MH. Susceptibility or resilience to maltreatment can be explained by specific differences in brain network architecture. Biol psychiatry. 2019;85:690–702. doi: 10.1016/j.biopsych.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teicher MH, Anderson CM, Ohashi K, Polcari A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol psychiatry. 2014;76:297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA. 2012;109:E563–72. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders—clinician version (SCID-CV). Washington DC: American Psychiatric Press; 1997.
- 37.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured clinical interview for DSM–IV Axis II personality disorders. Washington DC;American Psychiatric Association; 1997.
- 38.Taylor RL, Cooper SR, Jackson JJ, Barch DM. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open. 2020;3:e2023774. doi: 10.1001/jamanetworkopen.2020.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufman AS, Kaufman NL. Kaufman brief intelligence Test, 2nd edition. In: Reynolds CR, Vannest KJ, Fletcher-Janzen E, editors. Encyclopedia of special education 4th ed. Hoboken (NJ): John Wiley & Sons, Inc; 2014. 10.1002/9781118660584.ese1325.
- 40.Teicher MH, Parigger A. The ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PloS one. 2015;10:e0117423. doi: 10.1371/journal.pone.0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bond T, Fox CM. Applying the Rasch model: fundamental measurement in the human sciences. 2 ed. Mahwah (NJ): Lawrence Erlbaum; 2007.
- 42.Khan A, McCormack HC, Bolger EA, McGreenery CE, Vitaliano G, Polcari A, et al. Childhood maltreatment, depression, and suicidal ideation: critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Front Psychiatry. 2015;6:42. doi: 10.3389/fpsyt.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 44.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. Proc Intl Soc Mag Reson Med. 2007;15:3720. [Google Scholar]
- 46.Schalinski I, Teicher MH, Nischk D, Hinderer E, Muller O, Rockstroh B. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16:295. doi: 10.1186/s12888-016-1004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schalinski I, Teicher MH, Rockstroh B. Early neglect is a key determinant of adult hair cortisol concentration and is associated with increased vulnerability to trauma in a transdiagnostic sample. Psychoneuroendocrinology. 2019;108:35–42. doi: 10.1016/j.psyneuen.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Teicher MH, Anderson CM, Ohashi K, Khan A, McGreenery CE, Bolger EA, et al. Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage. 2018;169:443–52.. doi: 10.1016/j.neuroimage.2017.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J, Lowen SB, Anderson CM, Ohashi K, Khan A, Teicher MH. Association of prepubertal and postpubertal exposure to childhood maltreatment with adult amygdala function. JAMA psychiatry. 2019. [DOI] [PMC free article] [PubMed]
- 50.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–44. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schalinski I, Breinlinger S, Hirt V, Teicher MH, Odenwald M, Rockstroh B. Environmental adversities and psychotic symptoms: the impact of timing of trauma, abuse, and neglect. Schizophr Res. 2019;205:4–9. doi: 10.1016/j.schres.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 52.Tomoda A, Polcari A, Anderson CM, Teicher MH. Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PloS one. 2012;7:e52528. doi: 10.1371/journal.pone.0052528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 54.Kellner R. A symptom questionnaire. J Clin Psychiatry. 1987;48:268–73.. [PubMed] [Google Scholar]
- 55.De Bellis MD, Keshavan MS. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci Biobehav Rev. 2003;27:103–17. doi: 10.1016/s0149-7634(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 56.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol psychiatry. 1999;45:1271–84. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 57.Kinoshita Y, Ohnishi A, Kohshi K, Yokota A. Apparent diffusion coefficient on rat brain and nerves intoxicated with methylmercury. Environ Res. 1999;80:348–54. doi: 10.1006/enrs.1998.3935. [DOI] [PubMed] [Google Scholar]
- 58.Wei L, Hao J, Lacher RK, Abbott T, Chung L, Colangelo CM, et al. Early-life stress perturbs key cellular programs in the developing mouse hippocampus. Dev Neurosci. 2015;37:476–88. doi: 10.1159/000430861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanti A, Kim JJ, Wakid M, Davoli MA, Turecki G, Mechawar N. Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol Psychiatry. 2018;23:2018–28.. doi: 10.1038/mp.2017.231. [DOI] [PubMed] [Google Scholar]
- 60.Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26:1439–47. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paus T, Toro R. Could sex differences in white matter be explained by g ratio? Front Neuroanat. 2009;3:14. doi: 10.3389/neuro.05.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pesaresi M, Soon-Shiong R, French L, Kaplan DR, Miller FD, Paus T. Axon diameter and axonal transport: in vivo and in vitro effects of androgens. Neuroimage. 2015;115:191–201. doi: 10.1016/j.neuroimage.2015.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teicher MH. Scars that won’t heal: the neurobiology of child abuse. Sci Am. 2002;286:68–75. doi: 10.1038/scientificamerican0302-68. [DOI] [PubMed] [Google Scholar]
- 64.Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016;57:241–66. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–66. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 66.Teicher MH, Ito Y, Glod CA, Schiffer F, Gelbard HA. Neurophysiological mechanisms of stress response in children. In: Pfeffer C, editor. Severe stress and mental disturbance in children. Washington DC: American Psychiatric Association Press; 1996. p. 59–84.
- 67.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–6. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 69.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–53. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 70.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578–91. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andersen SL, Tomoda A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2020;177:20–36. doi: 10.1176/appi.ajp.2019.19010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trickett PK, Noll JG, Putnam FW. The impact of sexual abuse on female development: lessons from a multigenerational, longitudinal research study. Dev Psychopathol. 2011;23:453–76. doi: 10.1017/S0954579411000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scott KM, Smith DR, Ellis PM. Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Arch Gen Psychiatry. 2010;67:712–9. doi: 10.1001/archgenpsychiatry.2010.71. [DOI] [PubMed] [Google Scholar]
- 75.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156:1223–9. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- 76.Ajnakina O, Trotta A, Oakley-Hannibal E, Di Forti M, Stilo SA, Kolliakou A, et al. Impact of childhood adversities on specific symptom dimensions in first-episode psychosis. Psychol Med. 2016;46:317–26. doi: 10.1017/S0033291715001816. [DOI] [PubMed] [Google Scholar]
- 77.Storvestre GB, Jensen A, Bjerke E, Tesli N, Rosaeg C, Friestad C, et al. Childhood trauma in persons with schizophrenia and a history of interpersonal violence. Front Psychiatry. 2020;11:383. doi: 10.3389/fpsyt.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Struck N, Krug A, Yuksel D, Stein F, Schmitt S, Meller T, et al. Childhood maltreatment and adult mental disorders–the prevalence of different types of maltreatment and associations with age of onset and severity of symptoms. Psychiatry Res. 2020;293:113398. doi: 10.1016/j.psychres.2020.113398. [DOI] [PubMed] [Google Scholar]
- 79.Thomas S, Hofler M, Schafer I, Trautmann S. Childhood maltreatment and treatment outcome in psychotic disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2019;140:295–312. doi: 10.1111/acps.13077. [DOI] [PubMed] [Google Scholar]
- 80.Agnew-Blais J, Danese A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:342–9. doi: 10.1016/S2215-0366(15)00544-1. [DOI] [PubMed] [Google Scholar]
- 81.Duarte D, Belzeaux R, Etain B, Greenway KT, Rancourt E, Correa H, et al. Childhood-maltreatment subtypes in bipolar patients with suicidal behavior: systematic review and meta-analysis. Braz J Psychiatry. 2020;42:558–67.. doi: 10.1590/1516-4446-2019-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Post RM, Leverich GS, Xing G, Weiss RB. Developmental vulnerabilities to the onset and course of bipolar disorder. Dev Psychopathol. 2001;13:581–98. doi: 10.1017/s0954579401003091. [DOI] [PubMed] [Google Scholar]
- 83.Aguado-Gracia J, Mundo-Cid P, Lopez-Seco F, Acosta-Garcia S, Cortes-Ruiz MJ, Vilella E, et al. Lifetime victimization in children and adolescents with ADHD. J Interpers Violence. 2021;36:NP3241–NP62.. doi: 10.1177/0886260518771680. [DOI] [PubMed] [Google Scholar]
- 84.Boyd M, Kisely S, Najman J, Mills R. Child maltreatment and attentional problems: a longitudinal birth cohort study. Child Abus Negl. 2019;98:104170. doi: 10.1016/j.chiabu.2019.104170. [DOI] [PubMed] [Google Scholar]
- 85.Capusan AJ, Kuja-Halkola R, Bendtsen P, Viding E, McCrory E, Marteinsdottir I, et al. Childhood maltreatment and attention deficit hyperactivity disorder symptoms in adults: a large twin study. Psychol Med. 2016;46:2637–46. doi: 10.1017/S0033291716001021. [DOI] [PubMed] [Google Scholar]
- 86.Craig SG, Bondi BC, O’Donnell KA, Pepler DJ, Weiss MD. ADHD and exposure to maltreatment in children and youth: a systematic review of the past 10 Years. Curr Psychiatry Rep. 2020;22:79. doi: 10.1007/s11920-020-01193-w. [DOI] [PubMed] [Google Scholar]
- 87.Clark DB, Lesnick L, Hegedus AM. Traumas and other adverse life events in adolescents with alcohol abuse and dependence. J Am Acad Child Adolesc Psychiatry. 1997;36:1744–51. doi: 10.1097/00004583-199712000-00023. [DOI] [PubMed] [Google Scholar]
- 88.De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–70. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- 89.Potthast N, Neuner F, Catani C. The contribution of emotional maltreatment to alcohol dependence in a treatment-seeking sample. Addict Behav. 2014;39:949–58. doi: 10.1016/j.addbeh.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 90.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170:1114–33. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.