Abstract
Maternal obesity (MO) and gestational diabetes mellitus (GDM) are common in western societies, which impair fetal development and predispose offspring to metabolic dysfunction. Placenta is the organ linking the mother to her fetus, and MO suppresses the development of vascular system and expression of nutrient transporters in placenta, thereby affecting fetal development. For maintaining its proper physiological function, placenta is energy demanding, which is met through extensive oxidative phosphorylation. However, the oxidative capacity of placenta is suppressed due to MO and GDM. Recently, several studies showed that physical activity during pregnancy enhances oxidative metabolism and improves placental function, which might be partially mediated by exerkines, referring to cytokines elicited by exercise. In addition, as an endocrine organ, placenta secretes cytokines, termed placentokines, including apelin, superoxide dismutase 3 (SOD3), irisin and adiponectin, which mediate fetal development and maternal metabolism. Possible molecular mechanisms linking ME and placentokines to placental and fetal development are further discussed. As an emerging field, up to now, available studies are limited, mostly conducted in rodents. Given the epidemics of obesity and metabolic disorders, as well as the prevalence of maternal sedentary lifestyle, the effects of exercise of pregnant women on placental function and placentokine secretion, as well as their impacts on fetal development, need to be further examined.
Keywords: Development, exerkine, maternal exercise, metabolism, placenta
Graphical Abstract
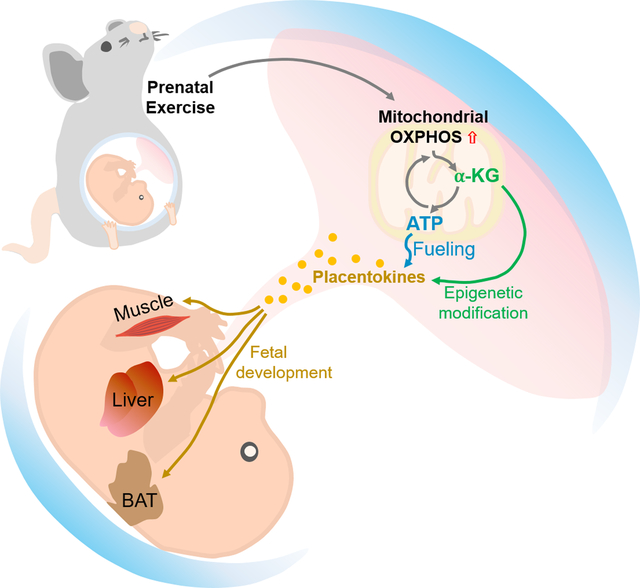
Placenta mediates the delivery of oxygen and nutrients from the mother to her fetuses, and also exerts endocrine functions. To maintain its physiological functions, placenta is energy demanding, which is mainly met by oxidative phosphorylation. Physical activity during pregnancy enhances placental oxidative metabolism and function, which is partially mediated by exercise-derived cytokines (exerkines). In addition, exercise stimulates the secretion of cytokines from placenta, termed as placentokines, which further mediate fetal development and may exert long-term effects on the metabolic health of offspring.
1. Introduction
The population of women at reproductive age with overweight and obesity is rapidly increasing [1], accompanied with increased cases and rates of gestational hypertension (number of cases: 122,667 cases; incidence rate: 29.7%) and preeclampsia (number of cases: 132,800; incidence rate: 32.1%) in the United States in 2004 [2, 3]. Even worse, these deleterious maternal disorders affect fetal development, which predisposes children to metabolic diseases later in life [4, 5]. Furthermore, gestational diabetes mellitus and preeclampsia during pregnancy negatively affect birth size [6]. A number of previous reports have been reviewed, showing the association between birth size and impairment of glucose and insulin metabolism [7]. In particular, low birth weight predisposes offspring to increased insulin resistance and decreased insulin sensitivity, thereby leading to increasing incidence of type 2 diabetes mellitus [8]. The accumulating evidence of developmental origins of adult diseases highlights the importance of gestational management.
Given placenta as a mediator between mother and fetus, a reasonable explanation of intergeneration effects is that the fetus absorbs more glucose and lipids delivered through the placenta, which promotes fetal white adipose tissue development and elicits chronic inflammation [9–11], while suppressing muscle and brown adipose tissue development [12, 13]. Another possibility is due to the decreased levels of cytokines released from placenta into the fetal circulation, affecting fetal development [14]. These placenta-oriented cytokines are named as placentokines (PLACENTa + cytOKINE) [15]. Several studies suggest the importance of placentokines in regulating placental and fetal development [16–18]. In addition to nutritional and hormonal changes, maternal obesity induces mitochondrial, dysfunction, which impairs the generation of adenosine triphosphate (ATP) by oxidative phosphorylation, a critical source of energy for placenta [19]. Inversely, these disorders can be effectively reversed by physical activity during pregnancy [20].
Exercise has been recognized as a medicine [21], which is partially mediated by exercise-induced cytokines, termed exerkines [22]. In addition, maternal exercise enhances the secretion of apelin, an exerkine, which improves placental and fetal development, partially through enhancing oxidative metabolism [16, 23]. Recent studies further suggest that mitochondrial oxidative metabolism has key roles in mediating placental function and placentokine secretion [24–26]. In this review, we focus on studies examining the importance of placental oxidative function in mediating fetal development. In addition, the endocrine roles of placenta, via placentokines, in mediating placental and fetal development are also discussed.
2. Placenta as a key regulator of fetal development and maternal/fetal interaction
2.1. Placental structure and vascular system
The placenta is a unique organ mediating maternal and fetal interaction. The chorionic villi which are made of two cellular layers: the outer syncytiotrophoblast layer and the inner cytotrophoblast layer, are the basic functional units of the placenta [27]. In the maternal side, the uterine and ovarian arteries deliver blood through the arcuate arteries, radial arteries and spiral arteries to the intervillous space [27, 28]. In the fetal side, a syncytiotrophoblast layer separates maternal blood from the fetal components, which contain additional cytotrophoblasts, connective tissue from extraembryonic mesoderm, and fetal capillary endothelium [27, 28]. Nutrients and oxygen in maternal blood crosses syncytiotrophoblasts into the fetal side, and then delivered to the fetus through the umbilical vein [27]. Insufficient oxygen delivery due to placental functional deficiency exposes fetuses to hypoxic stress and nutrient deficiency, restricting fetal development [29, 30]. The nutrient uptake by placenta is mediated by nutrient transporters, including glucose transporters (GLUTs), amino acid transporters and fatty acid transporters (FATPs) [31]. Glucose is a critical nutrient for fetal development [32], and fetal glucose uptake is mainly mediated by GLUT1 [33], which is highly expressed in placenta [34]. GLUT1 level is increased due to insulin-dependent gestational diabetes mellitus (GDM) [35], whereas oxidative stress and SIRT1 activation down-regulate GLUT1 expression [36]. Hypoxic environment stimulates GLUT1 expression in placenta, which is mediated by hypoxia inducible factor-1a (HIF-1a) [37].
Successful pregnancy requires the proper development of placental vascular systems, which mediates gas and nutrient exchanges between the mother and her fetus [38]. The placental vascular system is developed through vasculogenesis and angiogenesis [39]. In the early stage of placental development, under the stimulus of vascular endothelial growth factor (VEGFA) primarily secreted by cytotrophoblasts, mesenchymal cells in the villous core are differentiated into hemangioblasts, which are then differentiated into endothelial cells (ECs) and hematopoietic cells (HSs). Then, these ECs organized into new vessels, referred as vasculogenesis [40]. Afterwards, the vascular system expands through angiogenesis [41, 42]. The major regulators of angiogenesis include placental growth factor and angiopoietins, in addition to oxygen tension as a key mediator [43].
2.2. Placenta as an endocrine organ
Beside nutrient and gas exchange, the placenta has a key endocrine function by producing various peptides and lipid hormones/cytokines [44]. Syncytiotrophoblasts secrete numerous placental hormones: human chorionic gonadotropin (hCG), progesterone, estrogen, placental lactogen and growth hormone, which are critical for maintaining pregnancy [45].
Human chorionic gonadotropin (hCG) is a heterodimeric protein with two linked subunits: α and β, which is an important hormone during early pregnancy, synthesized by syncytiotrophoblasts [46]. hCG binds to luteinizing hormone/choriogonadotropin receptor (LHCGR), a G protein-coupled receptor (GPCR), which activates Gs subunit and adenylyl cyclase (AC), concomitant with activation of protein kinase A (PKA) [47]. At the early stage of pregnancy, hCG promotes differentiation of cytotrophoblasts into syncytiotrophoblasts via stimulating PKA signaling [48]. hCG-induced endothelial cell migration promotes capillary formation in placenta [49] by stimulating placental VEGF signaling pathway [50] and microvascular endothelial cell proliferation to form the vascular system [51]; it also mediates maternal immunotolerance via stimulating the proliferation of uterine natural killer cells [52].
Leptin, a peptide hormone mainly secreted by adipose tissue, was introduced as a novel placenta-derived hormone, which regulates placental development and function through para/autocrine actions [53]. Placenta is one of the dominant organs secreting and releasing leptin into bloodstream [54]. Leptin has a pivotal role in mediating the feto-placental interface during the first trimester [55], which promotes trophoblast cell proliferation [56] and prevents apoptosis of trophoblast cells [57]. Moreover, leptin fundamentally acts as a modulator of immune system in the placenta [58], leading to enhancing innate and adaptive immunity in the placenta [59]. However, mechanisms stimulating leptin secretion in placenta remains to be defined.
Progesterone is a steroid hormone synthesized by oxidation of cholesterol inside mitochondria of syncytiotrophoblasts [60], and an endosome protein, metastatic lymph node 64 (MLN64), mediates cholesterol transferring into mitochondria [61]. In the mitochondrial inner membrane, the type 1 3beta-hydroxysteroid dehydrogenase (3β-HSD) isomerase, stimulated by p38 phosphorylation [62], converts pregnenolone into progesterone [63]. Progesterone is required for maintenance of pregnancy [64, 65].
Placental estrogens, made up of four steroid hormones: estrone (E1), 17β-estradiol (E2), estriol (E3) and estetrol (E4), are produced by corpus luteum during the first week of gestation [66], and these hormones are then secreted by the placenta [67]. The estrogens mediate several hormonal events during gestation via their nuclear estrogen receptors, ERα and β [45]; in addition, estrogens also activate a G-protein coupled receptor which mediate intracellular Ca2+ mobilization and PKA activation [68, 69]. ERα and β in the placenta are differentially activated: ERα is mainly expressed in the cytotrophoblasts, but ERβ in syncytiotrophoblasts [70]. As the most abundant estrogen, estradiol not only stimulates proliferation of mammary epithelial cells for preparation of breastfeeding [71], but also inhibits lipolysis and elevates fat storage [72]. In addition to estrogens, the placental lactogen (PL) and growth hormone (PGH) are placental polypeptide hormones, which are mainly synthesized within syncytiotrophoblasts [73, 74].
2.3. Importance of placental mitochondriogenesis in mediating its nutrient transportation and endocrine function
Placental functions, including substrate exchange and endocrine functions, as well as its development, require energy [75]. As multifunctional organelles, mitochondria produce ATP by oxidative phosphorylation [76]. Although ATP is produced during placental glycolysis, mitochondrial oxidative phosphorylation provides most ATP. As a result, pregnant women with mitochondrial dysfunction have high risks of pregnancy complications including pre-eclampsia, GDM and miscarriage [77]. Thus, maintaining mitochondrial homeostasis and oxidative function have profound impacts on placental function.
Mitochondrial adaptation in placenta occurs during pregnancy to support fetal development [78], which correlates with peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) activation in the labyrinth zone of placenta [79]. Consistently, markers of placental mitochondrial biogenesis, including the expression of PGC-1α and mitochondrial transcription factor A (TFAM), are up-regulated in response to maternal caloric restriction [80], suggesting maternal nutrient-dependent mitochondrial adaptation. In addition, a hypoxic environment leads to placental angiogenesis, which is partially mediated by mitochondrial oxidative stress and the production of mitochondrial reactive oxygen species [79, 81]. Placental angiogenesis improves the placental vascular system, which facilitates oxygen and nutrient delivery [82]. In short, the mitochondria-vascular system axis indirectly enhances the efficiency of nutrient delivery of placenta.
Besides nutrient transport efficiency, mitochondrial adaptation further regulates endocrine function of the placenta [45, 79]. Obesity and metabolic dysfunction during pregnancy increase risks of pregnancy complications such as pre-eclampsia and GDM [83], which might be associated with mitochondrial dysfunction of the placenta [84]. High fat diet-induced obesity during pregnancy decreases mitochondrial biogenesis [85, 86], which impairs placental function and fetal development [86]. Mitochondrial oxidative stress negatively affects secretion of endocrine hormones by placental syncytiotrophoblasts [87]. In summary, placental mitochondrial oxidation is important for placental endocrine function, which is negatively affected by poor environmental conditions, including malnutrition, hypoxia and obesity during gestation [88].
3. Maternal exercise, cytokines and their impacts on placental development and function.
3.1. Maternal exercise, obesity and other physiological changes
The beneficial effects of exercise during pregnancy are affected by duration and types of physical activities [85, 89–93]. Depending on the duration and intensity, exercise can be systematically separated into aerobic and anaerobic. During aerobic exercise, ATP is generated through oxidative phosphorylation within mitochondria. Anaerobic exercise, on the other hand, utilizes glycolysis to provide energy. Furthermore, regular aerobic exercise enhances cardiovascular function and muscle endurance capacity [94], while anaerobic exercise stimulates muscle hypertrophy and strength, which is more beneficial to the secretion of myokines [22]. Aerobic exercise with 40 to 60% of maximal oxygen consumption rates (VO2max) for 60 to 150 min/week is recommended for pregnant women [95, 96]. For anaerobic exercise, resistance exercise at moderate intensity is recommended for pregnant women [97–99]. In animal studies, voluntary wheel running (VWR) and forced treadmill exercise are commonly used for studying maternal exercise and fetal development [90–92], and the intensity of treadmill exercise during pregnancy is recommended at 40 to 65% VO2max [14, 16, 85, 93]. In human studies, both aerobic and anaerobic exercise training with intensity ranging from light to high have been tested in pregnant women in previous studies [97, 100].
Generally, exercise is beneficial for individuals with metabolic diseases such as obesity, type 2 diabetes mellitus and cardiovascular disorders [101]. Consistently, the recent meta-analysis of clinical trials showed that both aerobic and anaerobic exercises during pregnancy reduce the risk of gestational obesity and diabetes [20]. Furthermore, maternal exercise (ME) reduces risk factors of macrosomia and fetal metabolic dysfunction [102]. Furthermore, maternal aerobic exercise improves infant physical capacity, rendering them to be physically more active and thereby reducing risk of obesity in the early life [103]. Offspring born to exercised mothers have lower body mass index (BMI) compared to sedentary mothers [104, 105].
Beside human studies, similar beneficial effects of ME were observed in animal studies [16, 106]. ME reduces body weight gain and improves glucose tolerance in both mothers and their offspring [91, 92]. These benefits might be due to enhanced energy expenditure, because ME has long-term effects in improving brown fat thermogenesis and skeletal muscle oxidative function of offspring [14, 85]. Consistently, accumulating data suggest that maternal exercise pre- and/or during gestation is beneficial for metabolic health of female and male offspring, improving glucose tolerance in tissues including liver, muscle and pancreas [20, 107].
Obesity and type 2 diabetes mellitus are recognized as risk factors for placental dysfunction [108], which predisposes metabolic dysfunctions in offspring, even when offspring has a healthy lifestyle [91, 92]. Indeed, mothers with pre-gestational obesity have higher risks for developing GDM and metabolic complications, which impairs placental exchange functions [109, 110]. Consistently, obese mothers show less efficient placental function and fetal macrosomia, likely due to excessive fatty acid supply [16, 111, 112]. Obesity severely impedes placental vascularization, which impairs oxygen and nutrient delivery into the fetus [16, 113]. Consistent with the previous studies reporting the beneficial effects of ME on placental development [114, 115], we found that gestational exercise enhanced placental vascularization and activated nutrient transportation, protecting against the adverse effect of maternal obesity on fetal development [16].
3.2. Definitions of exerkines
Organs and tissues secrete their unique cytokines which are termed as myokines (secreted by muscle), adipokines (by adipose tissue) and placentokines (by placenta) [22, 116]. Their secretion and biological functions can be stimulated by external factors such as physical activity [117]. Those cytokines secreted in response to exercise are named as exerkines (exercise + cytokines) [118]. Thus, myokines, adipokines and placentokines can also be exerkines when their secretion is stimulated by physical activities (Fig. 1). For example, irisin is not only a myokine, but also an adipokine [119]; its secretion is further elevated by exercise, functioning as an exerkine [120]. Thus, irisin is a myokine, an adipokine and an exerkine depending on the contexts.
Figure 1.
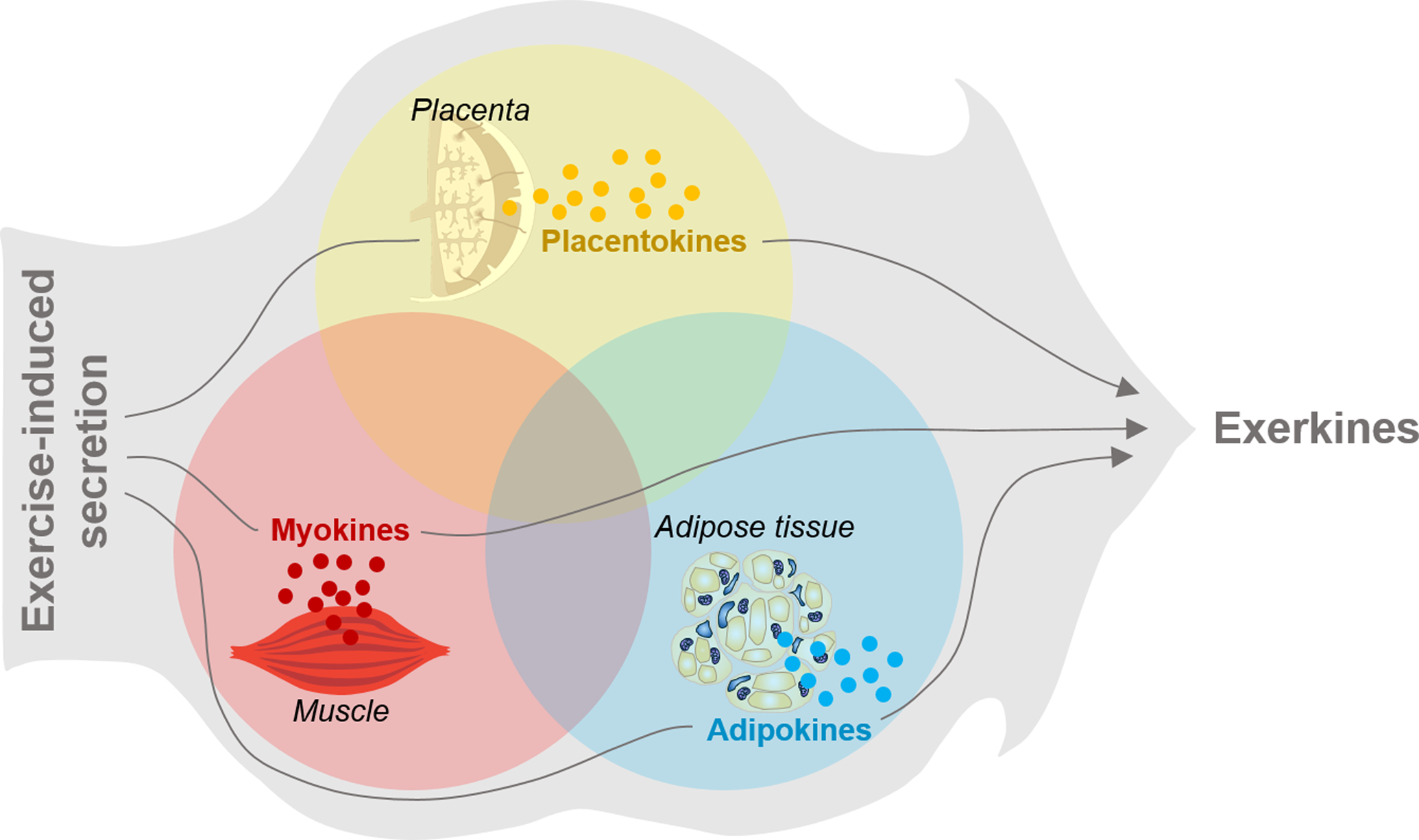
Diagram showing the definition of exerkines and organ/tissue secreted cytokines.
3.3. Exerkines regulating placental development
Maternal exercise is beneficial to placental development. In clinical trials, maternal aerobic exercise enhances placental functional and reduces the risk factors of metabolic dysfunction in pregnant women [121, 122]. Consistently, maternal strenuous exercise positively associates with placental amino acid metabolism and transport activity in a clinical study [123]. Simultaneously, maternally derived exerkines optimize placental development and function [23]. Here we reviewed key exerkines regulating placental development and function.
Apelin is a peptide hormone, which binds Gαi protein-coupled receptor, APJ [85, 124], activating AMP-activated kinase (AMPK) [125]. Exercise-induced apelin activation stimulates fatty acid oxidation and improves metabolic homeostasis [126–128]. Furthermore, apelin-APJ activation improves placental function [129, 130]. Maternal exercise stimulates apelin secretion by placenta, which enhances vascularization and nutrient delivery efficiency of placenta [16, 131]. However, the direct role of apelin in mediating the effects of maternal exercise on placental function remains to be examined.
Irisin is a cleaved form of fibronectin type III domain-containing 5 (FNDC5), which is up-regulated by the activation of PGC-1α [120]. Exercise activates PGC-1α, which stimulates irisin secretion. As a novel hormone, irisin enhances energy expenditure of skeletal muscle and adipose tissue, improving glucose homeostasis [132–134]. Interestingly, irisin also promotes trophoblast differentiation and placental development [135], and its level in circulation increases during gestation [135–137]. However, pregnancy complications such as preeclampsia and GDM are correlated with lower levels of circulatory irisin [136], suggesting that irisin may mediate placental function impaired due to obesity and GDM. Furthermore, irisin is highly secreted by ovary [138] and placenta themselves, suggesting its autocrine role for stimulating placental development [139].
Adiponectin, also known as AdipoQ, is an adipokine, with molecular weight at 30 kDa (ACRP30) [140]. Adiponectin is mainly secreted by white adipocytes [141]. Circulatory adiponectin level is negatively associated with obesity in both animals and humans [142, 143]. Exercise dramatically increases circulating adiponectin levels in patients with metabolic dysfunction, which might improve insulin sensitivity [144]. In addition, adiponectin is also secreted by placenta [145], which regulates cytotrophoblast differentiation [146] and potentially trophoblast invasion of the decidua [147]. Moreover, adiponectin administration during pregnancy reduces placental malfunction resulted from obesity [148] (Fig. 2).
Figure 2.
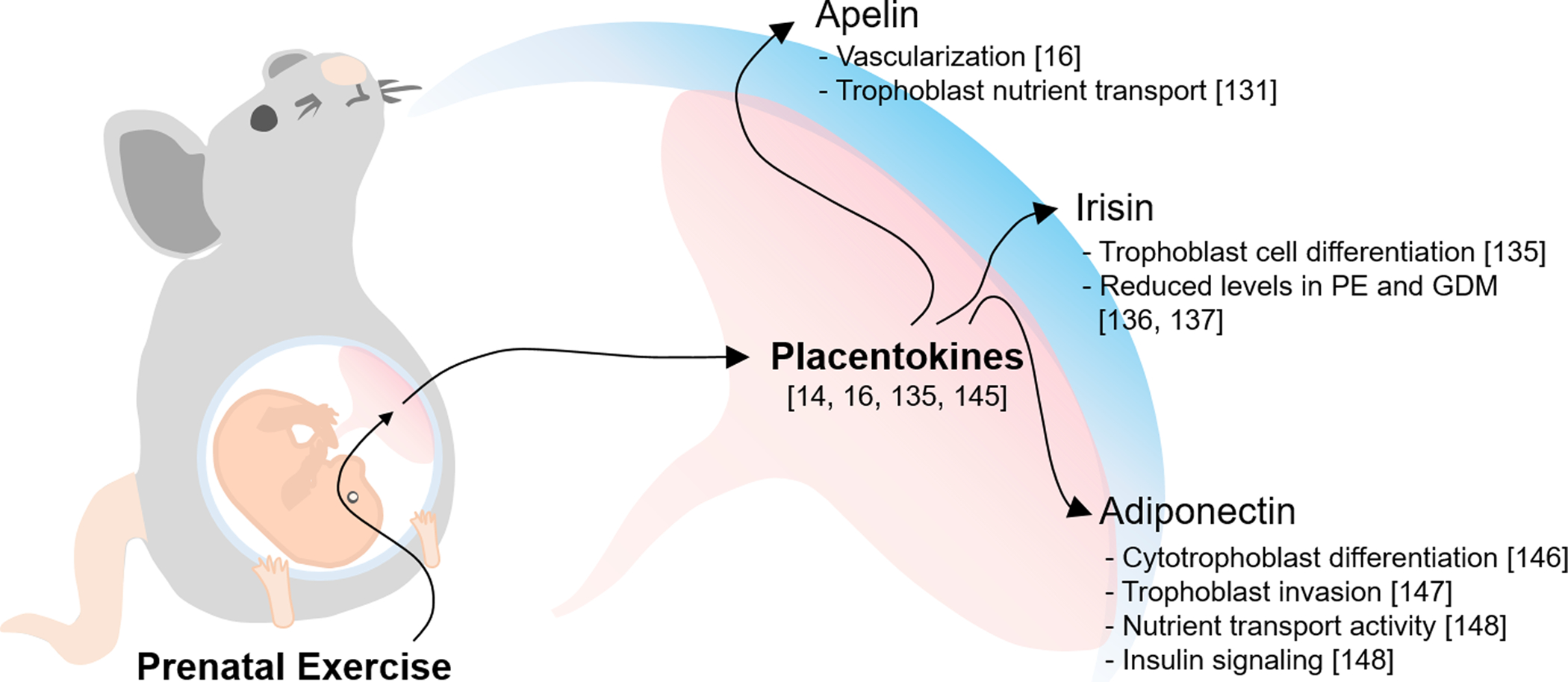
Exercise-induced placentokines regulating placental development. Exercise adaptation during pregnancy positively stimulates the secretion of placentokines including apelin, irisin and adiponectin.
3.4. Placentokines, fetal development and long-term effects on offspring
3.4.1. Maternal exercise-induced placentokines and fetal development
Placentokines enhance fetal development and exert long-term effects on offspring metabolic health [129, 130, 136], consistent with a recent study showing that ME increased placental weight which negatively associated with the risk of preterm birth [149]. Here, we briefly review three placentokines with potentials as therapeutic targets, including apelin, superoxide dismutase 3 (SOD3) and adiponectin, which improve fetal development.
Exercise during pregnancy elevates apelin levels in fetal circulation and stimulates placentokine apelin expression [14], which enhances mitochondrial biogenesis and the expression of PGC-1α, facilitating functional development in offspring muscle [85]. At the same time, apelin activates fetal brown adipogenesis, which persists in the BAT of offspring, improving their metabolic health when challenged by HFD [14, 85].
Besides, it was recently discovered that maternal exercise elevates serum and placental SOD3 in both mice and women [150]. Secreted SOD3 releases into the fetal liver and activates AMPK/isocitrate dehydrogenase (IDH)/α-Ketoglutarate (α-KG)/ten-eleven translocation (TET) axis [151], which results in hypomethylation of promoter regions of glucose metabolic genes, thereby leading to improvement of offspring metabolic homeostasis [150].
Consistent with the role of SOD3 as a mediator, adiponectin administration during pregnancy regulates fetal growth and glucose homeostasis, and protects offspring against adverse effects due to maternal obesity [148]. Similarly, irisin is activated by exercise, which improves metabolic health of women [152], and also reduces fetal growth abnormalities [153]. Furthermore, FNDC5 genetic polymorphisms in mothers are related with preterm birth [154], which are associated with elevation of infant mortality and morbidity [155]. Consistently, small for gestational age infants have lower serum irisin levels compared to counterparts [156].
In summary, current knowledge about placentokines stimulated by maternal exercise remains very limited. Additional placentokines, together with mechanisms linking placentokines to placental and fetal development, need to be further explored.
3.4.2. Maternal exercise and long-term improvement of metabolic health in progeny.
ME is effective in preventing metabolic dysfunction of offspring born to obese mothers [91]. Notably, middle-aged offspring born to mothers fed HFD during pregnancy show impaired glucose tolerance [91, 92]. The expression of PGC-1α, a key regulator of mitochondrial biogenesis, was down-regulated in offspring muscle due to maternal HFD intake, which was prevented by ME [90]. In addition, ME further enhances mitochondrial biogenesis in offspring skeletal muscle by persistent activation of apelin-APJ axis, which is associated with hypomethylation in the promoter of Ppargc1a gene in offspring muscle [85]. Up to now, studies on the long-term effects of ME on offspring metabolic health remain very limited and most available studies were conducted in animals; clinical studies are needed (Fig. 3).
Figure 3.
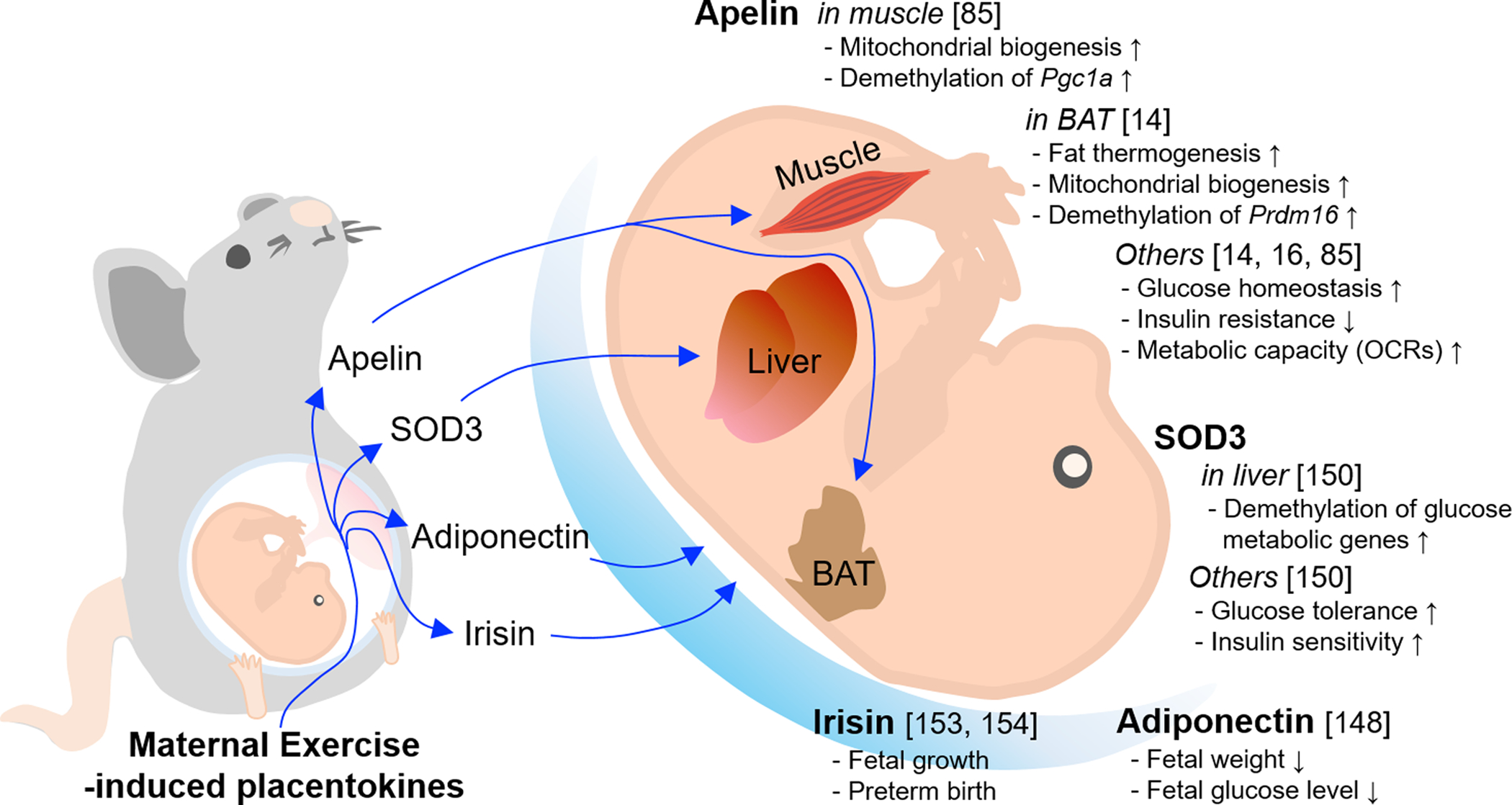
The effects of maternal exercise-derived placentokines on fetal development and its long-term effects on the offspring.
4. Placental oxidative metabolism in linking maternal exerkines, placentokines and placental function
Epigenetic modifications regulate gene expression [157]. DNA methylation and demethylation are key mechanisms regulating gene expression [158]. We recently found that ME activates AMPK and induces apelin secretion in placenta, which then releases into the fetal circulation to stimulate fetal brown fat and skeletal muscle development [14, 85]. Furthermore, we found that α-ketoglutarate (α-KG), a key mediator of the TCA cycle, mediates DNA demethylation the Prdm16 promoter via facilitating ten-eleven translocation (TET)-mediated DNA demethylation, which enhances fetal brown fat development [14, 85, 151]. Thus, activation of mitochondrial oxidative capacity not only provides energy for maintaining placental function, but may also facilitate epigenetic modifications in genes needed for placental vasculogenesis and nutrient delivery [16]; in addition, placenta secretes placentokines to regulate fetal development [14, 159]. Supportively, another possible placentokine, irisin, regulates the differentiation of placental trophoblast cells primarily via activating AMPK [135], and cord irisin levels were decreased in newborns of small for gestational age [153]. Up to now, potential roles of placentokines linking ME to placental and fetal development remain poorly defined.
Besides, fuel consumption of the placenta is essential for placental development and maintenance of its physiological functions, which is supported by oxidative phosphorylation in placental mitochondria [160]. Dysfunction of placental respiratory capacity caused by preeclampsia and GDM, indeed, can severely impair placental structure and function [160]. Although exercise improves mitochondrial adaptation and oxidative phosphorylation in different tissues such as skeletal muscle and adipose tissue [161, 162], the evidence supporting exercise in improving placental oxidative metabolism and fetal development remains weak, and more studies, especially, human studies are needed (Fig. 4).
Figure 4.
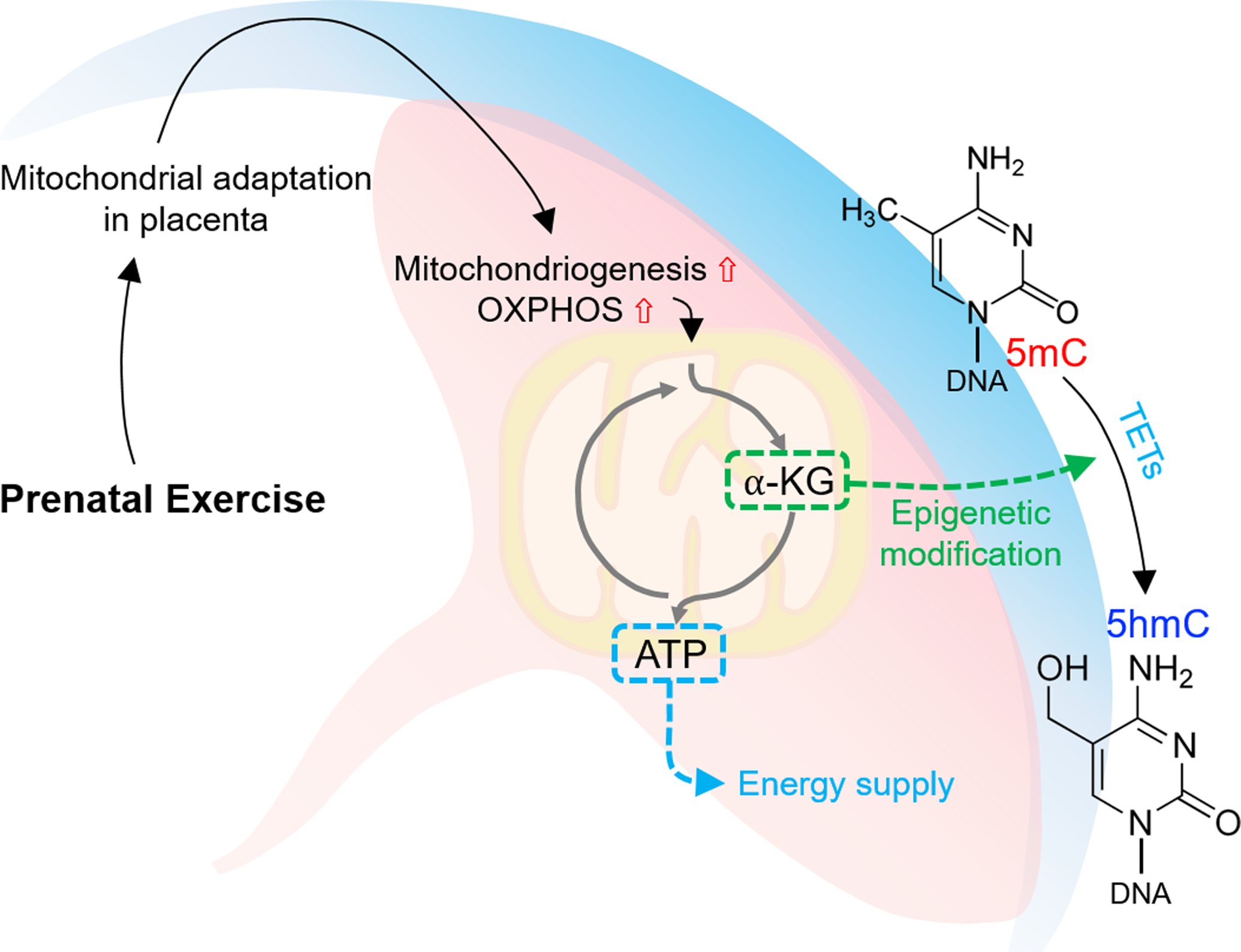
Potential mechanisms regulating epigenetic modifications of developmental genes in placenta in response to exercise during pregnancy. Prenatal exercise-dependently induced placental mitochondrial adaptation via providing α-ketoglutarate (α-KG), which facilitates TETs-mediated DNA demethylation [conversion of 5-methylcytosine (5-mC) to 5-hydroxy-methylcytosine (5-hmC)]).
5. Conclusion and future perspectives
The importance of intrauterine condition on fetal development has been well established [163, 164]. Placenta, the only tissue linking the mother to her fetus, is critical for proper fetal development [28]. As potential mediators for placental development, ME stimulates the secretion of exerkines facilitating placental development. Placental oxidative metabolism is critical for maintaining placental function, and ME enhances placental mitochondrial biogenesis and vascularization, which is partially mediated by placentokines. Placentokines in response to exercise during pregnancy regulate placental development and function, as well as fetal development, which may generate long-term effects on offspring metabolic health. However, additional placentokines need to be further identified and the types of placental cells secreting placentokines need to be further examined. Understanding molecular mechanisms linking exercise-induced placentokines to placental function and fetal development, as well as long-term metabolic health of offspring will help to identify therapeutic targets for improving both maternal and offspring health.
Acknowledgements
This work was supported by the National Institutes of Health (NIH R01-HD067449).
Abbreviations
- AC
Adenylyl cyclase
- ACRP30
adipokine with molecular weight at 30 kDa
- AMPK
AMP-activated kinase
- 3β-HSD
3beta-hydroxysteroid dehydrogenase
- BMI
body mass index
- E1
estrone
- E2
17β-estradiol
- E3
estriol
- E4
estetrol
- FNDC5
fibronectin type III domain-containing 5
- GDM
gestational diabetes mellitus
- GLUTs
glucose transporters
- GPCR
G protein-coupled receptor
- hCG
human chorionic gonadotropin
- HIF-1a
hypoxia inducible factor-1a
- LHCGR
luteinizing hormone/choriogonadotropin receptor
- ME
maternal exercise
- MO
maternal obesity
- VO2max
maximal oxygen consumption rates
- MLN64
metastatic lymph node 64
- SOD3
superoxide dismutase 3
- TFAM
mitochondrial transcription factor A
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator-1α
- PL
placental lactogen
- PGH
placental growth hormone
- PKA
protein kinase A
- VEGFA
vascular endothelial growth factor
- VWR
voluntary wheel running
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Chen C, Xu X & Yan Y (2018) Estimated global overweight and obesity burden in pregnant women based on panel data model, PloS one. 13, e0202183–e0202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallis AB, Saftlas AF, Hsia J & Atrash HK (2008) Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004, Am J Hypertens. 21, 521–526. [DOI] [PubMed] [Google Scholar]
- 3.Abalos E, Cuesta C, Grosso AL, Chou D & Say L (2013) Global and regional estimates of preeclampsia and eclampsia: a systematic review, Eur J Obstet Gynecol Reprod Biol. 170, 1–7. [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM & Shankar K (2017) Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child, BMJ (Clinical research ed). 356, j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, Pemu A & Rankin J (2019) The association between maternal body mass index and child obesity: A systematic review and meta-analysis, PLoS medicine. 16, e1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prentice PM, Olga L, Petry CJ, Simmons D, Murphy HR, Hughes IA, Acerini CL, Ong KK & Dunger DB (2019) Reduced size at birth and persisting reductions in adiposity in recent, compared with earlier, cohorts of infants born to mothers with gestational diabetes mellitus, Diabetologia. 62, 1977–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R & Law CM (2003) Is birth weight related to later glucose and insulin metabolism?--A systematic review, Diabetic medicine : a journal of the British Diabetic Association. 20, 339–48. [DOI] [PubMed] [Google Scholar]
- 8.Veening MA, van Weissenbruch MM, Heine RJ & Delemarre-van de Waal HA (2003) Beta-cell capacity and insulin sensitivity in prepubertal children born small for gestational age: influence of body size during childhood, Diabetes. 52, 1756–60. [DOI] [PubMed] [Google Scholar]
- 9.Ozanne SE & Hales CN (1999) The long-term consequences of intra-uterine protein malnutrition for glucose metabolism, The Proceedings of the Nutrition Society. 58, 615–9. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H & Flier JS (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance, The Journal of clinical investigation. 116, 3015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scifres CM, Chen B, Nelson DM & Sadovsky Y (2011) Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts, The Journal of clinical endocrinology and metabolism. 96, E1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du M, Yan X, Tong JF, Zhao J & Zhu MJ (2010) Maternal obesity, inflammation, and fetal skeletal muscle development, Biology of reproduction. 82, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Symonds ME, Pope M, Sharkey D & Budge H (2012) Adipose tissue and fetal programming, Diabetologia. 55, 1597–606. [DOI] [PubMed] [Google Scholar]
- 14.Son JS, Zhao L, Chen Y, Chen K, Chae SA, de Avila JM, Wang H, Zhu MJ, Jiang Z & Du M (2020) Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice, Science advances. 6, eaaz0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad KP & Benyo DF (1997) Placental cytokines and the pathogenesis of preeclampsia, American journal of reproductive immunology : AJRI : official journal of the American Society for the Immunology of Reproduction and the International Coordination Committee for Immunology of Reproduction. 37, 240–9. [DOI] [PubMed] [Google Scholar]
- 16.Son JS, Liu X, Tian Q, Zhao L, Chen Y, Hu Y, Chae SA, de Avila JM, Zhu MJ & Du M (2019) Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth, The Journal of physiology. 597, 3333–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang LJ, Xie Q, Tang CS & Zhang AH (2017) Expressions of irisin and urotensin II and their relationships with blood pressure in patients with preeclampsia, Clinical and experimental hypertension (New York, NY : 1993). 39, 460–467. [DOI] [PubMed] [Google Scholar]
- 18.Nogues P, Dos Santos E, Jammes H, Berveiller P, Arnould L, Vialard F & Dieudonné MN (2019) Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta, Clinical epigenetics. 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandò C, Anelli GM, Novielli C, Panina-Bordignon P, Massari M, Mazzocco MI & Cetin I (2018) Impact of Obesity and Hyperglycemia on Placental Mitochondria, Oxid Med Cell Longev. 2018, 2378189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusuyama J, Alves-Wagner AB, Makarewicz NS & Goodyear LJ (2020) Effects of maternal and paternal exercise on offspring metabolism, Nat Metab. 2, 858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin M & Jacobs I (2017) Exercise Is Medicine, But Does It Interfere With Medicine?, Exerc Sport Sci Rev. 45, 127–135. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen BK & Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ, Nature reviews Endocrinology. 8, 457–65. [DOI] [PubMed] [Google Scholar]
- 23.Dube C, Aguer C, Adamo K & Bainbridge S (2017) A role for maternally derived myokines to optimize placental function and fetal growth across gestation, Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 42, 459–469. [DOI] [PubMed] [Google Scholar]
- 24.Kusuyama J, Alves-Wagner AB, Conlin RH, Makarewicz NS, Albertson BG, Prince NB, Kobayashi S, Kozuka C, Møller M, Bjerre M, Fuglsang J, Miele E, Middelbeek RJW, Xiudong Y, Xia Y, Garneau L, Bhattacharjee J, Aguer C, Patti ME, Hirshman MF, Jessen N, Hatta T, Ovesen PG, Adamo KB, Nozik-Grayck E & Goodyear LJ (2021) Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health, Cell metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myatt L & Maloyan A (2016) Obesity and Placental Function, Seminars in reproductive medicine. 34, 42–9. [DOI] [PubMed] [Google Scholar]
- 26.Aye I, Aiken CE, Charnock-Jones DS & Smith GCS (2020) Placental energy metabolism in health and disease-significance of development and implications for preeclampsia, American journal of obstetrics and gynecology. [DOI] [PubMed] [Google Scholar]
- 27.Lu M & Sferruzzi-Perri AN (2020) Placental mitochondrial function in response to gestational exposures, Placenta. 104, 124–137. [DOI] [PubMed] [Google Scholar]
- 28.Guttmacher AE, Maddox YT & Spong CY (2014) The Human Placenta Project: placental structure, development, and function in real time, Placenta. 35, 303–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun C, Groom KM, Oyston C, Chamley LW, Clark AR & James JL (2020) The placenta in fetal growth restriction: What is going wrong?, Placenta. 96, 10–18. [DOI] [PubMed] [Google Scholar]
- 30.Meher S, Hernandez-Andrade E, Basheer SN & Lees C (2015) Impact of cerebral redistribution on neurodevelopmental outcome in small-for-gestational-age or growth-restricted babies: a systematic review, Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 46, 398–404. [DOI] [PubMed] [Google Scholar]
- 31.Lager S & Powell TL (2012) Regulation of nutrient transport across the placenta, Journal of pregnancy. 2012, 179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hay WW Jr. (2006) Placental-fetal glucose exchange and fetal glucose metabolism, Transactions of the American Clinical and Climatological Association. 117, 321–39; discussion 339–40. [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann MU, Deborde S & Illsley NP (2002) Placental glucose transfer and fetal growth, Endocrine. 19, 13–22. [DOI] [PubMed] [Google Scholar]
- 34.Ericsson A, Hamark B, Powell TL & Jansson T (2005) Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta, Hum Reprod. 20, 521–30. [DOI] [PubMed] [Google Scholar]
- 35.Jansson T, Wennergren M & Powell TL (1999) Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes, American journal of obstetrics and gynecology. 180, 163–8. [DOI] [PubMed] [Google Scholar]
- 36.Lappas M, Andrikopoulos S & Permezel M (2012) Hypoxanthine-xanthine oxidase down-regulates GLUT1 transcription via SIRT1 resulting in decreased glucose uptake in human placenta, The Journal of endocrinology. 213, 49–57. [DOI] [PubMed] [Google Scholar]
- 37.Baumann MU, Zamudio S & Illsley NP (2007) Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1, American journal of physiology Cell physiology. 293, C477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gude NM, Roberts CT, Kalionis B & King RG (2004) Growth and function of the normal human placenta, Thromb Res. 114, 397–407. [DOI] [PubMed] [Google Scholar]
- 39.Jauniaux E, Hempstock J, Greenwold N & Burton GJ (2003) Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies, The American journal of pathology. 162, 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A & Jaffe RB (2002) Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2, The Journal of clinical endocrinology and metabolism. 87, 4213–24. [DOI] [PubMed] [Google Scholar]
- 41.Depoix CL, Colson A, Hubinont C & Debieve F (2020) Impaired vascular endothelial growth factor expression and secretion during in vitro differentiation of human primary term cytotrophoblasts, Angiogenesis. 23, 221–230. [DOI] [PubMed] [Google Scholar]
- 42.Pavlov N, Frendo JL, Guibourdenche J, Degrelle SA, Evain-Brion D & Badet J (2014) Angiogenin expression during early human placental development; association with blood vessel formation, BioMed research international. 2014, 781632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burton GJ, Charnock-Jones DS & Jauniaux E (2009) Regulation of vascular growth and function in the human placenta, Reproduction. 138, 895–902. [DOI] [PubMed] [Google Scholar]
- 44.Jones CT (1989) Endocrine function of the placenta, Bailliere’s clinical endocrinology and metabolism. 3, 755–80. [DOI] [PubMed] [Google Scholar]
- 45.Costa MA (2016) The endocrine function of human placenta: an overview, Reprod Biomed Online. 32, 14–43. [DOI] [PubMed] [Google Scholar]
- 46.Cole LA (1997) Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites, Clinical chemistry. 43, 2233–43. [PubMed] [Google Scholar]
- 47.Choi J & Smitz J (2014) Luteinizing hormone and human chorionic gonadotropin: origins of difference, Mol Cell Endocrinol. 383, 203–13. [DOI] [PubMed] [Google Scholar]
- 48.Pidoux G, Gerbaud P, Tsatsaris V, Marpeau O, Ferreira F, Meduri G, Guibourdenche J, Badet J, Evain-Brion D & Frendo JL (2007) Biochemical characterization and modulation of LH/CG-receptor during human trophoblast differentiation, Journal of cellular physiology. 212, 26–35. [DOI] [PubMed] [Google Scholar]
- 49.Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, Rao CV, Lang U & Preissner KT (2002) Characterization of human chorionic gonadotropin as a novel angiogenic factor, The Journal of clinical endocrinology and metabolism. 87, 5290–6. [DOI] [PubMed] [Google Scholar]
- 50.Islami D, Bischof P & Chardonnens D (2003) Modulation of placental vascular endothelial growth factor by leptin and hCG, Molecular human reproduction. 9, 395–8. [DOI] [PubMed] [Google Scholar]
- 51.Herr F, Baal N, Reisinger K, Lorenz A, McKinnon T, Preissner KT & Zygmunt M (2007) HCG in the regulation of placental angiogenesis. Results of an in vitro study, Placenta. 28 Suppl A, S85–93. [DOI] [PubMed] [Google Scholar]
- 52.Kane N, Kelly R, Saunders PT & Critchley HO (2009) Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor, Endocrinology. 150, 2882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T & Nakao K (1997) Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans, Nature medicine. 3, 1029–33. [DOI] [PubMed] [Google Scholar]
- 54.Lin KC (1999) Increase of maternal plasma leptin concentrations during pregnancy: comparison with nonpregnant women, The Kaohsiung journal of medical sciences. 15, 640–5. [PubMed] [Google Scholar]
- 55.Pérez-Pérez A, Toro A, Vilariño-García T, Maymó J, Guadix P, Dueñas JL, Fernández-Sánchez M, Varone C & Sánchez-Margalet V (2018) Leptin action in normal and pathological pregnancies, Journal of cellular and molecular medicine. 22, 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magariños MP, Sánchez-Margalet V, Kotler M, Calvo JC & Varone CL (2007) Leptin promotes cell proliferation and survival of trophoblastic cells, Biology of reproduction. 76, 203–10. [DOI] [PubMed] [Google Scholar]
- 57.Pérez-Pérez A, Maymó J, Dueñas JL, Goberna R, Calvo JC, Varone C & Sánchez-Margalet V (2008) Leptin prevents apoptosis of trophoblastic cells by activation of MAPK pathway, Archives of biochemistry and biophysics. 477, 390–5. [DOI] [PubMed] [Google Scholar]
- 58.Lappas M, Yee K, Permezel M & Rice GE (2005) Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies, The Journal of endocrinology. 186, 457–65. [DOI] [PubMed] [Google Scholar]
- 59.Pérez-Pérez A, Vilariño-García T, Fernández-Riejos P, Martín-González J, Segura-Egea JJ & Sánchez-Margalet V (2017) Role of leptin as a link between metabolism and the immune system, Cytokine & growth factor reviews. 35, 71–84. [DOI] [PubMed] [Google Scholar]
- 60.Sugawara T, Holt JA, Driscoll D, Strauss JF 3rd, Lin D, Miller WL, Patterson D, Clancy KP, Hart IM, Clark BJ & et al. (1995) Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13, Proc Natl Acad Sci U S A. 92, 4778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL, Rio MC, Baker ME & Strauss JF 3rd (1997) MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis, Proc Natl Acad Sci U S A. 94, 8462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa MA, Fonseca BM, Mendes A, Braga J, Teixeira NA & Correia-da-Silva G (2016) The endocannabinoid 2-arachidonoylglycerol dysregulates the synthesis of proteins by the human syncytiotrophoblast, Biochim Biophys Acta. 1861, 205–12. [DOI] [PubMed] [Google Scholar]
- 63.Tuckey RC (2005) Progesterone synthesis by the human placenta, Placenta. 26, 273–81. [DOI] [PubMed] [Google Scholar]
- 64.Brar AK, Frank GR, Kessler CA, Cedars MI & Handwerger S (1997) Progesterone-dependent decidualization of the human endometrium is mediated by cAMP, Endocrine. 6, 301–7. [DOI] [PubMed] [Google Scholar]
- 65.Halasz M & Szekeres-Bartho J (2013) The role of progesterone in implantation and trophoblast invasion, Journal of reproductive immunology. 97, 43–50. [DOI] [PubMed] [Google Scholar]
- 66.Loriaux DL, Ruder HJ, Knab DR & Lipsett MB (1972) Estrone sulfate, estrone, estradiol and estriol plasma levels in human pregnancy, The Journal of clinical endocrinology and metabolism. 35, 887–91. [DOI] [PubMed] [Google Scholar]
- 67.Napso T, Yong HEJ, Lopez-Tello J & Sferruzzi-Perri AN (2018) The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation, Frontiers in physiology. 9, 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagrange AH, Ronnekleiv OK & Kelly MJ (1997) Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A, Mol Pharmacol. 51, 605–12. [DOI] [PubMed] [Google Scholar]
- 69.Conde K, Meza C, Kelly MJ, Sinchak K & Wagner EJ (2016) Estradiol Rapidly Attenuates ORL-1 Receptor-Mediated Inhibition of Proopiomelanocortin Neurons via Gq-Coupled, Membrane-Initiated Signaling, Neuroendocrinology. 103, 787–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bechi N, Ietta F, Romagnoli R, Focardi S, Corsi I, Buffi C & Paulesu L (2006) Estrogen-like response to p-nonylphenol in human first trimester placenta and BeWo choriocarcinoma cells, Toxicological sciences : an official journal of the Society of Toxicology. 93, 75–81. [DOI] [PubMed] [Google Scholar]
- 71.Pang WW & Hartmann PE (2007) Initiation of human lactation: secretory differentiation and secretory activation, Journal of mammary gland biology and neoplasia. 12, 211–21. [DOI] [PubMed] [Google Scholar]
- 72.Butte NF (2000) Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus, The American journal of clinical nutrition. 71, 1256s–61s. [DOI] [PubMed] [Google Scholar]
- 73.Kliman HJ, Nestler JE, Sermasi E, Sanger JM & Strauss JF 3rd (1986) Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae, Endocrinology. 118, 1567–82. [DOI] [PubMed] [Google Scholar]
- 74.Lacroix MC, Guibourdenche J, Fournier T, Laurendeau I, Igout A, Goffin V, Pantel J, Tsatsaris V & Evain-Brion D (2005) Stimulation of human trophoblast invasion by placental growth hormone, Endocrinology. 146, 2434–44. [DOI] [PubMed] [Google Scholar]
- 75.Desforges M & Sibley CP (2010) Placental nutrient supply and fetal growth, The International journal of developmental biology. 54, 377–90. [DOI] [PubMed] [Google Scholar]
- 76.Ermak G & Davies KJ (2002) Calcium and oxidative stress: from cell signaling to cell death, Molecular immunology. 38, 713–21. [DOI] [PubMed] [Google Scholar]
- 77.Karaa A, Elsharkawi I, Clapp MA & Balcells C (2019) Effects of mitochondrial disease/dysfunction on pregnancy: A retrospective study, Mitochondrion. 46, 214–220. [DOI] [PubMed] [Google Scholar]
- 78.Holland OJ, Hickey AJR, Alvsaker A, Moran S, Hedges C, Chamley LW & Perkins AV (2017) Changes in mitochondrial respiration in the human placenta over gestation, Placenta. 57, 102–112. [DOI] [PubMed] [Google Scholar]
- 79.Sferruzzi-Perri AN, Higgins JS, Vaughan OR, Murray AJ & Fowden AL (2019) Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth, Proceedings of the National Academy of Sciences of the United States of America. 116, 1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayeur S, Lancel S, Theys N, Lukaszewski MA, Duban-Deweer S, Bastide B, Hachani J, Cecchelli R, Breton C, Gabory A, Storme L, Reusens B, Junien C, Vieau D & Lesage J (2013) Maternal calorie restriction modulates placental mitochondrial biogenesis and bioenergetic efficiency: putative involvement in fetoplacental growth defects in rats, American journal of physiology Endocrinology and metabolism. 304, E14–22. [DOI] [PubMed] [Google Scholar]
- 81.Reichard A & Asosingh K (2019) The role of mitochondria in angiogenesis, Molecular biology reports. 46, 1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zygmunt M, Herr F, Münstedt K, Lang U & Liang OD (2003) Angiogenesis and vasculogenesis in pregnancy, Eur J Obstet Gynecol Reprod Biol. 110 Suppl 1, S10–8. [DOI] [PubMed] [Google Scholar]
- 83.Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S & Gillman MW (2016) Preconceptional and maternal obesity: epidemiology and health consequences, The lancet Diabetes & endocrinology. 4, 1025–1036. [DOI] [PubMed] [Google Scholar]
- 84.Mandò C, De Palma C, Stampalija T, Anelli GM, Figus M, Novielli C, Parisi F, Clementi E, Ferrazzi E & Cetin I (2014) Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia, American journal of physiology Endocrinology and metabolism. 306, E404–13. [DOI] [PubMed] [Google Scholar]
- 85.Son JS, Chae SA, Wang H, Chen Y, Bravo Iniguez A, de Avila JM, Jiang Z, Zhu MJ & Du M (2020) Maternal Inactivity Programs Skeletal Muscle Dysfunction in Offspring Mice by Attenuating Apelin Signaling and Mitochondrial Biogenesis, Cell reports. 33, 108461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borengasser SJ, Faske J, Kang P, Blackburn ML, Badger TM & Shankar K (2014) In utero exposure to prepregnancy maternal obesity and postweaning high-fat diet impair regulators of mitochondrial dynamics in rat placenta and offspring, Physiological genomics. 46, 841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker OS, Ragos R, Wong MK, Adam M, Cheung A & Raha S (2020) Reactive oxygen species from mitochondria impacts trophoblast fusion and the production of endocrine hormones by syncytiotrophoblasts, PloS one. 15, e0229332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu M & Sferruzzi-Perri AN (2021) Placental mitochondrial function in response to gestational exposures, Placenta. 104, 124–137. [DOI] [PubMed] [Google Scholar]
- 89.Carter LG, Lewis KN, Wilkerson DC, Tobia CM, Ngo Tenlep SY, Shridas P, Garcia-Cazarin ML, Wolff G, Andrade FH, Charnigo RJ, Esser KA, Egan JM, de Cabo R & Pearson KJ (2012) Perinatal exercise improves glucose homeostasis in adult offspring, American journal of physiology Endocrinology and metabolism. 303, E1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laker RC, Lillard TS, Okutsu M, Zhang M, Hoehn KL, Connelly JJ & Yan Z (2014) Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1alpha gene and age-dependent metabolic dysfunction in the offspring, Diabetes. 63, 1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stanford KI, Lee MY, Getchell KM, So K, Hirshman MF & Goodyear LJ (2015) Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring, Diabetes. 64, 427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stanford KI, Takahashi H, So K, Alves-Wagner AB, Prince NB, Lehnig AC, Getchell KM, Lee MY, Hirshman MF & Goodyear LJ (2017) Maternal Exercise Improves Glucose Tolerance in Female Offspring, Diabetes. 66, 2124–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chae SA, Son JS, Zhu M-J, de Avila JM & Du M (2020) Treadmill Running of Mouse as a Model for Studying Influence of Maternal Exercise on Offspring, Bio-protocol. 10, e3838–e3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mann S, Beedie C & Jimenez A (2014) Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations, Sports medicine (Auckland, NZ). 44, 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zavorsky GS & Longo LD (2011) Exercise guidelines in pregnancy: new perspectives, Sports Med. 41, 345–60. [DOI] [PubMed] [Google Scholar]
- 96.Savvaki D, Taousani E, Goulis DG, Tsirou E, Voziki E, Douda H, Nikolettos N & Tokmakidis SP (2018) Guidelines for exercise during normal pregnancy and gestational diabetes: a review of international recommendations, Hormones (Athens, Greece). 17, 521–529. [DOI] [PubMed] [Google Scholar]
- 97.Davenport MH, Ruchat SM, Poitras VJ, Jaramillo Garcia A, Gray CE, Barrowman N, Skow RJ, Meah VL, Riske L, Sobierajski F, James M, Kathol AJ, Nuspl M, Marchand AA, Nagpal TS, Slater LG, Weeks A, Adamo KB, Davies GA, Barakat R & Mottola MF (2018) Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis, British journal of sports medicine. 52, 1367–1375. [DOI] [PubMed] [Google Scholar]
- 98.Ming WK, Ding W, Zhang CJP, Zhong L, Long Y, Li Z, Sun C, Wu Y, Chen H, Chen H & Wang Z (2018) The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis, BMC pregnancy and childbirth. 18, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Wen D, Liu X & Liu Y (2019) Impact of exercise on maternal gestational weight gain: An updated meta-analysis of randomized controlled trials, Medicine. 98, e16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beetham KS, Giles C, Noetel M, Clifton V, Jones JC & Naughton G (2019) The effects of vigorous intensity exercise in the third trimester of pregnancy: a systematic review and meta-analysis, BMC pregnancy and childbirth. 19, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schellenberg ES, Dryden DM, Vandermeer B, Ha C & Korownyk C (2013) Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis, Ann Intern Med. 159, 543–51. [DOI] [PubMed] [Google Scholar]
- 102.Moyer C, Reoyo OR & May L (2016) The Influence of Prenatal Exercise on Offspring Health: A Review, Clin Med Insights Womens Health. 9, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McMillan AG, May LE, Gaines GG, Isler C & Kuehn D (2019) Effects of Aerobic Exercise during Pregnancy on 1-Month Infant Neuromotor Skills, Medicine and science in sports and exercise. 51, 1671–1676. [DOI] [PubMed] [Google Scholar]
- 104.Mourtakos SP, Tambalis KD, Panagiotakos DB, Antonogeorgos G, Arnaoutis G, Karteroliotis K & Sidossis LS (2015) Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: a study of 5,125 children, BMC pregnancy and childbirth. 15, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Y, Ma G, Hu Y, Yang Q, Deavila JM, Zhu MJ & Du M (2021) Effects of Maternal Exercise During Pregnancy on Perinatal Growth and Childhood Obesity Outcomes: A Meta-analysis and Meta-regression, Sports medicine (Auckland, NZ). [DOI] [PubMed] [Google Scholar]
- 106.Lahti-Pulkkinen M, Bhattacharya S, Wild SH, Lindsay RS, Räikkönen K, Norman JE, Bhattacharya S & Reynolds RM (2019) Consequences of being overweight or obese during pregnancy on diabetes in the offspring: a record linkage study in Aberdeen, Scotland, Diabetologia. 62, 1412–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harris JE, Baer LA & Stanford KI (2018) Maternal Exercise Improves the Metabolic Health of Adult Offspring, Trends in endocrinology and metabolism: TEM. 29, 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bedell S, Hutson J, de Vrijer B & Eastabrook G (2021) Effects of Maternal Obesity and Gestational Diabetes Mellitus on the Placenta: Current Knowledge and Targets for Therapeutic Interventions, Current vascular pharmacology. 19, 176–192. [DOI] [PubMed] [Google Scholar]
- 109.Bianchi C, Taricco E, Cardellicchio M, Mandò C, Massari M, Savasi V & Cetin I (2020) The role of obesity and gestational diabetes on placental size and fetal oxygenation, Placenta. 103, 59–63. [DOI] [PubMed] [Google Scholar]
- 110.Scifres CM, Parks WT, Feghali M, Caritis SN & Catov JM (2017) Placental maternal vascular malperfusion and adverse pregnancy outcomes in gestational diabetes mellitus, Placenta. 49, 10–15. [DOI] [PubMed] [Google Scholar]
- 111.Ouyang F, Parker MG, Luo ZC, Wang X, Zhang HJ, Jiang F, Wang X, Gillman MW & Zhang J (2016) Maternal BMI, gestational diabetes, and weight gain in relation to childhood obesity: The mediation effect of placental weight, Obesity (Silver Spring, Md). 24, 938–46. [DOI] [PubMed] [Google Scholar]
- 112.Fattuoni C, Mandò C, Palmas F, Anelli GM, Novielli C, Parejo Laudicina E, Savasi VM, Barberini L, Dessì A, Pintus R, Fanos V, Noto A & Cetin I (2018) Preliminary metabolomics analysis of placenta in maternal obesity, Placenta. 61, 89–95. [DOI] [PubMed] [Google Scholar]
- 113.Hu C, Yang Y, Li J, Wang H, Cheng C, Yang L, Li Q, Deng J, Liang Z, Yin Y, Xie Z & Tan C (2019) Maternal Diet-Induced Obesity Compromises Oxidative Stress Status and Angiogenesis in the Porcine Placenta by Upregulating Nox2 Expression, Oxid Med Cell Longev. 2019, 2481592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mangwiro YT, Briffa JF, Gravina S, Mahizir D, Anevska K, Romano T, Moritz KM, Cuffe JS & Wlodek ME (2018) Maternal exercise and growth restriction in rats alters placental angiogenic factors and blood space area in a sex-specific manner, Placenta. 74, 47–54. [DOI] [PubMed] [Google Scholar]
- 115.Mangwiro YTM, Cuffe JSM, Briffa JF, Mahizir D, Anevska K, Jefferies AJ, Hosseini S, Romano T, Moritz KM & Wlodek ME (2018) Maternal exercise in rats upregulates the placental insulin-like growth factor system with diet- and sex-specific responses: minimal effects in mothers born growth restricted, The Journal of physiology. 596, 5947–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fasshauer M & Blüher M (2015) Adipokines in health and disease, Trends in pharmacological sciences. 36, 461–70. [DOI] [PubMed] [Google Scholar]
- 117.Hansen D, Meeusen R, Mullens A & Dendale P (2012) Effect of acute endurance and resistance exercise on endocrine hormones directly related to lipolysis and skeletal muscle protein synthesis in adult individuals with obesity, Sports medicine (Auckland, NZ). 42, 415–31. [DOI] [PubMed] [Google Scholar]
- 118.Safdar A & Tarnopolsky MA (2018) Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise, Cold Spring Harbor perspectives in medicine. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J & Mantzoros CS (2018) Irisin in metabolic diseases, Endocrine. 59, 260–274. [DOI] [PubMed] [Google Scholar]
- 120.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP & Spiegelman BM (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis, Nature. 481, 463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jackson MR, Gott P, Lye SJ, Ritchie JW & Clapp JF 3rd (1995) The effects of maternal aerobic exercise on human placental development: placental volumetric composition and surface areas, Placenta. 16, 179–91. [DOI] [PubMed] [Google Scholar]
- 122.Clapp JF (2006) Influence of endurance exercise and diet on human placental development and fetal growth, Placenta. 27, 527–34. [DOI] [PubMed] [Google Scholar]
- 123.Day PE, Ntani G, Crozier SR, Mahon PA, Inskip HM, Cooper C, Harvey NC, Godfrey KM, Hanson MA, Lewis RM & Cleal JK (2015) Maternal Factors Are Associated with the Expression of Placental Genes Involved in Amino Acid Metabolism and Transport, PloS one. 10, e0143653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H & Fujino M (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor, Biochemical and biophysical research communications. 251, 471–6. [DOI] [PubMed] [Google Scholar]
- 125.Yue P, Jin H, Xu S, Aillaud M, Deng AC, Azuma J, Kundu RK, Reaven GM, Quertermous T & Tsao PS (2011) Apelin decreases lipolysis via G(q), G(i), and AMPK-Dependent Mechanisms, Endocrinology. 152, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Attané C, Foussal C, Le Gonidec S, Benani A, Daviaud D, Wanecq E, Guzmán-Ruiz R, Dray C, Bezaire V, Rancoule C, Kuba K, Ruiz-Gayo M, Levade T, Penninger J, Burcelin R, Pénicaud L, Valet P & Castan-Laurell I (2012) Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice, Diabetes. 61, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buléon M, Cani PD, Attané C, Guigné C, Carpéné C, Burcelin R, Castan-Laurell I & Valet P (2008) Apelin stimulates glucose utilization in normal and obese insulin-resistant mice, Cell metabolism. 8, 437–445. [DOI] [PubMed] [Google Scholar]
- 128.Castan-Laurell I, Dray C, Knauf C, Kunduzova O & Valet P (2012) Apelin, a promising target for type 2 diabetes treatment?, Trends in endocrinology and metabolism: TEM. 23, 234–41. [DOI] [PubMed] [Google Scholar]
- 129.Malamitsi-Puchner A, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D & Briana DD (2007) Circulating apelin concentrations in mother/infant pairs at term, Acta paediatrica (Oslo, Norway : 1992). 96, 1751–4. [DOI] [PubMed] [Google Scholar]
- 130.Ho L, van Dijk M, Chye STJ, Messerschmidt DM, Chng SC, Ong S, Yi LK, Boussata S, Goh GH, Afink GB, Lim CY, Dunn NR, Solter D, Knowles BB & Reversade B (2017) ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice, Science (New York, NY). 357, 707–713. [DOI] [PubMed] [Google Scholar]
- 131.Vaughan OR, Powell TL & Jansson T (2019) Apelin is a novel regulator of human trophoblast amino acid transport, American journal of physiology Endocrinology and metabolism. 316, E810–e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crujeiras AB, Pardo M, Arturo RR, Navas-Carretero S, Zulet MA, Martínez JA & Casanueva FF (2014) Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women, American journal of human biology : the official journal of the Human Biology Council. 26, 198–207. [DOI] [PubMed] [Google Scholar]
- 133.Arhire LI, Mihalache L & Covasa M (2019) Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome, Front Endocrinol (Lausanne). 10, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paris MT, Bell KE & Mourtzakis M (2020) Myokines and adipokines in sarcopenia: understanding cross-talk between skeletal muscle and adipose tissue and the role of exercise, Current opinion in pharmacology. 52, 61–66. [DOI] [PubMed] [Google Scholar]
- 135.Drewlo S, Johnson E, Kilburn BA, Kadam L, Armistead B & Kohan-Ghadr HR (2020) Irisin induces trophoblast differentiation via AMPK activation in the human placenta, Journal of cellular physiology. 235, 7146–7158. [DOI] [PubMed] [Google Scholar]
- 136.Garcés MF, Peralta JJ, Ruiz-Linares CE, Lozano AR, Poveda NE, Torres-Sierra AL, Eslava-Schmalbach JH, Alzate JP, Sánchez AY, Sanchez E, Angel-Müller E, Ruíz-Parra AI, Diéguez C, Nogueiras R & Caminos JE (2014) Irisin levels during pregnancy and changes associated with the development of preeclampsia, The Journal of clinical endocrinology and metabolism. 99, 2113–9. [DOI] [PubMed] [Google Scholar]
- 137.Ebert T, Stepan H, Schrey S, Kralisch S, Hindricks J, Hopf L, Platz M, Lossner U, Jessnitzer B, Drewlo S, Blüher M, Stumvoll M & Fasshauer M (2014) Serum levels of irisin in gestational diabetes mellitus during pregnancy and after delivery, Cytokine. 65, 153–8. [DOI] [PubMed] [Google Scholar]
- 138.Poretsky L, Islam J, Avtanski D, Lin YK, Shen YL, Hirth Y, Lesser M, Rosenwaks Z & Seto-Young D (2017) Reproductive effects of irisin: Initial in vitro studies, Reproductive biology. 17, 285–288. [DOI] [PubMed] [Google Scholar]
- 139.Chen J, Li Q & Ma J (2019) Maternal serum, placental, and umbilical venous blood irisin levels in intrahepatic cholestasis of pregnancy, The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet, 1–8. [DOI] [PubMed] [Google Scholar]
- 140.Scherer PE, Williams S, Fogliano M, Baldini G & Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes, J Biol Chem. 270, 26746–9. [DOI] [PubMed] [Google Scholar]
- 141.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T & Matsuzawa Y (2000) Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients, Arteriosclerosis, thrombosis, and vascular biology. 20, 1595–9. [DOI] [PubMed] [Google Scholar]
- 142.Hu E, Liang P & Spiegelman BM (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity, J Biol Chem. 271, 10697–703. [DOI] [PubMed] [Google Scholar]
- 143.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T & Matsuzawa Y (2003) Association of hypoadiponectinemia with coronary artery disease in men, Arteriosclerosis, thrombosis, and vascular biology. 23, 85–9. [DOI] [PubMed] [Google Scholar]
- 144.Blüher M, Bullen JW Jr., Lee JH, Kralisch S, Fasshauer M, Klöting N, Niebauer J, Schön MR, Williams CJ & Mantzoros CS (2006) Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training, The Journal of clinical endocrinology and metabolism. 91, 2310–6. [DOI] [PubMed] [Google Scholar]
- 145.Shen L, Zhu Y, Xiao J, Qian B, Jiang T, Deng J, Peng G, Yu S, Cao S, Zuo Z, Ma X, Zhong Z, Ren Z, Wang Y, Zhou Z, Liu H, Zong X & Hu Y (2020) Relationships between placental adiponectin, leptin, visfatin and resistin and birthweight in cattle, Reproduction, fertility, and development. 32, 402–408. [DOI] [PubMed] [Google Scholar]
- 146.McDonald EA & Wolfe MW (2009) Adiponectin attenuation of endocrine function within human term trophoblast cells, Endocrinology. 150, 4358–65. [DOI] [PubMed] [Google Scholar]
- 147.Benaitreau D, Dos Santos E, Leneveu MC, De Mazancourt P, Pecquery R & Dieudonné MN (2010) Adiponectin promotes syncytialisation of BeWo cell line and primary trophoblast cells, Reproductive biology and endocrinology : RB&E. 8, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aye IL, Rosario FJ, Powell TL & Jansson T (2015) Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth, Proceedings of the National Academy of Sciences of the United States of America. 112, 12858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang L, Fan L, Ding P, He YH, Xie C, Niu Z, Tian FY, Yuan SX, Jia DQ & Chen WQ (2019) Maternal exercise during pregnancy reduces the risk of preterm birth through the mediating role of placenta, The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 32, 109–116. [DOI] [PubMed] [Google Scholar]
- 150.Kusuyama J, Alves-Wagner AB, Conlin RH, Makarewicz NS, Albertson BG, Prince NB, Kobayashi S, Kozuka C, Møller M, Bjerre M, Fuglsang J, Miele E, Middelbeek RJW, Xiudong Y, Xia Y, Garneau L, Bhattacharjee J, Aguer C, Patti ME, Hirshman MF, Jessen N, Hatta T, Ovesen PG, Adamo KB, Nozik-Grayck E & Goodyear LJ (2021) Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health, Cell metabolism. 33, 939–956.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, Berim A, Zhang S, Wang S, Wang B, Foretz M, Viollet B, Gang DR, Rodgers BD, Zhu MJ & Du M (2016) AMPK/alpha-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis, Cell metabolism. 24, 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cao RY, Zheng H, Redfearn D & Yang J (2019) FNDC5: A novel player in metabolism and metabolic syndrome, Biochimie. 158, 111–116. [DOI] [PubMed] [Google Scholar]
- 153.Shan D, Liu X, Cai Y, Qiao X, Xu L & Zou L (2020) Irisin level and neonatal birthweight: A systematic review and meta-analysis, Eur J Obstet Gynecol Reprod Biol. 254, 25–32. [DOI] [PubMed] [Google Scholar]
- 154.Salem H, Yatchenko Y, Anosov M, Rosenfeld T, Altarescu G, Grisaru-Granovsky S & Birk R (2018) Maternal and neonatal irisin precursor gene FNDC5 polymorphism is associated with preterm birth, Gene. 649, 58–62. [DOI] [PubMed] [Google Scholar]
- 155.Goldenberg RL, Culhane JF, Iams JD & Romero R (2008) Epidemiology and causes of preterm birth, Lancet (London, England). 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Joung KE, Park KH, Filippaios A, Dincer F, Christou H & Mantzoros CS (2015) Cord blood irisin levels are positively correlated with birth weight in newborn infants, Metabolism: clinical and experimental. 64, 1507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nelissen EC, van Montfoort AP, Dumoulin JC & Evers JL (2011) Epigenetics and the placenta, Hum Reprod Update. 17, 397–417. [DOI] [PubMed] [Google Scholar]
- 158.Gupta R, Nagarajan A & Wajapeyee N (2010) Advances in genome-wide DNA methylation analysis, BioTechniques. 49, iii–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Thompson LP & Al-Hasan Y (2012) Impact of oxidative stress in fetal programming, Journal of pregnancy. 2012, 582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hebert JF & Myatt L (2021) Placental mitochondrial dysfunction with metabolic diseases: Therapeutic approaches, Biochimica et biophysica acta Molecular basis of disease. 1867, 165967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meinild Lundby AK, Jacobs RA, Gehrig S, de Leur J, Hauser M, Bonne TC, Flück D, Dandanell S, Kirk N, Kaech A, Ziegler U, Larsen S & Lundby C (2018) Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis, Acta physiologica (Oxford, England). 222. [DOI] [PubMed] [Google Scholar]
- 162.Stanford KI, Middelbeek RJ & Goodyear LJ (2015) Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations, Diabetes. 64, 2361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Parrettini S, Caroli A & Torlone E (2020) Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes, Front Endocrinol (Lausanne). 11, 611929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moreno-Fernandez J, Ochoa JJ, Lopez-Frias M & Diaz-Castro J (2020) Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review, Nutrients. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]


