Chronic pain is associated with increased sensitivity to non-nociceptive modalities including light, sound, and olfaction [7,13,26,36,41,60,63,66,88]. Hypersensitivity (i.e., greater sensitivity) to disparate sensory modalities also occurs in individuals with chronic pelvic pain (CPP) who report increased visceral sensitivity with concomitant mechanical and thermal hypersensitivity across non-pelvic sites [37,39,56,77]. Given that visceral and widespread somatic hypersensitivity are CPP hallmarks, understanding the neural mechanisms responsible could identify therapeutic targets [65,88,97].
Prior work suggests that central nervous system mechanisms of pain sensitization [5,15,80] involving broad abnormalties in gray matter volume, function, and connectivity [e.g., 12,51–54,64,68] may underlie multimodal hypersensitivity [24]. For example, fibromyalgia patients report increased unpleasantness during visual or multimodal stimulation with greater insula activation [36,60] and decreased visual cortex activity [60] compared to pain-free controls. Multimodal hypersensitivity has been observed after acute sensitization of the skin, resulting in enhanced event-related potentials from non-nociceptive stimuli, including vibrotactile [11] and visual [92] stimulation. Very little work has attempted to look at the co-presence of visceral and visual hypersensitivity under this shared sensitization hypothesis. Also, it remains unclear whether increased sensitivity in chronic pain from repeated pain exposure (e.g., episodic menstrual pain) is due to increased activity in primary sensory circuits or involves downstream cortical integration.
To address these questions, we have identified a cohort of women with dysmenorrhea (episodic menstrual pain) that despite being free of other chronic pain symptoms, harbor many symptom and physiological features of CPP. Notably, they have visceral hypersensitivity and mechanical hypersensitivity in non-pelvic sites [39] that correlate with mild preclinical symptoms of CPP [38,96,97]. We evaluated participants’ visceral sensitivity using our validated noninvasive bladder-filling task [96], and menstrual pain was assessed via self-report. We evaluated visual sensitivity by presenting participants with an aversive but nonpainful visual stimulus while recording scalp electroencephalography (EEG) and perceived unpleasantness. Pattern-reversal visual stimuli elicit a steady-state visual evoked potential (SSVEP) evident in the broadband EEG spectra at the presentation frequency [71,98]. SSVEPs are robust measures of stimulus driven synaptic activity [90,104] with sources principally located in primary (V1) and motion sensitive visual cortex [21].
Given previous literature linking visual discomfort and cortical measures [34,72,76], we hypothesized that brightness intensity would modulate SSVEP amplitudes, particularly in V1 (electrode Oz). Considering the comorbidity of visceral and widespread somatic hypersensitivity in CPP [88], we hypothesized that the relationship between visual unpleasantness and SSVEP amplitudes would be affected by relative bladder pain sensitivity. We accounted for somatic symptoms and menstrual pain to differentiate the contributions of these common co-occuring symptoms [100] to behavioral and neural measures of visual sensitivity. Analyzing the relationship of self-reported responses to visceral and visual provocation with cortical recordings allowed us to address the following questions: 1) Does presenting increasing brightness of a visual stimulus coincide with increased stimulus driven V1 oscillatory activity? 2) Do women who exhibit increased visceral sensitivity (provoked bladder pain) report heightened unpleasantness during visual stimulation? 3a) Is visual unpleasantness associated with increased stimulus driven V1 oscillatory activity? 3b) If so, is this relationship moderated by visceral sensitivity?
Method
Participants
The present investigation was part of a larger clinical trial “Deciphering the Hormonal and Nociceptive Mechanisms Underlying Bladder Pain” (NCT02214550) that enrolled 378 reproductive-age (18-45) women between 2014-2020. The larger clinical trial (NCT02214550) evaluated the effects of hormonal suppression on bladder sensitivity in women with moderate to severe dysmenorrhea. Women not meeting criteria for dysmenorrhea were used for comparison. The sample used in the present investigation pooled across these categories, yielding a broad range of menstrual pain for dimensional analysis. This dimensional strategy is recommended to avoid artificial boundaries and artifacts associated with categorical diagnostic criteria, increase relevance to preclinical forms of disease, and improve statistical power [14,84,107].
Participants were recruited by flyers posted on local college campuses, advertisements on public transportation, and by referral from nearby gynecology clinics. Potential participants who passed an eligibility screening over the phone were scheduled for an initial screening visit. Participants with chronic pain conditions other than dysmenorrhea were excluded from the present study to avoid potential confounding of the many factors (e.g., anxiety, depression, catastrophizing) that are heightened in chronic pain [27]. Exclusion criteria for the present study included: a) presence of active pelvic or abdominal malignancies, b) absence of regular menses, c) active genitourinary infection in the last four weeks, d) unwilling to undergo pelvic examination/testing, e) presence of hypertension or risk for developing hypertension, f) unwilling to withdraw from oral contraceptives for two months before the study visit, g) inadequate visual acuity to identify 3mm letters on a monitor 1 m away, or h) hairstyles that precluded EEG cap placements.
From the 378 enrolled participants, 93 with chronic pain diagnoses (e.g., bladder pain syndrome, chronic pain) were excluded from the present investigation, 95 did not complete the assessment visit, 40 were excluded due to technical difficulties (e.g., poor EEG quality resulting from equipment malfunction, capping difficulties due to hairstyle, etc.), two declined to participate in the EEG portion of the study due to migraine sensitivity, and one was excluded due to recreational/illicit substance use during the testing appointment; therefore, data from a total of 147 women were included in the present investigation. We preferentially recruited women with moderate to severe dysmenorrhea (81% of the cohort but about 25% of the general population [86,110]) to study women potentially at greater risk for meeting CPP criteria [57,58,100]. To confirm increased risk for CPP, one-year follow-up data were available from 106 of 147 participants (82 participants that met criteria for moderate to severe dysmenorrhea and 24 that did not). Whereas 30% of participants with dysmenorrhea met criteria for CPP via either the ROME III questionnaire [25] or ICSI questionnaire for bladder pain syndrome [73] one year later, only 8% of participants without dysmenorrhea did (Fisher’s Exact Test p = .035). Therefore, women with dysmenorrhea in this cohort were more likely to meet criteria in validated questionnaires for CPP. All participants provided written consent and were monetarily compensated for their time. All procedures followed the principles and guidelines stated in the Declaration of Helsinki and were approved by the NorthShore University HealthSystem’s Institutional Review Board.
Procedure
At an initial screening session, participants completed questionnaire measures encompassing medical, surgical, psychological, and gynecological history. A full list of all questionnaires administered in the larger clinical trial are reported elsewhere [95]; however, those pertinent in the present investigation are described here and reported in Table 1. In particular, a subset of questions from the Brief Symptom Inventory [BSI; 19] representing the somatic symptom subscale evaluated the psychological distress related to the perception of bodily discomfort. Participants rated on a 5-point scale of distress a series of questions that assessed perceptions of bodily pain, such as “Faintness or dizziness”, “Pains in heart or chest”, “Numbness or tingling in parts of your body”, etc. Scores were summed across these questions for each participant and provided a total BSI score, hereafter referred to as “somatic symptoms.”
Table 1.
Participant Demographics
| Measure | Response | N | % of N or M (SD) | Range |
|---|---|---|---|---|
| Age (years) | 147 | 24.1 (6.3) | 18-43 | |
|
| ||||
| Race | White | 87 | 59.2% | |
| Black or African American | 13 | 8.8% | ||
| Asian | 32 | 21.8% | ||
| Native Hawaiian or Pacific Islander | 0 | 0.0% | ||
| American Indian or Alaskan Native | 1 | 0.7% | ||
| Multi-Racial | 13 | 8.8% | ||
| Did not respond | 1 | 0.7% | ||
|
| ||||
| Ethnicity | Hispanic or Latino | 23 | 15.6% | |
| Not Hispanic or Latino | 124 | 84.4% | ||
|
| ||||
| Average number of days bleeding# | 146 | 5.7 (1.3) | 3-10 | |
| Questions for participants with painful periods only: | 116 | 78.9% | ||
|
| ||||
| Did period pain start at the time of menarche? * | Yes | 60 | 51.7% | |
| Age (years) that painful periods began (if not at menarche)? | 56 | 16.3 (2.9) | 10-28 | |
|
| ||||
| Years since menarche (first period) without a period? * | Never, always had a regular period | 67 | 57.8% | |
| Less than 1 year | 32 | 27.6% | ||
| 1 | 8 | 6.9% | ||
| 2 | 3 | 2.6% | ||
| 3 | 2 | 1.7% | ||
| 5 to 10 | 3 | 2.6% | ||
| More than ten years | 1 | 0.9% | ||
|
| ||||
| Days of pelvic menstrual pain/month * | 116 | 3.7 (2.0) | 1-10 | |
| Days of school or work missed in last 3 months * | 116 | 2.2 (3.3) | 0-25 | |
Note.
participants were administered this question if they answered Yes to painful periods (> 5 out of 10);
one participant was not administered this question because she did not report having a period for the last 6 months due to birth control pill use although she had a period before the EEG visit.
A standardized pelvic exam was performed by a gynecologist (FFT) to identify potential causes of menstrual pain on the first 98 participants [38]. Potential clinical exam findings were only observed in eight participants and followed up with ultrasonography. Among these eight participants, three participants had small pelvic cysts (<2.5 x 3.0 cm), and one had subserosal and intramural leiomyomata (<2.5 x 2.5 cm). We discontinued performing pelvic exams to limit potential discomfort and inconvenience, given that most recruited participants had exam profiles consistent with primary dysmenorrhea.
Eligible participants were subsequently scheduled for a mid-luteal phase assessment visit (approximately 17-25 days post-onset of menses). Participants used ovulation tests on days 10-17 to detect luteinizing hormone surges and confirm the menstrual cycle phase [31]. Participants were asked to rate the “average amount of cramping or pain you have experienced during your menstrual period over the past 3 months when not taking any painkillers and on the worst day of your period” using a 0-100 Visual Analog Scale (VAS; 0 – no pain, 100 – worst pain imaginable) [40]. This question was asked during the luteal phase and thus avoided complications due to variable painkiller use. We confirmed the average intensity of menstrual pain using electronic daily diaries over a full menstrual cycle before the assessment visit [38].
During their mid-luteal phase assessment visit, participants were asked to avoid taking short-acting, over-the-counter analgesics (e.g., ibuprofen, acetaminophen), short-acting opioids (e.g., hydrocodone or oxycodone), and caffeine for at least six hours before arrival. Participants were instructed to also avoid longer-acting over-the-counter analgesics (e.g., naproxen) for at least twelve hours before arrival. We performed comprehensive quantitative sensory testing [39] and noninvasive experimental bladder distension on all participants to assess their visceral sensitivity [38,96]. Our bladder test mimics clinical retrograde cystometry, starting with an emptied bladder. After oral ingestion of 20 oz of water, participants were instructed to report when they reached three standard levels of bladder urgency: first sensation, first urge to void, and maximal capacity [1]. At baseline and each of these time points, we obtained three-dimensional sonographic measurements of the bladder (GE Voluson 750, Wauwatosa, WI), and participants rated their bladder pain and urgency on a 10 cm VAS using a tablet computer. Experimental bladder pain assessment was capped at two hours, even if participants did not reach maximal capacity. Previous investigations from our laboratory have demonstrated that bladder pain ratings at first urge to void is a specific sign of additional bladder pain hypersensitivity that is observed on retrospective surveys and diaries correlated with clinical markers of bladder pain [38,39]; therefore, the first urge bladder pain ratings from the bladder distension task, referred to hereafter as “provoked bladder pain”, were used as a moderating factor in regression modeling. After completing this task, we confirmed that provoked bladder pain had returned to baseline levels before beginning the EEG task.
EEG Instrumentation
Participants were then prepared for EEG recording. Simultaneously, the room was darkened to less than 10 lux ambient light to allow the participant to adapt sufficiently for the visual task. Participants were instrumented with 32 Ag/AgCl active electrodes arranged in the International 10-20 montage (Brain Vision ActiCap). EEG was recorded at 500Hz (1 Hz high-pass and 250 Hz low-pass 20 dB/decade Butterworth filter) using a Brain Vision actiChamp 24-bit A/D amplifier with Pycorder software (BrainVision, NC). Facial and eye movements were recorded from electrodes placed above the right eye, below the left eye, and in the middle of the brow. Electrode impedances were kept below manufacturer guidelines for active electrodes (25 kΩ). The left mastoid served as the online reference, and FPz served as the ground electrode. Participants were instructed to avoid clenching, blinking, speaking, and any facial muscle activity. Standard technical quality inspections were also performed (e.g., requesting participants to blink and verifying signal changes in real-time) throughout the recording. During recording, EEG data were examined to evaluate whether participants were compliant with instructions.
EEG Experimental Task
We verified sufficient acuity for the visual stimulation task by asking participants to identify eight lines each containing six letters ranging in size from 5 mm to 2 mm. Participants performed the task seated one meter from a 41x30 cm computer monitor with a 100 Hz refresh rate at 23° viewing angle. The same monitor and distance was used for the visual task described below. Stimulus presentation and onset of physiological data collection were controlled by E-prime 2.0 (Psychology Software Tools, PA).
Five blocks of a blue/yellow checkerboard with contrasts (positive and negative flips) alternating at 25 Hz were presented for 20 seconds each [36]. This 25Hz stimulus frequency was selected because it aligned with the monitor’s refresh rate, produced an irritating flicker, and resided within the frequency range shown to produce robust SSVEPs [108]. Each block contained a single maximal brightness intensity (1, 30, 60, 90 or 120 lux) obtained by adjusting the contrast of the image focused on a fixed crosshair centered in the middle of a solid background. Block order was randomized across participants. Participants were instructed to focus on a fixed crosshair centered in the middle of the screen. After each block, participants rated the unpleasantness of the stimuli using the Gracely Box Scale [29,79] that lists the numbers 0 to 20 in descending order next to a set of verbal anchors with logarithmically placed validated positions (see Figure 1).
Figure 1. Visual stimulation task presented during EEG recording and designed to elicit an SSVEP.
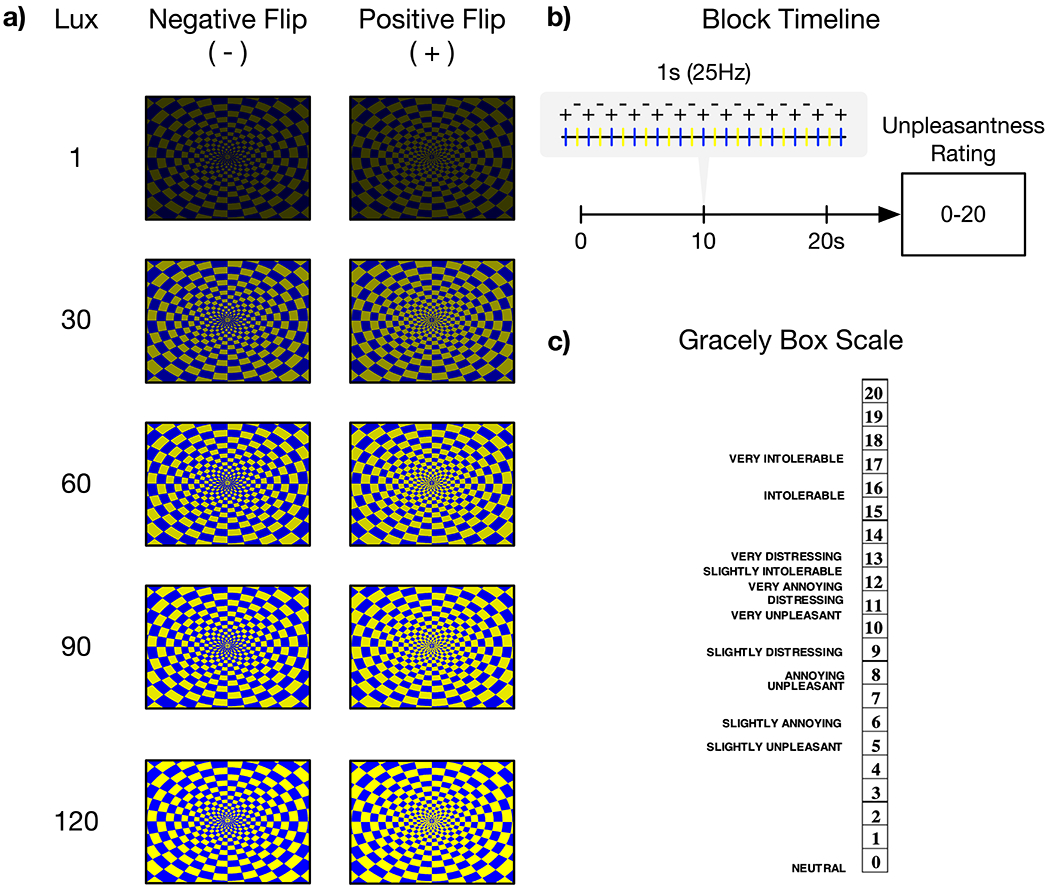
a) Participants viewed an alternating blue-yellow checkerboard pattern with positive and negative reversals across five different brightness intensities modulated with monotonically increasing lux. b) Checkerboards alternated at 25Hz and were presented for 20 seconds before an unpleasantness rating for each brightness intensity. Block order was randomized across participants. c) Participants’ unpleasantness ratings were measured using the Gracely Box Scale with textual descriptors.
EEG Data Reduction
EEG data were processed in MATLAB using the EEGLAB toolbox [17]. EEG data were re-referenced to an averaged mastoid reference, down-sampled to 256Hz, and digitally filtered using a 1Hz Hamming windowed sinc finite impulse response (FIR) high-pass filter (-6 dB half-amplitude cutoff, 2Hz transition bandwidth). Line noise (i.e., 60Hz) was removed using the Cleanline EEGLAB plugin [67]. Preprocessed data were then visually inspected and noisy sections of continuous EEG were removed. Clean segments of continuous EEG were submitted to an infomax independent component analysis (ICA) using the “runica” algorithm in EEGLAB [62]. Artifactual components were identified (p > .6) and removed automatically by the Multiple Artifact Rejection Algorithm [MARA; 103] EEGLAB plugin. Continuous EEG was then reconstructed from the remaining independent components and noisy channels spherically interpolated if they maintained uncharacteristic signals throughout the recording (e.g., disconnected channels). Two-second epochs with one-second overlap were extracted from each of the five 20 second stimulation blocks and subjected to artifact rejection using a ±100µV threshold that excluded only 11 epochs (< 1% of eligible epochs). To reduce the spreading of the signal on the scalp due to volume conduction effects and increase topographical specificity, we applied a surface Laplacian spatial filter using the CSD MATLAB Toolbox [49,50] on the extracted epochs before spectral power calculations that utilized Fast Fourier Transform with Hamming window taper. Power spectral density (PSD) estimates at 25Hz, which was our experimentally controlled visual stimulation frequency, were averaged across the 2-second epochs within each of the five stimulation brightness intensities for the visual task. Therefore, each participant had five PSD estimates used for regression modeling.
Statistical Analyses
These data were analyzed using multilevel models (MLMs; i.e., linear mixed models) with random intercepts and slopes to model brain-behavior relationships using a model comparison approach [45]. Clinical studies often differentiate participants into groups (e.g., chronic pain vs. controls) based on metrics with cutoffs that are arbitrary or differ across investigations; however, MLMs provide more flexibility in examining continuous moderating estimates (e.g., provoked bladder pain) that allow for a more sensitive and predictive analysis. The following analyses examined brain-behavior relationships and how these relationships change depending on additional continuous measures, such as pain ratings and somatic symptoms. Importantly, this statistical design accounts for variance at the individual level (i.e., random intercepts and slopes) while estimating between-subject differences, thus providing an optimal method for modeling visceral-visual sensitivity. All significance testing was two-tailed.
Analysis 1: Validating the Anticipated Effect of Visual Stimulation on Evoked Brain Activity
Analysis 1 addressed question #1 regarding whether presenting increasing brightness intensities of a visual stimulus coincided with evoked cortical activity. For each participant and each electrode separately, first level models estimated cortical PSD at 25Hz as a function of linearly increasing brightness:
| (Eq. 1) |
Where i refers to an individual participant and j refers to brightness intensity. Values for increasing brightness intensity were mean centered: −2, −1, 0, 1, 2. The participants’ regression estimates from first level models were used as dependent variables in second level models that estimated variations in intercepts:
| (Eq. 2) |
and slopes:
| (Eq. 3) |
Regression parameters in second levels are designated with γ and subscripts that denote the estimated parameter from level one and level two, respectively. Estimation of the intercept (γ00) reflects the average PSD across brightness intensities, while estimation of the slope (γ10) reflects the change in PSD per each increase in brightness intensity.
Analysis 2: Examining the Relationship Between Visual Unpleasantness and Evoked Cortical Activity with Moderating Factors of Provoked Bladder Pain, Somatic Symptoms, and Menstrual Pain
Analysis 2 addressed questions #2, #3a, and #3b as outlined in the introduction. Put otherwise, we evaluated the relationships between task evoked cortical activity and visual unpleasantness and how these relationships were moderated by menstrual pain, provoked bladder pain, and somatic symptoms (see Figure 2). For each participant and each electrode separately, first level models estimated reported unpleasantness as a function of increasing brightness intensity and cortical activity measured via 25Hz PSD estimates (i.e., SSVEP amplitude):
| (Eq. 4) |
Where i refers to each participant, while j refers to brightness intensity. Both brightness intensity and PSD were mean centered. Regression estimates from first level models were used as dependent variables in second level models that additionally included the mean centered moderating variables of menstrual pain, provoked bladder pain, and somatic symptoms. Previous studies have examined the relationship between neural activity and menstrual pain [94,99], bladder pain [20], or somatization [10] and discovered significant effects. Our study builds on these findings by incorporating all three variables simultaneously to identify the fundamental factors responsible for altered neural mechanisms. The first “Intercept Model” estimated moderating effects on the intercept (b0i) that represented average unpleasantness ratings collapsed across brightness intensity:
| (Eq. 5) |
Estimation of the intercept (γ00) reflected mean unpleasantness ratings at average levels of menstrual pain, somatic symptoms, and provoked bladder pain. Estimation of moderating slopes reflected changes in unpleasantness ratings with changes in menstrual pain (γ01), somatic symptoms (γ02), and provoked bladder pain (γ03). The moderating variable of provoked bladder pain addressed question #2.
Figure 2. Multilevel modeling allows for comprehensive analysis of brain-behavior relationships accounting menstrual pain, somatic symptoms, provoked bladder pain.
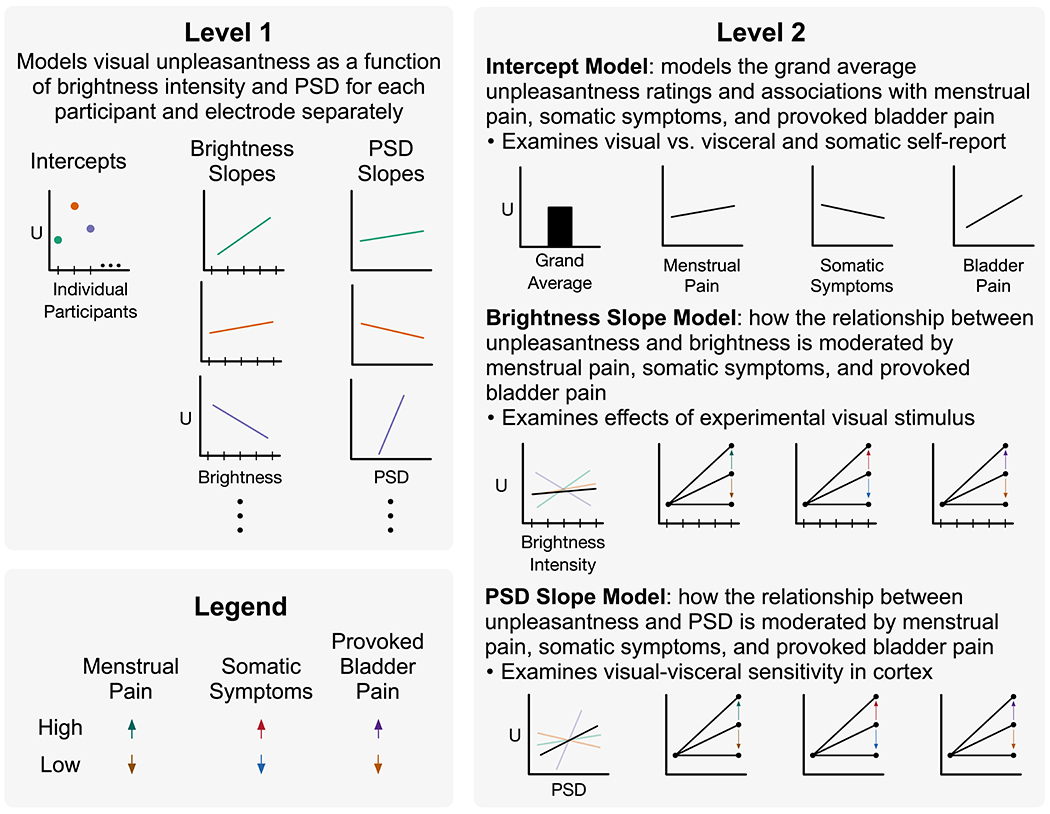
In level 1, participant unpleasantness ratings (U) from visual stimulation were modeled as a function of brightness intensity and cortical activity from 25Hz power spectral density (PSD) estimates. Each participant and electrode were modeled individually, allowing for random intercepts and slopes. Ellipses indicate all participants are included in this model. Intercepts, brightness slopes, and PSD slopes were modeled separately in level 2 as a function of participants’ prior menstrual pain, somatic symptoms, and provoked bladder pain. This was modeled separately for each electrode but across participants. Moderating effects are demonstrated with arrows depicting high vs. low reported pain/symptoms. Therefore, level 2 brightness and PSD slopes depict positive moderating effects; however, negative moderating effects are also possible (not shown). Data presented are fictional and shown for illustrative purposes.
The second “Brightness Slope Model” estimated moderating effects on the brightness slopes (b1i) that represented the change in unpleasantness ratings with increases in brightness intensity:
| (Eq. 6) |
Estimation of the intercept (γ10) reflected mean brightness slopes at average menstrual pain, somatic symptoms, and provoked bladder pain. Estimation of moderating slopes reflected changes in brightness slopes with changes in menstrual pain (γ11), somatic symptoms (γ12), and provoked bladder pain (γ13).
The third “PSD Slope Model” estimated moderating effects on PSD slopes (b2i) that represented the change in unpleasantness ratings with increases in cortical activity:
| (Eq. 7) |
Estimation of the intercept (γ20), which addressed question #3a, reflected mean PSD slopes at average levels of menstrual pain, somatic symptoms, and provoked bladder pain. Estimation of moderating slopes, which addressed question #3b, reflected changes in PSD slopes with changes in menstrual pain (γ21), somatic symptoms (γ22), and provoked bladder pain (γ23).
We hypothesized that unpleasantness ratings would be modulated by brightness intensity and cortical activity. Additionally, we hypothesized that these relationships would be moderated by visceral hypersensitivity, i.e., provoked bladder pain controlling for menstrual pain and non-specific somatic symptoms. We predicted the primary effect of moderation would occur at Oz, given that the neural generators of pattern reversal SSVEPs are located in primary visual cortex (V1) and areas that are sensitive to motion (V5/MT) [21,71]. Analyses that included the remaining 31 electrode sites were exploratory and were corrected for multiple comparisons by controlling for false discovery rate [FDR; 6]. Model assumptions and multicollinearity were examined for violations and deviations from normality.
Statistical analyses were performed in R [version 3.6.3; ,81] and RStudio (version 1.2.5033) using the dplyr [102], broom [83], and lmSupport [16], and performance [61] packages, whereas figures were prepared using ggplot2 [101], patchwork [78], and RColorBrewer [70].
Results
Analysis 1 (Question #1): Visual Stimulation Modulated 25Hz SSVEP PSD
As expected, the visual task effectively elicited a 25Hz SSVEP that increased in PSD with increasing brightness intensities, demonstrating robust stimulation of visual cortex (Figure 3). Specifically, for every increase in brightness intensity we observed an average increase of 2.1 95% CI [1.9, 2.2] dB of 25Hz PSD at Oz (p < .001; see Table 2). Put differently, participants’ 25Hz PSD increased an average of 10dB across all brightness intensities (1-120 lux). This effect was expected and largest at Oz (partial eta-squared [] = .78). Significant linear increases in 25Hz PSD occurred at every electrode site across the scalp even when correcting for multiple comparisons (see Supplementary Table 1). Thus, our visual task effectively evoked widespread cortical activity precisely synchronized to our stimulus frequency in proportion to stimulus brightness intensity.
Figure 3. The unpleasant checkerboard stimuli presented at 25Hz evoked widespread robust SSVEPs focused at Oz.
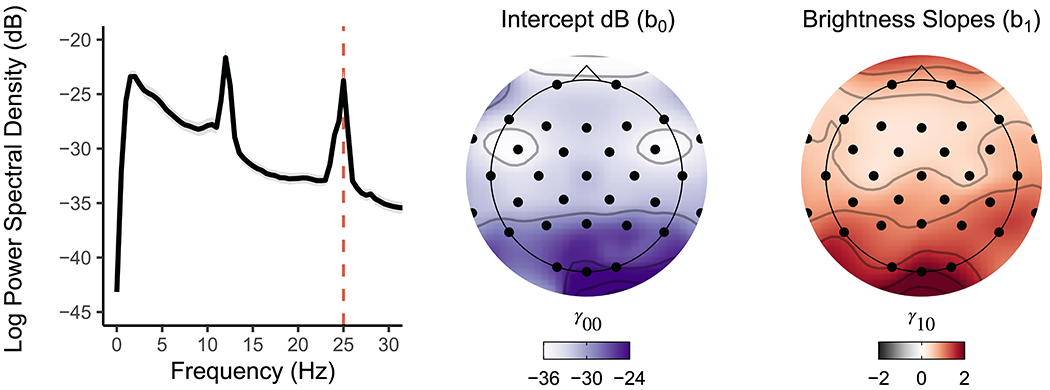
Left. Broadband PSD averaged across the five presented brightness intensities for all participants shows a clear peak at the 25Hz SSVEP alternating checkerboard frequency at Oz. Grey shading denotes 95% confidence interval. Middle. Topographically plotted intercepts demonstrated elevated PSD estimates toward occipital electrode sites. Right. Topographically plotted regression slopes show scalp-wide positive slopes, especially at occipital sites and Oz, demonstrating an increase in SSVEP PSD estimates with increasing brightness intensities. All topographic sites for intercept and slope effects were significant after correcting for multiple comparisons (pFDR < .001).
Table 2.
Multilevel Modeling Results at Electrode Oz
| 95% CI |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis/Question | Model | Source | b | LL | UL | SE | SS | MSE | F | p | |
| 1/1 | Intercept | Intercept | −23.76 | −24.68 | −22.84 | 0.47 | 82992.47 | 31.92 | 2600.06 | < .001 | 0.95 |
|
| |||||||||||
| 1/1 | Intercept | Brightness | 2.05 | 1.87 | 2.23 | 0.09 | 617.64 | 1.22 | 504.68 | < .001 | 0.78 |
|
| |||||||||||
| 2 | Intercept | Intercept | 8.00 | 7.37 | 8.63 | 0.32 | 9401.60 | 14.92 | 629.97 | < .001 | 0.81 |
|
| |||||||||||
| 2 | Intercept | Menstrual Pain | 0.01 | −0.01 | 0.04 | 0.01 | 13.99 | 14.92 | 0.94 | 0.335 | 0.01 |
| 2 | Intercept | Somatic Symptoms | −0.14 | −0.41 | 0.14 | 0.14 | 14.13 | 14.92 | 0.95 | 0.332 | 0.01 |
| 2/2 | Intercept | Provoked Bladder Pain | 0.06 | 0.02 | 0.11 | 0.02 | 134.33 | 14.92 | 9.00 | 0.003 | 0.06 |
|
| |||||||||||
| 2 | Brightness | Intercept | 0.36 | 0.23 | 0.49 | 0.07 | 18.75 | 0.66 | 28.62 | < .001 | 0.17 |
|
| |||||||||||
| 2 | Brightness | Menstrual Pain | 0.01 | < .001 | 0.01 | 0.003 | 3.32 | 0.66 | 5.07 | 0.026 | 0.03 |
| 2 | Brightness | Somatic Symptoms | −0.03 | −0.09 | 0.03 | 0.03 | 0.80 | 0.66 | 1.22 | 0.271 | 0.01 |
| 2 | Brightness | Provoked Bladder Pain | −0.003 | −0.01 | 0.01 | 0.004 | 0.27 | 0.66 | 0.42 | 0.519 | 0.003 |
|
| |||||||||||
| 2/3a | PSD | Intercept | 0.11 | 0.04 | 0.17 | 0.03 | 1.68 | 0.17 | 9.87 | 0.002 | 0.06 |
|
| |||||||||||
| 2 | PSD | Menstrual Pain | −0.001 | −0.004 | 0.002 | 0.001 | 0.12 | 0.17 | 0.70 | 0.404 | 0.005 |
| 2 | PSD | Somatic Symptoms | −0.005 | −0.03 | 0.02 | 0.01 | 0.02 | 0.17 | 0.10 | 0.750 | < .001 |
| 2/3b | PSD | Provoked Bladder Pain | 0.01 | < .001 | 0.01 | 0.002 | 0.83 | 0.17 | 4.88 | 0.029 | 0.03 |
Note. Degrees of freedom (numerator, denominator): analysis 1 (1, 146) and analysis 2 (1, 143), respectively; CI = confidence interval; LL = lower level; UL = upper level.
Analysis 2: Brain-Behavior Relationship Moderated by Prior Menstrual Pain, Somatic Symptoms, and Provoked Bladder Pain
The MLM regression procedure modeled brain-behavior relationships and how these relationships were moderated by visceral pain and somatic symptoms. Menstrual pain, somatic symptoms, and provoked bladder pain were positively correlated with each other (see Table 3 for descriptive statistics) and demonstrated variability across their respective ranges (see Supplementary Figure 1).
Table 3.
Correlations and Descriptive Statistics of Level 2 Moderating Variables
| Bootstrapped 95% CI |
Variable 1 Descriptive Statistics |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable 1 | Variable 2 | r | Lower Level | Upper Level | p | M | SD | SEM | MIN | MAX |
| Menstrual Pain | Provoked Bladder Pain | 0.28 | .13 | .42 | <.001 | 62.5 | 26.1 | 2.15 | 0 | 100 |
| Provoked Bladder Pain | Somatic Symptoms | 0.28 | .12 | .42 | <.001 | 12.2 | 16 | 1.32 | 0 | 59 |
| Somatic Symptoms | Menstrual Pain | 0.16 | −.002 | .31 | .053 | 2.37 | 2.4 | 0.198 | 0 | 15 |
Intercept Model (Question #2): Visual Unpleasantness is Associated with Provoked Bladder Pain
The intercept model estimated how the participants’ unpleasantness ratings following visual stimulation were moderated by self-reported menstrual pain, somatic symptoms, and provoked bladder pain (Table 2). Collapsing across all brightness intensities, participants rated the unpleasantness associated with visual stimulation as a Gracely Box Scale (GBS) rating of 8.0 [7.4, 8.6] corresponding with the descriptors “annoying” and “unpleasant”. Menstrual pain and somatic symptoms did not moderate mean unpleasantness ratings. However, we observed a positive linear association between provoked bladder pain and mean unpleasantness ratings, suggesting that participants exhibiting visceral hypersensitivity rated the visual stimulation as more unpleasant (see Figure 4a). On average, participants’ unpleasantness ratings increased by 1.0 GBS point per 15-point increase in provoked bladder pain ( = .06, p = .003).
Figure 4. The relationships between visual unpleasantness and brightness intensity/cortical activity were moderated by menstrual pain, somatic symptoms and provoked bladder pain.
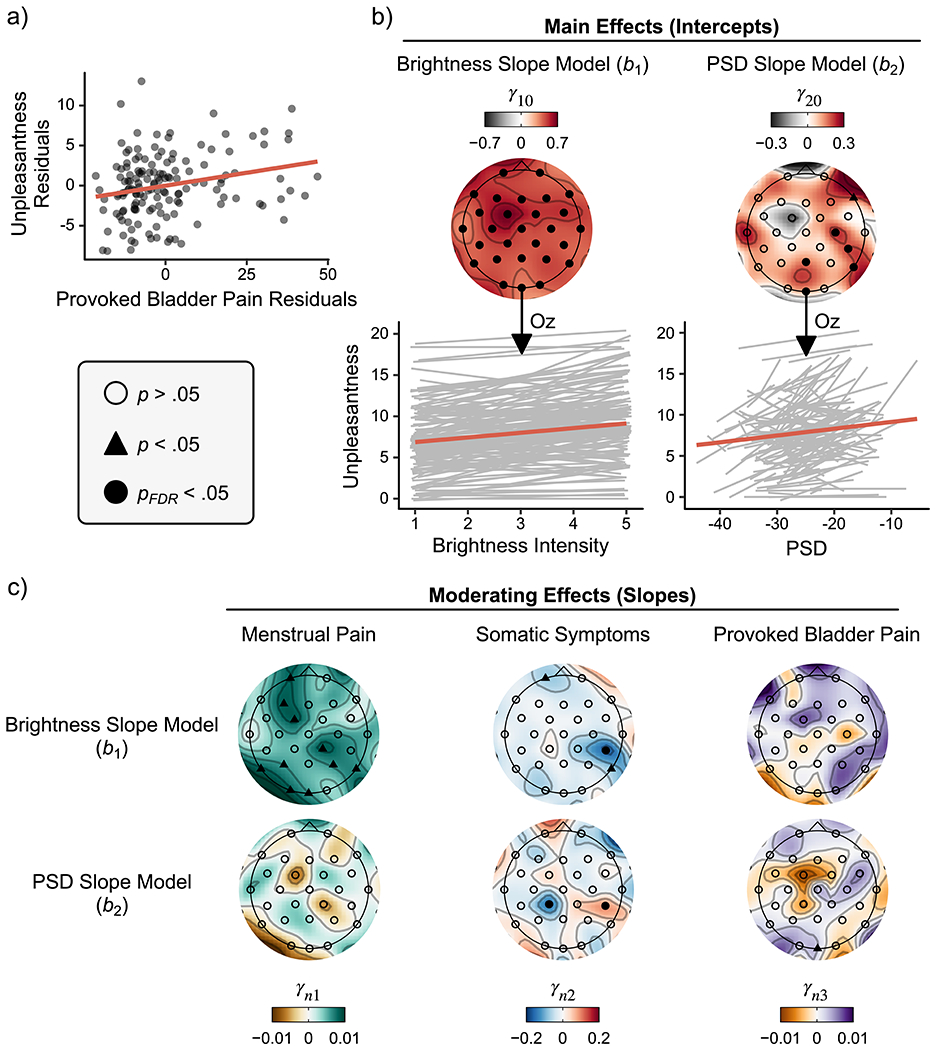
a) Partial regression scatter plots depict the positive relationship between provoked bladder pain and participants’ mean unpleasantness ratings averaged across brightness intensities accounting for menstrual pain and somatic symptoms. b) Topographic plots of regression slopes testing the intercepts from the brightness and PSD models. Oz was our a priori electrode of interest. Raw (grey) and averaged (red) slopes across all participants plotted below demonstrate that increases in brightness intensity and 25Hz PSD resulted in concomitant increases in participant unpleasantness ratings when accounting for one another. c) Scalp topographies of moderating slopes from second level multilevel modeling results. Given that positive relationships were observed between unpleasantness ratings and brightness/PSD in b, positive slopes here depict an increasing positive relationship between moderating variables, while negative slopes depict an increasing negative relationship. Menstrual pain ratings moderated the positive relationship between unpleasantness ratings and brightness, but not 25 Hz PSD at Oz (a priori chosen) and several other exploratory electrode sites. Somatic symptoms did not moderate these relationships at Oz; however, somatic symptoms moderated brightness and PSD slopes at a right posterior site (CP6; pFDR < .05 corrected), despite conflicting directions of moderation. In contrast, provoked bladder pain moderated the positive relationship between unpleasantness and PSD, but not brightness. Level two regression parameter notation n denotes both the brightness (n=1) and PSD (n=2) slope models. PSD = power spectral density; FDR = false discovery rate.
Brightness Slope Model: The Association Between Unpleasantness and Brightness Intensity is Moderated by Menstrual Pain and Somatic Symptoms
The brightness model estimated whether participants’ slopes predicting unpleasantness as a function of brightness intensity differed significantly from zero on average (i.e., intercepts) and whether this relationship was moderated by self-reported menstrual pain, somatic symptoms, and provoked bladder pain. Given that PSD was estimated alongside brightness in level 1 models (see Eq. 4), brightness slopes are controlled for PSD at each electrode site (i.e., the relationship of unpleasantness and brightness at average PSD). It is for this reason that brightness slopes vary across electrodes because each participant varied in their average PSD for each electrode. After correcting for multiple comparisons, the relationship between unpleasantness ratings and brightness intensity was positive on average across participants at Oz (see Table 2) and across all electrode sites (see Supplementary Table 2), suggesting that participants experienced a monotonic increase in unpleasantness with each increasing brightness intensity (see Figure 4b). More specifically, at average intensities of menstrual pain, provoked bladder pain, and somatic symptoms, the participants reported an increase of .36 [.23, .49] GBS points per increasing intensity level of brightness (p < .001), or nearly 2 GBS points from the least to greatest intensity of brightness ( = .17).
The relationship between unpleasantness and brightness was moderated by participants’ self-reported menstrual pain at Oz ( = .03) and additional exploratory sites including left posterior, right parietal, and left frontal electrodes ( = .03-.06). Increases in menstrual pain strengthened the positive relationship (i.e., steeper slopes) between unpleasantness ratings and brightness intensities (see Figure 4c). In other words, participants with the greatest menstrual pain experienced more unpleasantness at equivalent intensities of brightness compared to those with less menstrual pain (see Supplementary Figure 2).
Somatic symptoms moderated the relationship between unpleasantness and brightness at a left frontal electrode (Fp1; =.03, p = .048) and a right posterior parietal site (P8, = .04, p = .01). A right posterior parietal site (CP6) was significant even after corrections for multiple comparisons ( = .09, pfdr = .008). Given its negative slope, this finding suggests that increases in somatic symptoms are associated with an increased negative relationship between unpleasantness ratings and brightness intensities.
Although average unpleasantness ratings were moderated by provoked bladder pain in the intercept model (Figure 5a), the relationship between brightness intensity and unpleasantness was not significantly moderated by provoked bladder pain at any electrode sites (p > .2).
Figure 5. At equivalent amounts of primary visual cortex activity, individuals with greater provoked bladder pain experience greater visual discomfort.
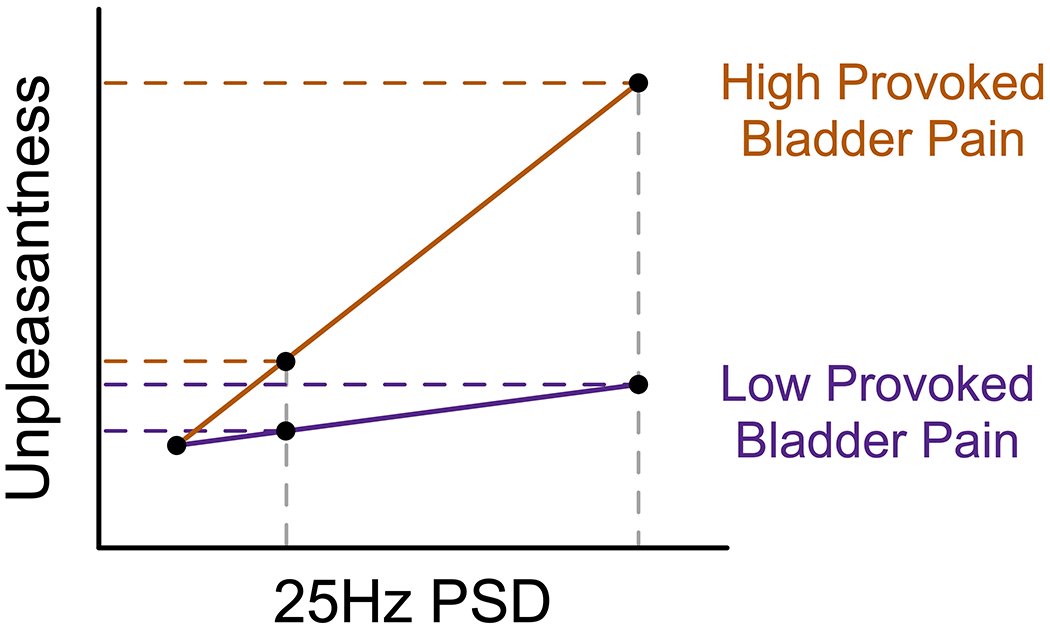
Conceptual line plots demonstrate the moderating effect of provoked bladder pain on the relationship between unpleasantness ratings and cortical activity measured via 25Hz power spectral density (PSD) estimates electrode Oz. This moderating effect implies that when stimulus driven oscillatory activity in visual cortex is low, high and low provoked bladder pain individuals experience similar discomfort. However, individuals with greater provoked bladder pain report more unpleasantness when activity increases. Activity in primary visual cortex is therefore not greater in individuals with visceral hypersensitivity; rather, downstream interpretation of this signal is likely amplified in women with comorbid visceral hypersensitivity.
PSD Slope Model (Question #3a): The Association Between Evoked Cortical Activity and Unpleasantness
The PSD model estimated unpleasantness as a function of 25Hz PSD (i.e., evoked cortical activity). Simultaneously, this model also evaluated whether this relationship was moderated by self-reported menstrual pain, somatic symptoms, and provoked bladder pain. After correcting for multiple comparisons, the relationship between unpleasantness ratings and PSD was positive on average across participants at Oz (see Table 2) and several other central and right parieto-temporal sites (see Supplementary Table 2), suggesting participants experienced a monotonic increase in unpleasantness with increases in 25Hz SSVEP PSD (see Figure 4b). More specifically, the participants reported an increase of .11 [.04, .17] GBS points per increase in 1 dB of 25Hz power at Oz (p = .002), or nearly 1.1 GBS points per 10 dB PSD increase in our observed SSVEP ( = .07). Thus, the relative amount of evoked activity at Oz, even accounting for simulation intensity, is a key predictor of evoked unpleasantness.
PSD Slope Model (Question #3b): The Association Between Unpleasantness and Cortical Activity is Moderated by Provoked Bladder Pain and Somatic Symptoms
To determine whether provoked bladder pain specifically moderated the cortical contribution to visual unpleasantness, we examined relationships in a final set of models controlling for menstrual pain and somatic symptoms (Figure 4c). Although menstrual pain did not moderate this relationship, somatic symptoms did. Somatic symptoms demonstrated conflicting moderating effects on PSD slopes at two parietal electrode sites that survived corrections for multiple comparisons: relationships were both positive (CP6, = .08, pfdr = .015) and negative (CP1, = .06, pfdr = .043). We are cautious about interpreting these effects further given that electrode CP6, an electrode that was not defined a priori, negatively moderated brightness slopes (see above) but positively moderated PSD slopes. However, given that electrode CP6 survived multiple comparison corrections in both brightness (see above) and PSD models, we believe this right lateralized effect may represent a cortical phenomenon that differentially responds to brightness and cortical activity independently of each other.
In the final moderation analysis, we observed that provoked bladder pain positively moderated this relationship, suggesting that women with visceral hypersensitivity had a stronger positive relationship between unpleasantness and cortical activity at Oz ( = .03). In other words, women with visceral hypersensitivity reported greater unpleasantness ratings at equivalent amounts of cortical response in primary visual cortex (see Supplementary Figure 2). See Table 4 for a summary of Analysis 2 findings.
Table 4.
Analysis 2 Regression Coefficient Explanations and Result Summary
| Parameter |
|||||
|---|---|---|---|---|---|
| Level One | Level Two | Hypothesis | Explanation | Electrode | Result |
| Intercept b0i | Intercept γ00 | Grand average unpleasantness ratings | All | M = 8, p<.001 | |
|
| |||||
| Menstrual Pain γ01 | Association between unpleasantness and menstrual pain | All | ns | ||
|
| |||||
| Somatic Symptoms γ02 | Association between unpleasantness and somatic symptoms | All | ns | ||
|
| |||||
| Provoked Bladder Pain γ03 | 2 | Association between unpleasantness and provoked bladder pain | All | ηp2=.06, p=.003 | |
|
| |||||
| Brightness Slope b1i | Intercept γ10 | Association between unpleasantness and brightness intensity | Oz | ηp2=.17, p<.001 | |
|
| |||||
| Menstrual Pain γ11 | How menstrual pain affects the association between unpleasantness and brightness intensity | Oz | ηp2=.03, p=.03 | ||
|
| |||||
| Somatic Symptoms γ12 | How somatic symptoms affect the association between unpleasantness and brightness intensity | Oz | ns | ||
| CP6 | ηp2=.09, pfdr=.01 | ||||
|
| |||||
| Provoked Bladder Pain γ13 | How provoked bladder pain affects the association between unpleasantness and brightness intensity | Oz | ns | ||
|
| |||||
| PSD Slope b2i | Intercept γ20 | 3a | Association between unpleasantness and cortical activity | Oz | ηp2=.07, p=.002 |
|
| |||||
| Menstrual Pain γ21 | How menstrual pain affects the association between unpleasantness and cortical activity | Oz | ns | ||
|
| |||||
| Somatic Symptoms γ22 | How somatic symptoms affect the association between unpleasantness and cortical activity | Oz | ns | ||
| CP1 | ηp2=.06, pfdr=.04 | ||||
| CP6 | ηp2=.08, pfdr=.02 | ||||
|
| |||||
| Provoked Bladder Pain γ23 | 3b | How provoked bladder pain affects the association between unpleasantness and cortical activity | Oz | ηp2=.03, p=.03 | |
Note. Unpleasantness refers to ratings of visual unpleasantness during the visual task. ns = not significant; fdr = false discovery rate.
Discussion
This study demonstrated an association between behavioral-cortical responses to visual stimulation and visceral sensitivity in a cohort of women who report symptoms of dysmenorrhea. Presenting increasing brightness intensities of a pattern-reversal checkerboard stimulus evoked increased cortical activity, especially at our central occipital site (Oz) of interest (Question #1). Provoked bladder pain predicted the degree of stimulus evoked visual unpleasantness, consistent with the broad notion that a centralized mechanism of sensory hypersensitivity encompasses both visceral and visual sensation. We observed a positive relationship between perceived visual unpleasantness and SSVEP amplitude. We therefore hypothesize that increased synaptic activity within primary visual cortex [90,104], as well as other cortical areas, contributes to increased visual discomfort. Importantly, this relationship was moderated by participants’ rating of provoked bladder pain when accounting for somatic symptoms and menstrual pain; at equivalent activity in primary visual cortex, women with increased visceral sensitivity experienced increased visual discomfort. In other words, the increased visual unpleasantness in individuals with concomitant visceral hypersensitivity was not driven by increased activity in primary visual cortex. Together with prior literature, we theorize that mechanisms in association cortex are responsible for amplifying signals from primary sensory cortex (e.g., visual cortex), resulting in multimodal (e.g., visceral-visual) hypersensitivity.
Provoked Bladder Pain Predicts Visual Discomfort (Question #2)
Participants with greater provoked bladder pain rated the visual stimulation as more unpleasant, even after accounting for menstrual pain and somatic symptoms. This finding demonstrates a relationship between disparate sensory modalities—visceral and visual—in this cohort. Although visual stimulation is associated with increased unpleasantness in other conditions, like migraine [18] and fibromyalgia [36], our study specifically identifies provoked bladder pain as an additional predictor of visual sensitivity. Provoked bladder pain was also related to mechanical and thermal hypersensitivity across non-pelvic sites and correlated with the severity of self-reported bladder pain, bowel pain, and intercourse pain in this cohort [39], and in participants with severe chronic pain [97]. Given the relationship between bladder pain and other sensory modalities, including vision, we recommend expanding the modalities comprising multimodal hypersensitivity to include the viscera (e.g., bladder pain).
Stimulus Driven Oscillatory Activity in Cortex Predicts Visual Discomfort (Question #3a)
We observed a robust brain-behavior relationship that may further support the role of cortical activity in perceived visual unpleasantness. The participants’ perceived unpleasantness to visual stimulation was positively associated with SSVEP amplitude across all electrodes. These findings replicate previous investigations that demonstrated associations between unpleasantness and increased cortical activity in response to aversive visual stimulation [2,34,42,76]. Our visual task showed that SSVEPs, a measure of stimulus driven oscillatory activity in cortex, were effectively modulated and related to self-reported measures of unpleasantness, thus promoting the use of SSVEPs to the study of visual sensitivity. Our study supports evidence that increased unpleasantness to a stimulus is associated with increased sensory pathway activity [15], even in a non-somatic pathway such as vision.
Bladder Pain Sensitivity Moderates the Relationship between Visual Unpleasantness and Cortical Activity
The robust association between visual unpleasantness and cortical activity was positively moderated by provoked bladder pain. A steeper positive relationship between unpleasantness and SSVEP amplitude was observed in women with greater provoked bladder pain (see Figure 5). This moderation accounted for variance associated with menstrual pain and somatic symptoms, suggesting that the bladder provocation response was the predominant factor in our model associated with neural correlates of visual unpleasantness. Moreover, this effect was observed in our a priori electrode of interest (Oz), where SSVEP amplitudes are generally observed and principally arise from primary visual cortex [71,98]. At average primary visual cortex activity, women with visceral hypersensitivity experienced more visual discomfort than women with less visceral sensitivity. Otherwise stated, at low oscillatory activity there were only slight differences in visual unpleasantness, while these differences were more pronounced at increased brightness intensities, with more unpleasantness experienced by women with visceral hypersensitivity.
This finding implies that primary visual cortex activity is not greater in individuals with visceral hypersensitivity, thus implicating the alternative explanation that downstream interpretation of this signal is likely amplified in women with visceral hypersensitivity. Harte and colleagues [36] observed increased insular activation in response to aversive visual stimulation related to pain intensity in fibromyalgia, suggesting the insular cortex as a downstream contributor of integrative processing and sensory amplification. Lόpez-Solà and colleagues [60] also observed this amplification in insular cortex and downstream cortical areas; however, concomitant reductions in primary visual and auditory cortex activity were observed in fibromyalgia patients. In chronic pain conditions, altered neural processing in primary (sensory) cortex, both hypoactivations [60] and hyperactivations [55,66], is commonly observed [4]. We provide contrasting evidence that primary visual cortex activity is not increased in a cohort of women with varying menstrual and bladder pain comorbidity. This discrepancy may emerge from the millisecond temporal resolution of EEG that is methodologically suited to detect the short latency excitation (<50 ms) in primary cortices, unlike fMRI that typically averages signals over several seconds [28].
Somatic Symptoms Moderate the Relationship between Visual Unpleasantness and Parietal Cortical Activity
Electrode positions outside our a priori region of interest (Oz) did not demonstrate moderation effects between perceived unpleasantness and cortical activity for either menstrual pain or provoked bladder pain. Somatic symptoms, however, moderated this association across cortical sites putatively associated with somatosensory processing. Although many different cortical regions could be responsible for these effects at the observed central parietal electrode locations (CP6 and CP1), we can infer potential contributors. The 10-20 system of electrode placement positions CP6 above the angular gyrus (AG) corresponding to Brodmann area 39 [74,75]. The AG has emerged as a major hub that integrates multisensory information to provide a high-level interpretation of our environment [89]. In contrast, electrode CP1 is positioned near the left somatosensory association cortex that plays a vital role in deciphering the context of multimodal percepts and emotional processing [3]. Anterior regions, including the prefrontal cortex, insula, and anterior cingulate cortex, have also demonstrated altered activity during peripheral/visceral sensitization induced by acute inflammation [8,35,46]. It would be valuable for future investigations to establish whether functional communication between parietal cortex and anterior integrative cortex is predictive for the development of chronic pain.
Strengths and Limitations
Some study limitations may qualify our interpretations of the results and generalizability. The initial power analysis to plan the broader clinical trial [39] did not account for the present investigation. Despite this, our sample size was sufficiently powered (80%) to detect a small-to-medium effect ( = .051). These results should be verified in a cohort of severe chronic pain participants and in a comparable risk-enhanced male cohort to assess generalizability across sex.
Although we have previously identified that participants with provoked bladder pain also have increased sensitivity in other nociceptive modalities, such as pressure and cold pain sensitivity [39], other sensory components were not experimentally measured in the current investigation, such as auditory and olfactory sensitivity. Cognitive and affective components, like attention [33] and fear [47], modulate SSVEPs, but were not assessed during the visual task; therefore, we cannot rule out their potential influence. Likewise, our study design and statistical procedure precluded inferences regarding the underlying causes of multimodal hypersensitivity. Rather, we established brain-behavior relationships between disparate sensory systems that inform mechanisms of hypersensitivity that warrant further study. Therefore, future studies would be well served to assess how the underlying dysfunction that we observe as visceral hypersensitivity influences responses of other sensory modalities to broaden our mechanistic understanding of multimodal hypersensitivity.
A key strength of EEG is that it allows for precise temporal measurement of early cortical synaptic activity [90,104]. Prior research has mainly relied on questionnaires to evaluate multimodal hypersensitivity rather than experimental methods [44,59,105,106,109], despite the susceptibility of behavioral ratings of discomfort to retrospective biases [82]. Given the visual task’s relatively brief administration (~2.5 minutes), other studies can readily extend our findings to clarify pathophysiology through experimental evaluation of brain-behavior relationships in conjunction with questionnaire-based assessments. Given these advantages, the visual task is well suited for simultaneous neuromodulation strategies, such as transcranial magnetic or electric stimulation and neurofeedback [48,69,91].
Conclusion
Our findings demonstrated a relationship between visceral and visual sensitivity in a cohort of women with dysmenorrhea. Brain-behavior relationships between stimulus driven cortical activity and visual discomfort were moderated by visceral and somatic sensitivity. These results are congruent with previous work [36,60] that sensory hypersensitivity likely results from aberrant sensory integration rather than increased synaptic activity in primary cortex. This evidence emphasizes the need for effective interventions targeting how sensory information is cortically integrated. Mindfulness interventions that decrease general distress also affect top-down modulation [9,23,30,43,93] and even reduce SSVEP amplitude [87]. A future enhanced mindfulness strategy combining simultaneous neurofeedback [22] from the visual task itself could be used to reprogram the neural circuitry responsible for multimodal hypersensitivity. A key next step is determining whether other extant chronic pain treatments—transcranial magnetic stimulation [32], anticonvulsants [36], or antidepressants [85]—could reduce multimodal hypersensitivity and these associated neural mechanisms in at-risk patients. The paradigm presented here provides a useful experimental method to further evaluate multimodal hypersensitivity and its neural correlates.
Supplementary Material
Acknowledgements
The authors thank Dr. G.F. Gebhart for his sagacious advice and editorial assistance, and Ekarin E. Pongpipat for statistical guidance. They are grateful to Ellen Garrison and Nicole Steiner for technical assistance. This study was funded by the National Institute of Child and Human Development (R01HD098193) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100368). The authors have no conflicts of interest to declare.
Data and Code Availability
The data that support the findings of this study and all code used in data processing and analysis are available on Open Science Framework (https://osf.io/yrzac/?view_only=1231254dc96c4999832ea0c8056374fa); DOI 10.17605/OSF.IO/YRZAC
References
- [1].Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116–126. [DOI] [PubMed] [Google Scholar]
- [2].Adjamian P, Holliday IE, Barnes GR, Hillebrand A, Hadjipapas A, Singh KD. Induced visual illusions and gamma oscillations in human primary visual cortex. Eur J Neurosci 2004;20:587–592. [DOI] [PubMed] [Google Scholar]
- [3].Anders S, Birbaumer N, Sadowski B, Erb M, Mader I, Grodd W, Lotze M. Parietal somatosensory association cortex mediates affective blindsight. Nat Neurosci 2004;7:339–340. [DOI] [PubMed] [Google Scholar]
- [4].Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9:463–463. [DOI] [PubMed] [Google Scholar]
- [5].Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Drewes AM. Assessment and manifestation of central sensitisation across different chronic pain conditions. 2017:26. [DOI] [PubMed] [Google Scholar]
- [6].Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol 1995;57:289–300. [Google Scholar]
- [7].Bennett RM. Emerging Concepts in the Neurobiology of Chronic Pain: Evidence of Abnormal Sensory Processing in Fibromyalgia. Mayo Clin Proc 1999;74:385–398. [DOI] [PubMed] [Google Scholar]
- [8].Benson S, Rebernik L, Wegner A, Kleine-Borgmann J, Engler H, Schlamann M, Forsting M, Schedlowski M, Elsenbruch S. Neural circuitry mediating inflammation-induced central pain amplification in human experimental endotoxemia. Brain Behav Immun 2015;48:222–231. [DOI] [PubMed] [Google Scholar]
- [9].Berkovich-Ohana A, Glicksohn J, Goldstein A. Mindfulness-induced changes in gamma band activity - implications for the default mode network, self-reference and attention. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 2012;123:700–710. [DOI] [PubMed] [Google Scholar]
- [10].Boeckle M, Schrimpf M, Liegl G, Pieh C. Neural correlates of somatoform disorders from a meta-analytic perspective on neuroimaging studies. 2016:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van den Broeke EN, Mouraux A. High-frequency electrical stimulation of the human skin induces heterotopical mechanical hyperalgesia, heat hyperalgesia, and enhanced responses to nonnociceptive vibrotactile input. J Neurophysiol 2014;111:1564–1573. [DOI] [PubMed] [Google Scholar]
- [12].Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 2014;44:68–75. [DOI] [PubMed] [Google Scholar]
- [13].Clark JR, Nijs J, Yeowell G, Holmes P, Goodwin PC. Trait Sensitivity, Anxiety, and Personality Are Predictive of Central Sensitization Symptoms in Patients with Chronic Low Back Pain. Pain Pract 2019;19:800–810. [DOI] [PubMed] [Google Scholar]
- [14].Cohen J The Cost of Dichotomization. Appl Psychol Meas 1983;7:249–253. [Google Scholar]
- [15].Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central Hypersensitivity in Chronic Pain: Mechanisms and Clinical Implications. Phys Med Rehabil Clin N Am 2006;17:287–302. [DOI] [PubMed] [Google Scholar]
- [16].Curtin J lmSupport: Support for Linear Models. R package version 2.9.13, 2018. Available: https://CRAN.R-project.org/package=lmSupport. [Google Scholar]
- [17].Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- [18].Demarquay G, Mauguière F. Central Nervous System Underpinnings of Sensory Hypersensitivity in Migraine: Insights from Neuroimaging and Electrophysiological Studies. Headache J Head Face Pain 2016;56:1418–1438. [DOI] [PubMed] [Google Scholar]
- [19].Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13:595–605. [PubMed] [Google Scholar]
- [20].Deutsch G, Deshpande H, Frölich MA, Lai HH, Ness TJ. Bladder Distension Increases Blood Flow in Pain Related Brain Structures in Subjects with Interstitial Cystitis. J Urol 2016;196:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Di Russo F, Pitzalis S, Aprile T, Spitoni G, Patria F, Stella A, Spinelli D, Hillyard SA. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Hum Brain Mapp 2007;28:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dunham CM, Burger AL, Hileman BM, Chance EA, Hutchinson AE, Kohli CM, DeNiro L, Tall JM, Lisko P. Brainwave Self-Regulation During Bispectral IndexTM Neurofeedback in Trauma Center Nurses and Physicians After Receiving Mindfulness Instructions. Front Psychol 2019;10:2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci 2007;2:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fitzcharles M-A, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. The Lancet 2021;397:2098–2110. [DOI] [PubMed] [Google Scholar]
- [25].Ford AC, Bercik P, Morgan DG, Bolino C, Pintos–Sanchez MI, Moayyedi P. Validation of the Rome III Criteria for the Diagnosis of Irritable Bowel Syndrome in Secondary Care. Gastroenterology 2013;145:1262–1270.e1. [DOI] [PubMed] [Google Scholar]
- [26].Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A Psychophysical Study of Auditory and Pressure Sensitivity in Patients With Fibromyalgia and Healthy Controls. J Pain 2008;9:417–422. [DOI] [PubMed] [Google Scholar]
- [27].Geisser ME, Robinson ME, Keefe FJ, Weiner ML. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain 1994;59:79–83. [DOI] [PubMed] [Google Scholar]
- [28].Ghuman AS, Martin A. Dynamic Neural Representations: An Inferential Challenge for fMRI. Trends Cogn Sci 2019;23:534–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gracely RH, Kwilosz DM. The Descriptor Differential Scale: applying psychophysical principles to clinical pain assessment: Pain 1988;35:279–288. [DOI] [PubMed] [Google Scholar]
- [30].Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain 2011;152:150–156. [DOI] [PubMed] [Google Scholar]
- [31].Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ, Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132 Suppl 1:S26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Grisaru N, Amir M, Cohen H, Kaplan Z. Effect of transcranial magnetic stimulation in posttraumatic stress disorder: a preliminary study. Biol Psychiatry 1998;44:52–55. [DOI] [PubMed] [Google Scholar]
- [33].Gulbinaite R, Roozendaal DHM, VanRullen R. Attention differentially modulates the amplitude of resonance frequencies in the visual cortex. NeuroImage 2019;203:116146. [DOI] [PubMed] [Google Scholar]
- [34].Haigh SM, Barningham L, Berntsen M, Coutts LV, Hobbs EST, Irabor J, Lever EM, Tang P, Wilkins AJ. Discomfort and the cortical haemodynamic response to coloured gratings. Vision Res 2013;89:47–53. [DOI] [PubMed] [Google Scholar]
- [35].Hannestad J, Subramanyam K, DellaGioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, Carson RE. Glucose Metabolism in the Insula and Cingulate Is Affected by Systemic Inflammation in Humans. J Nucl Med 2012;53:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Harte SE, Ichesco E, Hampson JP, Peltier SJ, Schmidt-Wilcke T, Clauw DJ, Harris RE. Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula: PAIN 2016;157:1933–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Harte SE, Schrepf A, Gallop R, Kruger GH, Lai HHH, Sutcliffe S, Halvorson M, Ichesco E, Naliboff BD, Afari N, Harris RE, Farrar JT, Tu F, Landis JR, Clauw DJ, for the MAPP Research Network. Quantitative assessment of nonpelvic pressure pain sensitivity in urologic chronic pelvic pain syndrome: a MAPP Research Network study. Pain 2019;160:1270–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hellman KM, Datta A, Steiner ND, Kane Morlock JN, Garrison EF, Clauw DJ, Tu FF. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol 2018;219:84.e1–84.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hellman KM, Roth GE, Dillane KE, Garrison EF, Oladosu FA, Clauw DJ, Tu FF. Dysmenorrhea subtypes exhibit differential quantitative sensory assessment profiles: PAIN 2020;161:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S, European Palliative Care Research Collaborative (EPCRC). Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011;41:1073–1093. [DOI] [PubMed] [Google Scholar]
- [41].Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, Siddiqi MQ, Maixner W. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: An evaluation of the generalized hypervigilance hypothesis: Pain 2009;141:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huang J, Zong X, Wilkins A, Jenkins B, Bozoki A, Cao Y. fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia 2011;31:925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jacobs GD, Benson H, Friedman R. Topographic EEG mapping of the relaxation response. Biofeedback Self-Regul 1996;21:121–129. [DOI] [PubMed] [Google Scholar]
- [44].Jones R, Latinovic R, Charlton J, Gulliford M. Physical and psychological co-morbidity in irritable bowel syndrome: a matched cohort study using the General Practice Research Database. Aliment Pharmacol Ther 2006;24:879–886. [DOI] [PubMed] [Google Scholar]
- [45].Judd CM, McClelland GH, Ryan CS. Data Analysis: A Model Comparison Approach to Regression, ANOVA, and Beyond. Third Edition. New York, NY: Routledge, 2017. [Google Scholar]
- [46].Karshikoff B, Jensen KB, Kosek E, Kalpouzos G, Soop A, Ingvar M, Olgart Höglund C, Lekander M, Axelsson J. Why sickness hurts: A central mechanism for pain induced by peripheral inflammation. Brain Behav Immun 2016;57:38–46. [DOI] [PubMed] [Google Scholar]
- [47].Kastner-Dorn AK, Andreatta M, Pauli P, Wieser MJ. Hypervigilance during anxiety and selective attention during fear: Using steady-state visual evoked potentials (ssVEPs) to disentangle attention mechanisms during predictable and unpredictable threat. Cortex 2018;106:120–131. [DOI] [PubMed] [Google Scholar]
- [48].Kayiran S, Dursun E, Dursun N, Ermutlu N, Karamürsel S. Neurofeedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Appl Psychophysiol Biofeedback 2010;35:293–302. [DOI] [PubMed] [Google Scholar]
- [49].Kayser J Current source density (CSD) interpolation using spherical splines - CSD Toolbox (Version 1.1). New York State Psychiatric Institute: Division of Cognitive Neuroscience, 2009. Available: http://psychophysiology.cpmc.columbia.edu/Software/CSDtoolbox. [Google Scholar]
- [50].Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol 2006;117:348–368. [DOI] [PubMed] [Google Scholar]
- [51].Kilpatrick Lisa A, Kutch Jason J, Kirsten Tillisch, Naliboff Bruce D, Labus Jennifer S, Zhiguo Jiang, Farmer Melissa A., Vania Apkarian A., Sean Mackey, Martucci Katherine T., Clauw Daniel J., Harris Richard E., Georg Deutsch, Ness Timothy J., Yang Claire C., Kenneth Maravilla, Chris Mullins, Mayer Emeran A. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. J Urol 2014;192:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kutch JJ, Ichesco E, Hampson JP, Labus JS, Farmer MA, Martucci KT, Ness TJ, Deutsch G, Apkarian AV, Mackey SC, Klumpp DJ, Schaeffer AJ, Rodriguez LV, Kreder KJ, Buchwald D, Andriole GL, Lai HH, Mullins C, Kusek JW, Landis JR, Mayer EA, Clemens JQ, Clauw DJ, Harris RE, for the MAPP Research Network. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain 2017;158:1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kutch JJ, Labus JS, Harris RE, Martucci KT, Farmer MA, Fenske S, Fling C, Ichesco E, Peltier S, Petre B, Guo W, Hou X, Stephens AJ, Mullins C, Clauw DJ, Mackey SC, Apkarian AV, Landis JR, Mayer EA, for the MAPP Research Network. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain 2017;158:1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C, Farmer MA, Johnson KA, Ness TJ, Deutsch G, Harris RE, Apkarian AV, Clauw DJ, Mackey SC, Mullins C, Mayer EA. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP. NeuroImage Clin 2015;8:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lang E, Kaltenhauser M, Neundorfer B, Seidler S. Hyperexcitability of the primary somatosensory cortex in migraine—a magnetoencephalographic study. Brain 2004;127:2459–2469. [DOI] [PubMed] [Google Scholar]
- [56].Larsson MBO, Tillisch K, Craig AD, Engström M, Labus J, Naliboff B, Lundberg P, Ström M, Mayer EA, Walter SA. Brain Responses to Visceral Stimuli Reflect Visceral Sensitivity Thresholds in Patients With Irritable Bowel Syndrome. 2012;142:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li R, Kreher DA, Jusko TA, Chapman BP, Bonham AD, Seplaki CL. Prospective Association between Dysmenorrhea and Chronic Pain Development in Community-Dwelling Women. J Pain 2021. doi: 10.1016/j.jpain.2021.03.139. [DOI] [PubMed] [Google Scholar]
- [58].Li R, Li B, Kreher DA, Benjamin AR, Gubbels A, Smith SM. Association between dysmenorrhea and chronic pain: a systematic review and meta-analysis of population-based studies. Am J Obstet Gynecol 2020;223:350–371. [DOI] [PubMed] [Google Scholar]
- [59].Lionetti F, Aron A, Aron EN, Burns GL, Jagiellowicz J, Pluess M. Dandelions, tulips and orchids: evidence for the existence of low-sensitive, medium-sensitive and high-sensitive individuals. Transl Psychiatry 2018;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].López-Solà M, Pujol J, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodríguez O, Monfort J, Garcia-Fructuoso F, Deus J. Altered Functional Magnetic Resonance Imaging Responses to Nonpainful Sensory Stimulation in Fibromyalgia Patients: Brain Response to Nonpainful Multisensory Stimulation in Fibromyalgia. Arthritis Rheumatol 2014;66:3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lüdecke D, Makowski D, Waggoner P, Patil I. Assessment of Regression Models Performance. 2020. Available: https://easystats.github.io/performance/.
- [62].Makeig S, Jung T-P, Bell AJ, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proc Natl Acad Sci 1997;94:10979–10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Martenson ME, Halawa OI, Tonsfeldt KJ, Maxwell CA, Hammack N, Mist SD, Pennesi ME, Bennett RM, Mauer KM, Jones KD, Heinricher MM. A possible neural mechanism for photosensitivity in chronic pain. PAIN 2016;157:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Martucci KT, Shirer WR, Bagarinao E, Johnson KA, Farmer MA, Labus JS, Apkarian AV, Deutsch G, Harris RE, Mayer EA, Clauw DJ, Greicius MD, Mackey SC. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network—a resting-state study from the MAPP Research Network. Pain 2015;156:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995;109:40–52. [DOI] [PubMed] [Google Scholar]
- [66].Montoya P, Sitges C, García-Herrera M, Rodríguez-Cotes A, Izquierdo R, Truyols M, Collado D. Reduced brain habituation to somatosensory stimulation in patients with fibromyalgia. Arthritis Rheum 2006;54:1995–2003. [DOI] [PubMed] [Google Scholar]
- [67].Mullen T CleanLine EEGLAB plugin. San Diego, CA: Neuroimaging Informatics Tools and Resources Clearinghouse (NITRC)., 2012. [Google Scholar]
- [68].Napadow V, Kim J, Clauw DJ, Harris RE. Brief Report: Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 2012;64:2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Neeb L, Bayer A, Bayer K-E, Farmer A, Fiebach JB, Siegmund B, Volz MS. Transcranial direct current stimulation in inflammatory bowel disease patients modifies resting-state functional connectivity: A RCT. Brain Stimulat 2019;12:978–980. [DOI] [PubMed] [Google Scholar]
- [70].Neuwirth E RColorBrewer: ColorBrewer Palettes. R package version 1.1–2, 2014. Available: https://CRAN.R-project.org/package=RColorBrewer.
- [71].Norcia AM, Appelbaum LG, Ales JM, Cottereau BR, Rossion B. The steady-state visual evoked potential in vision research: A review. J Vis 2015;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].O’Hare L Steady-state VEP responses to uncomfortable stimuli. Eur J Neurosci 2017;45:410–422. [DOI] [PubMed] [Google Scholar]
- [73].O’Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology 1997;49:58–63. [DOI] [PubMed] [Google Scholar]
- [74].Pascual-Marqui RD. Review of Methods for Solving the EEG Inverse Problem. 1999;1:13. [Google Scholar]
- [75].Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol Off J Int Organ Psychophysiol 1994;18:49–65. [DOI] [PubMed] [Google Scholar]
- [76].Patterson Gentile C, Aguirre GK. A neural correlate of visual discomfort from flicker. J Vis 2020;20:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Payne LA, Rapkin A, Seidman L, Zeltzer L, Tsao J. Experimental and procedural pain responses in primary dysmenorrhea: a systematic review. J Pain Res 2017;Volume 10:2233–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pederson TL. patchwork: The Composer of Plots. R package version 1.0.0, 2019. Available: https://CRAN.R-project.org/package=patchwork.
- [79].Petzke F, Harris RE, Williams DM, Clauw DJ, Gracely RH. Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. Eur J Pain 2005;9:325–335. [DOI] [PubMed] [Google Scholar]
- [80].Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states - Maybe it is all in their head. Best Pract 2011:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria., 2020. Available: https://www.R-project.org/. [Google Scholar]
- [82].Redelmeier DA, Kahneman D. Patients’ memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain 1996;66:3–8. [DOI] [PubMed] [Google Scholar]
- [83].Robinson D, Hayes A. broom: Convert Statistical Analysis Objects into Tidy Tibbles. R package version 0.5.5., 2020. Available: https://CRAN.R-project.org/package=broom.
- [84].Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006;25:127–141. [DOI] [PubMed] [Google Scholar]
- [85].Sayar K, Barsky AJ, Gulec H. Does somatosensory amplification decrease with antidepressant treatment? Psychosomatics 2005;46:340–344. [DOI] [PubMed] [Google Scholar]
- [86].Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol 2019;220:569.e1–569.e7. [DOI] [PubMed] [Google Scholar]
- [87].Schöne B, Gruber T, Graetz S, Bernhof M, Malinowski P. Mindful breath awareness meditation facilitates efficiency gains in brain networks: A steady-state visually evoked potentials study. Sci Rep 2018;8:13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Schrepf A, Williams DA, Gallop R, Naliboff BD, Basu N, Kaplan C, Harper DE, Landis JR, Clemens JQ, Strachan E, Griffith JW, Afari N, Hassett A, Pontari MA, Clauw DJ, Harte SE. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP Research Network study. PAIN 2018;159:2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Seghier ML. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. The Neuroscientist 2013;19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Silberstein RB, Nunez PL, Pipingas A, Harris P, Danieli F. Steady state visually evoked potential (SSVEP) topography in a graded working memory task. Int J Psychophysiol 2001;42:14. [DOI] [PubMed] [Google Scholar]
- [91].Stokes DA, Lappin MS. Neurofeedback and biofeedback with 37 migraineurs: a clinical outcome study. Behav Brain Funct BBF 2010;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Torta DME, Van Den Broeke EN, Filbrich L, Jacob B, Lambert J, Mouraux A. Intense pain influences the cortical processing of visual stimuli projected onto the sensitized skin. Pain 2017;158:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Travis F, Haaga DAF, Hagelin J, Tanner M, Arenander A, Nidich S, Gaylord-King C, Grosswald S, Rainforth M, Schneider RH. A self-referential default brain state: patterns of coherence, power, and eLORETA sources during eyes-closed rest and Transcendental Meditation practice. Cogn Process 2010;11:21–30. [DOI] [PubMed] [Google Scholar]
- [94].Tu C-H, Niddam DM, Chao H-T, Liu R-S, Hwang R-J, Yeh T-C, Hsieh J-C. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. NeuroImage 2009;47:28–35. [DOI] [PubMed] [Google Scholar]
- [95].Tu FF, Datta A, Atashroo D, Senapati S, Roth G, Clauw DJ, Hellman KM. Clinical profile of comorbid dysmenorrhea and bladder sensitivity: a cross-sectional analysis. Am J Obstet Gynecol 2020;222:594.e1–594.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A Noninvasive Bladder Sensory Test Supports a Role for Dysmenorrhea Increasing Bladder Noxious Mechanosensitivity: Clin J Pain 2013;29:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tu FF, Kane JN, Hellman KM. Noninvasive experimental bladder pain assessment in painful bladder syndrome. BJOG Int J Obstet Gynaecol 2017;124:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Vialatte F-B, Maurice M, Dauwels J, Cichocki A. Steady-state visually evoked potentials: Focus on essential paradigms and future perspectives. Prog Neurobiol 2010;90:418–438. [DOI] [PubMed] [Google Scholar]
- [99].Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women: Pain 2011;152:1966–1975. [DOI] [PubMed] [Google Scholar]
- [100].Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am J Obstet Gynecol 2013;209:422.e1–422.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wickham H ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag, 2016. [Google Scholar]
- [102].Wickham H, François R, Henry L, Müller K. dplyr: A Grammar of Data Manipulation. R package version 0.8.5, 2020. Available: https://CRAN.R-project.org/package=dplyr.
- [103].Winkler I, Haufe S, Tangermann M. Automatic Classification of Artifactual ICA-Components for Artifact Removal in EEG Signals. Behav Brain Funct 2011;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wu Z Physical connections between different SSVEP neural networks. Sci Rep 2016;6:22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yano K Replication of the three sensitivity groups and investigation of their characteristics in Japanese samples. Curr Psychol n.d.:10. [Google Scholar]
- [106].Yavuz BG, Aydinlar EI, Dikmen PY, Incesu C. Association between somatic amplification, anxiety, depression, stress and migraine. J Headache Pain 2013;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yee CM, Javitt DC, Miller GA. Replacing DSM Categorical Analyses With Dimensional Analyses in Psychiatry Research: The Research Domain Criteria Initiative. JAMA Psychiatry 2015;72:1159–1160. [DOI] [PubMed] [Google Scholar]
- [108].Zhu D, Bieger J, Garcia Molina G, Aarts RM. A Survey of Stimulation Methods Used in SSVEP-Based BCIs. Comput Intell Neurosci 2010;2010:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zincir SB, Sunbul M, Sunbul EA, Dalkilic B, Cengiz F, Kivrak T, Durmus E. Evaluation of alexithymia, somatosensory sensitivity, and health anxiety levels in patients with noncardiac chest pain. BioMed Res Int 2014;2014:896183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zondervan KT, Yudkin PL, Vessey MP, Jenkinson CP, Dawes MG, Barlow DH, Kennedy SH. Chronic pelvic pain in the community—Symptoms, investigations, and diagnoses. Am J Obstet Gynecol 2001;184:1149–1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study and all code used in data processing and analysis are available on Open Science Framework (https://osf.io/yrzac/?view_only=1231254dc96c4999832ea0c8056374fa); DOI 10.17605/OSF.IO/YRZAC


