Abstract
The capability of most higher plants to tolerate environmental conditions strongly depends on their developmental stage. In addition, environmental factors have pleiotropic effects on many developmental processes. The interaction between plant development and environmental conditions implies that some genes must be regulated by both environmental factors and developmental cues. To understand their developmental regulation and obtain possible clues on their functions, we have isolated genomic clones for RCI2A and RCI2B, two genes from Arabidopsis ecotype Columbia (Col), whose expression is induced in response to low temperature, dehydration, salt stress, and abscisic acid. The promoters of RCI2A and RCI2B were fused to the uidA (GUS)-coding sequence and the resulting constructs used to transform Arabidopsis. GUS activity was analyzed in transgenic plants during development under both stressed and unstressed conditions. Transgenic plants with either the RCI2A or RCI2B promoter showed strong GUS expression during the first stages of seed development and germination, in vascular bundles, pollen, and most interestingly in guard cells. When transgenic plants were exposed to low temperature, dehydration, salt stress, or abscisic acid, reporter gene expression was induced in most tissues. These results indicate that RCI2A and RCI2B are regulated at transcriptional level during plant development and in response to different environmental stimuli and treatments. The potential role of RCI2A and RCI2B in plant development and stress response is discussed.
Many plants from temperate regions are able to increase their freezing tolerance in response to low, non-freezing temperatures. This adaptive process, known as cold acclimation (Levitt, 1980; Sakai and Larcher, 1987), involves a number of biochemical and physiological changes, including ultrastructural modifications in cellular organelles (Niki and Sakai, 1981; Fujikawa and Takabe, 1996), compositional changes in apoplastic solutions and plasma membrane (Steponkus, 1984; Yoshida, 1984; Zhou et al., 1994; Griffith and Antikainen, 1996), accumulation of intracellular compatible osmolytes (Hare et al., 1998), and increased rigidity of cell walls (Rajashekar and Lafta, 1996). Many of these alterations are regulated by low temperatures through changes in gene expression, and a number of cold-inducible genes have been isolated and characterized from several plant species (for review, see Hughes and Dunn, 1996; Thomashow, 1999). Although the expression of some cold-inducible genes seems to be specifically regulated by low temperature, most of them are also responsive to abscisic acid (ABA) and water stress (Hughes and Dunn, 1996; Thomashow, 1999). The physiological relevance of this observation is sustained by the fact that both ABA and water stress treatments reproduce the effects of low temperature by increasing freezing tolerance (Cloutier and Siminovitch, 1982; Chen and Gusta, 1983; Mäntylä et al., 1995). Furthermore, several cold-inducible genes have also been reported to be regulated by salt stress (Kurkela and Borg-Franck, 1992; Yamaguchi-Shinozaki and Shinozaki, 1994), pathogen infection (Molina and García-Olmedo, 1993), hypoxia (Jarillo et al., 1993), light stress (Leyva et al., 1995; Capel et al., 1998), or mechanical stress (Polisensky and Braam, 1996).
Considering that freezing tolerance of plant tissues is affected by their developmental stage (Wang and Cutler, 1995) and low temperatures have pleiotropic effects on many developmental processes (Thomashow, 1999), it is expected that some cold-inducible genes are also regulated by intrinsic developmental cues. Thus, it is surprising that, despite the number of cold-inducible genes that have been identified, the information regarding their regulation during plant growth and development is still very limited. Studies on the developmental regulation of genes whose expression is induced by low temperatures not only may help to reveal how environmental conditions interact with developmental processes, but also may provide clues to their function by uncovering where and when they are required. Moreover, if these genes are going to be expressed in heterologous plants to increase cold or freezing tolerance, their developmental expression must be fully characterized. Analyses of promoter-GUS fusions have shown that the Arabidopsis ecotype Columbia (Col), cold-inducible RD29A, COR15A, KIN1, and COR6.6 genes are regulated during plant development under both stressed (cold) and unstressed conditions (Yamaguchi-Shinozaki and Shinozaki, 1993; Baker et al., 1994; Wang and Cutler, 1995). The expression of BLT101, BLT4.9, and BLT14, three cold-inducible genes from barley, has also been reported to be regulated during development in cold-acclimated plants (Pearce et al., 1998). In addition, TCH3 and TCH4, two genes from Arabidopsis encoding calmodulin- and xyloglucan endotransglycosylase-related proteins, are also regulated not only in response to low temperature but also during plant development under unstressed conditions (Sistrunk et al., 1994; Xu et al., 1995). It is interesting that most of these genes show highly specific expression patterns during plant development, suggesting that the molecular mechanisms of cold acclimation differ markedly between tissues.
In a previous work (Capel et al., 1997), we described the isolation and molecular characterization of RCI2A and RCI2B, two homologous rare cold inducible genes that constitute a new small family of low temperature regulated genes in Arabidopsis. The expression of these genes is transiently regulated during cold acclimation and is also induced by ABA and water stress. Furthermore, RCI2A and RCI2B expression is up-regulated by low temperature in ABA-deficient (aba) and -insensitive (abi) mutants of Arabidopsis, suggesting that both ABA-dependent and -independent pathways regulate their low-temperature responsiveness. RCI2A and RCI2B encode small (54 residues), highly hydrophobic proteins with two potential transmembrane domains. The absence of signals for organelle targeting allowed to propose that both proteins could be localized in the plasma membrane, which is considered a primary site of injury during freezing (Lyons, 1973). A role for RCI2A and RCI2B in maintaining membrane function and/or integrity in water stress situations triggered by low temperatures, freezing, or other environmental conditions that reduce water availability was suggested accordingly. It was proposed, alternatively, that they could interact with other membrane proteins to maintain the hydric equilibrium of the cells (Capel et al., 1997).
To investigate the regulation of RCI2A and RCI2B during plant development and to gain new insights on the role of these genes in low temperature response, we isolated and characterized the corresponding genomic clones. The promoter regions were fused to the uidA (GUS) reporter gene, and the levels of GUS activity were analyzed quantitatively in Arabidopsis transgenic plants carrying the resulting constructs under different treatments known to be related to cold stress. In addition, the developmental profiles of RCI2A-GUS and RCI2B-GUS expression was examined by histochemical analysis of GUS activity in the transgenic plants under both stressed and unstressed conditions.
RESULTS
Isolation and Molecular Characterization of RCI2A and RCI2B Genomic Clones
The RCI2A cDNA (Capel et al., 1997) was used as a probe to screen an Arabidopsis genomic library. Five positives recombinant phages were isolated and analyzed. Two of them contained an identical EcoRI fragment of approximately 4.5 kb that showed a strong hybridation signal to both RCI2A and RCI2B cDNAs. This fragment was subcloned and sequenced, revealing that it contained both the RCI2A and RCI2B genes. These genes are thus tandemly arranged in the genome of Arabidopsis, RCI2B being located 1 kb upstream from the RCI2A transcription initiation site (Fig. 1A). The sequence of the 4.5-kb genomic fragment has been deposited in the GenBank database with the accession number AF264749.
Figure 1.
Molecular characterization of RCI2A and RCI2B genes. A, Organization of RCI2A and RCI2B in the 4.5-kb EcoRI genomic fragment. Exons are shown as white boxes and introns as black boxes. Transcriptional orientation is indicated by arrows. B, Sequence comparison of RCI2A and RCI2B promoter regions. Transcription start sites are indicated by +1 and underlined. The initiation ATGs codons, and putative TATA boxes are double underlined. G-Box (CACGTG) and related sequences are shown in gray boxes. Black boxes highlight LTRE/DRE/C-repeat (CCGAC) and related sequences. MYB-like sequences (TAACCA) are indicated as open boxes. The MYC-like sequence (CACATG) is shaded. The pollen-specific regulatory element AAATGA is underlined. Identical nucleotides are indicated by a line. Points represent gaps inserted into the sequences for optimal alignment.
Comparison of the genomic and cDNA sequences showed that RCI2A and RCI2B contain two introns each, the first being located into their coding regions while the second into their 3′-untranslated regions. The introns are slightly longer in RCI2A (136/415 bp) than in RCI2B (109/384 bp). The RCI2A and RCI2B coding regions are 84% identical, whereas the introns are 40.4% and 29.9% identical depending on their location into coding or untranslated regions, respectively. The transcription initiation sites were determined by primer extension analysis (data not shown), and are indicated in the nucleotide sequences of the promoter regions (Fig. 1B) by position +1. The initiation ATG codons of RCI2A and RCI2B are 72 and 52 bp downstream from the transcription initiation sites, respectively. Putative TATA box sequences are located at positions −37 (TATAA) in RCI2A and −42 (TATATA) in RCI2B. A putative polyadenylation signal was found in the 3′-untranslated region of RCI2A at 230 bp from the stop codon, but no related signal could be identified in the 3′-untranslated region of RCI2B (data not shown). The predicted amino acid sequences from both RCI2A and RCI2B genomic clones (Fig. 2) are identical to those previously deduced from the corresponding cDNAs clones (Capel et al., 1997).
Figure 2.
Sequence similarity between RCI2 and related proteins. Alignment of amino acid sequences of RCI2 and similar proteins from Hordeum vulgare (BLT101; accession no. Z25537), L. elongatum (ESI3; accession no. U00966), Saccharomyces cerevisiae (PMP3; accession no. X91499), Synechocystis sp. (SSR1169; accession no. P74805), E. coli (YQAE; accession no. P77240), and Caenorhabditis elegans (T23F23; accession no. Q22700). Identical amino acid residues to RCI2A sequence are indicated by asterisks. Dashes represent gaps inserted into the sequences for optimal alignment. Gray boxes highlight the putative transmembrane domains. The number of residues of each sequence is indicated in the right side.
A search in the protein sequence databases revealed that in addition to the RCI2 plant relatives described in barley (BLT101; Goddard et al., 1993) and Lophopyrum elongatum (ESI3; Gulick et al., 1994), proteins showing high similarity (37%–78% sequence identity) with RCI2A and RCI2B have been found in yeast (PMP3; Navarre and Goffeau, 2000), cyanobacteria (SSR1169; GenBank database accession no. P74805), Escherichia coli (YQAE; GenBank database accession no. P77240), and nematodes (T23F23; GenBank database accession no. Q22700; Fig. 2). This suggests that these proteins fulfill an essential role that is conserved throughout evolution in living cells.
Sequence Analysis of RCI2A and RCI2B Promoter Regions
The isolated 4.5-kb genomic fragment containing the RCI2A and RCI2B genes included the whole promoter region of RCI2A (1,088 bp) and 600 bp of the RCI2B promoter. The comparison of these promoter regions revealed a moderate level (27%) of identity (Fig. 1B). Regulatory motifs similar to those described in other plant genes were identified in RCI2A and RCI2B promoters (Fig. 1B). Both promoters contain putative regulatory elements similar or identical to the G-box core element (CACGTG), which is involved in regulating gene expression in response to a variety of environmental stresses and ABA (Guiltinan et al., 1990; Schindler et al., 1992; Williams et al., 1992; Busk and Pages, 1998). Furthermore, a pentamer CCGAC, which corresponds to the core sequence of the low-temperature and drought response element (LTRE/DRE/C-repeat; Baker et al., 1994; Yamaguchi-Shinozaki and Shinozaki 1994; Jiang et al., 1996; Ouellet et al., 1998), was present at position −438 in the minus strand of the RCI2A promoter. Sequences that differ in only one nucleotide from the LTRE/DRE/C-repeat core motif, were also found in the same promoter. In contrast, the sequence CCTAC (−116) was the only motif related to the LTRE/DRE/C-repeat element that could be detected in the RCI2B promoter. Sequences resembling MYB (PyAACNPu; Biedenkapp et al., 1988; Nakagoshi et al., 1990) and MYC (CACATG; Abe et al., 1997) binding regions were also found in the RCI2A and RCI2B promoters. In addition, the pollen-specific regulatory element AAATGA (Weterings et al., 1995) was present once in the promoter of RCI2A and three times in that of RCI2B (Fig. 1B).
Expression of RC2A and RCI2B in Response to Stress Conditions as Quantitatively Determined by GUS Activity
The expression of RCI2A and RCI2B is induced in response to different treatments including low temperature, dehydration, and exogenous ABA (Capel et al., 1997). To elucidate whether the cis-elements involved in the regulation of these responses are located in the isolated promoter fragments, Arabidopsis plants were transformed with chimeric genes consisting of the isolated RCI2 promoter regions (−1,088 to +71 and −595 to +51 for RCI2A and RCI2B, respectively; Fig. 1B) fused to a GUS reporter gene. Stable transformant lines were obtained and three independent representative ones, all homozygous for each construct, were analyzed. Figure 3 shows the results obtained in these lines after exposure to low temperature, dehydration, ABA, and salt stress. For all treatments, GUS activity exhibited by the RCI2A-GUS plants was higher than that showed by the RCI2B-GUS ones. However, the relative increase of GUS activity obtained after treatments when compared with control values were similar in both types of transgenic plants. A 5-fold increase in GUS activity was observed in both RCI2A- and RCI2B-GUS transgenic lines upon exposure to 4°C for 24 h. In dehydrated transgenic plants, GUS activity values reached slightly higher levels than those obtained after low temperature exposure. Treatments with a 100-μm solution of ABA for 6 h were also effective in increasing GUS activity, although the attained levels were lower than those obtained in response to low temperature and dehydration. Regarding salt stress, it was previously reported (Capel et al., 1997) that the expression of RCI2 genes was not regulated by NaCl. However, RCI2 mRNAs accumulation in response to NaCl could be transient having escaped from detection in those experimental conditions. A time-course experiment revealed that, in fact, RCI2A and RCI2B transcript levels increased after exposing Arabidopsis plants for 12 h to 250 mm NaCl. Transcript levels subsequently started to decline demonstrating that transcript accumulation was transient (data not shown). When RCI2A- and RCI2B-GUS transgenic plants were exposed to the same stress conditions, a 3-fold increase in GUS activity was observed (Fig. 3). All these data suggest that the isolated promoter regions contain the cis-acting elements that are involved in the response of RCI2A and RCI2B to low temperature, dehydration, ABA, and salt stress. In addition, these results indicate that the expression of RCI2A and RCI2B in response to these treatments is regulated at the transcriptional level.
Figure 3.
GUS activity in transgenic seedlings of Arabidopsis carrying RCI2A- and RCI2B-GUS fusions, exposed to different treatments. Ten-day-old seedlings were used as controls (C), exposed to 4°C for 24 h (COLD), dehydrated till losing 50% of fresh weight (DH), treated with 100 μm ABA (ABA), and exposed to 250 mm NaCl for 12 h (NaCl). Data are expressed as means (n = 15; five plants of tree independent transformant lines). Bars indicate se. In all cases, values obtained from treated and control plants were significantly different (P < 0.05) as determined by Student's t test.
Expression of RCI2A and RCI2B during Plant Development as Histochemically Determined by GUS Activity
Once proved that the isolated promoter regions of RCI2A and RCI2B were able to drive the transcription of the GUS gene in response to low temperature, dehydration, ABA, and high salinity, this reporter gene assay was used to follow the expression of each RCI2 gene in situ, and thus to investigate whether their expression was also regulated during plant development. It is worthy to note that, despite the expected variability due to the position effect, within each set of transgenic lines (RCI2A- or RCI2B-GUS) most individual plants displayed a similar GUS expression pattern.
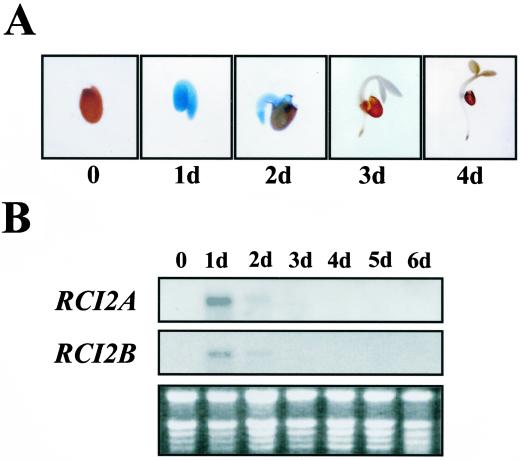
Seed germination was studied as representative of the early stages of plant development. Histochemical analysis of GUS activity showed no staining in both RCI2A- and RCI2B-GUS mature seeds (Fig. 4A). Seeds that were cut to facilitate the penetration of the GUS substrate did not show any GUS activity either (data not shown). After 1 d of germination, RCI2A- and RCI2B-GUS transgenic seedlings showed a strong GUS staining in the cotyledons and radicle. This staining progressively decreased during germination, particularly in cotyledons, disappearing after the 4th d (Fig. 4A). The transient accumulation of RCI2 transcripts during seed germination was verified by RNA-blot hybridizations with total RNA extracted from mature seeds and 1- to 6-d-old seedlings (Fig. 4B).
Figure 4.
Expression of RCI2A and RCI2B during seed germination. A, Histochemical localization of GUS activity in germinating transgenic seeds containing the RCI2A-GUS fusion. Seeds were placed on filter paper soaked with distilled water and stained for GUS activity 0, 1, 2, 3, and 4 d after germination. Identical results were obtained with RCI2B-GUS germinating seeds. B, RNA-blot analysis of RCI2A and RCI2B genes in germinating seeds. Total RNA (10 μg) obtained from matured (0) and 1 to 6 d (1d–6d) germinating seeds was loaded on each line. The specific probes for RCI2A and RCI2B are described in Capel et al. (1997). The ethidium bromide-stained gel is shown at bottom as a control of equal RNA loading.
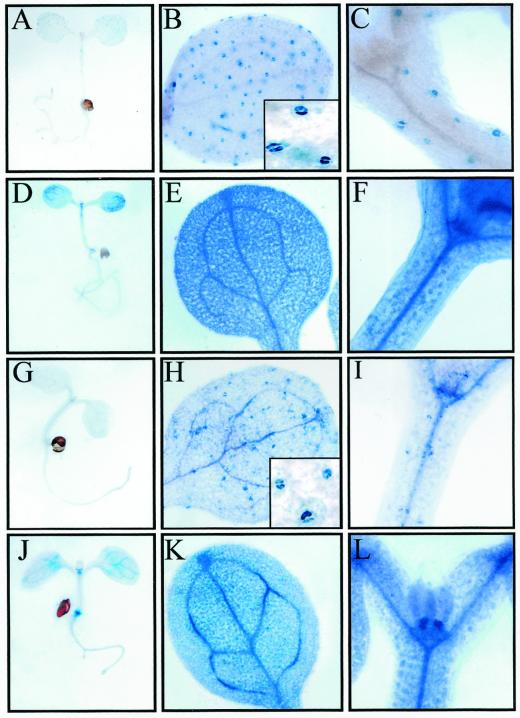
After seed germination, GUS activity was analyzed in 7-d-old RCI2A- and RCI2B-GUS seedlings grown under control, unstressed conditions (Fig. 5, A–C and G–I). Cotyledons, hypocotyls, and roots from both types of transgenic seedlings only showed a weak GUS staining (Fig. 5, A and G). It is interesting that microscopic analysis of cotyledons and hypocotyls uncovered that their GUS activity was mainly due to a strong staining of the guard cells (Fig. 5, B, C, H, and I). The vascular tissues of cotyledons and hypocotyls, as well as the shoot apical meristem and the root-hypocotyl transition zone were more intensely stained in RCI2B-GUS than in RCI2A-GUS seedlings (Fig. 5, B, C, H, and I). When 7-d-old transgenic seedlings were exposed to low temperature, dehydration, ABA, or salt stress, the patterns of GUS activity were very similar, the levels of GUS staining being higher than those described for control plants in all cases. The results obtained in cold-stressed seedlings are shown as a representative example (Fig. 5, D–F and J–L). High GUS activity was detected in all tissues of the cotyledons, hypocotyls, and roots, particularly in vascular tissues and root tips (Fig. 5, D and J). The differences in GUS staining intensities already observed between RCI2A-GUS and RCI2B-GUS seedlings under control conditions were also observed in the stressed samples (Fig. 5, E, F, K, and L).
Figure 5.
Histochemical localization of GUS activity in transgenic Arabidopsis seedlings containing RCI2A- and RCI2B-GUS fusions grown under control conditions or exposed to 4°C for 24 h. A through C, Seven-day-old RCI2A-GUS seedlings grown under control conditions. A, Whole seedling; B, cotyledon. The close up shows GUS staining in guard cells. C, Hypocotyl; D through F, 7-d-old RCI2A-GUS seedlings exposed to 4°C for 24 h; D, whole seedling; E, cotyledon; F, hypocotyl; G through I, 7-d-old RCI2B-GUS seedlings grown under control conditions; G, whole seedling; H, cotyledon. The close up shows strong GUS staining in guard cells. I, Hypocotyl; J through L, 7-d-old RCI2B-GUS seedlings exposed to 4°C for 24 h. J, Whole seedling; K, cotyledon; L, hypocotyl.
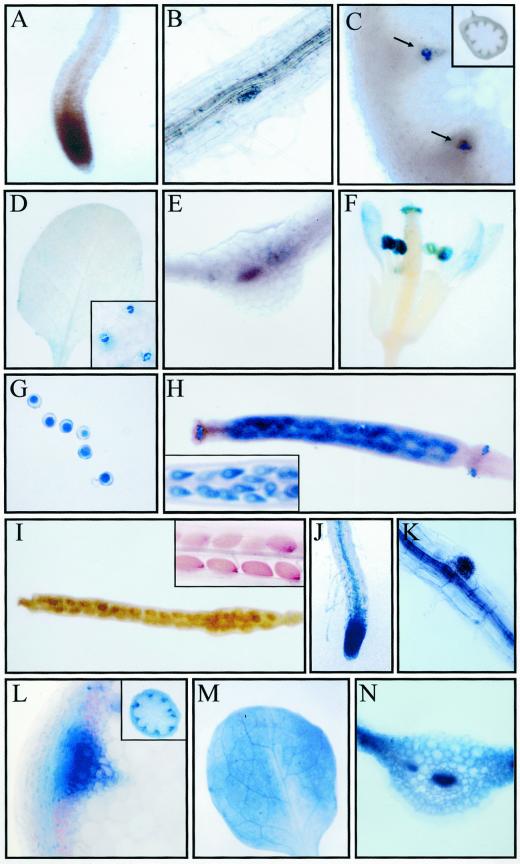
Finally, GUS activity in fully-developed RCI2A- and RCI2B-GUS transgenic plants was studied. Under control, unstressed conditions their GUS staining patterns were almost identical. The results obtained in RCI2A-GUS plants are shown (Fig. 6, A–I). Roots revealed a very low GUS activity, if any (Fig. 6, A and B). In stems, examination of tissue sections showed that a faint GUS expression was closely associated with the vascular system, being confined to protoxylem cells (Fig. 6C). RCI2A and RCI2B promoters only provided a very weak GUS expression in control leaves that, as in the case of seedlings, was restricted to guard cells (Fig. 6D). Transversal leaf sections did not reveal any GUS staining in vascular or other internal tissues (Fig. 6E). Flowers showed GUS activity in pollen and petals, anthers, and stigmatic papillae with pollen (Fig. 6, F and G). GUS staining was also observed in the stigmas of immature siliques (shorter than 5 mm), in their ovules, and in the abscission zones (Fig. 6H). Mature siliques (longer than 7 mm) did not show GUS activity at all (Fig. 6I). When full-developed transgenic plants containing the RCI2A-GUS and RCI2B-GUS fusions were exposed to low temperature, dehydration, ABA, or high salt, the patterns of GUS expression were very similar in all cases regardless of the treatment or the transgenic line analyzed. The results obtained in RCI2A-GUS cold-treated plants are shown as a representative example (Fig. 6, J–N). GUS staining was detected in both lateral and main roots, markedly in the root vascular cylinder, root primordia, and root tips (Fig. 6, J and K). Analyses of cross-sections from stressed stems revealed a strong GUS activity in the vascular system, namely in phloem and xylem cells. The cortical cells were also stained (Fig. 6L). In treated leaves, RCI2A and RCI2B promoters induced GUS expression in all tissues and cells, being especially strong in vascular bundles (Fig. 6, M and N). Histochemical GUS staining of transgenic flowers and siliques exposed to low temperature, dehydration, ABA, or salt stress showed a pattern almost identical to that observed in flowers from untreated transgenic plants (Fig. 6, F and I). Taken together, all these results indicate that the expression of RCI2A and RCI2B is regulated during seed germination and plant development and that this regulation occurs at the transcriptional level.
Figure 6.
Histochemical localization of GUS activity in adult transgenic Arabidopsis plants containing RCI2A- and RCI2B-GUS fusions grown under control conditions or exposed to 4°C for 24 h. A through I, RCI2A-GUS plants grown under control conditions. A, Root; B, root primordia; C, cross-section of a stem. Arrows indicate GUS staining in protoxylem cells. The inside shows a general view of the section. D, Leaf. The close up shows GUS staining in guard cells. E, Cross-section of a leaf; F, flower; G, pollen grains; H, inmature silique. The close up shows GUS staining in ovules. I, Mature silique. The close up shows mature seeds. J through N, RCI2A-GUS plants exposed to 4°C for 24 h. J, Root; K, root primordia; L, cross-section of a stem. The inside shows a general view of the section. M, Leaf; N, cross-section of a leaf. Identical results were obtained with RCI2B-GUS plants.
DISCUSSION
In this work, we have used promoter-GUS chimeric fusions and a transgenic approach to investigate the expression of RCI2A and RCI2B, two cold-inducible genes from Arabidopsis (Capel et al., 1997), during plant development under both unstressed and stressed conditions related to low temperatures. To isolate the RCI2A and RCI2B promoters, we identified a genomic fragment that contained both genes. Sequence analysis indicated that RCI2B and RCI2A are organized in tandem, in that order, in the Arabidopsis genome. Each gene comprises two exons of conserved lengths and two introns. The high similarity between RCI2A and RCI2B (65% identity among their whole sequences), together with their tandem disposition and their same transcriptional orientations, clearly suggests a common origin, probably by duplication of an ancestral gene and subsequent divergence through mutations. It is interesting that the same origin has been proposed for the members of other five families of low-temperature responsive genes from Arabidopsis, whose homologous genes are tandemly arranged in the genome (LTI78/RD29A/COR78 and LTI65/RD29B, Nordin et al., 1991; Horvath et al., 1993; Yamaguchi-Shinozaki and Shinozaki, 1993; KIN1 and COR6.6/KIN2, Gilmour et al., 1992; Kurkela and Borg-Franck, 1992; COR15A and COR15B, Wilhelm and Thomashow, 1993; LTI45/LTI29 and COR47, Welin et al., 1994; and CBF1, CBF2, and CBF3, Medina et al., 1999).
When transgenic lines were exposed to low temperature, dehydration, ABA, or salt stress, GUS activity driven by RCI2A and RCI2B promoters was induced at significant levels (Fig. 3), closely coinciding with the accumulation patterns of endogenous RCI2 mRNAs (Capel et al., 1997). This indicates that the isolated promoter regions contain all the cis-acting elements involved in their up-regulation by low temperature, dehydration, ABA, or salt stress, and demonstrate that the induction of RCI2A and RCI2B expression by these treatments is regulated at the transcriptional level. It is interesting that for all treatments the GUS activity exhibited by RCI2A-GUS transgenic plants was higher than that showed by RCI2B-GUS ones. This could reflect not only qualitative but also quantitative (i.e. number of regulatory motifs) differences among the RCI2 promoter regions used in this study. In fact, the consensus LTRE/DRE/C-repeat core sequence (CCGAC), which is essential for low-temperature and drought induced gene expression through the CBF/DREB genes (Jagglo-Ottosen et al., 1998; Liu et al., 1998; Kasuga et al., 1999), as well as other related elements, are present in the promoter of RCI2A. By contrast, in the case of RCI2B promoter only the sequence CCTAC is related to the LTRE/DRE/C-repeat motif. Other motifs (G-box, MYC, and MYB) that have been involved in inducing gene expression in response to dehydration, ABA, and NaCl (Guiltinan et al., 1990; Williams et al., 1992; Abe et al., 1997; Busk and Pages, 1998) are also present in higher number in RCI2A promoter than in RCI2B one.
Histochemical determination of GUS activity in Arabidopsis transgenic plants containing the RCI2A- or RCI2B-GUS fusions allowed to perform a detailed analysis of RCI2A and RCI2B expression during plant development (Figs. 4–6). Transient GUS staining could be detected in 1-d-old transgenic seedlings, indicating that RCI2A and RCI2B expression is temporarily induced during seed germination. Although several genes whose expression is up-regulated by gibberellins have been described to be transiently induced during seed germination (Casacuberta et al., 1992; Cooley et al., 1999), measurements of GUS activity in transgenic lines containing the RCI2 promoter-GUS fusions after being treated with a 100 μm solution of GA3 indicated that RCI2 genes are not regulated by giberellins (data not shown). After germination, in 7-d-old seedlings, RCI2A and RCI2B promoters were active in the guard cells of cotyledons and hypocotyls. The RCI2B promoter, but not the RCI2A one, was also significantly active in the vascular bundles, the shoot apical meristem, and the root-hypocotyl transition zone. These differences between RCI2A and RCI2B expression seem to be specific of this developmental stage since they disappeared when plant development proceeded. In fact, adult transgenic plants containing RCI2A- or RCI2B-GUS fusions showed almost identical GUS staining patterns. However, the possibility that the differences found between RCI2A and RCI2B expression during development are due to the lack of appropriated regulatory sequences in the isolated promoter fragments cannot be discarded. In fully developed plants, RCI2A and RCI2B promoters provided GUS expression in guard cells, in the vascular system of stems, namely in protoxylem cells, in pollen grains, in developing seeds, and in the abscission zone of petals and sepals. Although there is little information on cis-elements able to confer tissue expression patterns as those displayed by RCI2 genes, the sequence AAATGA, which has been described as being capable of driving pollen-specific expression independently of its orientation (Weterings et al., 1995), is present in both RCI2A and RCI2B promoters. In any case, all these data clearly indicate that the expression of RCI2 genes is regulated at the transcriptional level during Arabidopsis development.
The regulation of cold-inducible genes during plant development has been studied in a few cases, and results indicate that expression of these genes is regulated in different tissues and cells under both stressed and unstressed conditions (Horvath et al., 1993; Yamaguchi-Shinozaki and Shinozaki, 1993; Baker et al., 1994; Sistrunk et al., 1994; Wang and Cutler, 1995; Xu et al., 1995; Pearce et al., 1998). Genes whose expression is induced by environmental stresses different from low temperatures have also been described to be regulated during plant development (Michel et al., 1993; Leyva et al., 1995; Rouse et al., 1996; Nakashima et al., 1997; Capel et al., 1998; Nakashima et al., 1998; Colmenero-Flores et al., 1999). It is tempting to speculate that the regulation of gene expression during plant development could be a common feature among genes that are induced in response to environmental stresses. In fact, taking into consideration that developmental processes in plants are strongly influenced by environmental conditions, it can be anticipated that many genes regulated by environmental factors are also regulated by developmental cues.
The observation that RCI2A and RCI2B are expressed in several types of tissues during Arabidopsis development probably implies that their products should play a role under normal growth conditions. On the other hand, the fact that RCI2A and RCI2B show very similar expression patterns in response to different treatments indicates that their roles may be related to a common effect caused by the treatments. RCI2A and RCI2B belong to a family of proteins widely distributed among living organisms (Fig. 2). Members of this family are characterized by a small molecular size and an extreme degree of hydrophobicity (Capel et al., 1997). The high level of sequence conservation existing among the RCI2-related proteins (Fig. 2) suggests a conserved and important role for them throughout evolution. Recent data reported by Navarre and Goffeau (2000) may contribute significantly to understand the function of these proteins. These authors have isolated and characterized a RCI2-related gene from yeast, named PMP3, whose deduced protein shows 37% and 43% sequence identity with RCI2A and RCI2B, respectively, being a residue longer than these proteins. PMP3 contains two membrane-spanning domains and, as predicted for RCI2 proteins, is located in the plasma membrane. Functional analyses showed that deletion of PMP3 results in an increase in Na+ and K+ uptake due to an hyperpolarization of the membrane potential, which suggests a role for PMP3 in regulating the potential of plasma membrane. It is interesting that the expression of RCI2A in yeast can substitute the loss of PMP3 (Navarre and Goffeau, 2000), indicating that both proteins are functionally interchangeable. These data suggest that PMP3 and RCI2 proteins would have a common role in the regulation of membrane potential, a conserved function that might be of crucial importance in Arabidopsis tolerance to different environmental stresses including low temperatures, dehydration, and high salt. Complementary genetic and biochemical analysis in yeast and plants will provide definitive evidences on the function of RCI2 proteins, on their modes of action, and on their role during plant development and in response to stress conditions.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Treatments
Seeds from Arabidopsis ecotype Columbia (Col) were purchased from Lehle Seeds (Tucson, AZ). Seeds, seedlings, and plants were used for the experiments. For seedlings, seeds were sown under sterile conditions in Petri dishes containing mineral nutrient solution (Haughn and Somerville, 1986), solidified with 0.8% (w/v) agar. For plants, seeds were sown in pots containing a mixture of soil and vermiculite (3:1 w/w) and irrigated with water and mineral nutrient solution (Haughn and Somerville, 1986) once a week. Both, plants and seedlings were grown at 22°C under long-day photoperiods (16 h of cool-white fluorescent light, photon flux of 70 μmol m−2 s−1).
Low temperature treatments were performed by transferring plants and seedlings to a growth chamber set to 4°C for different periods of time under the light and photoperiodic conditions described above. For ABA treatments, plants and seedlings were sprayed with 100 μm ABA and harvested 6 h later. The ABA stock solution (100 mm) was prepared in dimethyl sulfoxide, and control treatments were given by spraying plants and seedlings with water containing the same final concentration of the ABA solvent. Water stress was induced by transferring plants and seedlings to Petri dishes without agar medium and allowing them to lose 50% of their fresh weight. For NaCl treatments, plants were watered with 250 mm NaCl and leaves were harvested 12 h later. In the case of seedlings, NaCl treatment was performed by transferring them to new Petri dishes containing the agar medium plus 250 mm NaCl and maintaining seedlings on this medium for 12 h. After the treatments, seedlings and leaves of plants were immediately frozen in liquid N2 and stored at −80°C until their use.
Isolation of Genomic Clones, DNA-Blot Hybridizations, and Sequence Analysis
An Arabidopsis genomic library, Col ecotype, in bacteriophage λGEM11 was screened by using the RCI2A cDNA (Capel et al., 1997) as a probe following standard methods (Sambrook et al., 1989). Two of the five recombinant phages that hybridized with the probe, λ-clones pf1 and pf4, also hybridized with the RCI2B cDNA (Capel et al., 1997) and were selected for further analyses. DNA from pf1 and pf4 was isolated, digested with different restriction enzymes, and hybridized with RCI2A and RCI2B cDNA probes after being electrophoresed in agarose gels and transferred to nylon membranes, as described by Sambrook et al. (1989). Both λ-clones contained an identical 4.5-kb EcoRI fragment that hybridized with both probes and was subcloned into the EcoRI site of the pBluescript SK vector (Stratagene, La Jolla, CA). The nucleotide sequence of this fragment was determined on both strands by using specific oligonucleotide primers and a semi-automatic DNA sequencer (model 373A, Perkin Elmer, San Jose, CA).
Databases were searched for sequence similarities using the BLAST (BLAST) program of the National Center for Biotechnology Information (Altschul et al., 1997). The comparison of the nucleotide and amino acid sequences was performed with the software package DNASTAR.
Isolation of RNA and RNA-Blot Hybridizations
Total RNA was isolated from 4-week-old Arabidopsis plants and from seeds at different stages of germination according to the method described by Nagy et al. (1988). RNA-blot hybridizations were performed as previously described (Medina et al., 1999). DNA probes were radioactively labeled with [α-32P]dCTP using the Megaprime kit (Amersham, Buckinghamshire, UK). The RCI2A- and RCI2B-specific probes were 0.3- and 0.2-kb fragments from the 3′-non-coding regions, respectively (Capel et al., 1997). Equal RNA loading was controlled by using a 0.3-kb EcoRI fragment from the 18Sr DNA as a probe (Tremousaygue et al., 1992).
Mapping the Transcription Start Sites by Primer Extension
Primer extension experiments were performed according to the method of Ausubel et al. (1992), using the [γ-32P]ATP-labeled oligonucleotides 5′-TTTTCTAAGCTCTTTGAAAG- 3′, and 5′-TTTCAAGCTCTTCAAATTTCA- 3′, which correspond to the complementary sequences upstream of the coding region of the RCI2A (+52 to +71) and RCI2B (+31 to +51), respectively. The RNA for the primer extension was extracted from 4-week-old Arabidopsis plants exposed at 4°C for 1 d. The products for the reverse transcription reactions were resolved in a 6% (w/v) acrylamide sequencing gel, and the start points of transcription were determined by comparing to sequencing reactions performed with the genomic clones using the same primers. Sequencing was carried out by the dideoxy method (Sanger et al., 1977) using the Sequenase 2.0 kit (United States Biochemical, Cleveland).
Transgenic Plants
A PCR-based cloning procedure was used to make RCI2A and RCI2B promoter-GUS transcriptional fusions. By using the primers 5′-CCGGGATCCCGTTTTCTAAGCTCTTTGAAAG- 3′ and 5′ -TGCGGTCGACTATACTTTCTGCTGA- 3′, a 1.2-kb fragment immediately upstream of the RCI2A coding region was amplified, introducing a BamHI site at the ATG initiation codon and a SalI site at the 5′ end. This RCI2A promoter fragment was ligated into the corresponding sites of pBI101.2 (Jefferson, 1987), yielding the RCI2A-GUS construct. By using the primers 5′-CCGGGATCCCGTTTCAAGCTCTTCAAATTTC- 3′ and T7, a 0.6-kb fragment immediately upstream of the RCI2B coding region was amplified, introducing a BamHI site at the ATG initiation codon. The amplified 0.6-kb RCI2B promoter fragment was double digested with BamHI and SalI restriction enzymes and ligated into the corresponding sites of pBI101.2 (Jefferson, 1987), yielding the RCI2B-GUS construct. These constructs, once verified by DNA sequencing, were transferred to Agrobacterium tumefaciens LBA4404 (Hoekema et al., 1983). Transformation of Arabidopsis, Col ecotype, was performed by vacuum infiltration (Bechtold et al., 1993).
Assays of GUS Activity and Histochemical Staining
GUS activity was assayed in whole extracts from 4-week-old plants by fluorometric determination of 4-methyl-umbelliferone production from the glucoronide precursor using the protocol described by Jefferson (1987). Histochemical localization of GUS activity was performed by incubating samples from transgenic seedlings or plants in 100 mm sodium phosphate buffer (pH 7.0), 0.02% (w/v) NaN3, 0.1% (w/v) Triton X-100, and 1 mm 5-bromo-4-chloro-3-indolyl-β-glucuronic acid for 3 to 12 h at 37°C. Samples were fixed with formaldehyde:acetic acid:40% (v/v) ethanol (5:5:90), and then removing chlorophyll by incubation in a 50% to 100% ethanol series. To allow a better penetration of the substrate, vacuum was applied during 30 s. For hand-cut sections, samples were embedded in 4% (w/v) agarose.
ACKNOWLEDGMENTS
We thank Dr. Gabriel Salcedo and José J. Sánchez-Serrano for helpful discussions and critical reading of the manuscript. We are grateful to E. Rodríguez and A. Redondo for their technical assistance.
Footnotes
This work was supported by the European Union (grant no. BIO CT96–0101), by Comision Interministerial de Ciencia y Tecnologia (grant no. BIO98–0189), by a fellowship from de Comunidad Autónoma de Madrid (to J.M.), and by a fellowship from Instituto Nacional de Investigacion y Tecnologia Agraria y Alimentaria (to R.C.).
LITERATURE CITED
- Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. Role of Arabidopsis MYC and MYB homologous in drought and abscisic acid-regulated gene expression. Plant Cell. 1997;9:1859–1868. doi: 10.1105/tpc.9.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology, Suppl 20. New York: John Wiley & Sons; 1992. [Google Scholar]
- Baker SS, Wilhelm KS, Thomashow MF. The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol. 1994;24:701–713. doi: 10.1007/BF00029852. [DOI] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci Paris, Sci la Vie/Life Sci. 1993;316:1194–1199. [Google Scholar]
- Biedenkapp H, Borgmeyer U, Sippel AE, Klempnauer KH. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988;335:835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- Busk PK, Pages M. Regulation of abscisic acid-induced transcription. Plant Mol Biol. 1998;37:425–435. doi: 10.1023/a:1006058700720. [DOI] [PubMed] [Google Scholar]
- Capel J, Jarillo JA, Madueño F, Jorquera MJ, Martinez-Zapater JM, Salinas J. Low temperature regulates Arabidopsis Lhcb gene expression in a light-independent manner. Plant J. 1998;13:411–418. doi: 10.1046/j.1365-313x.1998.00039.x. [DOI] [PubMed] [Google Scholar]
- Capel J, Jarillo JA, Salinas J, Martinez-Zapater JM. Two homologous low-temperature-inducible genes from Arabidopsis encode highly hydrophobic proteins. Plant Physiol. 1997;115:569–576. doi: 10.1104/pp.115.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casacuberta JM, Raventos D, Puigdomenech P, San Segundo B. Expression of the gene encoding the PR-like protein PRms in germinating maize embryos. Mol Gen Genet. 1992;234:97–104. doi: 10.1007/BF00272350. [DOI] [PubMed] [Google Scholar]
- Chen THH, Gusta LV. Abscisic acid induced freezing resistance in cultured plant cells. Plant Physiol. 1983;73:71–75. doi: 10.1104/pp.73.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier Y, Siminovitch D. Correlation between cold- and drought-induced frost hardiness in winter wheat and rye varieties. Plant Physiol. 1982;69:256–258. doi: 10.1104/pp.69.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero-Flores JM, Moreno LP, Smith CE, Covarrubias A. Pvlea-18, a member of a new late-embryogenesis-abundant protein family that accumulates during water stress and in the growing regions of well-irrigated bean seedlings. Plant Physiol. 1999;120:93–103. doi: 10.1104/pp.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley MB, Yang H, Dahal P, Mella RA, Downie AB, Haigh AM, Bradford KJ. Vacuolar H+-ATPase is expressed in response to gibberellin during tomato seed germination. Plant Physiol. 1999;121:1339–1348. doi: 10.1104/pp.121.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa S, Takabe K. Formation of multiplex lamellae by equilibrium slow freezing of cortical parenchyma cells of mulberry and its possible relationship to freezing tolerance. Protoplasma. 1996;190:189–203. [Google Scholar]
- Gilmour SJ, Artus NN, Thomashow MF. cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol Biol. 1992;18:13–21. doi: 10.1007/BF00018452. [DOI] [PubMed] [Google Scholar]
- Goddard NJ, Dunn MA, Zhang L, White PL, Jack PL, Hughes MA. Molecular analysis and spatial expression pattern of a low-temperature-specific barley gene, blt101. Plant Mol Biol. 1993;23:871–879. doi: 10.1007/BF00021541. [DOI] [PubMed] [Google Scholar]
- Griffith M, Antikainen M. Extracellular ice formation in freezing-tolerant plants. In: Steponkus PL, editor. Advances in Low-Temperature Biology. Vol. 3. London: JAI; 1996. pp. 107–139. [Google Scholar]
- Guiltinan MJ, Marcotte WR, Quatrano RS. A plant leucine zipper protein that recognizes an abscisic responsive element. Science. 1990;250:267–271. doi: 10.1126/science.2145628. [DOI] [PubMed] [Google Scholar]
- Gulick PJ, Shen W, An H. ESI3, a stress-induced gene from Lophopyrum elongatum. Plant Physiol. 1994;104:799–800. doi: 10.1104/pp.104.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare PD, Cress WA, van Staden J. Dissectin the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998;21:535–553. [Google Scholar]
- Haughn G, Somerville C. Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet. 1986;204:430–434. [Google Scholar]
- Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA. A binary plant vector strategy based on separation of vir- and T- region of the Agrobacterium tumefaciens Ti-plasmid. Nature. 1983;303:179–180. [Google Scholar]
- Horvath DP, McLarney BK, Thomashow MF. Regulation of Arabidopsis thaliana L. (Heyn) cor78 in response to low temperature. Plant Physiol. 1993;103:1047–1053. doi: 10.1104/pp.103.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MA, Dunn MA. The molecular biology of plant acclimation to low temperature. J Exp Bot. 1996;47:291–305. [Google Scholar]
- Jagglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexperssion induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Jarillo JA, Leyva A, Salinas J, Martinez-Zapater JM. Low temperature induces the accumulation of alcohol dehydrogenase mRNA in Arabidopsis thaliana, a chilling-tolerant plant. Plant Physiol. 1993;101:833–837. doi: 10.1104/pp.101.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- Jiang C, Iu B, Singh J. Requirement of a CCGAC cis-acting element for cold induction of the BN115 gene from winter Brassica napus. Plant Mol Biol. 1996;30:679–684. doi: 10.1007/BF00049344. [DOI] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnol. 1999;17:287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- Kurkela S, Borg-Franck M. Structure and expression of Kin2, one of two cold- and ABA-induced genes of Arabidopsis thaliana. Plant Mol Biol. 1992;19:689–692. doi: 10.1007/BF00026794. [DOI] [PubMed] [Google Scholar]
- Levitt J. Responses of Plants to Environmental Stresses: Chilling, Freezing and High Temperature Stresses. New York: Academic Press; 1980. [Google Scholar]
- Leyva A, Jarillo JA, Salinas J, Martinez-Zapater JM. Low temperature induces the accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNAs of Arabidopsis thaliana, in a light dependent manner. Plant Physiol. 1995;108:39–46. doi: 10.1104/pp.108.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 andDREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM. Chilling injury in plants. Annu Rev Plant Physiol. 1973;24:445–466. [Google Scholar]
- Mäntylä E, Lang V, Palva T. Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LTI78 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol. 1995;107:141–148. doi: 10.1104/pp.107.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Bargues M, Terol J, Perez-Alonso, Salinas J. The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol. 1999;119:463–469. doi: 10.1104/pp.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel D, Salamini F, Bartels D, Dale P, Baga M, Szalay A. Analysis of a desiccation and ABA-responsive promoter isolated from the resurrection plant Craterostigma plantagineum. Plant J. 1993;4:29–40. doi: 10.1046/j.1365-313x.1993.04010029.x. [DOI] [PubMed] [Google Scholar]
- Molina A, García-Olmedo F. Developmental and pathogen-induced expression of three barley genes encoding lipid transfer proteins. Plant J. 1993;4:983–991. doi: 10.1046/j.1365-313x.1993.04060983.x. [DOI] [PubMed] [Google Scholar]
- Nagy F, Kay SA, Chua N-H. Analysis of gene expression in transgenic plants. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–29. [Google Scholar]
- Nakagoshi H, Nagase T, Kanei-Ishii C, Ueno Y, Ishii S. Binding of the c-myb proto-oncogene product to the simian virus 40 enhancer stimulates transcription. J Biol Chem. 1990;265:3479–3483. [PubMed] [Google Scholar]
- Nakashima K, Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K. A nuclear gene, erd1, encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. Plant J. 1997;12:851–861. doi: 10.1046/j.1365-313x.1997.12040851.x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Satoh R, Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K. A gene encoding proline dehydrogenase is not only induced by proline and hypoosmolarity, but is also developmentally regulated in the reproductive organs of Arabidopsis. Plant Physiol. 1998;118:1233–1241. doi: 10.1104/pp.118.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre C, Goffeau A. Membrane hyperpolarization and salt sensitivity induced by deletion of PMP3, a highly conserved small protein of yeast plasma membrane. EMBO J. 2000;19:2515–2524. doi: 10.1093/emboj/19.11.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki T, Sakai A. Ultrastructural changes related to frost hardiness in the cortical parenchyma cells from mulberry twigs. Plant Cell Physiol. 1981;22:171–183. [Google Scholar]
- Nordin K, Heino P, Palva ET. Separate signal pathways regulate the expression of a low-temperature-induced gene in Arabidopsis thaliana (L.) Heynh. Plant Mol Biol. 1991;187:169–183. doi: 10.1007/BF00016077. [DOI] [PubMed] [Google Scholar]
- Ouellet F, Vazquez-Tello A, Sarhan F. The wheat wcs120 promoter is cold-inducible in both monocotyledonous and dicotyledonous species. FEBS Lett. 1998;423:324–328. doi: 10.1016/s0014-5793(98)00116-1. [DOI] [PubMed] [Google Scholar]
- Pearce RS, Houlston CE, Atherton KM, Rixon JE, Harrison P, Hughes MA, Alison Dunn M. Localization of expression of three cold-induced genes, blt101, blt4.9, and blt14, in different tissues of the crown and developing leaves of cold-acclimated cultivated barley. Plant Physiol. 1998;117:787–795. doi: 10.1104/pp.117.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisensky DH, Braam J. Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating intracellular calcium levels. Plant Physiol. 1996;111:1271–1279. doi: 10.1104/pp.111.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekar CB, Lafta A. Cell-wall changes and cell tension in resoponse to cold acclimation and exogenous abscisic acid in leaves and cells cultures. Plant Physiol. 1996;111:605–612. doi: 10.1104/pp.111.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse DT, Marotta R, Parish RW. Promoter and expression studies on an Arabidopsis thaliana dehydrin gene. FEBS Lett. 1996;381:252–256. doi: 10.1016/0014-5793(96)00051-8. [DOI] [PubMed] [Google Scholar]
- Sakai A, Larcher W. Responses and Adaptation to Freezing Stress. Berlin: Springer-Verlag; 1987. Frost survival of plants. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanger F, Nicklen S, Coulsen AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U, Terzaghi W, Beckmann H, Kadesch T, Cashmore AR. DNA binding site preferences and transcriptional activation properties of the Arabidopsis transcription factor GBF1. EMBO J. 1992;11:1275–1289. doi: 10.1002/j.1460-2075.1992.tb05171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistrunk ML, Antosiewicz DM, Purugganan MM, Braam J. Arabidopsis TCH3 encodes a novel Ca2+ binding protein and shows environmentally induced and tissue-specific regulation. Plant Cell. 1994;6:1553–1565. doi: 10.1105/tpc.6.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus PL. Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol. 1984;35:543–584. [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Tremousaygue D, Laudie M, Grellet F, Dalseny M. The Brassica oleracea rDNA spacer revisited. Plant Mol Biol. 1992;18:1013–1018. doi: 10.1007/BF00019222. [DOI] [PubMed] [Google Scholar]
- Wang H, Cutler AJ. Promoters from kin1 and cor6.6, two Arabidopsis thaliana low-temperature- and ABA-inducible genes, direct strong beta-glucuronidase expression in guard cells, pollen and young developing seeds. Plant Mol Biol. 1995;28:619–634. doi: 10.1007/BF00021188. [DOI] [PubMed] [Google Scholar]
- Welin BV, Olson A, Nylander M, Palva ET. Characterization and differential expression of dhn/lea/rab-like genes during cold acclimation and drought stress in Arabidopsis thaliana. Plant Mol Biol. 1994;26:131–144. doi: 10.1007/BF00039526. [DOI] [PubMed] [Google Scholar]
- Weterings K, Schrauwen J, Wullems G, Twell D. Functional dissection of the promoter of the pollen-specific gene NTP303 reveals a novel pollen-specific, and conserved cis-regulatory element. Plant J. 1995;8:55–63. doi: 10.1046/j.1365-313x.1995.08010055.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm KS, Thomashow MF. Arabidopsis thaliana cor l5b, an apparent homologue of cor l5a, is strongly responsive to cold and ABA, but not drought. Plant Mol Biol. 1993;23:1073–1077. doi: 10.1007/BF00021822. [DOI] [PubMed] [Google Scholar]
- Williams ME, Foster R, Chua NH. Sequences flanking the hexameric G-box core CACGTG affect the specifity of protein binding. Plant Cell. 1992;4:485–496. doi: 10.1105/tpc.4.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell. 1995;7:1555–1567. doi: 10.1105/tpc.7.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet. 1993;236:331–340. doi: 10.1007/BF00277130. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Chemical and biophysical changes in the plasma membrane during cold acclimation of mulberry bark cells (Morus bombycis Koidz. cv Goroji) Plant Physiol. 1984;76:257–265. doi: 10.1104/pp.76.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BL, Arakawa K, Fujikawa S, Yoshida S. Cold-induced alterations in plasma membrane proteins that are specifically related to the development of freezing tolerance in cold-hardy winter wheat. Plant Cell Physiol. 1994;35:175–182. [Google Scholar]