Dynamic rearrangement of the actin cytoskeleton drives a myriad of processes in eukaryotic cells, such as cell migration and vesicle trafficking, and its dysregulation is deeply associated with various diseases, including cancer, immune deficiency, and neurological disorders. Members of the Wiskott-Aldrich Syndrome Protein (WASP) family are ubiquitous regulators of actin dynamics, including WASP, N-WASP, WAVE, WASH, WHAMM, JMY, and the recently identified WHIMP. Although each WASP-family protein uses a different regulatory mechanism and participates in distinct cellular processes, they all act by integrating various upstream signals and transmitting them to the C-terminal WCA (WH2-Central-Acidic, with WH2 representing WASP Homology 2) domain, which stimulates the actin nucleation activity of the Arp2/3 complex to promote formation of new filaments from existing ones, creating branched actin networks crucial to dynamic deformations of membranes.
Among the WASP-family proteins, WAVE (WASP family Verprolin homolog—also known as SCAR for Suppressor of cAMP Receptor) is uniquely regulated through the constitutive incorporation into a large protein assembly of ~400 kDa, known as the WAVE Regulatory Complex (WRC). Since the Kirschner lab first discovered it in 2002 by biochemical purification, the WRC has attracted major attention from biologists and biochemists to understand its structure, regulation, and function. This is not only because the WRC is essential to membrane protrusion and cell migration and is widely implicated in human disease, but also because the size and complexity of the WRC make it a fascinating model for understanding membrane-to-actin signaling and allosteric regulation.
A series of tour de force studies in recent years combining cell biology, biochemistry, and structural biology have elucidated several fundamental mechanisms about the WRC and established it as a major signaling hub between the plasma membrane and actin in diverse processes. It is widely accepted that in its basal state, the WRC is autoinhibited in the cytosol, but a large variety of membrane ligands, including GTPases, phospholipids, membrane receptors, and kinases, can act cooperatively to activate and/or recruit the complex to the plasma membrane through direct interactions, which, in turn, stimulates the Arp2/3 complex to polymerize actin. Many mechanistic questions, however, still remain unanswered, and new functions and mechanisms of the WRC are emerging. Below, we summarize our current knowledge about the WRC and discuss major questions and challenges to be addressed for fully understanding its regulation and function.
Composition and assembly
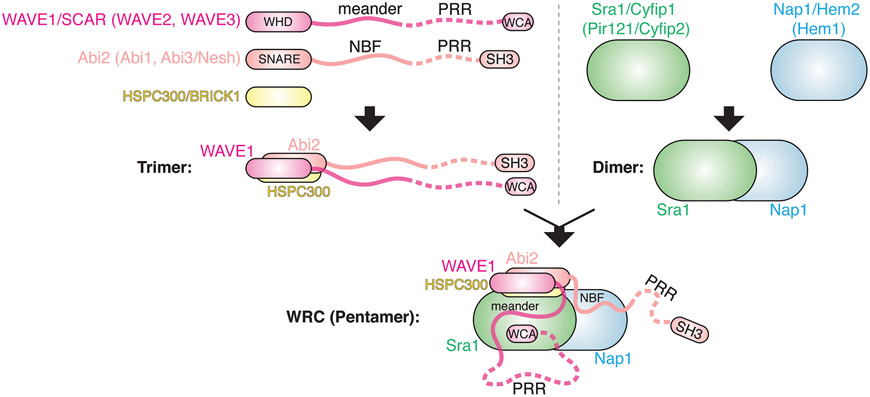
The WRC is assembled from five different protein subunits which are conserved in most eukaryotic species, including animals, plants, slime molds, and many protists and single-cell algae. For each subunit, multicellular plants and vertebrates usually encode several orthologs in their genomes (Fig. 1), including Sra1/Cyfip1 (or its ortholog Pir121/Cyfip2), Nap1/Hem2 (or its ortholog Hem1), Abi2 (or its orthologs Abi1 and Abi3/Nesh), HSPC300/BRICK1, and WAVE1/SCAR (or its orthologs WAVE2 and WAVE3). For simplicity, we use nomenclature based on murine or human WRC, particularly the one containing Sra1, Nap1, Abi2, HSPC300, and WAVE1, which is a major form in the human brain and had led to the first crystal structure of the complex.
Figure 1. Composition and in vitro assembly of WRC.
Schematic showing how the WRC is assembled from five different subunits through an in vitro biochemical reconstitution. Curved lines represent flexible sequences. Dotted lines represent unstructured sequences containing proline-rich regions (PRR) and not resolved in crystal structures. NBF: Nap1 Binding Fragment; WHD: WAVE Homology Domain; WCA: WH2-Central-Acidic domain. Names of homologous proteins in vertebrates are shown in parentheses.
It is remarkable that the composition and assembly of the WRC are highly conserved across species. First, ortholog subunits in a given organism can be exchanged to form different WRCs through mix and match, even though different orthologs may have specific tissue distribution, interact with distinct regulatory molecules, or exhibit different kinetics in promoting Arp2/3-mediated actin polymerization. Second, the five subunits appear to have co-existed through evolution: an organism either contains all five components, or none of them (such as yeast). Third, knocking out or suppressing expression of a single subunit in cells often eliminates or reduces the expression of all other subunits, disrupting WRC-mediated processes in a manner similar to removing WAVE. Together, the above evidence emphasizes the importance of an intact, fully assembled WRC to its function. Although individual subunits have been suggested to participate in other protein complexes, such as Abi binding to N-WASP and Sra1 binding to FMRP and eIF4E, these interactions either need more experimental validations or may have evolved as a function peripheral to their essential roles in WRC.
How the WRC is assembled from newly synthesized subunits in cells and how the cell precisely balances protein levels of the five subunits remain intriguing questions. In vitro, individually purified subunits are either sticky, aggregated, or unstable (which could cause various artifacts if a study only uses individual subunits as the experimental material—see next section). This indicates that in cells, individual subunits or partially assembled subcomplexes may be unstable, requiring certain stabilization mechanisms before being assembled into WRC. For example, HSPC300 was shown to form a homotrimer prior to entering the WRC. In addition, Nudel was suggested to serve as a chaperon binding to and stabilizing subcomplexes including Sra1-Nap1-Abi1 and HSPC300.
Although little is known about WRC assembly in cells, based on in vitro reconstitution and its crystal structures, the WRC can be readily dissected into two parts: a large elongated dimer of ~10x10x20 nm formed by Sra1 and Nap1, and a smaller trimer formed by WAVE1, Abi2, and HSPC300 (Fig. 1). Nearly the whole Sra1/Nap1 dimer is made of α-helices, without clearly distinguishable subdomains. It is worth noting that despite the low sequence similarity between Sra1 and Nap1, they share distant homology and define a protein family together with several remotely related proteins, including SWIP and Strumpellin (subunits of the related WASH Regulatory Complex), and the recently identified Rac1-binding protein, CYRI. In contrast, the WAVE1/Abi2/HSPC300 trimer consists of a 4-helix bundle, which is immediately followed by two long tails extending from WAVE1 and Abi2, respectively. The helix bundle, aligned along the long axis of the Sra1/Nap1 dimer, is formed by the N-terminal WHD (WAVE Homology Domain) domain of WAVE1, the N-terminal SNARE-like helices of Abi2, and the entire helix of HSPC300 (Fig. 1).
The long tail extending from WAVE1 consists of three parts: a sequence of ~90 a.a. known as the meander region, a long unstructured sequence of ~300 a.a. containing multiple proline-rich regions (PRR), and the WCA domain of ~75 a.a. at the C-terminus. The long Abi2 tail also consists of three parts: a sequence of ~40 a.a. named as NBF (Nap1 Binding Fragment), a long unstructured sequence of ~250 a.a. rich in prolines (PRR), and a C-terminal SH3 domain (Fig. 2). The meander region in WAVE1 literally “meanders” across the surface of Sra1 as a loose collection of loops and helices, which together with Sra1 plays a key role in stabilizing WCA domain binding. In comparison, the seemingly unstructured NBF sequence in Abi2 “crawls” along the surface of Nap1, forming an extensive interaction with the latter. The unstructured PRR sequences in both WAVE1 and Abi2 provide many potential binding sites for various SH3- or EVH1-domain containing proteins, such as Eps8, p47phox, and Ena/VASP (Fig. 2). Similarly, the SH3 domain of Abi2 can bind to proteins containing PRR sequences, such as Abl family kinase. Finally, as the defining feature of WASP-family proteins, the WCA domain serves as the output module of the entire complex. The W region is an actin-binding module found in various actin binding proteins, and the C and A regions are critical for binding and activating the Arp2/3 complex. W and C elements form two short helices, which are sequestered by multiple interactions with both Sra1 and the meander region of WAVE1, keeping the WRC inhibited. Activation of the WRC disrupts these interactions and releases the WCA sequence, allowing the latter to bind and activate the Arp2/3 complex to promote actin polymerization.
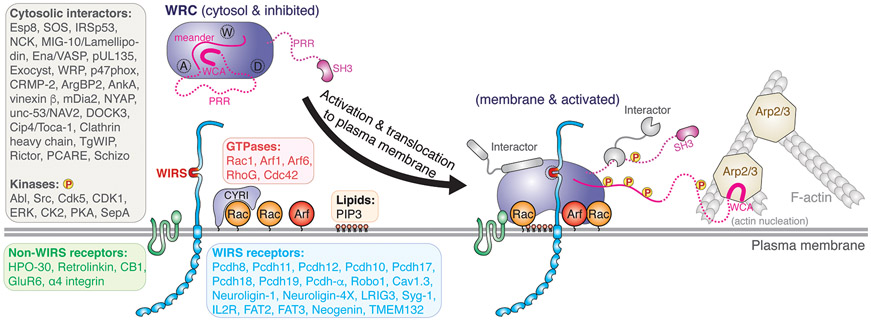
Figure 2. Activation and Regulation of WRC at the Plasma Membrane.
Schematic depicting concomitant activation of the WRC and its translocation to the plasma membrane by cooperative actions of major groups of membrane ligands, leading to release of the WCA, which in turn binds to the Arp2/3 complex, to stimulate actin nucleation and formation of branched actin networks. A, D, and W Sites are indicated by dotted circles on the WRC. Each text box shows a ligand group and a list of representative ligands. Ligands that bind to individual subunits of the WRC, but do not bind to the fully assembled WRC are not listed, which include N-WASP, FMRP, and eIF4E.
Regulation mechanisms
Studies in the past 20 years have identified a large number of molecules that interact with the WRC, ranging from GTPases, inositol phospholipids, membrane receptors, and kinases, to various cytosolic proteins (with caveats discussed below), highlighting both the complexity and the central role of WRC regulation in linking diverse signaling pathways to actin (Fig. 2). By contrast, we are just at the beginning of precisely understanding their underlying mechanisms.
Central to WRC regulation is its activation. The Rho-family GTPase Rac1 is the ubiquitous activator of WRC—this connection was established for individual subunits even before the WRC was identified. The essential role of the Rac1-WRC-Arp2/3 axis in actin regulation is further underlined by the recent discovery of the Rac1-binding protein CYRI, which acts by specifically disrupting Rac1-WRC interactions using its A-Site-analogous domain (also known as DUF1394) (Fig. 2). Despite the importance of the Rac1-WRC interaction, several obstacles have stymied a mechanistic understanding of WRC activation for many years, which include the low affinity of Rac1 binding the WRC, the cooperativity of Rac1 with other ligands, and the difficulty of WRC purification. Several conflicting models were not reconciled until the successful purification of the recombinant WRC by the Rosen lab, which subsequently led to a series of biochemical and structural studies establishing that the WRC is autoinhibited in the resting state, and Rac1 binding activates it by allosterically releasing the WCA. Structural work identified two distinct Rac1 binding sites located at the opposite sides of the Sra1 subunit, named the A Site and D Site (for Adjacent or Distant to the WCA binding site), respectively (Fig. 2). Neither site overlaps with the WCA binding region, suggesting an allosteric activation mechanism. Biochemical studies showed that two Rac1 molecules can simultaneously bind to both sites, with the A Site having ~40 times lower affinity than the D Site and both sites being essential to WRC activation. In contrast, cell biological studies in both mammalian and Dictyostelium cells found that only the A Site was crucial to WRC activation, whereas the D Site served to promote or optimize WRC’s function. The mixed consistency and discrepancy between biochemical and cellular studies suggest further complexity for WRC regulation in cells and the necessity of developing new experiments and tools to further understand the contribution and mechanism of the two Rac1 binding sites both in vitro and in vivo.
Aside from Rac1, other GTPases have also been suggested to contribute to WRC activation, such as the Arf-family GTPases Arf1 and Arf6, or tune its activity, as in case of the Rho-GTPases RhoG and Cdc42 (Fig. 2). Not sufficient for activating the WRC on their own, these GTPases might act cooperatively with Rac1 to optimize WRC activity, which may be important for linking Rac1-WRC-Arp2/3 signaling to processes mainly regulated by these GTPases, including vesicle trafficking and pathogen invasion. How these GTPases cooperate with Rac1 to drive WRC activation or output is mostly unknown. Furthermore, kinases also play an important role in activating the WRC (Fig. 2). Both WAVE and Abi have many phosphorylation sites targeted by various kinases, such as Abl, Src, and Cdk5. Phosphorylation in their unstructured PRR regions may regulate interactions with auxiliary proteins, whereas phosphorylation in the meander region of WAVE can directly destabilize WCA binding and evoke activation. Moreover, the WCA region is often found phosphorylated, which can fine-tune its kinetics in promoting Arp2/3-mediated actin polymerization.
Concomitant with activation, the WRC is translocated to and stays associated with the plasma membrane to sustain Arp2/3 complex activation. Rac1 (or its orthologs Rac2 and Rac3) plays a key role in this process. In addition, phospholipids, PIP3 and many transmembrane proteins may provide more precise, perhaps spatiotemporal control of WRC localization at membranes (Fig. 2). PIP3 is known to enhance WRC activation by Rac1, likely by binding to the positively charged surface of the WRC. Various transmembrane receptors with diverse functions can directly recruit the WRC to membranes by using a peptide motif named WIRS (WRC Interacting Receptor Sequence), which binds to a surface pocket on the WRC (which we name the W or WIRS Site) (Fig. 2). The W Site comprises residues from Sra1 and Abi2 using amino acids 100% conserved in animals, suggesting that these WIRS-receptors connect intact WRCs, instead of individual subunits, to processes specific to animals, such as immune responses or morphogenesis and functions of neurons (Fig. 3). Notably, sequences flanking WIRS motifs can also modulate WRC activity, either by directly interacting with the complex or by recruiting regulatory molecules such as Rac GEFs, which can synergistically promote WRC activation by increasing local Rac concentrations.
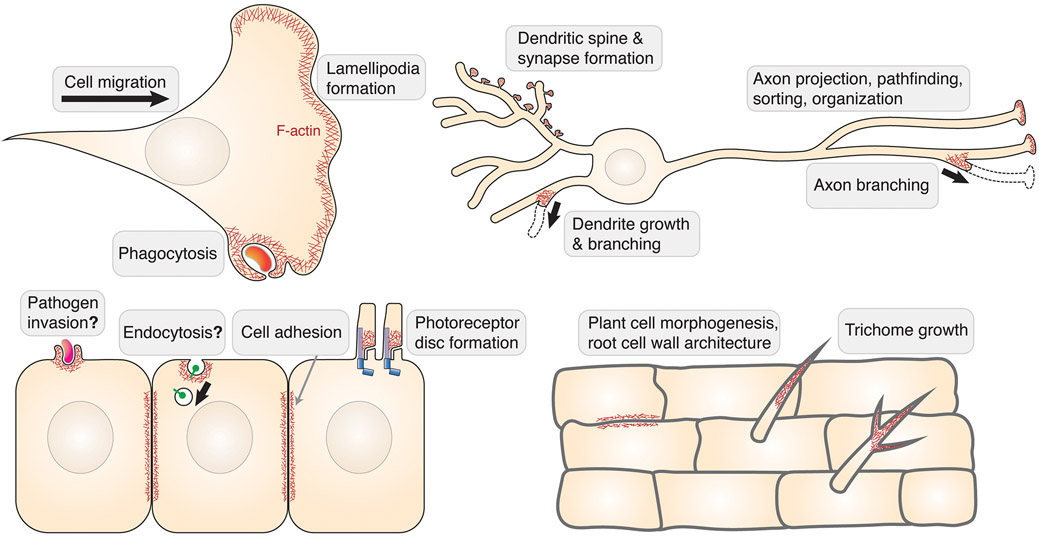
Figure 3. Biological functions of WRC.
Schematic showing major cellular processes, indicated by grey text boxes, which are regulated by WRC-mediated actin network formation (represented by F-actin in red; WRC not shown). Processes that are still controversial or in which a general function for WRC remains to be confirmed are indicated by question marks.
Finally, some membrane receptors not containing a WIRS motif and many cytosolic proteins may interact with WRC, which can potentially link the WRC and actin regulation to a much broader range of cellular activities (Fig. 2). In addition to host proteins, pathogen effectors may also interact with the complex, to hijack actin regulation and facilitate pathogen entry or immune evasion, such as pUL135 from the Human Cytomegalovirus (HCMV), AnkA from Anaplasma phagocytophilum, and TgWIP from Toxoplasma gondii. In most cases, however, the precise mechanism and function of aforementioned interactions warrant careful further examinations, both in vitro and in cells. One major caveat to bear in mind is that many of these interactions were identified and characterized by using isolated WRC subunits, which—apart from Abi-SH3 and Abi- and WAVE-PRR—are unstable and tend to form aggregates and non-specific interactions. It will thus be critical to further examine these interactions in the context of fully assembled WRC in the future.
Biological/organismic functions
Parallel with the long list of WRC-interacting ligands and the importance of Rac-WRC-Arp2/3 signaling in actin dynamics, the WRC performs many important functions in biology. In most cases, the WRC is crucial for morphogenetic processes that rely on dynamic rearrangements of Arp2/3-mediated actin networks formed at the plasma membrane (Fig. 3).
Best known among these processes in animal cells are lamellipodia protrusion and membrane ruffling, which can significantly enhance the efficiency of cell migration in various cell types and tissue contexts. The WRC unequivocally drives these processes in many different model systems, ranging from migration of melanoma or immune cells in mice, cell motility in zebrafish, fruit fly and nematode worms, to pseudopod-mediated crawling in Dicytostelium discoideum. In neurons, WRC’s function is essential to various cell protrusions rich in actin filaments, which can generate dendritic spines and neuromuscular synapse junctions, drive branch formation of dendrites and axons to establish complex neural circuits, and guide axon growth, organization, and precise projection to target tissues.
Other processes involving Rac-mediated actin remodeling, such as cell-cell adhesion, phagocytosis, and photoreceptor disc formation, also require WRC function (Fig. 3). Furthermore, the complex was implicated in pathogen invasion, including gram-positive Listeria and gram-negative Rickettsia and Salmonella, albeit to variable extents in different studies, warranting additional, more detailed investigations. Interestingly, the WRC was even implicated in specific types of endocytosis, which is usually considered as a major function of N-WASP. The endocytosis required direct binding of the WRC to receptors through WIRS (IL-2R) or non-WIRS (Retrolinkin) interactions. Additional examples will be required in the future to examine if the WRC plays a general role in endocytosis.
The importance of the WRC is reflected by the severe phenotypes of deleting individual WRC subunits in animals. Deleting ubiquitously expressed subunits in mammals, such as Sra1, WAVE2, Nap1, and Abi1, was always embryonically lethal, whereas deleting more tissue-specific orthologs, such as WAVE1 (nervous system) and Hem1 (hematopoietic cells), produces offspring with significant defects in neural or immune functions. In contrast, deleting Cyfip2, which is enriched in the brain, is perinatally lethal, but reducing Cyfip2 dose in heterozygous animals or specifically deleting it in postnatal forebrain excitatory neurons causes neurobehavioral phenotypes, consistent with various Cyfip2 mutations in human patients (see next section).
The WRC also plays many important roles in plant cells, but very little is known about the molecular or biochemical mechanisms due to the functional redundancy of many orthologs of WRC subunits in plants and lack of biochemical reconstitution of a plant WRC (Fig. 3). Although plant cells lack dynamic membrane protrusions, they rely on proper remodeling of actin networks in the cytosol to regulate transport of polysaccharides to their cell wall destinations. It is well established that by controlling actin polymerization required for plant wall biosynthesis, the WRC regulates various aspects of plant development and cell function, ranging from cell division, cell morphology, trichome growth, root rigidity, drought response, and cell-cell junction to symbiosis and host-pathogen interactions.
Implications in diseases
Given the broad functions of the WRC in various cell types, especially in the immune and nervous system, it is not surprising that perturbations of WRC subunits or its many ligands are associated with various genetic diseases, including neurological disorders, immune deficiencies, and cancer.
Since the WRC carries out its function as an integral complex and the expression level between subunits is tightly regulated, mutations affecting the expression of a single subunit can readily disturb the total levels of WRC, leading to disease. Copy number variations (CNVs) arising from chromosome microdeletions, nonsense mutations or gene duplications, regardless if they reduce or elevate WRC levels, are common causes of WRC-associated disorders. For example, the 15q11.2 region of the human genome, which contains Cyfip1 (encoding Sra1), is a hot spot of chromosome microdeletions or duplications. Anomalies in this region are heavily associated with neurological disorders, including autism spectrum disorder (ASD), schizophrenia, epilepsy, and intellectual disability (ID). Similarly, heterozygous deletions or pre-mature truncations of Cyfip2, Nap1, WAVE1, or Hem1 are tightly associated with early-onset epileptic encephalopathy (EOEE), Alzheimer, ASD, ID, seizures, immune difficiency, or autoimmunity. In addition, overexpression (sometimes deletion) of WRC components, including WAVE1/2/3, Sra1, Nap1, Hem1, Abi1/3, and HSPC300, are frequently found in various types of cancers (breast, ovary, lung, etc.) and are often associated with tumor invasiveness and poor prognosis.
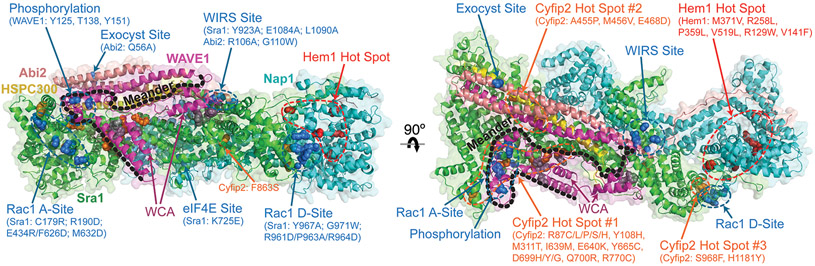
Compared to mutations causing changes in expression levels, missense mutations in patients are more rare, but provide invaluable opportunities for understanding the regulation mechanisms of the WRC and developing therapeutic strategies to target these mechanisms. Crystal structures shown in Figure 4 summarize an up-to-date collection of missense mutations associated with human diseases (orange and red in Fig. 4). As a reference point, amino acids previously identified to be important to WRC regulation are also labeled, including residues key to ligand binding, phosphorylation sites, and mutations designed through structural, biochemical, and cell biological analyses (blue and grey in Fig. 4). It is intriguing that most missense mutations are clustered at a few spatially concentrated hot spots in the WRC structure, even though their positions in the primary sequence may be distant. Structural analysis suggests these mutations either act by destabilizing protein folding to cause WRC degradation or by disrupting WRC autoinhibition. Cyfip2 contains three hot spots. These mutations are typically involved in neurodevelopmental disorders such as EOEE, ID, seizures, hypotonia, West Syndrome, eating disorders, and altered drug addiction. It is worth noting that the hot spot #1 is located close to the A Site or the meander sequence, and the hot spot #3 is underneath the D Site, suggesting these mutations act by disrupting autoinhibition and/or modulating Rac1 binding. Hem1 contains one hot spot. These mutations were identified to cause novel syndromes that combine immunodeficiency and autoimmunity. Most mutations in Hem1 seem to disrupt Hem1 structure, leading to WRC degradation, except M371V, which was suggested to disrupt Arf1-WRC signaling.
Figure 4. Regulatory sites and missense mutations in WRC.
Cartoon representation of the WRC crystal structure in the inhibited state (PDB: 3P8C). For clarity, the meander sequence in WAVE1 is traced by black dotted lines. Amino acids important for regulation, or previously mutated to disrupt ligand binding or to cause diseases are shown in spheres. Regulatory sites, including phosphorylation sites and binding sites for Rac1, WIRS receptors, Exocyst, and eIF4E are blue (eIF4E site is buried and not compatible with the WRC assembly). Shown in orange are amino acid sequence changes in Cyfip2 (Ref sequence: NP_001032410.1), which were identified to cause neurodevelopmental disorders, including the West Syndrome, early-onset epileptic encephalopathy, intellectual disability, seizures, drug addiction, and binge eating. Shown in red are amino acid sequence changes in Hem1 (Ref sequence: NP_005328.2), which led to immunological disorders, including immunodeficiency, lymphoproliferation, and autoimmunity. Mutations previously designed to disrupt WCA inhibition are colored in grey (but not labeled), including Sra1: L697D, Y704D, L841A, F844A, W845A, F686E, and WAVE1: W161E, K162D.
Outlook and future directions
Many important questions remain to be answered for truly understanding the function and regulation of WRC, from both a biochemical/structural and cell biological point of view. The key question remains as to how the WRC interacts with or is activated by various ligands, including Rac1, Arf1, PIP3 and many other molecules, both individually and cooperatively, and both in vitro and at the plasma membrane of cells. As example, it has remained unclear whether WRC activation can be separated from its membrane recruitment. Answering all these questions will promote the development of inhibitors, activators, and chemical or optogenetic tools to control or track WRC functions in cells, which will be of both scientific and potential medical relevance. Such tools might also extend our capabilities to unravel additional, perhaps less canonical functions than the ones summarized above. How different WRC variants containing distinct combinations of subunits are differentially regulated in cells is emerging as yet another exciting future topic. Additional questions that have remained nearly entirely unanswered include the regulation of WRC assembly, recycling and degradation, as well as biochemical mechanisms of WRC regulation in plants. Last, not least, it will be essential for a full understanding of WRC regulation and function to establish how its individual subunits also participate in other complexes, such as Sra1 with FMRP-eIF4E, and how cells balance all these individual subunit activities in normal development and disease.
ACKNOWLEDGEMENTS
KR and TEBS acknowledge funding of their research by the Deutsche Forschungsgemeinschaft (DFG) and the Helmholtz Society, and BC by the National Institute of Health NIGMS and the American Heart Association.
Footnotes
DECLARATION OF INTERESTS STATEMENT
The authors have no financial interests, patents or positions to declare, and are not members of the journal’s advisory board.
FURTHER READING
- 1.Begemann A, Sticht H, Begtrup A, Vitobello A, Faivre L, Banka S, Alhaddad B, Asadollahi R, Becker J, Bierhals T, et al. (2020). New insights into the clinical and molecular spectrum of the novel CYFIP2-related neurodevelopmental disorder and impairment of the WRC-mediated actin dynamics. Genet Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen B, Brinkmann K, Chen Z, Pak CW, Liao Y, Shi S, Henry L, Grishin NV, Bogdan S, and Rosen MK (2014). The WAVE regulatory complex links diverse receptors to the actin cytoskeleton. Cell 156, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen B, Chou HT, Brautigam CA, Xing W, Yang S, Henry L, Doolittle LK, Walz T, and Rosen MK (2017). Rac1 GTPase activates the WAVE regulatory complex through two distinct binding sites. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Borek D, Padrick SB, Gomez TS, Metlagel Z, Ismail AM, Umetani J, Billadeau DD, Otwinowski Z, and Rosen MK (2010). Structure and control of the actin regulatory WAVE complex. Nature 468, 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook SA, Comrie WA, Poli MC, Similuk M, Oler AJ, Faruqi AJ, Kuhns DB, Yang S, Vargas-Hernandez A, Carisey AF, et al. (2020). HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science 369, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fort L, Batista JM, Thomason PA, Spence HJ, Whitelaw JA, Tweedy L, Greaves J, Martin KJ, Anderson KI, Brown P, et al. (2018). Fam49/CYRI interacts with Rac1 and locally suppresses protrusions. Nat Cell Biol 20, 1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautreau A, Ho HY, Li J, Steen H, Gygi SP, and Kirschner MW (2004). Purification and architecture of the ubiquitous Wave complex. Proc Natl Acad Sci U S A 101, 4379–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Innocenti M, Gerboth S, Rottner K, Lai FP, Hertzog M, Stradal TE, Frittoli E, Didry D, Polo S, Disanza A, et al. (2005). Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol 7, 969–976. [DOI] [PubMed] [Google Scholar]
- 9.Koronakis V, Hume PJ, Humphreys D, Liu T, Horning O, Jensen ON, and McGhie EJ (2011). WAVE regulatory complex activation by cooperating GTPases Arf and Rac1. Proc Natl Acad Sci U S A 108, 14449–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebensohn AM, and Kirschner MW (2009). Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell 36, 512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin H, Lu S, Thangaraju M, and Cowell JK (2019). Wasf3 Deficiency Reveals Involvement in Metastasis in a Mouse Model of Breast Cancer. Am J Pathol 189, 2450–2458. [DOI] [PubMed] [Google Scholar]
- 12.Salzer E, Zoghi S, Kiss MG, Kage F, Rashkova C, Stahnke S, Haimel M, Platzer R, Caldera M, Ardy RC, et al. (2020). The cytoskeletal regulator HEM1 governs B cell development and prevents autoimmunity. Sci Immunol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaks M, Singh SP, Kage F, Thomason P, Klunemann T, Steffen A, Blankenfeldt W, Stradal TE, Insall RH, and Rottner K (2018). Distinct Interaction Sites of Rac GTPase with WAVE Regulatory Complex Have Non-redundant Functions in Vivo. Curr Biol 28, 3674–3684 e3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Ma L, Wu Y, Zeng R, and Zhu X (2012). Nudel is crucial for the WAVE complex assembly in vivo by selectively promoting subcomplex stability and formation through direct interactions. Cell Res 22, 1270–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki D, Suetsugu S, Miki H, Kataoka Y, Nishikawa S, Fujiwara T, Yoshida N, and Takenawa T (2003). WAVE2 is required for directed cell migration and cardiovascular development. Nature 424, 452–456. [DOI] [PubMed] [Google Scholar]