Abstract
The need for new coronavirus disease 2019 (COVID-19) therapeutic strategies continues, especially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants emerge. Zhang and colleagues elegantly engineered a mutant angiotensin-converting enzyme 2 (ACE2) that competitively binds SARS-CoV-2 spike protein, reduces viral uptake by human lung cells, and ameliorates SARS-CoV-2-induced lung injury in mice expressing human ACE2.
Keywords: ARDS, viral pneumonia, COVID-19 therapy
COVID-19 caused by SARS-CoV-2 continues to be a pandemic with a very significant health, social, and economic impact globally, including a tenfold increase in acute respiratory failure from severe pneumonia and acute respiratory distress syndrome (ARDS) in the USA [1]. At an unprecedented pace, several effective vaccines against SARS-CoV-2 were developed, approved, and deployed globally over the past year that prevent COVID-19. However, the emergence of more infectious and transmissible SARS-CoV-2 variants, causing infection in vaccinated and unvaccinated individuals, has prompted the testing of variant-specific vaccines. Accordingly, there is still a need for new therapeutics to combat SARS-CoV-2 infections in unvaccinated individuals as well as vaccinated individuals with breakthrough infections, especially as new variants emerge.
The SARS-CoV-2 spike (S) protein binds ACE2 on the cell surface. This enables viral entry into the cell, where it can replicate and initiate an infection. Thus, blocking the binding of the viral S protein to ACE2 on the surface of cells lining the lung airways and airspaces, in particular lung alveolar epithelial cells, is one approach to potentially prevent or attenuate SARS-CoV-2 infection and COVID-19. To this end, Zhang and colleagues [2] used deep mutagenesis to identify a mutant ACE2, referred to as sACE2.v2.4, that binds the viral S protein with 35-fold higher affinity than wild type (WT) ACE2. They determined that higher binding affinity is in part the result of conformational stability that allows the mutant sACE2.v2.4 to exist in a form that readily binds to the viral S protein. As a result, preincubation with dimeric sACE22.v2.4 reduced the entry of SARS-CoV-2 pseudovirus into ACE2-expressing human lung endothelial and epithelial cells in vitro.
To test sACE22.v2.4 in vivo, Zhang and colleagues used transgenic mice expressing human ACE2 via the epithelial-specific K18 protomer (K18-hACE2) that were infected with live SARS-CoV-2. K18-hACE2 mice express human ACE2 on the epithelium, thus enabling SARS-CoV-2 entry into the lung epithelial cells, which are known to be injured in COVID-19 patients [3] (Figure 1 ). This model has lung histologic and other features similar to severe clinical COVID-19 [4]. Zhang and colleagues found that K18-hACE2 mice intranasally inoculated with SARS-CoV-2 WA-1 strain developed increased lung vascular permeability to protein, pulmonary edema, weight loss, and mortality. For analysis in the disease model, the decoy sACE22.v2.4 was fused to the Fc of IgG1 to increase its serum stability. Stable concentrations of the sACE22.v2.4-IgG1 fusion protein could be established in the lungs by intravenous infusion or inhalation using this construct. Intravenous infusion of the sACE22.v2.4-IgG1 initiated either 12 or 24 hours post-inoculation reduced lung injury and improved survival 7 days following WA-1 infection. The primary physiological endpoints focused on the lung endothelium with measures of transvascular permeability to protein and lung wet/dry ratio that showed sACE22.v2.4-IgG1 ameliorated lung injury and promoted recovery of lung vascular barrier integrity.
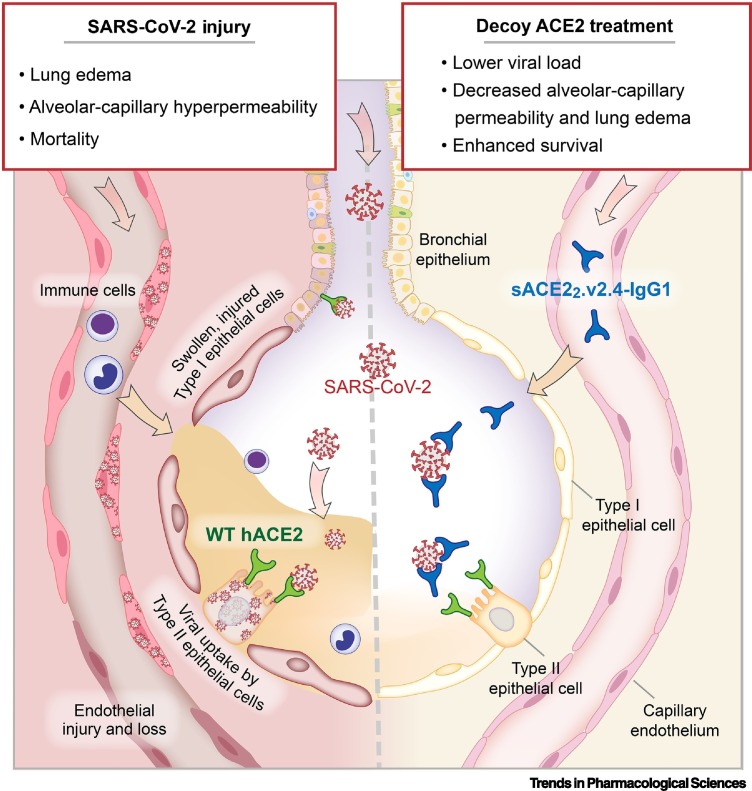
Figure 1.
A decoy angiotensin-converting enzyme 2 (ACE2) receptor mutant, sACE22.v2.4-IgG1, prevents severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uptake by lung epithelial cells and ameliorates lung injury caused by SARS-CoV-2 infection in mice expressing human ACE2 (hACE2).
SARS-CoV-2 in the pulmonary airways and alveolar airspaces binds wild type (WT) hACE2 expressed on the surface of epithelial cells to gain entry and initiate an infection, leading to lung epithelial and endothelial injury that culminates in lung edema, alveolar-capillary hyperpermeability, and mortality characterizing coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome (ARDS) in patients. A soluble decoy ACE2 receptor mutant, sACE22.v2.4-IgG1, competitively binds SARS-CoV-2 spike protein with greater affinity than WT hACE2 and prevents viral uptake by lung epithelial cells. In K18-hACE2 mice expressing WT hACE2 on the lung epithelium and infected with SARS-CoV-2, intravenously infused sACE22.v2.4-IgG1 fusion protein achieves stable concentration in the lungs and reduces lung edema and lung viral load, returns alveolar-capillary barrier permeability to a more normal level, and improves survival.
Engineered ACE2-Fc decoys have been reported to improve lung histopathology [5] and macroscopic lung damage and systemic manifestations [6] in hamster models of COVID-19. Reduced pulmonary SARS-CoV-2 pseudovirus transduction with an ACE2-Fc decoy in K18-hACE2 mice has also been reported, with an Fc variant possessing enhanced effector functions to promote immune clearance of the virus [7]. Importantly, Zhang and colleagues examined functional variables directly relevant to pulmonary microvascular injury in clinical ARDS and COVID-19 [3], including assays of endothelial barrier dysfunction, VE-cadherin integrity, and pulmonary edema formation. Inclusion of such functional analyses is a strength of this study compared with others that have also investigated mutant ACE2 receptor decoys.
Since the investigators focused on the beneficial effects of the decoy strategy on the lung endothelium, it is unclear from this study whether sACE22.v2.4-IgG1 treatment also reduced injury to the lung epithelium, as measures of lung epithelial injury were not done. The extended time (14 days rather than 7 days) of treatment needed for clearance of edema fluid and for the lung wet/dry ratio to normalize suggests that the lung epithelium has an important role in recovery in these mice, consistent with prior studies of experimental influenza pneumonia [8]. The restoration of weight following the overall improvement in survival suggests that epithelial injury was likely also improved by sACE22.v2.4-IgG1 treatment.
Zhang and colleagues also determined that the binding affinity of the decoy sACE22.v2.4-IgG1 to S protein is comparable with that of some monoclonal antibodies targeting SARS-CoV-2, which are currently in clinical use. Both strategies aim to directly neutralize the virus by binding and preventing its docking and uptake by ACE2 on the cell surface. Monoclonal antibodies also promote viral clearance by the immune system. Intravenous infusion of sACE22.v2.4-IgG1 reduced lung viral load in this study, an effect that can be attributed to the IgG1 Fc fragment as part of the fusion construct, which enables immune clearance of free virions and SARS-CoV-2-infected cells [7]. The infusion of functional human ACE2 as part of the decoy did not have a deleterious effect in lungs of K18-hACE2 mice, but it raises concern for aberrant ACE2 activity or interference with endogenous ACE2 signaling in the clinical setting. However, adverse hemodynamic changes were not observed in a human clinical trial in which WT soluble ACE2 was administered [9], and ACE2-like enzymatic activity even ameliorated SARS-CoV-2-induced lung injury in mice and hamsters [10]. Another concern is whether a decoy could trigger an autoimmune reaction when infused into patients, which would need to be determined in early phase clinical trials.
To address the applicability of the decoy strategy to more aggressive SARS-CoV-2 variants of concern, Zhang and colleagues inoculated K18-hACE2 mice with the more transmissible and lethal P.1 variant and found that sACE22.v2.4-IgG1 prevented death only when administered 12 but not 24 hours post-inoculation. This finding suggests that infection with a more lethal variant would require earlier treatment than might sometimes be possible in patients. Additionally, the investigators showed in vitro that sACE2.v2.4 binds more strongly than WT ACE2 to the S protein of several other SARS-CoV-2 variants. Nonetheless, sACE2.v2.4 was not studied here with the Omicron variant, which contains a highly mutated S protein that has the ability to escape neutralization by monoclonal antibodies that have been therapeutic against other previously emerged variants [11]. While the Omicron S protein binds WT ACE2 more strongly than S protein of other COVID-19 variants, sACE2.v2.4 has been reported to also neutralize the Omicron variant in vitro [12]. Thus, the sACE2.v2.4 decoy strategy shows promise to remain effective against lung injury induced by Omicron and, perhaps, other variants that may yet emerge.
Declaration of interests
No interests are declared.
References
- 1.Wick K.D., et al. Promises and challenges of personalized medicine to guide ARDS therapy. Crit. Care. 2021;25:404. doi: 10.1186/s13054-021-03822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., et al. Engineered ACE2 decoy mitigates lung injury and death induced by SARS-CoV-2 variants. Nat. Chem. Biol. 2022;18:342–351. doi: 10.1038/s41589-021-00965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middleton E.A., Zimmerman G.A. COVID-19-associated acute respiratory distress syndrome: lessons from tissues and cells. Crit. Care Clin. 2021;37:777–793. doi: 10.1016/j.ccc.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yinda C.K., et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higuchi Y., et al. Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2. Nat. Commun. 2021;12:3802. doi: 10.1038/s41467-021-24013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari M., et al. Characterization of a novel ACE2-based therapeutic with enhanced rather than reduced activity against SARS-CoV-2 variants. J. Virol. 2021;95 doi: 10.1128/JVI.00685-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., et al. Engineered ACE2-Fc counters murine lethal SARS-CoV-2 infection through direct neutralization and Fc-effector activities. bioRxiv. 2021 doi: 10.1101/2021.11.24.469776. Published online November 24, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan M.C.W., et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2016;113:3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan A., et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi T., et al. ACE2-like carboxypeptidase B38-CAP protects from SARS-CoV-2-induced lung injury. Nat. Commun. 2021;12:6791. doi: 10.1038/s41467-021-27097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VanBlargan L.A., et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022 doi: 10.1038/s41591-021-01678-y. Published online January 19, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikemura N., et al. Engineered ACE2 counteracts vaccine-evading SARS-CoV-2 Omicron variant. bioRxiv. 2021 doi: 10.1101/2021.12.22.473804. Published online December 23, 2021. [DOI] [Google Scholar]