Abstract
We showed recently that antisense plants with decreased activity of the plastidic ATP/ADP-transporter protein exhibit drastically reduced levels of starch and a decreased amylose/amylopectin ratio, whereas sense plants with increased activity of the transporter possessed more starch than wild-type plants and an increased amylose/amylopectin ratio. In this paper we investigate the effect of altered plastidic ATP/ADP-transporter protein expression on primary metabolism and granule morphology in more detail. Tuber tissues from antisense and sense plants exhibited substantially increased respiratory activity compared with the wild type. Tubers from antisense plants contained markedly increased levels of free sugars, UDP-Glc, and hexose phosphates, whereas phosphoenolpyruvate, isocitrate, ATP, ADP, AMP, UTP, UDP, and inorganic pyrophosphate levels were slightly decreased. In contrast, tubers from sense plants revealed a slight increase in adenine and uridine nucleotides and in the levels of inorganic pyrophosphate, whereas no significant changes in the levels of soluble sugars and metabolites were observed. Antisense tubers contained 50% reduced levels of ADP-Glc, whereas sense tubers contained up to 2-fold increased levels of this sole precursor for starch biosynthesis. Microscopic examination of starch grain morphology revealed that the size of starch grains from antisense tubers was substantially smaller (50%) compared with the wild type. The large starch grains from sense tubers appeared of a more angular morphology, which differed to the more ellipsoid shape of wild type grains. The results suggest a close interaction between plastidial adenylate transport and starch biosynthesis, indicating that ADP-Glc pyrophosphorylase is ATP-limited in vivo and that changes in ADP-Glc concentration determine starch yield, as well as granule morphology. Possible factors linking starch synthesis and respiration are discussed.
The pathway of Suc to starch conversion has been intensively investigated in growing potato (Solanum tuberosum cv Desirée) tubers (for review see, ap Rees, 1988; Kruger, 1997). After entering the cytosol of tuber parenchyma cells via plasmodesmata (Oparka et al., 1992), Suc is converted by Suc synthase (SuSy; Zrenner et al., 1995) to Fru and UDP-Glc. Fru is subsequently phosphorylated to Fru6P (Renz et al., 1993), and UDP-Glc is converted to Glc1P via a pyrophosphate- (PPi) dependent reaction catalyzed by UDP-Glc pyrophosphorylase (UGPase; Zrenner et al., 1993). After interconversion of hexose-phosphates, Glc6P or Glc1P enter the amyloplast by the action of a hexose-phosphate/phosphate antiporter (Schott et al., 1995; Naem et al., 1997; Kammerer et al., 1998). Within the amyloplast, Glc1P serves as the substrate for the first committed step in starch synthesis, catalyzed by ADP-Glc pyrophosphorylase (AGPase; Preiss, 1988). The final polymerizing steps are catalyzed by different classes of starch synthases, soluble-, or granule-bound isoforms, which incorporate the Glc moiety of ADP-Glc into the elongating glucan chains of the granule (see Martin and Smith, 1995; Smith et al., 1997).
Our knowledge about the regulation of Suc to starch conversion in potato tubers is still limited. Most of the previous molecular studies focused on the analysis of transformants with decreased expression of individual enzymes of the pathway from Suc to starch (Müller-Röber et al., 1992; Zrenner et al., 1993, 1995; Marshall et al., 1996; Geigenberger et al., 1999a, 1999b), or on evaluation of fine control mechanisms acting on AGPase (Stark et al., 1992; Geigenberger et al., 1997, 1998).
Even less attention has been paid to the possibility that carbon fluxes may be restricted by the levels of nucleotide cofactors. Suc degradation via the reversible reaction of SuSy (Geigenberger and Stitt, 1993) requires uridine nucleotides. The concentration of UDP determined in potato tubers (Geigenberger et al., 1993, 1994; Loef et al., 1999) are well below the Km of potato tuber SuSy for UDP (Avigad, 1982) and could limit the rate of Suc degradation. Recent experiments using potato tuber slices showed that Suc degradation and starch synthesis are stimulated when the overall uridine nucleotide pool is increased by feeding orotate, an intermediate of the de novo pathway of purine synthesis (Loef et al., 1999).
Conversion of Suc to starch is ATP dependent. The ATP required for the AGPase reaction is imported into the plastid via an ATP/ADP transport protein (AATP) located on the inner-envelope membrane (Heldt, 1969; Schünemann et al., 1993; Kampfenkel et al., 1995). Tjaden et al. (1998) recently showed that a relatively small decrease in ATP/ADP transporter activity leads to a reduced level of total starch content and a lower amylose to amylopectin ratio. In contrast, increased transporter activity correlated with higher starch contents and a higher amylose to amylopectin ratio. In total, these observations indicated that the rate of ATP import exerts considerable control on the rate of starch synthesis and affects the molecular composition of starch in potato tubers.
Given that adenylate transport exhibits considerable impact on the yield and molecular structure of potato tuber starch, it is important to understand the metabolic interaction between the rate of ATP consumption and the processes leading to ATP production.
To do this we examined the respiratory gas exchange of tuber tissues, the tuber levels of adenine and uridine nucleotides, as well as the amounts of sugars, glycolytic intermediates, and organic acids. To further quantify effects on starch yield, the number and size distribution of starch grains were analyzed by polarized-light microscopy and laser diffraction spectroscopy.
RESULTS
Influence of Altered Plastidic Adenylate Transporter Activity on Respiratory Gas Exchange
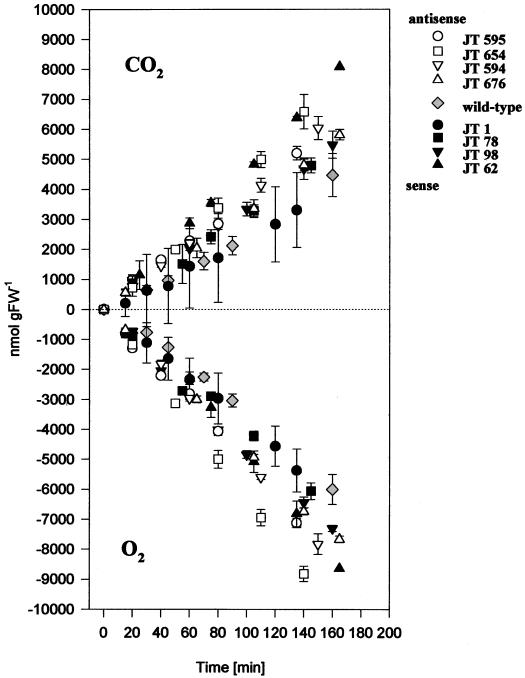
We showed recently that antisense tubers with decreased activity of the AATP exhibited drastically reduced levels of starch, whereas sense tubers with increased transporter activity contained more tuber starch than the wild-type plant (Tjaden et al., 1998). To analyze for a putative interaction between plastidial ATP import and consumption and respiratory activity, we quantified the rates of oxygen uptake and CO2 release in slices dissected from wild-type or transgenic tubers using a Warburg apparatus (Fig. 1).
Figure 1.
Respiration rates in slices from tubers with altered expression of the AATP. Slices were incubated in 100 mm Suc at a temperature of 25°C. Data points are means ± sd of three independent samples. Each sample represents four discs taken from four individual tubers of a line.
In wild-type tissue the mean oxygen consumption was 33.8 nmol O2 g fresh weight−1 min−1 and CO2 evolution was 23.6 nmol O2 g fresh weight−1 min−1 (calculated from the gradients of the data points given in Fig. 1). These values are in close agreement with previous studies that measured tuber respiration from slices in a standard oxygen electrode (25–30 nmol O2 g fresh weight−1 min−1; Geiger et al., 1998; Loef et al., 1999) or from intact tubers in an infrared gas-exchange system (about 20 nmol CO2 g fresh weight−1 min−1; Trethewey et al., 1998). It is interesting that CO2 evolution was about 30% lower than O2 consumption.
In discs dissected from tubers with increased AATP expression, the rates of oxygen consumption and CO2 production were increased compared with the wild type. The mean rate of oxygen consumption was 33.8, 38.1, 41.8, 46.0, and 50.5, CO2 evolution 23.6, 24.4, 31.3, 33.5, and 47.3, and the CO2/O2 ratios were 0.69, 0.64, 0.75, 0.73, and 0.93 in wild-type, JT 1, JT 78, JT 98, and JT 62 plants, respectively (rates are given in nanomoles per grams of fresh weight per minute and were calculated from the gradients of the data points given in Fig. 1). Stimulation of respiration was especially marked for lines JT 98 and JT 62, and was largest in line JT 62, which also exhibited the strongest increase in transporter activity and starch content (see Tjaden et al., 1998). Since data points were taken at different time intervals, the significance of the changes could not be analyzed.
It was unexpected that there was also an up to 2-fold increase in respiration rates in slices dissected from antisense tubers with decreased activity of the AATP and decreased ATP requirement due to less starch (Tjaden et al., 1998). Mean rates of oxygen consumption were 33.8, 46.5, 52.1, 62.9, and 52.7, CO2 evolution was 23.6, 34.3, 37.6, 45.3, and 35.6, and the CO2/O2 ratios were 0.69, 0.74, 0.72, 0.72, and 0.676 in wild-type, JT 676, JT 594, JT 654, and JT 595 plants, respectively (rates in nanomoles per grams of fresh weight per minute calculated from the gradients of the data points of Fig. 1).
Levels of Sugars, Glycolytic Intermediates, and Organic Acids in Tubers with Altered Expression of the Plastidic Adenylate Transporter
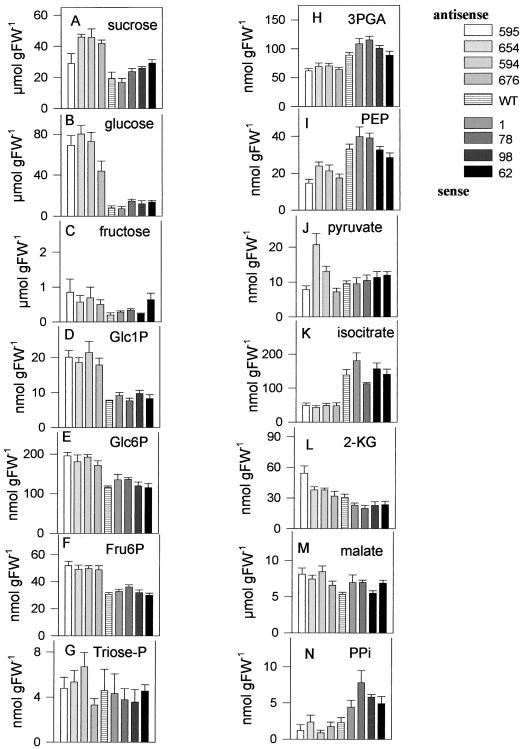
To establish the consequences of increased respiratory activity on primary metabolism we quantified several key metabolites in extracts from corresponding tubers (Fig. 2). In tubers with increased AATP expression Suc levels were not significantly changed compared with the wild type (Fig. 2A). There were also no significant changes in the levels of Glc, Fru, hexose phosphates, triose phosphates, glycerate-3-phosphate (3PGA), phosphoenolpyruvate (PEP), pyruvate, isocitrate, α-ketoglutarate, or malate compared with the wild type (Fig. 2, B–M). These results clearly indicate that supply and initial mobilization of Suc do not restrict the rate of respiration and starch synthesis in sense tubers.
Figure 2.
Sugars and metabolite levels in tubers with altered expression of the AATP. Tuber material was frozen in liquid nitrogen and then extracted in trichloroacetic acid to analyze the levels of Suc (A), Glc (B), Fru (C), Glc1P (D), Glc6P (E), Fru6P (F), triose-P (G), 3PGA (H), PEP (I), pyruvate (J), isocitrate (K), α-ketoglutarate (L), malate (M), and PPi (N). Results are means ± se (n = 5).
In antisense tubers with reduced AATP expression, levels of soluble sugars increased markedly compared with the wild type. This was reflected by an up to 2.5-fold increase of Suc (significant for JT 676, JT 594, and JT 654; Fig. 2A), an up to 10-fold significant increase of Glc (to about 80 μmol g fresh weight−1; Fig. 2B), and an up to 4-fold, but non-significant, increase of Fru (Fig. 2C). These changes were paralleled by a more than 2-fold significant increase in Glc1P (Fig. 2D), a 1.7-fold significant increase in Glc6P (Fig. 2E), and a 1.7-fold significant increase in Fru6P (Fig. 2F). There were no significant changes in the levels of triose phosphates (Fig. 2G) and pyruvate (Fig. 2J), with the exception of line JT 654, which had significantly increased pyruvate levels. The levels of 3PGA (Fig. 2H), PEP (Fig. 2I), and isocitrate (Fig. 2K) decreased significantly by up to 23%, 50%, and 70%, respectively. Also the PEP/pyruvate ratio decreased by approximately 50% (calculated from Fig. 2, I and J), indicating a stimulation of PEP-utilizing reactions (Geigenberger and Stitt, 1991; Hatzfeld and Stitt, 1991). The marked and significant drop in isocitrate was accompanied by an up to 40% increase in the levels of α-ketoglutarate (significant for JT 595; Fig. 2L) and malate (significant for JT 594, JT595, and JT 654; Fig. 2 m). The ratio between α-ketoglutarate and isocitrate increased significantly up to 5-fold (compare Fig. 2, L and K), indicating activation of isocitrate dehydrogenase. Similar changes in sugar and metabolite levels occurred in transgenic tubers with decreased expression of AGPase (Geigenberger et al., 1999).
Levels of Nucleotides and PPi in Tubers with Altered Expression of the Plastidic Adenylate Transporter
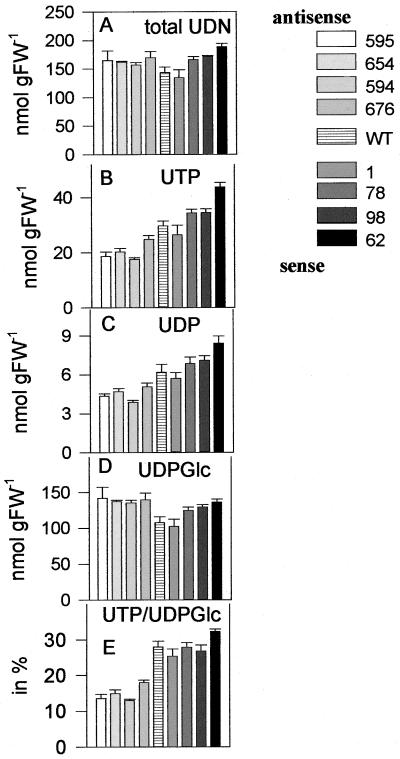
Suc mobilization in potato tubers requires uridine nucleotides and PPi as cofactors (see above). The overall levels of uridine nucleotides (Fig. 3A) were slightly increased in sense and antisense transformants (significant only for line JT 62). Tubers with increased AATP expression had up to 40% higher levels of UTP (Fig. 3B) and UDP (Fig. 3C), and an up to 26% increased levels of UDP-Glc (Fig. 3D; only significant for line JT 62). In contrast to this, the 15% increase in the overall uridine nucleotide content in the antisense tubers (Fig. 3A) was mainly due to a 25% increase in the levels of UDP-Glc (significant for lines JT 594 and JT 654; Fig. 3D). The levels of UTP and UDP dropped in these tubers by 50% (significant for lines JT 594, JT 654, and JT 595; Fig. 3, B and C), resulting in a 2-fold and significant decline in the ratio of UTP to UDP-Glc (Fig. 3E) compared with the wild type.
Figure 3.
Uridine nucleotide levels in tubers with altered expression of the AATP. The same extracts as in Figure 2 were used to analyze total uridine nucleotide levels (A), which is the sum of UTP (B), UDP (C), and UDP-Glc (D). The ratio of UTP/UDP-Glc (E) is also shown. Results are means ± se (n = 5).
PPi is produced in various cellular reactions, including starch synthesis, and could also serve as an alternative energy donor during Suc mobilization and glycolysis (Stitt, 1998). In sense tubers with increased AATP expression there was a 2- to 3-fold significant increase in PPi levels, whereas antisense tubers with decreased AATP expression exhibited up to 50% reduced levels of PPi compared with the wild type, which was significant only for JT 594 (Fig. 2N). It is interesting that a similar decrease in PPi level has been observed in tubers with reduced expression of AGPase (Farré et al., 2000).
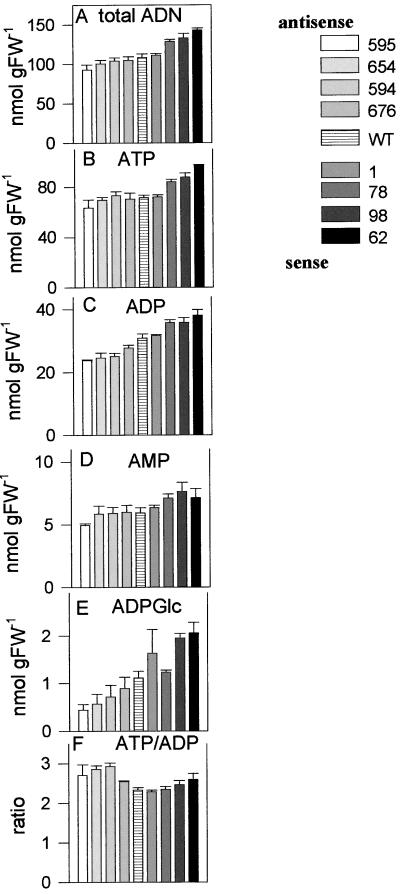
Adenine nucleotides are involved in respiratory energy production as well as in biosynthetic processes like anabolic, energy consuming processes such as starch biosynthesis. In sense tubers with increased AATP activity there was a slight increase in the overall level of adenine nucleotides compared with the wild type, which was significant for JT 78, JT 98, and JT 62 (Fig. 4A). In these lines there was an up to 1.4-fold significant increase in the level of ATP (Fig. 4B), a 1.3-fold significant increase in the level of ADP (Fig. 4C), a 1.3-fold significant increase in the level of AMP (Fig. 4D), and a slight, but not significant, increase in the overall ATP/ADP ratio (Fig. 4F).
Figure 4.
Adenine nucleotide levels in tubers with altered expression of the plastidic ATP/ADP translocator. The same extracts as in Figure 2 were used to analyze total adenine nucleotide levels (A), which is the sum of ATP (B), ADP (C), AMP (D), and ADP-Glc (E). The ratio of ATP/ADP (F) is also shown. Results are means ± se (n = 5).
This contrasts with the situation in antisense tubers, which exhibited an up to 20% decrease in the overall level of adenine nucleotides, which was however non-significant compared with the wild type (Fig. 4A). This was reflected by an up to 20% decrease in ATP (Fig. 4B), an up to 30% decrease in ADP (significant for lines JT 594, JT 654, and JT 595; Fig. 4C), and an up to 20% decrease in AMP (Fig. 4D). The overall ATP/ADP ratio increased slightly compared with the wild type (Fig. 4F).
These results indicate that alterations in the activity of the plastidic adenylate transporter affect the overall levels of adenine and uridine nucleotides, as well as the level of PPi. The reason for this is unknown. However, previous studies on potato tubers document that the levels of adenine and uridine nucleotides are regulated in parallel with changes in Suc and starch metabolism to avoid a limitation of Suc mobilization and starch biosynthesis (Geigenberger et al., 1994; Loef et al., 1999; Geigenberger and Stitt, 2000).
Levels of ADP-Glc in Tubers with Altered Expression of the Plastidic Adenylate Transporter
ADP-Glc is the product of the ATP-dependent AGPase reaction in the plastid and it represents the sole precursor of starch biosynthesis. The level of ADP-Glc was up to 2-fold higher in tubers with increased AATP expression compared with the wild type (Fig. 4E), the increase being significant for lines JT 98 and JT 62. In transgenic line JT 62 the ADP-Glc concentration was above 2 nmol g fresh weight−1 compared with 1.1 nmol g fresh weight−1 in the wild type. In contrast to this, antisense repression of AATP resulted in a 25% to 70% decrease of the ADP-Glc level compared with the wild type, which was significant for lines JT 595 and JT 654 and lead to values of 0.4 nmol g fresh weight−1 in line JT 595 (Fig. 4E). The contents of ADP-Glc in the transgenic tubers are in the range where the rate of starch synthesis is strongly dependent on the ADP-Glc content (Geigenberger et al., 1997, 1998).
Analysis of Enzyme Activities in Tubers with Altered Expression of the Plastidic Adenylate Transporter
To investigate whether the alterations in primary metabolism of the transgenic tubers are accompanied by pleiotropic changes in enzyme activities we analyzed the maximum activities of several enzyme in extracts from corresponding tubers under optimized assay conditions (Table I). There were no significant changes in the activities of Suc synthase and UGPase in extracts made from sense or antisense AATP expressing tubers compared with the wild type. In contrast to this, there was a 2-fold increase in alkaline invertase and a 3- to 7-fold increase in acid invertase activity in antisense tubers with decreased AATP expression (significant, P < 0.05), whereas no significant changes in invertase activities could be observed in sense tubers with increased AATP expression (Table I).
Table I.
Enzyme activities assayed in extracts from antisense (JT 676, JT 594, JT 654, and JT 595) and sense (JT 1, JT 78, JT 98, and JT 62) AATP-tubers
| Enzymes | WT | JT 676 | JT 594 | JT 654 | JT 595 | JT 1 | JT 78 | JT 98 | JT 62 |
|---|---|---|---|---|---|---|---|---|---|
| Suc synthase | 1,901 ± 211 | 1,941 ± 110 | 2,261 ± 143 | 2,337 ± 111 | 2,079 ± 300 | 1,626 ± 130 | 2,538 ± 62 | 2,253 ± 89 | 2,541 ± 200 |
| UDPGlc pyrophosphorylase | 1,397 ± 70 | 1,640 ± 25 | 1,668 ± 18 | 1,435 ± 51 | 1,629 ± 17 | 1,318 ± 58 | 1,401 ± 31 | 1,473 ± 52 | 1,389 ± 63 |
| Alkaline invertase | 25.6 ± 1.1 | 35.7 ± 0.6 | 57.7 ± 4.3 | 60.9 ± 2.9 | 51.1 ± 4.1 | 24.5 ± 1.5 | 27.6 ± 1.9 | 28.0 ± 1.2 | 30.9 ± 3.5 |
| Acid invertase | 6.1 ± 0.4 | 17.6 ± 2.0 | 33.5 ± 4.5 | 41.9 ± 8.5 | 43.3 ± 12.2 | 10.6 ± 5.7 | 7.1 ± 1.4 | 7.1 ± 1.6 | 9.8 ± 1.6 |
| SPS (Vmax) | 506 ± 34 | 453 ± 84 | 492 ± 37 | 447 ± 41 | 575 ± 68 | 435 ± 67 | 493 ± 34 | 486 ± 34 | 379 ± 94 |
| SPS (Vsel) | 32.2 ± 8.3 | 49.8 ± 5.5 | 52.5 ± 6.4 | 57.4 ± 3.4 | 70.0 ± 10 | 25.9 ± 4.2 | 36.2 ± 14 | 24.4 ± 7.1 | 27.2 ± 1.0 |
| SPS (Vsel/Vmax) | 6.0 ± 1.3 | 14.6 ± 5.0 | 10.5 ± 0.6 | 13.1 ± 0.8 | 12.3 ± 1.1 | 6.6 ± 1.6 | 6.9 ± 2.3 | 5.3 ± 1.5 | 7.2 ± 0.3 |
| Glc6P-dehydrogenase (G6PDH) | 1,437 ± 105 | 1,383 ± 73 | 1,521 ± 8 | 1,467 ± 103 | 1,639 ± 41 | 1,398 ± 57 | 1,319 ± 54 | 1,408 ± 140 | 1,939 ± 216 |
| 6-Phosphogluconate-DH | 270 ± 16 | 381 ± 54 | 327 ± 12 | 347 ± 8 | 358 ± 3 | 273 ± 12 | 276 ± 12 | 315 ± 11 | 343 ± 30 |
| Phosphofruktokinase (PFK) | 101 ± 6 | 76 ± 4 | 110 ± 9 | 129 ± 16 | 104 ± 10 | 98 ± 7 | 119 ± 6 | 105 ± 10 | 109 ± 9 |
| Pyrophosphate: Fru-6-phosphate: phosphotransferase (PFP) | 1,125 ± 69 | 926 ± 9 | 1,152 ± 48 | 940 ± 101 | 1,084 ± 60 | 1,089 ± 69 | 1,112 ± 41 | 1,160 ± 123 | 1,265 ± 90 |
| Glycerinaldehydephosphate dehydrogenase (GAP-DH) | 2,087 ± 255 | 1,616 ± 115 | 2,153 ± 245 | 2,094 ± 271 | 2,209 ± 97 | 1,959 ± 232 | 2,049 ± 304 | 1,698 ± 256 | 2,350 ± 346 |
| Pyruvatekinase | 379 ± 48 | 340 ± 38 | 418 ± 17 | 313 ± 36 | 281 ± 35 | 423 ± 65 | 528 ± 46 | 333 ± 93 | 376 ± 58 |
| PEP carboxylase | 97 ± 10 | 172 ± 18 | 186 ± 14 | 191 ± 16 | 232 ± 18 | 99 ± 7 | 129 ± 19 | 141 ± 11 | 131 ± 5 |
| PEP phosphatase | 517 ± 66 | 388 ± 47 | 608 ± 33 | 858 ± 120 | 608 ± 21 | 474 ± 44 | 698 ± 12 | 470 ± 101 | 479 ± 69 |
| ADPGlc pyrophosphorylase | 893 ± 75 | 723 ± 37 | 686 ± 35 | 812 ± 38 | 680 ± 51 | 857 ± 152 | 1,088 ± 29 | 1,024 ± 94 | 998 ± 66 |
Results (nmol g fresh wt−1 min−1) are the mean ± se (n = 5 separate tubers from different plants). Suc phosphate synthase (SPS) was measured under two different assay conditions: Vmax assay (12 mm Fru6P, 36 mm Glc6P, and 6 mm UDPGLc), or Vsel assay (2 mm Fru6P, 6 mm Glc6P, 6 mm UDPGlc, and 5 mm Pi).
Suc mobilization in potato tubers is catalyzed by a cycle of synthesis and degradation (Geigenberger and Stitt, 1993). We, therefore, also measured the activity of SPS in the corresponding extracts. When assayed under optimized conditions (Vmax), no significant changes in SPS activity were observed compared with the wild type. When the activity was assayed in the presence of limiting substrate concentrations (termed Vsel assay), antisense tubers revealed 2-fold increased SPS activities (significant for lines JT 654 and JT 595), whereas a slight, but non-significant, decline in activity was observed in sense tubers compared with the wild type. The Vsel/Vmax ratio was significantly increased in antisense tubers, whereas no significant changes were observed in sense tubers (Table I). Changes in the Vsel/Vmax ratio are indicative for changes in the phosphorylation status of potato tuber SPS (Reimholz et al., 1994). A similar increase in SPS activation was also observed in tubers with decreased expression of AGPase (Geigenberger et al., 1999a; Sweetlove et al., 1999). It is striking that the increase in the activities of SPS, alkaline invertase, and acid invertase in tubers with decreased AATP expression (Table I) coincided with a marked increase of Suc and an up to 10-fold increase in hexose levels (see Fig. 2, A–C), indicating an increased recycling of hexose phosphates to Suc and Glc in these tubers.
There were no substantial changes in the activities of G6P-DH, PFK, PFP, GAP-DH, pyruvatekinase, and PEP phosphatase in sense and antisense tubers compared with the wild type (Table I). Sense tubers revealed a slight, but non-significant, increase in 6-phosphogluconatedehydrogenase (6-phosphogluconate-DH) and PEP carboxylase activities. In antisense tubers, 6-phosphogluconate-DH increased up to 1.4-fold (significant for lines JT 594, JT 595, and JT 654), and PEP carboxylase increased significantly up to 2.4-fold, correlating with the decrease in the PEP/pyruvate ratio (see Fig. 2, I and J) and the accompanying increase in respiration rates in these lines (see Fig. 1). No substantial changes were observed in the maximal activity of AGPase in the corresponding tubers.
Starch Grain Morphology and Physical Properties of Starch in Tubers with Altered Expression of the Plastidic Adenylate Transporter
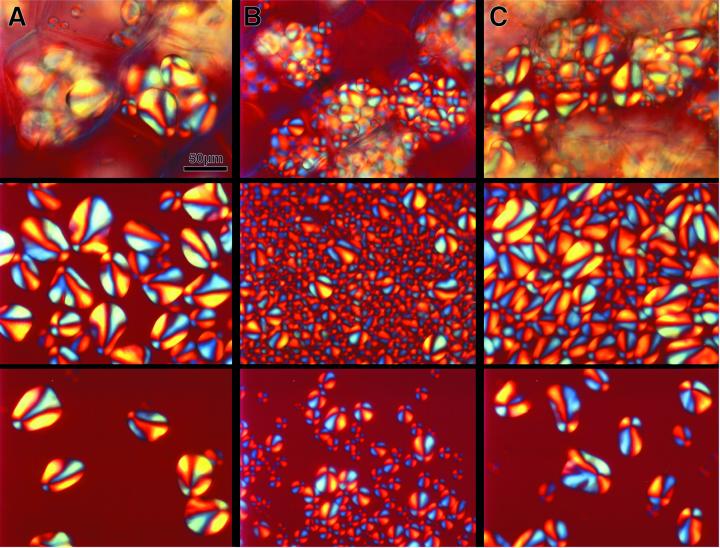
A polarization-light microscopic examination of starch grains revealed marked alterations of size and morphology of grains from wild-type and transgenic tissues (Fig. 5). The generally radial orientation of the birefringent, semi-crystalline amylopectin molecules (Wang et al., 1998), however, is not changed in the transformants, as indicated in all preparations by the blue color in the northeast and southwest sectors of each starch grain and by the yellow in the northwest and southeast sectors, respectively (Fig. 5).
Figure 5.
Microscopic examination of starch grains using polarized light. Hand sections of potato tubers (top panel), accumulation of isolated starch grains (middle panel), and single isolated starch grains (bottom panel) are depicted from wild type (A), antisense JT 654 (B), and sense JT 62 (C) transformants.
In wild-type cells about five to seven starch grains of ellipsoidal shape are present (Fig. 5A, top panel). The ellipsoidal shape of wild-type starch grains, which exhibit a diameter of about 40 to 50 μm, is easier to follow by microscopic examination of isolated grains (Fig. 5A, middle and bottom panel). In strong contrast, the total number of grains in antisense cells (data from line JT 654 is shown, but is typical of results obtained from other lines) is significantly increased (Fig. 5B, top panel), and the size is substantially smaller (Fig. 5B, middle and bottom panel). The major difference in starch grains obtained from plants containing a sense construct is that several large starch grains exhibit an angular shape (Fig. 5C, bottom panel), which seem to be different to the ellipsoidal wild-type grains (see above).
To quantify differences in granule size we extracted starch mechanically and carried out laser-diffraction spectroscopy (Table II). Ten percent of the wild-type grains exhibited a size of up to 20.7 μm (Table II). In contrast, 10% of grains from all four antisense lines examined were not more than 15 μm, corresponding to an average decrease in size of about 30%. Ninety percent of the wild-type grains exhibited a size of up to 67.1 μm, whereas 90% of the antisense grains do not exceed a size of more than 43 μm (Table II). No substantial differences in granule size distribution were found between wild type and the four sense lines (data not shown).
Table II.
Distribution of starch grain size
| Line/Genotype | Distribution of Starch Grain Size
|
||
|---|---|---|---|
| 10% | 50% | 90% | |
| μm | |||
| Wild type | 20.7 | 40.8 | 67.1 |
| JT 676 | 14.0 | 26.7 | 42.6 |
| JT 594 | 12.5 | 24.3 | 38.6 |
| JT 654 | 13.6 | 22.4 | 34.1 |
| JT 595 | 14.7 | 25.7 | 38.4 |
The analysis was carried out using laser-diffraction spectroscopy.
DISCUSSION
Alterations in Plastidic Adenylate Transporter Activity Lead to Increased Respiratory Activity in Potato Tubers
In potato tubers ATP is synthesized in the mitochondria, subsequently transported into the cytosol, and from there into the amyloplast via an ATP/ADP translocator (Heldt, 1969; Schünemann et al., 1993; Kampfenkel et al., 1995; Tjaden et al., 1998). We showed recently that transgenic tubers with decreased activity of AATP due to the insertion of an antisense ATP/ADP translocator construct exhibit drastically reduced levels of starch, whereas starch yield was increased in tubers of plants that contained a sense construct and that demonstrated increased transporter activity (Tjaden et al., 1998). In the present paper we demonstrate that tissues from antisense and sense tubers exhibit increased respiratory activity (Fig. 1). These results indicate that alterations in the ATP/ADP exchange rate between cytosol and plastid affect starch accumulation, as well as the rate of respiration.
It has been debated whether mitochondrial electron transport in plants is limited by the availability of carbon substrate, the cytosolic phosphorylation potential, or the absolute cytosolic concentration of ADP (Moore, 1992). In tubers with increased AATP expression, stimulation of respiratory activity occurred without major changes in the levels of soluble sugars, phosphorylated intermediates, or organic acids (Fig. 2), indicating that stimulation of respiration is not due to increased availability of carbon substrate. As the increased ATP/ADP exchange across the amyloplast envelope leads to stimulation of ATP-consuming processes in the plastid (increased levels of starch; Tjaden et al., 1998), it is more likely that elevated ADP levels appearing in the cytosol induced high respiratory activity. Tubers with increased AATP activity revealed slightly, but significantly, increased levels of ADP. Most importantly, there was a positive correlation between the increase in starch level (see Tjaden et al., 1998), the stimulation of respiration (Fig. 1), and the increase in ADP level (Fig. 4B) in individual AATP-overexpressing lines (data not shown). In addition to this, it has been shown recently that increased levels of adenine nucleotides (including ADP) after feeding adenine to potato tuber discs resulted in a stimulation of respiration (Loef et al., 2001). It must be noted, however, that the reported ADP levels are overall levels and do not necessarily reflect cytosolic concentrations. Subcellular analysis of leaves showed that during the dark period most of the ADP is located in the plastid, rather than in the cytosol (Lilley et al., 1982; Stitt et al., 1982).
In antisense tubers with decreased AATP expression, induced respiration was accompanied by markedly increased levels of free sugars and hexose-phosphates (Fig. 2), whereas ADP levels decreased slightly (Fig. 4C). The more than 4-fold increase in free sugars and the 2-fold increase in hexose-phosphate levels were probably a result of limited carbon usage for starch biosynthesis. Glc levels increased up to 10-fold (Fig. 2B), which coincided with increased invertase activities (Table I). A similar increase of Glc and stimulation of respiration can also be induced by overexpression of a heterologous invertase in the cytosol of potato tubers (Trethewey et al., 1998). Respiration can also be induced by short-term feeding of external Glc to wild-type tuber slices (Geiger et al., 1998). The reason(s) for the stimulation of respiration in AATP antisense lines are not clear. There are three possible explanations. First, inhibition of starch accumulation results in increased availability of carbon substrates for respiratory metabolism. However, pyruvate, α-ketoglutarate, and malate increased only slightly, and isocitrate actually decreased (see Fig. 2). Second, increased hexose-phosphate levels lead to a stimulation of SPS activity and induction of futile Suc cycling (see Table I; Geigenberger et al., 1997; Trethewey et al., 1999), resulting in increased cellular energy consumption. It is now generally accepted that Glc acts as a metabolic signal regulating carbohydrate metabolism in plant tissues (Koch, 1996). Therefore, another possibility would be that invertase, increased Glc levels, or increased rates of Glc metabolism act as signal(s) leading to stimulation of respiratory activity. In this context it will be interesting in the very near future to deepen our understanding about concomitant changes in mitochondrial metabolism in these transgenic plants.
Alterations in Plastidic Adenylate Transporter Activity Modulate Starch Synthesis by Regulating ADP-Glc Pyrophosphorylase
As demonstrated previously, plants exhibiting increased plastidic adenylate transporter activity accumulate significantly higher amounts of starch than wild-type plants, whereas antisense plants contain substantially reduced levels of starch (Tjaden et al., 1998). By comparing the in vivo levels of ADP-Glc in wild-type and transgenic plants (Fig. 4E) and the corresponding starch levels in the individual plant lines (Tjaden et al., 1998), a correlation coefficient (r2) of 0.93 is present (data not shown). Therefore, the data presented here provide striking evidence that the level of ADP-Glc, the sole precursor for starch biosynthesis, governs starch accumulation in potato tubers. This conclusion is further strengthened by data from transgenic potato tubers containing decreased AGPase activity and less starch (Müller-Röber et al., 1992), because a similar decrease in ADP-Glc was observed (P. Geigenberger, unpublished data).
The good in vivo correlation between the levels of ADP-Glc and starch in tubers with different AATP expression further indicates that the rate of starch accumulation was modified by regulating AGPase activity. This suggests a close interaction between ATP availability in the plastid, AGPase activity, and starch biosynthesis. Since overall AGPase activity was not substantially changed (Table I), we can rule out that modulation of starch synthesis was due to changes in the expression of AGPase in these lines. However, there are also further factors that can alter AGPase activity in potato tubers. AGPase from potato tubers is allosterically activated by 3PGA competitively to Pi (Preiss, 1988). Previous studies document the in vivo significance of this mechanism in the regulation of starch synthesis in potato tubers (Stark et al., 1992; Geigenberger et al., 1997, 1998). Since 3PGA did not change significantly in tubers with increased AATP activity compared with the wild type, it is very unlikely that changes in 3PGA are responsible for the stimulation of AGPase in these lines. An alternative and more likely explanation is that AGPase is restricted in vivo by the plastidic adenylate transporter activity and the plastidic concentration of ATP. This conclusion is further strengthened by recent studies showing that an increase in ATP level is responsible for stimulation of starch synthesis in wild-type tuber slices after feeding adenine (Loef et al., 2001). In antisense AATP tubers, however, a slight (20%), but significant, decrease of 3PGA was observed, which could provide an additional explanation for the inhibition of AGPase in these lines. However, it is unclear whether the decrease in 3PGA is counterbalanced by a possible decrease in Pi due to sequestration in hexose phosphates (the latter increased 2-fold in these lines).
The situation will be different in the seed endosperm of some cereals where AGPase is also present in the cytosol in addition to its plastidic location (for review, see Smith et al., 1997). A putative ADP-Glc/adenylate transporter has recently been identified in maize, which could mediate the transport of cytosolic ADP-Glc into the plastid (Sullivan and Kaneko, 1995; Shannon et al., 1998). Operation of this transporter during endosperm development could provide an additional capacity to supply ADP-Glc in situations where a strong demand for starch synthesis is present.
Alterations in Plastidic Adenylate Transporter Activity Lead to Changes in Granule Growth and Morphology
ADP-Glc is the product of the ATP-dependent AGPase reaction in the plastid, and the ultimate precursor of starch grain biosynthesis (Preiss, 1988). Transgenic tubers with increased plastidic ATP/ADP transporter activity contained increased levels of starch, whereas antisense tubers with reduced activity of the transporter contained reduced starch levels (Tjaden et al., 1998). It is interesting that not only the total starch was altered, but also the amylose to amylopectin ratio. This ratio was increased in sense plants and decreased in antisense plants (Tjaden et al., 1998). So far the interaction between starch levels and starch composition, and the structure of starch grains has received very little attention in the literature.
The morphological examination of starch grains (Fig. 5) and the quantitative analysis of starch grain size (Table II) unequivocally demonstrate that antisense tubers contain starch grains with much smaller diameter and more grains per cell. Lloyd et al. (1996) showed that in pea, starch mutants exhibit a similar correlation between starch level and size of starch grains. However, the amount of starch is not the only parameter that seems to control the grain size. A Chlamydomonas mutant lacking granule-bound starch synthase activity and no change in total starch content also has smaller grains, which is most likely due to the total absence of amylose (Buléon et al., 1997).
Therefore, it is now clear that altered ATP provision to potato amyloplasts not only influences tuber shape, starch level, and starch composition (Tjaden et al., 1998), but also starch grain morphology (Fig. 5; Table II). The latter observation is consistent with and extends previous studies on Chlamydomonas mutants defective in the 3PGA activation of AGPase (Van den Koornhuyse et al., 1996), and on transgenic potato tubers with decreased expression of AGPase (Lloyd et al., 1999). The results suggest that changes in ADP-Glc concentration determine starch yield and structure, as well as granule morphology. It will be interesting to study, in addition to the morphology, how far the physico-chemical properties of starch isolated from these sense and antisense plants are affected by starch gain size and the amylose-to-amylopectin ratio.
MATERIALS AND METHODS
Plant Material
Potato (Solanum tuberosum cv Desirée) plants were grown in soil (10-L pots) in a greenhouse (ambient light was supplemented with Philips Sont-Agro lights at 200 μmol photons m−2 s−1 irradiance, 16-h/8-h day/night regime, 20°C –23°C, 70%–80% relative humidity). Growing tubers from 12- to 15-week-old plants that were watered daily were used for the experiments.
Metabolite Analysis
Tissue slices were cut from intact growing tubers and frozen in liquid nitrogen, then extracted with trichloroacetic acid as given in Jelitto et al. (1992). Hexose-phosphates, 3PGA, pyruvate, isocitrate, α-ketoglutarate, and malate were quantified as given in Merlo et al. (1993). Adenine and uridine nucleotides were quantified in trichloroacetic acid extracts by high-pressure liquid chromatography using a Kontron HPLC-system (Kontron Instruments, Eiching, Germany) fitted with a Partisil-SAX10 anion-exchange column (Geigenberger et al., 1997). The recovery of small, representative amounts of each metabolite through the extraction, storage, and assay procedures has been documented previously (see Jelitto et al., 1992; Merlo et al., 1993; Geigenberger et al., 1994; Hajirezaei et al., 1994; Farré et al., 2000).
Enzyme Analysis
Tissue slices were cut from intact growing tubers, frozen in liquid nitrogen, then extracted, spin-desalted, and SPS was immediately assayed via the anthrone test as in Geigenberger et al. (1997). Aliquots of the extract were snap-frozen in liquid nitrogen and assayed for Suc synthase, acid invertase, alkaline invertase, UDP-Glc pyrophosphorylase, PFP, PFK, pyruvate kinase, PEP phosphatase, PEP carboxylase, and AGPase as in Merlo et al. (1993), GAP-DH according to Trethewey et al. (1998), and Glc6P-DH and 6-phosphogluconat-DH according to Bergmeyer (1987).
Analysis of Respiratory Activity
Four potato tuber discs (200 mg fresh weight) were transferred into the measuring chamber of a Warburg respirometer and supplied with Suc (100 mm) to measure O2 consumption and CO2 production (at 25°C). Four tuber discs were collected from four individual plants of a transgenic line or wild types. Tubers chosen were of equal size and exhibited about 6 cm in longitudinal diameter.
Microscopic Analysis of Grain Morphology
Hand sections of potato tubers were prepared in water and subject to polarization microscopy. Preparation of isolated starch grains was done by scraping material from a tuber with a razor blade, transferring it onto a microscope slide, and adding a drop of water. Starch grains are birefringent showing a Maltese cross when observed with crossed Polaris's. With addition of a λ-retardation plate in northeast-southwest direction, birefingent structures show up bright blue or yellow on a pink background dependent on their axis, oriented parallel (blue), or perpendicular (yellow) to the λ-plate.
Starch Grain Size Distribution Analysis
The size distribution of isolated starch grains was estimated by laser diffraction spectroscopy (type Mastersizer S, version 2.15, Malvern Instruments, UK). The instrument was equipped with a flow-through geometry unit for liquids and a 300-mm range lens. Beam length was 2.4 mm. Particle size distribution (0.05–900 μm) was registered using 64 information channels. The results of the measurements are expressed as the volume percentage of starch in particles smaller or equal to the threshold value of the channel. The starch powder was thoroughly suspended in water by stirring and was immediately added to the analytical water circuit of the instrument. This technique detects particle size, but cannot discriminate between single granules and possible granule clusters.
Statistical Analysis of Data
Statistics were calculated using the t test. The word significant is used in the text when the change in question has been confirmed to be statistically significant (P < 0.05).
ACKNOWLEDGMENT
We would like to thank Gudrun Henrichs (Universität Osnabrück, Germany) for expert technical assistance during the course of this work.
Footnotes
This work was supported by the Sonderforschunsgbereich 431 (Teilprojekt K7 to H.E.N.), by the Fonds der Chemischen Industrie (grant to H.E.N.), and by the Deutsche Forschungsgemeinschaft (grant to P.G.).
LITERATURE CITED
- ap Rees T. Hexose phosphate metabolism in non-photosynthetic tissues of higher plants. In: Preiss J, editor. The Biochemistry of Plants. Vol. 14. New York: Academic Press; 1988. pp. 1–33. [Google Scholar]
- Avigad G. Sucrose and other disaccharides. In: Loewus TA, Tanner W, editors. Encyclopedia of Plant Physiology. 18A. Heidelberg: Springer-Verlag; 1982. pp. 217–347. [Google Scholar]
- Bergmeyer HU. Methods of Enzymatic Analysis. Weinheim, Germany: VCH; 1987. [Google Scholar]
- Buléon A, Gallant DJ, Bouchet B, Mouille M, D'Hulst C, Kossmann J, Ball S. Starches from A to C: Chlamydomonas reinhardtii as a model system to investigate the biosynthesis of the plant amylopectin crystal. Plant Physiol. 1997;115:949–957. doi: 10.1104/pp.115.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré EM, Geigenberger P, Willmitzer L, Trethewey RN. A possible role for pyrophosphate in the coordination of cytosolic and plastidial carbon metabolism within the potato tuber. Plant Physiol. 2000;123:681–688. doi: 10.1104/pp.123.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Geiger M, Stitt M. High-temperature inhibition of starch synthesis is due to inhibition of ADPGlc pyrophosphorylase by decreased levels of 3PGA in growing potato tubers. Plant Physiol. 1998;117:1307–1317. doi: 10.1104/pp.117.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D, Heldt HW, Stitt M. Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta. 1993;190:446–453. [Google Scholar]
- Geigenberger P, Merlo L, Reimholz R, Stitt M. When growing potato tubers are detached from their mother plant there is a rapid inhibition of starch synthesis, involving inhibition of ADP-glucose pyrophosphorylase. Planta. 1994;193:486–493. [Google Scholar]
- Geigenberger P, Müller-Röber B, Stitt M. Contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis is decreased by water stress in growing potato tubers. Planta. 1999a;209:338–345. doi: 10.1007/s004250050641. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Reimholz R, Deiting U, Sonnewald U, Stitt M. Decreased expression of sucrose phosphate synthase strongly inhibits the water stress-induced synthesis of sucrose in growing potato tubers. Plant J. 1999b;19:119–129. doi: 10.1046/j.1365-313x.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta. 1997;201:502–518. [Google Scholar]
- Geigenberger P, Stitt M. Regulation of carbon partitioning between sucrose and nitrogen assimilation in cotyledons of germinating Ricinus communis L. seedlings. Planta. 1991;185:563–568. doi: 10.1007/BF00202967. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M. Sucrose synthase catalyzes a readily reversible reaction in developing potato tubers and other plant tissues. Planta. 1993;189:329–339. doi: 10.1007/BF00194429. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M. Diurnal changes in sucrose, nucleotides, starch synthesis, and AGPS transcript in growing potato tubers that are suppressed by decreased expression of sucrose phosphate synthase. Plant J. 2000;23:795–806. doi: 10.1046/j.1365-313x.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- Geiger M, Stitt M, Geigenberger P. Metabolism in potato tuber slices responds differently after addition of sucrose and glucose. Planta. 1998;206:245–252. [Google Scholar]
- Hajirezaei M, Sonnewald U, Viola R, Carlisle S, Dennis D, Stitt M. Transgenic potato plants with strongly decreased expression of pyrophosphate:fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta. 1994;192:16–30. [Google Scholar]
- Hatzfeld W-D, Stitt M. Regulation of glycolysis in heterotrophic cell suspension cultures of Chenopodium rubrum in response to proton fluxes at the plasmalemma. Physiol Plant. 1991;81:103–110. [Google Scholar]
- Heldt HW. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969;5:11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezaei MR, Stitt M. Inorganic pyrophosphate content and metabolites in leaves and tubers of potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta. 1992;188:238–244. doi: 10.1007/BF00216819. [DOI] [PubMed] [Google Scholar]
- Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge U-I. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose-6-phosphate/phosphate antiporter. Plant Cell. 1998;10:105–117. doi: 10.1105/tpc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K, Möhlmann T, Batz O, van Montagu M, Inze D, Neuhaus HE. Molecular cloning of an Arabidopsis thaliana cDNA encoding a novel putative adenylate translocator of higher plants. FEBS Lett. 1995;374:351–355. doi: 10.1016/0014-5793(95)01143-3. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Kruger NJ. Carbohydrate synthesis and degradation. In: Dennis DT, Turpin DH, Lefebvre DD, Layzell DB, editors. Plant Metabolism. Harlow, UK: Longman; 1997. pp. 83–104. [Google Scholar]
- Lilley RMcC, Stitt M, Mader G, Heldt HW. Rapid fractionation of wheat leaf protoplasts using membrane filtration. Plant Physiol. 1982;70:965–970. doi: 10.1104/pp.70.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Springer F, Buleon A, Müller-Röber B, Willmitzer L, Kossmann J. The influence of alterations in ADP-glucose pyrophosphorylase activities on starch structure and composition in potato tubers. Planta. 1999;209:230–238. doi: 10.1007/s004250050627. [DOI] [PubMed] [Google Scholar]
- Lloyd JR, Wang TL, Hedley CL. An analysis of seed development in Pisum sativum: XIX. Effect of mutant alleles at the r and rb loci on starch grain size and on the content and composition of starch in developing pea seeds. J Exp Bot. 1996;47:171–180. [Google Scholar]
- Loef I, Stitt M, Geigenberger P. Orotate leads to a specific increase in uridine nucleotide levels and a stimulation of sucrose degradation and starch synthesis in discs from growing potato tubers. Planta. 1999;209:314–323. doi: 10.1007/s004250050638. [DOI] [PubMed] [Google Scholar]
- Loef I, Stitt M, Geigenberger P (2001) Increased adenine nucleotide levels modify the interaction between respiration and starch synthesis when adenine is fed to discs of growing potato tubers. Planta (in press) [DOI] [PubMed]
- Marshall J, Sidebottom C, Debet M, Martin C, Smith A, Edwards A. Identification of the major soluble starch synthase in the soluble fraction of potato tubers. Plant Cell. 1996;8:1121–1135. doi: 10.1105/tpc.8.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Smith AM. Starch biosynthesis. Plant Cell. 1995;7:971–985. doi: 10.1105/tpc.7.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo L, Geigenberger P, Hajirezaei M, Stitt M. Changes of carbohydrates, metabolites and enzyme activities in potato tubers during development, and within a single tuber along a stolon-apex gradient. J Plant Physiol. 1993;142:392–402. [Google Scholar]
- Moore AL. Factors affecting the regulation of mitochondrial respiratory activity. In: Lambers H, van der Plas LHW, editors. Molecular, Biochemical and Physiological Aspects of Plant Respiration. The Hague, The Netherlands: SPB Academic Publishing; 1992. pp. 9–18. [Google Scholar]
- Müller-Röber BT, Sonnewald U, Willmitzer L. Inhibition of ADP-glucose pyrophosphorylase leads to sugar storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J. 1992;11:1229–1238. doi: 10.1002/j.1460-2075.1992.tb05167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naem M, Tetlow IJ, Emes M. Starch synthesis in amyloplasts purified from developing potato tubers. Plant J. 1997;11:1095–1103. [Google Scholar]
- Oparka K, Viola R, Wright K, Prior DAM. Sugar transport and metabolism in the potato tuber. In: Pollock CJ, Farrar JF, Gordon AJ, editors. Carbon Partitioning within and between Organisms. Oxford: BIOS Scientific Publishers; 1992. pp. 1–26. [Google Scholar]
- Preiss J. Biosynthesis of starch and its regulation. In: Preiss J, editor. The Biochemistry of Plants. Vol. 14. San Diego: Academic Press; 1988. pp. 181–254. [Google Scholar]
- Reimholz R, Geigenberger P, Stitt M. Sucrose-phosphate synthase is regulated via metabolites and protein phosphorylation in potato tubers, in a manner analogous to the enzyme in leaves. Planta. 1994;192:480–488. [Google Scholar]
- Renz A, Merlo L, Stitt M. Partial purification of three fructokinases and three hexokinases from potato tubers which show differing organ and developmental specificity. Planta. 1993;190:156–165. [Google Scholar]
- Schott K, Borchert S, Müller-Röber B, Heldt HW. Transport of inorganic phosphate, and C3- and C6-sugar phosphates across the envelope membranes of potato tuber amyloplasts. Planta. 1995;196:647–652. [Google Scholar]
- Schünemann D, Borchert S, Flügge U-I, Heldt HW. ATP/ADP translocator from pea root plastids: comparison with translocators from spinach chloroplasts and pea leaf mitochondria. Plant Physiol. 1993;103:131–137. doi: 10.1104/pp.103.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JC, Pien F-M, Cao H, Liu K-C. Brittle-1, an adenylate translocator, facilitates transfer of extra plastidial synthesized ADP-glucose into amyloplasts of maize endosperms. Plant Physiol. 1998;117:1235–1252. doi: 10.1104/pp.117.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Denyer K, Martin C. The synthesis of the starch granule. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:67–87. doi: 10.1146/annurev.arplant.48.1.67. [DOI] [PubMed] [Google Scholar]
- Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM. Regulation of the amount of starch in plant tissues by ADP-glucose pyrophosphorylase. Science. 1992;258:287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- Stitt M. Pyrophosphate as an alternative energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot Acta. 1998;111:167–175. [Google Scholar]
- Stitt M, Lilley RMcC, Heldt HW. Adenine nucleotide levels in the cytosol, chloroplasts and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982;70:971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T, Kaneko Y. The maize brittle1 gene encodes amyloplast membrane polypeptides. Planta. 1995;196:477–487. doi: 10.1007/BF00203647. [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Müller-Röber B, Willmitzer L, Hill SA. The contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis in potato tubers. Planta. 1999;209:330–337. doi: 10.1007/s004250050640. [DOI] [PubMed] [Google Scholar]
- Tjaden J, Möhlmann T, Kampfenkel K, Henrichs G, Neuhaus HE. Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of starch. Plant J. 1998;16:531–540. [Google Scholar]
- Trethewey RN, Geigenberger P, Hajirezaei M, Sonnewald U, Stitt M, Riesmeier J, Willmitzer L. Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J. 1998;15:109–118. doi: 10.1046/j.1365-313x.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Riesmeier J, Willmitzer L, Stitt M, Geigenberger P. Tuber-specific expression of a yeast invertase and a bacterial glucokinase in potato leads to an activation of sucrose phosphate synthase and the creation of a sucrose futile cycle. Planta. 1999;208:227–238. doi: 10.1007/s004250050554. [DOI] [PubMed] [Google Scholar]
- Van den Koornhuyse N, Libessart N, Delrue B, Zabawinski C, Decq A, Iglesias A, Carton A, Preiss J, Ball S. Control of starch composition and structure through substrate supply in the monocellular alga Chlamydomonas reinhardtii. J Biol Chem. 1996;271:16281–16287. doi: 10.1074/jbc.271.27.16281. [DOI] [PubMed] [Google Scholar]
- Wang TL, Bogracheva TY, Hedley CL. Starch: as simple as A, B, C? J Exp Bot. 1998;49:481–502. [Google Scholar]
- Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants. Plant J. 1995;7:97–107. doi: 10.1046/j.1365-313x.1995.07010097.x. [DOI] [PubMed] [Google Scholar]
- Zrenner R, Willmitzer L, Sonnewald U. Analysis of the expression of potato uridinediphosphoglucose pyrophosphorylase and its inhibition by antisense RNA. Planta. 1993;190:247–252. doi: 10.1007/BF00196618. [DOI] [PubMed] [Google Scholar]