Abstract
The use of lactic acid bacteria (LAB) and probiotic cultures in the breeding of animals such as poultry and swine are quite common. It is known that those strains can produce bacteriocins when grown in pure culture. However, the production of bacteriocin using co-culture of microorganisms has not been much studied so far. The present study contributes with innovation in this area by embracing the production of bacteriocin-like inhibitory substances (BLIS) by a newly isolated strain of Enterococcus faecium 135. Additionally, the co-cultivation of this strain with Ligilactobacillus salivarius and Limosilactobacillus reuteri was also investigated. The antimicrobial activity of the produced BLIS was evaluated against Listeria monocytogenes, Listeria innocua, Salmonella enterica, and Salmonella enterica serovar Typhimurium using two methods: turbidimetric and agar diffusion. In addition, the presence of enterocin genes was also evaluated. The BLIS produced showed a bacteriostatic effect against the bio-indicator strains, and the highest antimicrobial activities expressed by arbitrary units per mL (AU/mL) were obtained against L. monocytogenes in monoculture (12,800 AU/mL), followed by the co-culture of E. faecium with Limosilactobacillus reuteri (400 AU/mL). After concentration with ammonium sulfate, the antimicrobial activity raised to 25,600 AU/mL. Assays to determine the proteinaceous nature of the BLIS showed susceptibility to trypsin and antimicrobial activity until 90 °C. Finally, analysis of the presence of structural genes of enterocins revealed that four enterocin genes were present in E. faecium 135. These results suggest that BLIS produced by E. faecium 135 has potential to be a bacteriocin and, after purification, could potentially be used as an antimicrobial agent in animal breeding.
Keywords: Enterococcus faecium, Antimicrobial Activity, Co-culture, Foodborne pathogens
Introduction
The inappropriate and excessive use of antibiotics in the breeding of animals such as poultry and swine, associated with the increased resistance of bacteria to antibiotics, has led to major changes in policies to regulate the use of antibiotics, especially by the European Food Safety Authority (EFSA). These policies mainly imply the prohibition of certain antibiotics used in subtherapeutic doses to assist the growth of animals, in addition to the often inappropriate treatments by breeder [1]. With the mass production and processing of these meats, there is a great concern with the potential presence of pathogenic microorganisms (Salmonella spp., Listeria spp., and Campylobacter spp.), which has led to serious cleaning and disinfection measures in animal breeding, slaughtering, and meat handling [2]. Therefore, besides the classical methods of prevention such as sanitization and use of antibiotics, other techniques have also been used by breeders including vaccination, use of organic acids, lactic acid bacteria (LAB), probiotics, and bacteriocins [3, 4]. Some of the main reasons for the use of LAB and probiotics in the feeding of these animals are the prevention of growth of enteric pathogens and the improvement of the meat quality [5]. Usually, the administration of those probiotics and bacteriocins in animal breeding is made during the feed, and to secure the viability of the strains and action of the bacteriocins, the substances are commonly microencapsulated and administered with ration or water [6, 7].
Several microorganisms are known to show probiotic properties, including LAB, non-lactic acid bacteria, and some yeasts. From these microorganisms, LAB are the most studied and they generally belong to the genera Streptococcus spp., Lactococcus spp., Enterococcus and the family Lactobacillaceae [8–11]. Besides producing organic acids, these LAB can also produce other antimicrobial compounds such as hydrogen peroxide and bacteriocins [12]. Bacteriocins or bacteriocin-like inhibitory substances (BLIS) are peptides or proteins produced by the ribosomes and excreted into the extracellular environment, exerting antimicrobial activities against other bacteria [13, 14]. LAB strains that produce bacteriocins or BLIS include strains of the genera Enterococcus spp. [9, 15]. Among these, some strains of Enterococcus faecium have been reported to produce enterocins belonging to different classes, which are circular bacteriocins, members of class I or class II bacteriocins with low molecular weight (< 10 kDa) [13, 16] and excellent activities against Listeria monocytogenes, Salmonella enterica, and Escherichia coli [9, 17, 18]. From the Lactobacillaceae family, Ligilactobacillus salivarius is capable of producing salivaricin, a class II bacteriocin with activity against Salmonella sp., Campylobacter jejuni, and Listeria sp. [14], while Limosilactobacillus reuteri produces reuterin, a class II bacteriocin with an anti-listerical effect [19].
The use of co-cultures to produce bacteriocins is a strategy still not much investigated in the literature. Some authors reported that the production of nisin by Lactococcus lactis was increased by 85% when the strain was co-cultivated with Saccharomyces cerevisiae [20]. On the other hand, co-cultivation of Lactococcus lactis subsp. cremoris with a bacteriocin producer strain of E. faecium resulted in an interruption of the bacteriocin production [21]. In general, when bacteria are used in co-cultivation to produce bacteriocins, the success is strain related [22–24] and the inducing microorganism must be resistant to this particular bacteriocin [25–27].
Due to the potential of some LAB to produce BLIS or bacteriocins, this study aimed to evaluate the production of BLIS by a newly isolated strain of E. faecium 135, and its antimicrobial activity against Listeria monocytogenes, Listeria innocua, Salmonella enterica, and Salmonella enterica serovar Typhimurium. In addition, the presence of enterocin genes in E. faecium 135 DNA was investigated. The BLIS production by co-cultivation of E. faecium 135 with the two bacteriocin producers, Ligilactobacillus salivarius and Limosilactobacillus reuteri, was also evaluated, with its action being tested against the most common pathogens that cause concern to the breeding industry.
Materials and methods
Microorganisms
Enterococcus faecium 135 isolated from the intestines of a starfish (Order Forcipulatida) in Playa Unión, Rawson-Chubut (Patagonia, Argentina) and kindly donated by Prof. Marisol Vallejo, National University of Patagonia San Juan Bosco (Argentina), was used as BLIS producer. The following strains were used as bio-indicators: Salmonella enterica CECT 724 and Listeria monocytogenes CECT 934 (acquired from the Spain Collection of Cultures, Spain), Salmonella enterica serovar Typhimurium IOC 5551/16, and Listeria innocua CLIST 2711 (kindly provided by Fiocruz, Rio de Janeiro, Brazil). For the co-cultivation experiments, the bacteriocin producer strains Ligilactobacillus salivarius subsp. salicinius ATCC 11742 and Limosilactobacillus reuteri ATCC 23272 (acquired from André Tosello Foundation, Campinas, Brazil) were used.
The strains were cryopreserved in tubes containing 1 mL of cell culture with glycerol 20%, at − 76 °C.
Medium, inoculum, and cultivation conditions
MRS (de Man, Rogosa, and Sharpe) broth (Difco™, MD, USA) was the medium used for the bacteria cultivation. It was prepared following the manufacturer’s instructions and had the final pH adjusted to 6 by adding HCl 1 N.
The inoculum was prepared by adding 1 mL of stock culture of E. faecium 135 in a 250-mL Erlenmeyer flask containing 50 mL of MRS broth, followed by incubation on an orbital shaker (Tecnal Equipamentos Científicos, Piracicaba, Brazil) at 37 °C, 100 rpm, for 24 h. Then, 10 mL of the inoculum, with optical density (OD) at 600 nm wavelength adjusted to 0.8 (~ 7 log CFU/mL), was transferred to an Erlenmeyer flask containing 90 mL of MRS broth. In this step, the single cultivation of the strain was performed in a metabolic agitator under different conditions of temperature (30 and 35 °C) and agitation (100 and 150 rpm).
For the co-cultivation assays, the following strain mixtures were used: (i) Ligilactobacillus salivarius ATTC 11742 and Limosilactobacillus reuteri ATCC 23272 (5:5 mL); (ii) Ligilactobacillus salivarius ATTC 11742 and E. faecium 135 (5:5 mL); (iii) Limosilactobacillus reuteri ATCC 23272 and E. faecium 135 (5:5 mL); (iv) Ligilactobacillus salivarius ATTC 11742, Limosilactobacillus reuteri ATCC 23272, and E. faecium 135 (3.3: 3.3: 3.3 mL). Ligilactobacillus salivarius and Limosilactobacillus reuteri strains were activated in a similar way to that performed for E. faecium. For the experiments, all the strains had the OD adjusted to 0.8 (~ 7 log CFU/mL) in order to start with the same amount of cells. The co-cultivation assays were carried out in Erlenmeyer flasks containing 100 mL of MRS broth, at 35 °C, in an orbital shaker at 100 rpm for 24 h. At the end of the experiments, samples were taken and submitted to antimicrobial activity analysis.
Antimicrobial activity of potential BLIS
Determination of antimicrobial activity by turbidimetric analysis
Samples were taken at the end of the cultivations, centrifuged at 4470 g for 10 min, and the supernatant was filtered through 0.22 µm filter (Analítica, São Paulo, Brazil). Then, the cell-free supernatant (CFS) had the pH adjusted to 6.0–6.5 with NaOH 1 M and was thermically treated at 70 °C for 20 min to be used in the antimicrobial activity assays [28].
The antimicrobial activity was verified against the bio-indicator strains S. enterica, S. Typhimurium, and L. monocytogenes. The strains were previously grown in TSB (Tryptic Soy Broth) (Difco™, Le Pont de Claix, France) medium for 16 h. For the analysis, the cultures had the OD adjusted to 0.1 (S. enterica and S. Typhimurium) and 0.2 (L. monocytogenes).
Analyses were performed in 96-well plates using a microplate reader (Synergy HTX, Bio Tek, Winooski, USA). For analysis, the following volumes were added per well: 50 µL of the bio-indicator strain, 50 µL of the previously treated CFS, and 100 µL of TSB broth. Assays were also performed with a positive control, i.e., without BLIS. The microplates were maintained at 37 °C for 24 h. During this period, the OD (600 nm) was measured every 30 min (the plate was agitated at 50 rpm before every reading to homogenize the cell suspension). Analyses were performed in triplicate. After 24 h, a graphic comparing the positive control with the samples containing treated CFS was plotted to observe the action of BLIS on the growth of the bio-indicator strains.
Determination of antimicrobial activity through the agar diffusion method
After obtaining the CFS as described in the “Determination of antimicrobial activity by turbidimetric analysis,” BHI (Brain Heart Infusion) (Difco™, MD, USA) medium was used to grow L. monocytogenes, and TSB (Difco™, Le Pont de Claix, France) medium was used to grow S. enterica and S. Typhimurium. Initially, 108 CFU/mL of each strain were inoculated in 10 mL of BHI or TSB agar and then transferred to a Petri dish. After solidification, 10 μL of CFS was added and the plate was incubated at 37 °C for 18 h. The antimicrobial activity of the BLIS was expressed in arbitrary units per milliliter (AU/mL). The activity was measured by the dilution of the treated CFS in a twofold dilution using phosphate buffer 100 mM (pH 6.5). The highest dilution with a minimum halo of 2 mm of inhibition was considered for calculations using the Eq. (1), where a is the dilution factor, and b is the highest dilution with an inhibition halo; the obtained value is expressed in milliliters by multiplying per 100 [29].
| 1 |
Treated CFS from co-cultures of E. faecium, L. salivarius, and L. reuteri also had the antimicrobial activity tested using the method described above, being the activity also expressed in AU/mL.
BLIS concentration with ammonium sulfate
To concentrate the produced BLIS, 10 g of ammonium sulfate (Labsynth Produtos para Laboratórios Ltda, Diadema, Brazil) was added in tubes containing 20 mL of treated CFS (50% saturation), then the tubes were agitated vigorously for 1 min, followed by incubation at 10 °C, 100 rpm, for 1 h. Afterwards, the content of the tubes was centrifuged (4470 g, 4 °C, 30 min), the precipitate was recovered and resuspended with 10% (v/v) of the initial volume (20 mL) of 25 mM (pH 6.5) ammonium acetate (Labsynth Produtos para Laboratórios Ltda, Diadema, Brazil) solution, and filtered through 0.22 μm filter [29]. The antimicrobial activity of the concentrated BLIS was determined by the agar diffusion method.
Determination of the protein nature of the BLIS
The proteinaceous nature of the antimicrobial compound was determined by the agar diffusion method. Firstly, BLIS was obtained as described in the “Determination of antimicrobial activity by turbidimetric analysis.” Then, 10 µL of treated BLIS was poured in a Petri dish containing L. innocua as bio-indicator strain. The same volume of trypsin (10 µL) (Sigma-Aldrich, Saint Louis, USA) at 1 mg/mL was poured in the Petri dish in such a way that it would just partially cover the BLIS [29]. The proteinaceous nature of the BLIS was determined by the absence of antimicrobial activity affecting the formation of the inhibition halo.
Effect of salts, detergents, and temperature on BLIS stability
The method described by Todorov and Dicks [29] was used to verify the stability of the produced BLIS. For the assays, the BLIS liquid culture was treated with 1% (w/v) of the following reagents: NaCl (Cromoline Química Fina LTDA, Diadema, Brazil), EDTA, Triton 100x, SDS, Tween-20 (Inlab, Alamar Tecno Científica Ltda, São Paulo, Brazil), and Tween-80 (Labsynth Produtos para Laboratórios Ltda, Diadema, Brazil)) at 30 °C for 2 h. Reactions were performed in conical tubes with a working volume of 1 mL. In addition, BLIS was submitted to various heat treatments: 30, 50, 70, and 90 °C for 1 h, or 120 °C for 15 min. After treatment, the stability of the BLIS was verified through the agar diffusion method against L. innocua as bio-indicator strain.
Molecular identification of Enterococcus faecium 135 and amplification of enterocin genes
Genotypic identification of E. faecium 135 was confirmed through 16S rRNA technique. The DNA was extracted using a Promega Wizard Genomic DNA purification kit (Madison, WI, USA) following the manufacturer’s instructions. The protocols used in amplification were described by Jackson et al. [30] and the primers by Kariyama et al. [31]. Each assay had a negative control, and a positive control using a strain of E. faecium ATCC 19434. The PCR (polymerase chain reaction) was carried out in a thermocycler Mastercycler® (Eppendorf, Hamburg, Germany). Electrophoresis of the products from genetic amplification was performed in agarose gel 1.8% (w/v) (Sigma-Aldrich) at 70 V for 1 h using TAE (Tris, acetic acid, EDTA) (Sigma-Aldrich) buffer pH 8. To calculate the molecular size of the products from amplification, a molecular marker of 100–1000 bp (Inbio Highway, Buenos Aires, Argentina) was used. At the end of the run, the gel was transferred to a solution of TAE buffer and ethidium bromide (0.5 µg/mL) solution for 20 min. Then, the gel was visualized through UV light using a DNA light transilluminator U1000 (Labnet International Inc.) and photographed.
The presence of enterocin structural genes was also evaluated by PCR amplification. The primers and protocols used are listed in Table 1. PCR products were analyzed by gel electrophoresis as described above.
Table 1.
Primers used for PCR amplification of structural enterocin genes
| Enterocin | Target gene and primer sequence (5´- 3´) | PCR conditions | PCR positive control | Size (bp) | Reference | ||
|---|---|---|---|---|---|---|---|
| Temp (ºC) | Duration | No. of cycles | |||||
| Enterocin A | entA | 95 | 5 min. | 1 | [32] | ||
| f: GGTACCACTCATAGTGGAAA | 95 | 30 s | 30 | E. faecium ETW 20 | 138 | ||
| r: CCCTGGAATTGCTCCACCTAA | 95 | 30 s | |||||
| 72 | 5 min. | 1 | |||||
| Enterocin B | entB | ||||||
| f: CAAAATGTAAAAGAATTAAGTACG | 95 | 5 min. | 1 | 201 | |||
| r: AGAGTATACATTTGCTAACCC | 95 | 30 s | 30 | ||||
| 95 | 30 s | ||||||
| 72 | 5 min. | 1 | |||||
| Enterocin P | entP | ||||||
| f: GCTACGCGTTCATATGGTAAT | 95 | 5 min. | 1 | 87 | |||
| r: TCCTGCAATATTCTCTTTAGC | 95 | 30 s | 30 | ||||
| 95 | 30 s | ||||||
| Enterocin LB50A | 72 | 5 min. | 1 | 274 | |||
| entL50A | |||||||
| f: ATGGGAGCAATCGCAAAATTA | 95 | 5 min. | 1 | E. mundti STw60 | |||
| r: TTTGTTAATTGCCCATCCTTC | 95 | 30 s | 30 | ||||
| 95 | 30 s | ||||||
| 72 | 5 min. | 1 | |||||
| Enterocin LB50B | entL50B | ||||||
| f: ATGGGAGCAATCGCAAAATTA | 95 | 5 min. | 1 | E. faecium ETW20 | 274 | ||
| r: TAGCCATTTTTCAATTTGATC | 95 | 30 s | 30 | ||||
| 95 | 30 s | ||||||
| 72 | 5 min. | 1 | |||||
| Enterocin 96 | ent96 | 95 | 15 min. | 1 | |||
| f: GTGGAGAGGACGAAAGGAGA | 95 | 15 s | 40 | - | 291 | [33] | |
| r: TTGATTAGTGGAGAGGACGGATTA | 60 | 1 min. | |||||
| 72 | 1 min | 1 | |||||
| Enterocin 31 | Bact31 | 94 | 5 min. | 1 | E. faecalis FA 2-2 | ||
| f: CCTACGTATTACGGAAATGGT | 94 | 30 s | 35 | 130 | [34] | ||
| r: GCCATGTTGTACCCAACCATT | 58 | 30 s | |||||
| 72 | 45s | 1 | |||||
| Enterocin 1071 | Ent1071A/B | 97 | 2 min. | 1 | |||
| f: GGGGAGAGTCGGTTTTTAG | 94 | 45 s | 35 | 273 | [35] | ||
| r: ATCATATGCGGGTTGTAGCC | 55 | 30 s | - | ||||
| 72 | 45 s | ||||||
| 72 | 2 min. | 1 | |||||
| Enterocin Q | entqA | 97 | 2 min. | 1 | E. faecium L50 | [36] | |
| f: ATGAATTTTCTTCTTAAAAATGGTATCGCA | 94 | 1 min. | 35 | 105 | |||
| r: TTAACAAGAAATTTTTTCCCATGGCAA | 55 | 30 s | |||||
| 72 | 2 min. | 1 | |||||
| Mundticin KS | mun KS | 94 | 3 min. | 1 | E. mundti STw60 | [37] | |
| f: TGAGAGAAGGTTTAAGTTTTGAAGAA | 94 | 30 s | 30 | 379 | |||
| r: TCCACTGAAATCCATGAATGA | 53 | 30 s | |||||
| 72 | 1 min | 1 | |||||
| Hiracin JM79 | HirJm79 | 97 | 2 min. | 1 | E. hirae DCH5 | ||
| f: ATGAAAAAGAAAGTATTAAAACATTGTGTTATTCTAGG | 94 | 45 s | 35 | 250 | |||
| r:ATAAGTTAAGCTTGTACTACCTTCTAGGTGCCCATGGACC | 61 | 30 s | |||||
| 72 | 30 s | ||||||
| 72 | 7 min. | 1 | |||||
Statistical analysis
All the experiments were performed in triplicate and the results were evaluated by analysis of variance (ANOVA) using the software Statistica 12.0 (TIBCO, Palo Alto, CA, USA). The main values were compared using the Tukey test for a level of significance p < 0.05.
Results and discussion
Antimicrobial activity of potential BLIS produced by E. faecium 135
The results of antimicrobial activity through the turbidimetric method of the potential BLIS produced by E. faecium 135 against the bio-indicator strains Salmonella enterica (a), S. Typhimurium (b), and L. monocytogenes (c) are shown in Fig. 1. Assays against S. enterica revealed that the BLIS was able to reduce 37.7% of the OD when compared to the control; however, it was not able to fully inhibit the growth of this pathogen during the 24 h of assay. Similar results were observed against S. Typhimurium, with a reduction of 42.4% of OD when compared to the control; but not a complete inhibition of the growth of this pathogen during the 24 h assayed.
Fig. 1.

Antimicrobial activity of BLIS produced by E. faecium against the bio-indicator strains L. monocytogenes (a), S. enterica (b), and S. Typhimurium (c). Assays performed with positive controls (●). Samples cultivated at 30 °C (■) and 35 °C (
 )
)
Unlike the results obtained against Salmonella sp., the activity against L. monocytogenes showed a significant delay in the lag phase of this strain (Fig. 1c), with the OD value remaining at approx. 0.182 during 9 h and 14 h, for cultivations carried out at 30 and 35 °C, respectively. Only after these long periods of lag phase, the strain was able to reach the exponential phase, achieving an OD of 0.560 after 24 h, which revealed a bacteriostatic effect of the BLIS.
Figure 2 shows the results of antimicrobial activity obtained by the agar diffusion method. These results confirmed the previous obtained by turbidimetric assay, with the highest inhibitory action of BLIS (activity ranging between 6,400 and 12,800 AU/mL) being observed against L. monocytogenes (Fig. 2a). Antimicrobial activity was less pronounced against S. enterica (ranging between 200 and 400 AU/mL, Fig. 2b) and for S. Typhimurium 400 AU/mL, Fig. 2c) from E. faecium 135 cultures carried out at 35 °C, 100/150 rpm, and without activity at 30 °C. Overall, these results of antimicrobial activity are in agreement with other studies that have reported the production of BLIS and bacteriocins by other strains of E. faecium [9, 18, 38, 39], especially on the action against L monocytogenes, once the values of AU/mL were bigger against this strain, conforming the efficacy of the potential BLIS. Similar results were also reported by Baños et al. [40] for antimicrobial activity of E. faecalis UGRA10 enterocin against L. monocytogenes in refrigerated raw salmon meat. The antimicrobial activities against the strains of S. enterica and S. Typhimurium were less pronounced when compared to L. monocytogenes, probably because they are Gram-negative bacteria. According to some authors, it is usually difficult to bind the antimicrobial compound to the membrane of Gram-negative bacteria [15, 38, 39].
Fig. 2.
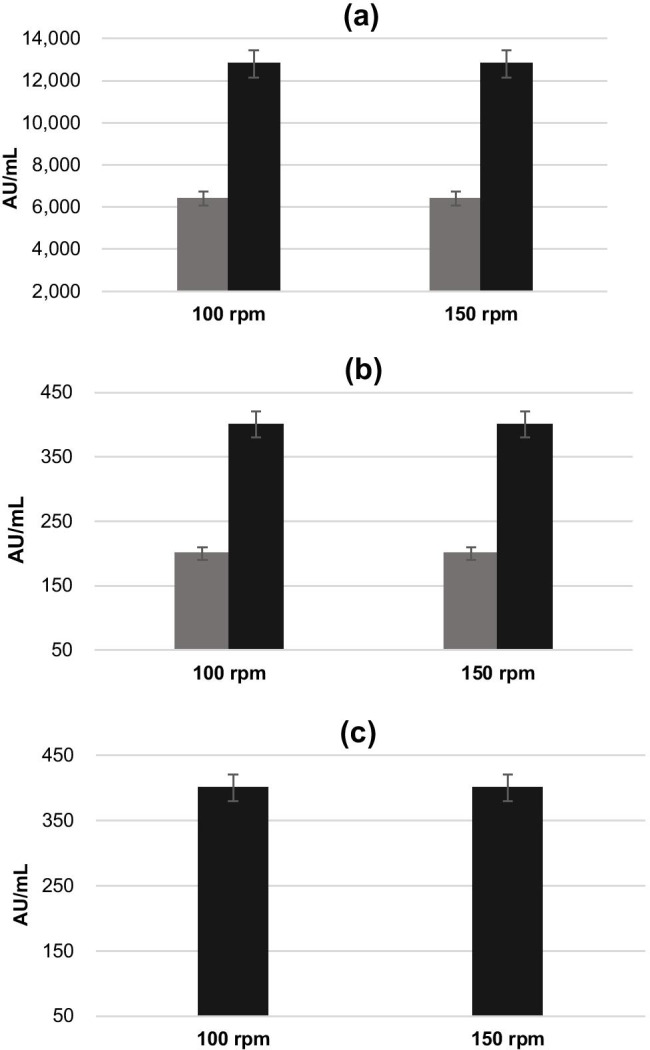
Antimicrobial activity of BLIS produced by E. faecium 135 at 30 °C (
 ) and 35 °C (■), 100 and 150 rpm, by the agar diffusion method against the strains L. monocytogenes (a), S. enterica (b), and S. Typhimurium (c). Results are expressed in arbitrary units AU/mL
) and 35 °C (■), 100 and 150 rpm, by the agar diffusion method against the strains L. monocytogenes (a), S. enterica (b), and S. Typhimurium (c). Results are expressed in arbitrary units AU/mL
L. monocytogenes, S. enterica, and S. Typhimurium are considered important pathogens in the meat-processing industry, mainly because the contamination can occur during the steps of cutting and evisceration where the pathogens mainly present in the intestinal contents could come into contact with the meat [4]. So a molecule that is capable of reducing the effect of these pathogens both in the animal’s gastrointestinal tract and at the time its meat is processed, is of great interest [40]. In this sense, the BLIS produced by E. faecium 135 presented activity against those pathogens, showing potential for future utilization by this industry. By applying a microencapsulation technique, the structure and activity of the BLIS could remain intact [7].
Figure 2 also shows that the results of antimicrobial activity were not significantly (p < 0.05) affected by the variations in temperature (30 or 35 °C) and agitation (100 or 150 rpm) evaluated for cultivation of E. faecium. Therefore, for the subsequent experiments, it was decided to perform the cultivations at 35 °C and 100 rpm since such conditions gave the best results by the turbidimetric assay (Fig. 1) and were also the only ones that showed antimicrobial activity against all the bio-indicator strains tested by the agar diffusion method (Fig. 2). After the positive results of antimicrobial activity, the treated CFS was now denominated as BLIS.
BLIS concentration with ammonium sulfate and its proteinaceous nature
In an attempt to increase the activity of the BLIS produced by E. faecium 135, the BLIS was concentrated with 50% (w/v) ammonium sulfate solution and then treated with trypsin (1 mg/mL). Results revealed that, after concentrated, the size of inhibition halos from BLIS against L. monocytogenes was 25,600 AU/mL, which represented an increase of 50% when compared to non-concentrated BLIS (12,800 AU/mL).
Concentration with ammonium sulfate is usually used to precipitate proteins [29] or as a pre-purification step for certain bacteriocins [18, 41]. In the present study, the isolation of proteins present in BLIS by treatment with ammonium sulfate resulted in a more active BLIS fraction against the bio-indicator strain since the interference of other compounds was reduced. It is worth mentioning that when the BLIS of E. faecium 135 was treated with trypsin (1 mg/mL), its antimicrobial activity ceased. This result indicates the proteinaceous nature of the antimicrobial compound present in the sample. Since trypsin is an enzyme with proteolytic activity, then the antimicrobial activity from E. faecium 135 probably came from the BLIS produced by this strain (remembering that BLIS and bacteriocins are small peptides). However, more tests using other proteases, as well as the application of more purification steps, would be useful to confirm the protein origin.
Stability of the BLIS produced by E. faecium 135
The stability of the BLIS is an important aspect to be evaluated since the use of salts and detergents during the purification of bacteriocins may interfere in the stability of the molecule [28]. Usually, solutions of 1% EDTA (w/v), SDS (w/v), Triton 100x (v/v), Tween-20 (v/v), or Tween-80 (v/v) present antimicrobial effect against the bio-indicator strain L. innocua CLIST 2711 [28, 42]. In fact, when the reagents SDS, Tween 20, Tween 80, and Triton 100x were incubated with BLIS from E. faecium 135, and a synergistic effect in the antimicrobial activity was observed (Table 2), with the halos increasing about 2/3 mm after BLIS interaction with those chemicals, when compared to the control. On the other hand, the presence of 1% NaCl did not affect the bio-indicator strain nor resulted in an additional effect when combined with BLIS from E. faecium 135. The same behavior was observed when EDTA was used, which was an unexpected result since EDTA usually has a certain antimicrobial activity because it is a chelating agent and has ability to destabilize cell membranes [43, 44].
Table 2.
Effect of detergents, salts, and temperature on the stability of the BLIS produced by Enterococcus faecium 135
| Treatment | Inhibition zone* |
|---|---|
| Listeria innocua CLIST 2711 | |
| Control | + + |
| Triton 100x | + + + |
| SDS | + + + |
| Tween-80 | + + + |
| Tween-20 | + + + |
| EDTA | + + |
| NaCl | + + |
| Thermic treatment: | |
| 30, 50, 70, or 90 °C for 1 h | + + |
| 120 °C for 15 min | - |
* (+ + +) > 12 mm, (+ +) 11.0–11.99 mm, ( +) 10.0–10.99 mm, and (-) did not show inhibition zone. Control: BLIS from E. faecium 135 without addition of any salt or detergent. The concentration of salts and detergents used for the experiments was of 1% (w/v) or (v/v)
Regarding the thermic treatment, the use of temperature up to 90 °C for 1 h did not affect the stability of the BLIS, revealing some heat stability of this biomolecule, which is an important information for future purification steps. A negative effect was only observed for higher temperature (120 °C), in this case, even for a shorter period (15 min only) (Table 2). Overall, the results presented in Table 2 were similar to those obtained by Todorov et al. [29, 42] when evaluating the stability of a bacteriocin produced by Lactobacillus spp.
Production of BLIS by microbial co-cultivation
E. faecium, Ligilactobacillus salivarius, and Limosilactobacillus reuteri, the strains used in co-cultivation in the present study, are commonly found as part of the microbiota of several animals and humans [9, 11, 15], which supports the idea that they could work in co-culture. The results of antimicrobial activity obtained from their co-cultivation are summarized in Table 3. As can be seen, when Ligilactobacillus salivarius and Limosilactobacillus reuteri were used in co-culture, no inhibitory activity was observed against any of the bio-indicator strains. However, the results were improved when E. faecium was used in co-cultivation with the other strains. The best results were obtained by co-cultivation of E. faecium with Limosilactobacillus reuteri, especially against L. monocytogenes (400 AU/mL) and S. enterica (200 AU/mL). Binary culture of E. faecium with Ligilactobacillus salivarius resulted in half of the antimicrobial activity against L. monocytogenes than the co-culture with Limosilactobacillus reuteri. Ternary culture of E. faecium, Limosilactobacillus reuteri, and Ligilactobacillus salivarius also gave lower (200 AU/mL) antimicrobial activity when compared to the binary culture of E. faecium and Limosilactobacillus reuteri (400 AU/mL), and when comparing the ternary culture with the binary culture of E. faecium and Ligilactobacillus salivarius, both presented a similar antimicrobial activity (200 AU/mL). None of the tested co-cultures showed antimicrobial activity against S. Typhimurium.
Table 3.
Inhibitory activity of BLIS produced by the binary and ternary cultures of E. faecium, L. salivarius, and L. reuteri against the bio-indicator strains L. monocytogenes, S. enterica, and S. Typhimurium. Results are expressed in arbitrary units per mL (AU/mL)
| BLIS producing strains | Bio-indicator strains | ||
|---|---|---|---|
| L. monocytogenes | S. enterica | S. Typhimurium | |
| L. salivarius + L. reuteri | - | - | - |
| E. faecium + L. reuteri | 400 a | 200 a | - |
| E. faecium + L. salivarius | 200 b | - | - |
| E. faecium + L. reuteri + L. salivarius | 200 b | - | - |
Different letters in the same column mean statistically different values according to Tukey’s test (p < 0.05)
Overall, the results obtained by co-cultures were less efficient than the antimicrobial activities obtained by monoculture. This would suggest that the strains compete by the carbon source. As it is known that the biosynthesis of many bacteriocins is regulated by quorum-sensing trigger [45, 46], rather than presence of other species, the carbon competition may have prevented bacterial cells from reaching a critical number that would trigger bacteriocin production. In addition, the strain itself may produce compounds that interfere with the growth of the other strain such as organic acids and even BLIS [22]. L. reuteri INIA P579 and L. salivarius SMXD5, for example, are producers of bacteriocins [19, 41]. Similar to the results obtained in the present study, Giraffa et al. [47] also observed a decrease in the production of enterocin when E. faecium 7C5 (an enterocin producer strain) was used in co-culture with S. thermophilus and L. bulgaricus (generally used in the preparation of cheeses and yogurts). According to the authors, when these three strains are used directly for food production, they can produce organic acids, which are able to inhibit the growth of pathogenic strains such as L. monocytogenes and S. enterica. Taking this into account, it is possible to conclude that if live strains were used in the present study instead of their BLIS, probably different results would have been obtained, since these strains are also able to produce organic acids [12] that have antimicrobial activity.
Molecular identification of enterocin genes in Enterococcus faecium 135
Analysis for molecular identification revealed that E. faecium 135 presents structural genes for the enterocins A (136 bp), B (198 bp), P (86 bp), and Mundticin KS (379 bp), which are classified as class II bacteriocins [48, 49]. Enterocin P is the most prevalent in Enterococcus species, followed by enterocin A, whose production is usually associated to enterocin B. Those enterocins are known for the high activity against L. monocytogenes [33, 37, 49]. Mundticin KS is produced by E. mundti NFRI 7393, for example, and it is known to have action against L. monocytogenes and Clostridium botulinum [49].
Other studies have also reported that some species of Enterococcus have more than one enterocin gene in their structure [33, 37]. However, the presence of different genes does not imply the production of different bacteriocins or even the production of bacteriocins at all [49, 50]. Liu et al. [51], for example, purified two different enterocins produced by E. faecium LM-2 with proved action against pathogens such as L. monocytogenes and Staphylococcus aureus. In another study, Liu et al. [50] characterized two bacteriocins (Ent7A and Ent7B) produced by E. faecalis 710C, which had action against Clostridium sporogenes and L. monocytogenes. The results obtained in the present study suggest that probably some of these four enterocin genes could have been produced by E. faecium 135, due to the excellent activity observed against L. monocytogenes. However, purification of the BLIS is necessary to verify if some of these enterocins are being produced.
Conclusion
This study demonstrated that a newly isolated strain of Enterococcus faecium 135 is able to produce BLIS with antimicrobial activity against L. monocytogenes and also against S. enterica and S. Typhimurium. In addition, the produced BLIS was stable under high temperatures (up to 90 °C) as well as in the presence of several salts and detergents used for purification purposes, and showed susceptibility to trypsin, which suggest a proteinaceous nature. The presence of four structural enterocin class II genes was confirmed, suggesting the production of some type of enterocin by E. faecium 135. Production of BLIS using monoculture of E. faecium 135 resulted in better antimicrobial activity compared to the production by some co-cultures tested. However, further studies are needed to elucidate the behavior of this new strain when used in co-culture. Studies on the characterization of this BLIS, as well as on the purification of this molecule, would also give better indications on the properties of this BLIS or potential bacteriocin, which could be a promising molecule with great applicability’s in animal breeding, and process of the meat, preventing contamination for the most common foodborne pathogens.
Acknowledgements
The authors also thank the Oswaldo Cruz Foundation (Fiocruz) for donating the Salmonella enterica serovar Typhimurium and Listeria innocua strains.
Author contribution
Anna Carolina Meireles Piazentin performed all the experiments and analyses of the data and wrote the first draft of this manuscript; Carlos Miguel Nóbrega Mendonça performed the microbial cultivation; Marisol Vallejo carried out the molecular identification of E. faecium; Solange I. Mussatto helped in the preparation of the manuscript and was responsible for the revision and correction of the text; and Ricardo Pinheiro de Souza Oliveira provided the equipment, reagents, and space to perform all the experimental analyses, and also helped in the revision and correction of the text.
Funding
This work was supported by São Paulo Research Foundation—FAPESP (grant #2018/25511–1), by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001, and by the National Council for Scientific and Technological Development – CNPq. Prof. Solange I. Mussatto acknowledges the support from the Novo Nordisk Foundation, Denmark (grant number NNF20SA0066233).
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Food Safety Authority, European Centre for Disease Prevention (2016) The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. EFSA J 14(2): 4380. 10.2903/j.efsa.2016.4380 [DOI] [PMC free article] [PubMed]
- 2.García-Sánchez L, Melero B, Jaime I, Hänninen ML, Rossi M, Rovira J. Campylobacter jejuni survival in a poultry processing plant environment. Food Microbiol. 2017;65:185–192. doi: 10.1016/j.fm.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Crotta M, Georgiev M, Guitian J. Quantitative risk assessment of Campylobacter in broiler chickens – assessing interventions to reduce the level of contamination at the end of the rearing period. Food Control. 2017;75:29–39. doi: 10.1016/j.foodcont.2016.12.024. [DOI] [Google Scholar]
- 4.Lakicevic B, Nastasijevic I. Listeria monocytogenes in retail establishments: contamination routes and control strategies. Food Rev Int. 2017;33:247–269. doi: 10.1080/87559129.2016.1175017. [DOI] [Google Scholar]
- 5.Johnson TJ, Shank JM, Johnson JG. Current and potential treatments for reducing Campylobacter colonization in animal hosts and disease in humans. Front Microbiol. 2017;8:1–14. doi: 10.3389/fmicb.2017.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strompfová V, Kubašová I, Ščerbová J. Oral administration of bacteriocin-producing and non-producingstrains of Enterococcus faecium in dogs. Appl Microbiol Biotechnol. 2019;103:4953–4965. doi: 10.1007/s00253-019-09847-3. [DOI] [PubMed] [Google Scholar]
- 7.Soltani S, Hammami R, Cotter PD. Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol Rev. 2021;45:1–24. doi: 10.1093/femsre/fuaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramchandran L, Shah NP. Characterization of functional, biochemical and textural properties of synbiotic low-fat yogurts during refrigerated storage. LWT - Food Sci Technol. 2010;43:819–827. doi: 10.1016/j.lwt.2010.01.012. [DOI] [Google Scholar]
- 9.Khan H, Flint S, Yu P-L. Enterocins in food preservation. Int J Food Microbiol. 2010;141:1–10. doi: 10.1016/j.ijfoodmicro.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Mitsuoka T. Development of functional foods. Biosci Microbiota, Food Heal. 2014;33:117–128. doi: 10.12938/bmfh.33.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O’Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Gänzle MG, Lebeer S. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 12.Gillor O, Etzion A, Riley MA. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol. 2008;81:591–606. doi: 10.1007/s00253-008-1726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter PD, Hill C, Ross RP. Food microbiology: bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 14.Messaoudi S, Manai M, Kergourlay G, Prévost H, Connil N, Chobert J-M, Dousset X. Lactobacillus salivarius: bacteriocin and probiotic activity. Food Microbiol. 2013;36:296–304. doi: 10.1016/j.fm.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Suvorov A. What is wrong with enterococcal probiotics? Probiotics Antimicrob Proteins. 2020;12:1–4. doi: 10.1007/s12602-020-09633-y. [DOI] [PubMed] [Google Scholar]
- 16.Acedo JZ, Chiorean S, Vederas JC, van Belkum MJ. The expanding structural variety among bacteriocins from Gram-positive bacteria. FEMS Microbiol Rev. 2018;42:805–828. doi: 10.1093/femsre/fuy033. [DOI] [PubMed] [Google Scholar]
- 17.Chakchouk-Mtibaa A, Sellem I, Kamoun Y, Smaoui S, Karray-Rebai I, Mellouli L. Safety aspect of Enterococcus faecium FL31 strain and antibacterial mechanism of its hydroxylated bacteriocin BacFL31 against Listeria monocytogenes. Biomed Res Int. 2018;2018:1–10. doi: 10.1155/2018/5308464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao X, Du R, Wang Y, Han Y, Zhou Z. Purification, characterization and mode of action of enterocin, a novel bacteriocin produced by Enterococcus faecium TJUQ1. Int J Biol Macromol. 2020;144:151–159. doi: 10.1016/j.ijbiomac.2019.12.090. [DOI] [PubMed] [Google Scholar]
- 19.Montiel R, Martín-Cabrejas I, Langa S, El Aouad N, Arqués JL, Reyes F, Medina M. Antimicrobial activity of reuterin produced by Lactobacillus reuteri on Listeria monocytogenes in cold-smoked salmon. Food Microbiol. 2014;44:1–5. doi: 10.1016/j.fm.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Liu Y, Liao W, Wen Z, Chen S. Simultaneous production of nisin and lactic acid from cheese whey: optimization of fermentation conditions through statistically based experimental designs. Appl Biochem Biotechnol - Part A Enzym Eng Biotechnol. 2004;114:627–638. doi: 10.1385/ABAB:114:1-3:627. [DOI] [PubMed] [Google Scholar]
- 21.Foulquié Moreno MR, Rea MC, Cogan TM, De Vuyst L. Applicability of a bacteriocin-producing Enterococcus faecium as a co-culture in Cheddar cheese manufacture. Int J Food Microbiol. 2003;81:73–84. doi: 10.1016/S0168-1605(02)00167-8. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado A, Ruiz-Barba JL, Jiménez-Diaaz R. Production of plantaricin NC8 by Lactobacillus plantarum NC8 is induced in the presence of different types of gram-positive bacteria. Arch Microbiol. 2004;181:8–16. doi: 10.1007/s00203-003-0606-8. [DOI] [PubMed] [Google Scholar]
- 23.Domínguez-Manzano J, Jiménez-Díaz R. Suppression of bacteriocin production in mixed-species cultures of lactic acid bacteria. Food Control. 2013;30:474–479. doi: 10.1016/j.foodcont.2012.09.014. [DOI] [Google Scholar]
- 24.Chanos P, Mygind T. Co-culture-inducible bacteriocin production in lactic acid bacteria. Appl Microbiol Biotechnol. 2016;100:4297–4308. doi: 10.1007/s00253-016-7486-8. [DOI] [PubMed] [Google Scholar]
- 25.Rojobezares B, Saenz Y, Navarro L, Zarazaga M, Ruizlarrea F, Torres C. Coculture-inducible bacteriocin activity of Lactobacillus plantarum strain J23 isolated from grape must. Food Microbiol. 2007;24:482–491. doi: 10.1016/j.fm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Kos B, Beganović J, Jurašić L, Švadumović M, Leboš Pavunc A, Uroić K, Šušković J. Coculture-inducible bacteriocin biosynthesis of different probiotic strains by dairy starter culture Lactococcus lactis. Mljekarstvo. 2011;61:273–282. [Google Scholar]
- 27.Gutiérrez-Cortés C, Suarez H, Buitrago G, Nero LA, Todorov SD. Enhanced bacteriocin production by Pediococcus pentosaceus 147 in co-culture with Lactobacillus plantarum LE27 on cheese whey broth. Front Microbiol. 2018;9:1–12. doi: 10.3389/fmicb.2018.02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabo ML, Murado MA, Gonzalez MP, Pastoriza L. A method for bacteriocin quantification. J Appl Microbiol. 1999;87:907–914. doi: 10.1046/j.1365-2672.1999.00942.x. [DOI] [PubMed] [Google Scholar]
- 29.Todorov SD, Dicks LMT. Screening for bacteriocin-producing lactic acid bacteria from boza, a traditional cereal beverage from Bulgaria. Process Biochem. 2006;41:11–19. doi: 10.1016/j.procbio.2005.01.026. [DOI] [Google Scholar]
- 30.Jackson CR, Fedorka-Cray PJ, Barrett JB. Use of a genus- and species-specific multiplex PCR for identification of enterococci. J Clin Microbiol. 2004;42:3558–3565. doi: 10.1128/JCM.42.8.3558-3565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kariyama R, Mitsuhata R, Chow JW, Clewell DB, Kumon H. Simple and reliable multiplex PCR assay for surveillance isolates of vancomycin-resistant enterococci. J Clin Microbiol. 2000;38:3092–3095. doi: 10.1128/jcm.38.8.3092-3095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Vuyst L. Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int J Food Microbiol. 2003;84:299–318. doi: 10.1016/S0168-1605(02)00425-7. [DOI] [PubMed] [Google Scholar]
- 33.Henning C, Gautam D, Muriana P. Identification of multiple bacteriocins in Enterococcus spp. using an enterococcus-specific bacteriocin PCR array. Microorganisms. 2015;3:1–16. doi: 10.3390/microorganisms3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Özdemir GB, Oryaşin E, Biyik HH, Özteber M, Bozdoǧan B. Phenotypic and genotypic characterization of bacteriocins in enterococcal isolates of different sources. Indian J Microbiol. 2011;51:182–187. doi: 10.1007/s12088-011-0143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martín M, Gutiérrez J, Criado R, Herranz C, Cintas LM, Hernández PE. Genes encoding bacteriocins and their expression and potential virulence factors of enterococci isolated from wood pigeons (Columba palumbus) J Food Prot. 2006;69:520–531. doi: 10.4315/0362-028X-69.3.520. [DOI] [PubMed] [Google Scholar]
- 36.Belgacem ZB, Abriouel H, Omar NB, Lucas R, Martínez-Canamero M, Gálvez A, Manai M. Antimicrobial activity, safety aspects, and some technological properties of bacteriocinogenic Enterococcus faecium from artisanal Tunisian fermented meat. Food Control. 2010;21:462–470. doi: 10.1016/j.foodcont.2009.07.007. [DOI] [Google Scholar]
- 37.Almeida T, Brandaão A, MuñOz-Atienza E, Gonçalves A, Torres C, Igrejas G, Hernández PE, Herranz C, Cintas LM, Poeta P. Identification of bacteriocin genes in enterococci isolated from game animals and saltwater fish. J Food Prot. 2011;74:1252–1260. doi: 10.4315/0362-028X.JFP-11-016. [DOI] [PubMed] [Google Scholar]
- 38.Lauková A, Guba P, Nemcová R, Vasilková Z. Reduction of Salmonella in gnotobiotic Japanese quails caused by the enterocin A-producing EK13 strain of Enterococcus faecium. Vet Res Commun. 2003;27:275–280. doi: 10.1023/A:1024027923824. [DOI] [PubMed] [Google Scholar]
- 39.Martínez PV, López AS, Omar NB, Abriouel H, López RL, Valdivia E, Belloso OM, Gálvez A. Enhanced bactericidal effect of enterocin AS-48 in combination with high-intensity pulsed-electric field treatment against Salmonella enterica in apple juice. Int J Food Microbiol. 2008;128:244–249. doi: 10.1016/j.ijfoodmicro.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Baños A, García-López JD, Núñez C, Martínez-Bueno M, Maqueda M, Valdivia E. Biocontrol of Listeria monocytogenes in fish by enterocin AS-48 and Listeria lytic bacteriophage P100. LWT - Food Sci Technol. 2016;66:672–677. doi: 10.1016/j.lwt.2015.11.025. [DOI] [Google Scholar]
- 41.Messaoudi S, Kergourlay G, Dalgalarrondo M, Choiset Y, Ferchichi M, Prévost H, Pilet MF, Chobert JM, Manai M, Dousset X. Purification and characterization of a new bacteriocin active against Campylobacter produced by Lactobacillus salivarius SMXD51. Food Microbiol. 2012;32:129–134. doi: 10.1016/j.fm.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Todorov SD, Furtado DN, Saad SMI, De Melo Franco BDG. Bacteriocin production and resistance to drugs are advantageous features for Lactobacillus acidophilus La-14, a potential probiotic strain. New Microbiol. 2011;34:357–370. [PubMed] [Google Scholar]
- 43.Mastromatteo M, Lucera A, Sinigaglia M, Corbo MR (2010) Synergic antimicrobial activity of Lysozyme, Nisin, and EDTA against Listeria monocytogenes in Ostrich Meat Patties. J Food Sci 75 (7): M422-M429 . 10.1111/j.1750-3841.2010.01732.x [DOI] [PubMed]
- 44.Khan A, Vu KD, Riedl B, Lacroix M. Optimization of the antimicrobial activity of nisin, Na-EDTA and pH against gram-negative and gram-positive bacteria. LWT - Food Sci Technol. 2015;61:124–129. doi: 10.1016/j.lwt.2014.11.035. [DOI] [Google Scholar]
- 45.Nes IF, Diep DB, Håvarstein LS, Brurberg MB, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek. 1996;70:113–128. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- 46.Diep DB, Håvarstein LS, Nes IF. A bacteriocin-like peptide induces bacteriocin synthesis in Lactobacillus plantarum C11. Mol Microbiol. 1995;18:631–639. doi: 10.1111/j.1365-2958.1995.mmi_18040631.x. [DOI] [PubMed] [Google Scholar]
- 47.Giraffa G, Carminati D, Tarelli GT. Inhibition of Listeria innocua in milk by bacteriocin-producing Enterococcus faecium 7C5. J Food Prot. 1995;58:621–623. doi: 10.4315/0362-028X-58.6.621. [DOI] [PubMed] [Google Scholar]
- 48.Nes IF, Diep DB, Holo H. Bacteriocin diversity in Streptococcus and Enterococcus. J Bacteriol. 2007;189:1189–1198. doi: 10.1128/JB.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franz CMAP, Van Belkum MJ, Holzapfel WH, Abriouel H, Gálvez A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol Rev. 2007;31:293–310. doi: 10.1111/j.1574-6976.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Vederas JC, Whittal RM, Zheng J, Stiles ME, Carlson D, Franz CMAP, McMullen LM, van Belkum MJ. Identification of an N-terminal formylated, two-peptide bacteriocin from Enterococcus faecalis 710C. J Agric Food Chem. 2011;59:5602–5608. doi: 10.1021/jf104751v. [DOI] [PubMed] [Google Scholar]
- 51.Liu G, Griffiths MW, Wu P, Wang H, Zhang X, Li P. Enterococcus faecium LM-2, a multi-bacteriocinogenic strain naturally occurring in “Byaslag”, a traditional cheese of Inner Mongolia in China. Food Control. 2011;22:283–289. doi: 10.1016/j.foodcont.2010.07.023. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


