Abstract
Fungal secondary metabolites with antimicrobial properties are used for biological pest control. Their production is influenced by several factors as environment, host, and culture conditions. In the present work, the secondary metabolites from fermented extracts of Beauveria bassiana PQ2 were tested as antifungal agents against Gibberella moniliformis LIA. The L18 (21 × 37) orthogonal array from Taguchi methodology was used to assess 8 parameters (pH, agitation, sucrose, yeast extract, KH2PO4, MgSO4, NH4NO3, and CaCl2) in B. bassiana PQ2 submerged fermentation. The ability of the fermented extracts to slow down the growth rate of G. moniliformis LIA was evaluated. The results from 18 trials were analyzed by Statistica 7 software by evaluating the signal-to-noise ratio (S/N) to find the lower-the-better condition. Optimal culture conditions were pH, 5; agitation, 250 rpm; sucrose, 37.5 g/L−1; yeast extract, 10 g/L−1; KH2PO4, 0.8 g/L−1; MgSO4, 1.2 g/L−1; NH4NO3, 0.1 g/L−1; and CaCl2, 0.4 g/L−1, being the agitation at the highest level the most significant factor. The optimal conditions were validated in a sparged bottle bioreactor resulting in a higher S/N value (12.48) compared to the estimate. The extract obtained has the capacity to inhibit the germination of G. moniliformis spores at 24 h. HPLC-ESI-MS2 allowed to identify the water-soluble red pigment as oosporein (m/z 304.9). The secondary metabolites from B. bassiana PQ2 are a suitable alternative to control the growth and sporulation of G. moniliformis.
Keywords: Biocontrol, Antifungal activity, Secondary metabolites, Oosporein, Taguchi DOE
Introduction
At present, interest in the search for new antimicrobial compounds from the production of secondary metabolites by microorganisms has been constant, mainly for use in biocontrol of pests because they are biodegradable, efficient, and safe compared to chemical pesticides [1]. At industrial scale, its production is less complex, being able to generate biomass and metabolites in a brief time [2]. Secondary metabolites are generally of low molecular weight and can be aromatic compounds, peptides, polyketides, or oligosaccharides that exhibit antibacterial, antifungal, anti-parasitic, and even antitumor activities [3]. It is known that about 1500 compounds have been identified from fungi, more than half with biological activity [4]. This diversity depends on the optimization of cultivation conditions [5]. Beauveria bassiana is an entomopathogenic fungus (EF) used worldwide due to its capacity as an insecticide. Currently, the secondary metabolites that this EF produces under in situ conditions favor infection in insects and have antimicrobial activity. The major secondary metabolites produced by B. bassiana include oosporein, tenellin, bassianin, beauvericin, bassianolide, and oxalic acid. The most studied have been beauvericin, oosporein, and tenellin obtained from liquid culture [6].
Among the most important phytopathogenic fungi in terms of economic losses is the genus Fusarium sp. that is estimated to comprise over 300 phylogenetically distinct species [7]. Some of the Fusarium species are plant pathogens with the ability to reproduce both sexually and asexually and are a great threat due to overcoming control methods based on fungicides and host resistance [8]. Gibberella moniliformis is the teleomorph of Fusarium verticillioides and is primarily a pathogen of maize[9]. However, it has also been reported affecting other crops such as banana [10], vanilla [11], pineapple [12], and onion [13]. The pathogenicity of G. moniliformis includes the contamination of food- and feedstuffs with the fumonisin mycotoxins, resulting in economically significant losses to both farmers and food processors [9]. In the present work, Taguchi method was used to evaluate the antifungal capacity of secondary metabolites produced by B. bassiana PQ2 against G. moniliformis LIA by manipulating the submerged culture conditions. The influence of different factors on the quality of the process was shown, and the best culture conditions were selected and validated in a bottle bioreactor. Also, the secondary metabolites produced from the fermented extract were identified by HPLC–MS/MS.
Materials and methods
Fungal strains and culture conditions
The strain of Beauveria bassiana PQ2 was obtained from the collection of the Food Analysis Laboratory, Instituto Tecnológico de Ciudad Valles, San Luis Potosí, Mexico.
The G. moniliformis LIA strain was provided by the Food Research Laboratory, Autonomous University of San Luis Potosí, and was isolated from a mango fruit according to Martínez-Bolaños et al. [14]. The fungal strain was identified on the basis of the ITS (internal transcribed spacer) fragment. The DNA was amplified using the polymerase chain reaction (PCR). The primers ITS4 (TCCTCCGCTTATTGATATGC) and ITS5 (GGAAGTAAAAGTCGTACAAGC) were used. The PCR amplification was performed by using 14.5 μL sterile deionized distilled cold water, buffer 3.5 μL (0.28 mM), 2 μL reverse primer (0.8 pM), 2 μL forward primer (0.8 pM), 0.5 μL of Taq DNA polymerase (0.1 U/ μL), and 2 μL of DNA template. A thermocycler (Veriti 96-well Thermal Cycler, Applied Biosystems) was used under the following program: 94 °C for 10 min, 35 cycles, 94 °C for 1 min; 55 °C for 1 min; 72 °C for 1 min; and final extension at 72 °C for 5 min. The DNA quantification was carried out in spectrophotometer (NANODROP 1000, Thermo Scientific). The nucleotide sequence of the strain was carried out by the dideoxynucleoside method using the genetic analyzer models 3500 and 3130 (Applied Biosystems, USA). The sequence was revised using the software BioEdit 7.2.5. The obtained sequence was compared with the reported sequences in GenBank database through BLAST tool (Basic Local Alignment Search Tool) from NCBI and also registered with the GenBank accession number OL672312.
The reactivation of each strain was carried out in potato dextrose agar from 7 days at 27 °C.
Medium optimization in liquid media
The L18 (21 × 37) orthogonal array was used to determine the optimal medium conditions for antifungal activity from the Taguchi design of experiment (DOE) using Statistica 7 software (Statsoft, Tulsa, OK). The culture media was the Czapek-Dox medium composed of KH2PO4, MgSO4, NH4NO3, and CaCl2, sucrose and yeast extract also, pH and agitation were considered (Table 1). One milliliter from B. bassiana PQ2 spore suspension (1 × 106 spores/mL) was inoculated in 500-mL Erlenmeyer flasks containing 50 mL of the culture medium previously autoclaved at 121 °C for 15 min in duplicate for each trial (Table 2) and placed in a rotary shaker (IKA KS 4000 i control) at 30 °C for 7 days. Once the fermentation time was finished, the extracts were filtered with sterile Whatman No. 1 filter paper using a vacuum pump and next with sterile membrane 0.45 μm (Millipore, Minisart, Sartorius Stedim Biotech). The filtered extracts were stored in 50-mL conical tubes at 4 °C until use.
Table 1.
Factors and levels considered for the fermentation process
| No | Factor | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|
| 1 | Initial pH | 5 | 6 | - |
| 2 | Agitation (rpm) | 150 | 200 | 250 |
| 3 | Sucrose (g/L) | 12.5 | 25.0 | 37.5 |
| 4 | Yeast extract (g/L) | 2.5 | 5.0 | 10.0 |
| 5 | KH2PO4 (g/L) | 0.2 | 0.4 | 0.8 |
| 6 | MgSO4 (g/L) | 0.3 | 0.6 | 1.2 |
| 7 | NH4NO3 (g/L) | 0.05 | 0.1 | 0.2 |
| 8 | CaCl2 (g/L) | 0.2 | 0.4 | 0.8 |
Table 2.
Experimental matrix and results for the orthogonal array L18 (21 × 37)
| Run | pH | Agitation | Sucrose | Yeast | Phosphate | Magnesium | Ammonium | Calcium | S/N (Signal/Noise) |
Growth rate (mm/h) | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8.55 | 0.37 | 0.999 |
| 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 8.96 | 0.36 | 0.994 |
| 3 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 9.00 | 0.35 | 0.996 |
| 4 | 1 | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 8.45 | 0.38 | 0.997 |
| 5 | 1 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 8.69 | 0.37 | 0.995 |
| 6 | 1 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 9.19 | 0.35 | 0.995 |
| 7 | 1 | 3 | 1 | 2 | 1 | 3 | 2 | 3 | 8.93 | 0.36 | 0.998 |
| 8 | 1 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 8.67 | 0.37 | 0.996 |
| 9 | 1 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 8.68 | 0.37 | 0.996 |
| 10 | 2 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 8.95 | 0.36 | 0.999 |
| 11 | 2 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 8.82 | 0.36 | 0.994 |
| 12 | 2 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 8.76 | 0.36 | 0.998 |
| 13 | 2 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 8.56 | 0.37 | 0.994 |
| 14 | 2 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 7.90 | 0.40 | 0.995 |
| 15 | 2 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 8.32 | 0.38 | 0.998 |
| 16 | 2 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 9.24 | 0.35 | 0.994 |
| 17 | 2 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 8.74 | 0.37 | 0.997 |
| 18 | 2 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 8.87 | 0.36 | 0.997 |
Antifungal activity of B. bassiana fermented extracts
At the end of the fermentation process, the antifungal activity of the fermented extracts was evaluated by poisoned media method proposed by Balouiri, Sadiki, and Ibnsouda [15], using the 20% extract to obtain 15 mL as the final volume (extract/culture medium). Then, 20 µL of G. moniliformis LIA spore suspension (1 × 105 spores/mL) was inoculated in the center of the PDA solidified medium. As a control, PDA medium was used with the same concentration of spores. The radial growth was measured using a rule in each treatment every 24 h for 9 days. All experiments were carried out in duplicates.
Experimental data analysis
Mathematical modeling of experimental data
The data obtained from the fungal radial growth against the time were adjusted with the primary model of Baranyi and Roberts [16] using the software DMFit (Microsoft Excel add in) (Institute of Food Research, Norwich Research Park, UK) in order to determine the growth rate according to the following equation:
| 1 |
where y (t) corresponds to the diameter of the colony (mm); t time (d); y0 concentration or initial diameter of the colony; μmax specific growth rate (h−1), m; v curvature parameters to characterize the transition of the exponential phase; and h0 dimensionless parameter that quantifies the initial physiological state of the cells.
Statistical analysis based on the Taguchi methodology
The data obtained were analyzed with the software Statistica 7 (Statsoft, Tulsa, OK). In the present work, the quality function used based on Taguchi methodology was smaller the better, in order to achieve the conditions to slow down growth rate, according to the following equation:
| 2 |
where the factor -10 ensures that this ratio measures the inverse of “bad quality,” y represents the experimental value obtained in each trial, and n is the number of samples.
Subsequently, an analysis of variance (ANOVA) was carried out to obtain the contribution percentage of each factor determined as follows:
| 3 |
where P is contribution percentage, SSi individual sum of squares, SST total sum of squares, MSi initial mean square, and dfi degrees of individual freedom.
Validation in sparged bottle bioreactor
To validate the optimal conditions, a sparged bottle bioreactor was used in duplicate. Three hundred fifty milliliters of culture medium (autoclaved at 15 psi for 15 min) was prepared, and 1 × 105 spores/mL of B. bassiana PQ2 was inoculated. The fermentation system was incubated at 30 °C and aeration rate at 4 volumes of air/volume of culture/min. The final time of the fermentation was after 92 h. The extracts were recovered through filtration, and the antifungal activity was evaluated in triplicate as was mentioned above.
Spore germination assay
The effect of the extract obtained in the validation step was evaluated on the spore germination of G. moniliformis LIA according to Costa et al. [17]. In triplicate, 100 μL of fermented extract (previously filtered) and 30 μL of spore suspension (1 × 105 spores/mL) were placed on glass slides and incubated at 25 °C/24 h. Then, two drops of lactophenol cotton blue stain were added and observed at 40 × under light microscope to evaluate 200 spores per slide to give a total of 600, considering that a conidium had germinated when the presence of its germinative tube was observed, regardless of its length. The results were expressed as percentage of spore germination according to the following equation:
| 4 |
where G (%) means the percentage of germination; EG the number of germinated conidia; and TE the total of conidia.
RP-HPLC analysis tandem mass spectrometry (HPLC–ESI–MS)
The filtered aqueous extract obtained in the validation was analyzed according to Aguilar-Zárate et al. [18] methodology, with some modifications, by reversed-phase high-performance liquid chromatography: autosampler (Varian ProStar 410, USA), ternary pump (Varian ProStar 230I, USA), and a PDA detector (Varian ProStar 330, USA). Sample (5 μL) was injected into a Denali C-18 column (150 mm × 2.1 mm, 3.1 μm, Grace, USA); the oven temperature was 30 °C. The elution gradient was formic acid (0.2% v/v, solvent A) and acetonitrile (solvent B) with initial gradient course of 3% B; 5–15 min, 16% B linear; and 15–45 min and 50% B linear. The flow rate was 0.2 mL/min, and the elution was monitored at 287 nm. A liquid chromatograph ion trap mass spectrometry (Varian 500-MS IT Mass Spectrometer, USA) equipped with an electrospray ion source was used. The MS analysis was performed in the negative mode [M-H]−1 using nitrogen as nebulizing gas and helium as damping gas. The parameters of the ion source were 5.0 kV spray voltage, 90.0 V capillary voltage, and 350 °C of temperature. Full-scan spectra were acquired in the m/z range 100–2000, and subsequently the MS/MS analyses were performed on a series of selected ions. The data was collected and processed using MS Workstation software (V 6.9).
Results and discussion
Evaluation of antifungal activity of fermented extracts
Beauveria bassiana PQ2 fermented extracts shown antifungal activity against G. moniliformis LIA in all the runs tested to reduce the growth rate from a range of 0.35 mm/h to 0.40 mm/h (Table 2); this could be due to the diversity of metabolites that were generated with the evaluated conditions based on the fact that the same strain can be metabolically diverse. However, it is important to note that from all the metabolite B. bassiana possesses, only oosporein and beauvericin have a moderate antifungal effect [19]. In each case, the signal-to-noise ratio was inversely proportional to growth rate; hence, of the total treatments, it was observed that only three of them (trials 3, 6, and 16) had the lowest growth rate (0.35 mm/h) and the highest signal-to-noise ratio (9, 9.19, and 9.24 S/N, respectively); the R2 value closer to 1 in all cases ensure the fit of the data obtained. Also, the trial number 14 (0.40 mm/h) had the lowest S/N value (7.90) than others and it is proportional with growth rate value in each case. Based on Taguchi’s L18 experimental design using the quality characteristic “smaller the better,” it has been possible to investigate the antifungal activity of fermented B. bassiana PQ2 extracts and find the conditions where the said extract has the capacity to reduce the mycelial growth of a phytopathogenic fungus. It is important to note that the higher the signal-to-noise (S/N) value, the lower the process variability and therefore the better the response [20].
It is important to mention that this is the first study where the antifungal effect on the production of secondary metabolites of an entomopathogenic fungus is considered since the use of the Taguchi experimental design has been carried out for biotechnological purposes in the optimization of biomass production [21], enzymes [22–24], synthesis of nanoparticles [25, 26], nano-emulsions [27], nutrient effect [28] and optimization of culture medium for the production of spores [29].
Analysis of individual factor contribution
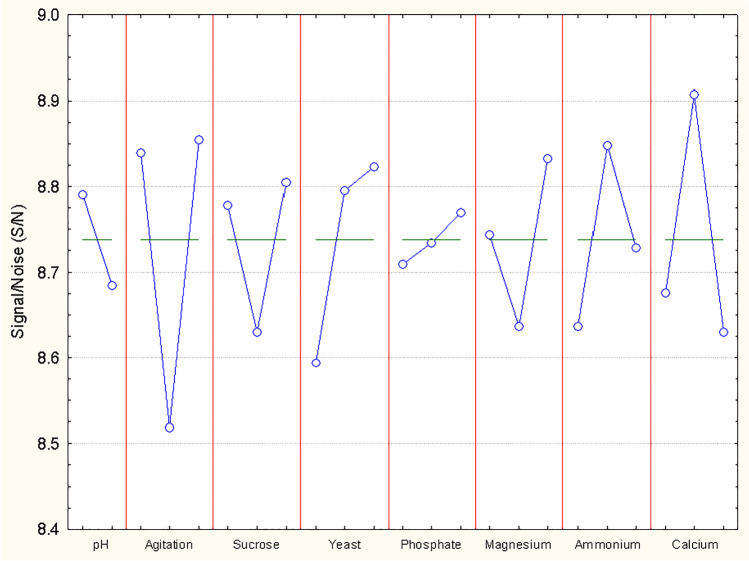
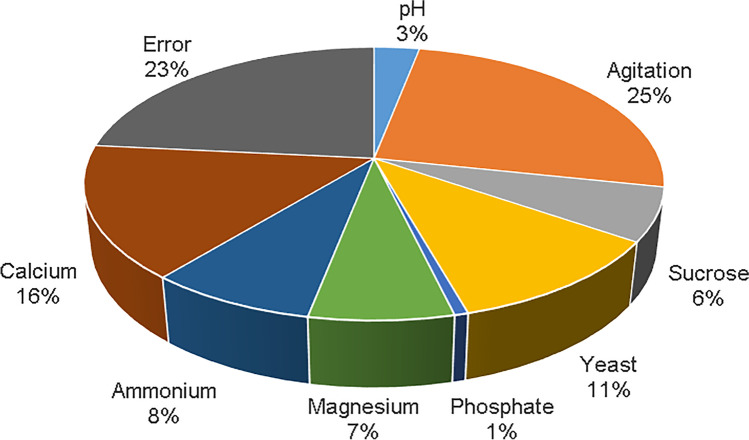
The influence of each factor evaluated show that calcium (level 2), agitation, sucrose, yeast, phosphate, and magnesium at high concentrations (level 3) contribute to the culture medium to achieve controlled culture conditions to offer a better antifungal effect against G. moniliformis LIA (Fig. 1). The relative influence of each factor is shown in Fig. 2. It is observed that there are factors that do not exert a significant effect, being to a lesser extent phosphate and pH. In relation to the error obtained (23.43%), Aguilar-Zarate et al. [24] mentioned that obtaining a high error value does not mean that the quality of the experiment is bad but there are factors that cannot be controlled or that have not been taken into account as factors to be evaluated. From the ANOVA analysis, the contribution percentage of each factor was calculated (Table 3). The agitation was the physical parameter that most influenced to the production of metabolites that slow down the growth rate of G. moniliformis contributing 25.34%, followed by calcium (15.65%) and the yeast extract (11.02%).
Fig. 1.
Influence of individual factors at different levels
Fig. 2.
Relative influence of the factors
Table 3.
Analysis of variance
| Serial | Factors | df | SS | MS | F | p | Percentage P (%) |
|---|---|---|---|---|---|---|---|
| 1 | pH | 1 | 0.051 | 0.051 | 0.26 | 0.662 | 3.01 |
| 2 | Agitation | 2 | 0.433 | 0.216 | 1.08 | 0.480 | 25.34 |
| 3 | Sucrose | 2 | 0.107 | 0.053 | 0.27 | 0.789 | 6.26 |
| 4 | Yeast | 2 | 0.188 | 0.094 | 0.47 | 0.680 | 11.02 |
| 5 | Phosphate | 2 | 0.011 | 0.005 | 0.03 | 0.973 | 0.64 |
| 6 | Magnesium | 2 | 0.115 | 0.057 | 0.29 | 0.776 | 6.76 |
| 7 | Ammonium | 2 | 0.134 | 0.067 | 0.34 | 0.748 | 7.88 |
| 8 | Calcium | 2 | 0.267 | 0.133 | 0.67 | 0.599 | 15.65 |
| Error | 2 | 0.400 | 0.200 | 23.43 | |||
| Total | 17 | 1.709 | 0.880 | 100 |
Some authors have reported that at high values of agitation a greater production of secondary metabolites in liquid medium is obtained [30–32] and therefore a better antimicrobial effect is generated [33]. On the other hand, calcium regulates, for example, the cell cycle in fungi [34], and it has been reported that at the concentration used in this study (0.4 g/L), high concentrations of biomass and blastospores of B. bassiana are obtained [35]. Nitrogen sources are a crucial factor not only for the growth of the fungus but also for the production of secondary metabolites [36]; according to Petlamul and Prasertsan [29], the combination of organic nitrogen (KNO3) as inorganic (yeast extract) contributes significantly in the production of B. bassiana spores in liquid medium (32.80% and 49.33%, respectively).
As for the rest of the parameters evaluated, their contribution, being less than 10%, is considered insignificant as they do not influence the process [37].
Submerged fermentation in sparged bottle bioreactor
The conditions of the optimal culture medium composition (Table 4) were reproduced in a sparged bottle bioreactor. It resulted in the production of a diffusible red pigment throughout the medium which was detected at the fourth day of fermentation (Fig. 3) as reported by Amin et al. [38], contrary to that observed in fermentation in the Erlenmeyer flask where the extracts were colorless. The crude fungal extract caused a reduction in the growth rate of G. moniliformis LIA (μ = 0.41 mm/h) compared to the control (μ = 0.45 mm/h). On the other hand, the experimental value of signal-to-noise (S/N) obtained was higher than expected by the Taguchi analysis (Table 4).
Table 4.
Optimum culture conditions
| Factors | Level | Value | Contribution |
|---|---|---|---|
| pH | 1 | 5 | 0.05 |
| Agitation | 3 | 250 | 0.12 |
| Sucrose (g/L−1) | 3 | 37.5 | 0.07 |
| Yeast extract (g/L−1) | 3 | 10 | 0.09 |
| KH2PO4 (g/L−1) | 3 | 0.8 | 0.03 |
| MgSO4 (g/L−1) | 3 | 1.2 | 0.10 |
| NH4NO3 (g/L−1) | 2 | 0.1 | 0.11 |
| CaCl2 (g/L−1) | 2 | 0.4 | 0.17 |
| Expected (S/N) | 9.47 | ||
| Experimental (S/N) | 12.48 | ||
Fig. 3.
Submerged fermentation in bottle bioreactor. On the left, the bottle bioreactor with parts. On the right, shows red pigment produced by Beauveria bassiana PQ2 on the fourth day of fermentation. In addition, abundant white mycelium is observed on the walls of the bottle
Zero percent of germination of the spores of G. moniliformis LIA was observed at 24 h with the red extract obtained in the validation. The pigment produced has been reported as oosporein, characteristic of Beauveria spp. [6]. It must be considered that oosporein has the capacity to react, through redox reactions, with proteins and amino acids through the change of -SH groups resulting in enzymatic malfunction [39]. It has also been reported that it inhibits the ATPase activity of erythrocytes with the consequent cell lysis [40]. Meazza, Dayan, and Wedge [41] studied the antifungal activity of quinones (1,4-naphthoquinones, 1,2-anthraquinones, and 1,4-benzoquinones) on Colletotrichum flagariae, C. gloeosporioides, and C. acutatum. Their results showed activity of sensitive to resistant, being the most resistant C. acutatum. In addition, they mentioned that the main function of quinones is to inhibit the transport of electrons, important in mitochondrial respiration. This is relevant due to the fact that oosporein is a quinone (1,4-dibenzoquinone) [42]. This is the first report of the effects of oosporein on G. moniliformis. Some authors have reported the antifungal effect of oosporein isolated from Verticillium psalliotae against Phytophthora infestans, Alternaria solani, and Fusarium oxysporum [43]; Chaetomium cupreum against Rhizoctonia solani and Pythium ultimum [44]; and Cochliobolus kusanoi against Candida albicans [45].
Characterization of compounds by HPLC–ESI–MS
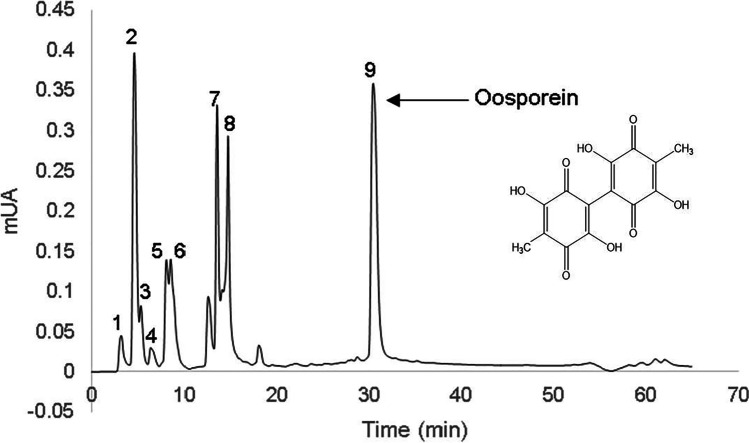
The extract was separated and characterized by RP-HPLC–ESI–MS in order to know the number of compounds present in the aqueous extract obtained in bioreactor (Table 5). The results obtained in the characterization of the compounds show nine peaks (Fig. 4) where oosporein was the only relevant metabolite detected. It was identified by information reported in the literature, with formula C14H10O8 [42, 46–48]. Also, maltose was detected. This could be due to the fact that Beauveria spp. can produce some soluble carbohydrates as an osmo-protection strategy or adaptation to stress [49]. It should be noted that the metabolites tenellin, bassianin, and beauvericin commonly produced by Beauveria species were not identified. This agrees with the results obtained by Strasser, Abendstein, et al. [46] who also consider that the production of oosporein is constitutive. However, the Beauveria genus is not the only one that produces it, since it has also been reported in some fungi such as V. psalliotae [43], C. kusanoi [45, 50], Lecanicillium aphanocladii [47], Tremella fuciformis [51], C. cupreum [44], and Isaria cicadae [52]. The composition of nutrients and the conditions of the culture medium influenced the antifungal activity of Beauveria bassiana PQ2.
Table 5.
Characterization of secondary metabolites by HPLC–ESI–MS analysis
| Peak no | Tentative identity | RT (min) | [M-H]− (m/z) | MS2 ion fragment (m/z) |
|---|---|---|---|---|
| 1 | Not identified | 3.77 | 352.8 | 334.8; 324.9; 316.9; 298.9; 260.9; 254.8; 219.1 |
| 2 | Maltose | 4.7 | 341.0 | 323.1; 179.1; 161.1; 143.1; 118 |
| 3 | Maltose | 5.31 | 341.0 | 323; 300.6; 179.2; 161.1 |
| 4 | Not identified | 7.17 | 290.0 | 254.1; 229.9; 214; 200; 128.1 |
| 5 | Not identified | 8.88 | 150.9 | 131.2 |
| 6 | Not identified | 9.55 | 242.9 | 199.9; 110 |
| 7 | Not identified | 14.23 | 282.0 | 150.1; 133 |
| 8 | Not identified | 15.52 | 385.9 | 343; 298.9; 297.9; 286 |
| 9 | Oosporein | 31.25 | 304.9 | 276.9; 262; 261; 249; 233; 217; 205; 189.1;161.1 |
Abbreviations: RT retention time
Fig. 4.
HPLC chromatogram of metabolites of Beauveria bassiana PQ2 extract. Nine peaks are shown where the latter is identified as oosporein
Conclusion
The optimal culture conditions based on the inhibition of the growth of G. moniliformis LIA by the effect of secondary metabolites was obtained. It was achieved to produce oosporein as the main secondary metabolite in a sparged bottle bioreactor. The water-soluble pigment was capable to inhibit the G. moniliformis LIA growth and spore germination. However, it is necessary to develop further experiments for the evaluation of antifungal activity and other bioactivities of purified oosporein.
Acknowledgements
The authors want to thank to Tecnológico Nacional de México and to the Autonomous University of Nuevo León for the financial support.
Author contribution
JGAV and MLCI performed the experimental work, data analysis, and wrote the manuscript. MRM, JEWP, DBMM, and RRM provided lab resources and reviewed the original draft. JAAV performed the chromatographic analysis. GCGMA and PAZ conceptualized the work, data analysis, and fund acquisition. All the authors approved the final manuscript.
Funding
The work was partially financed by the Autonomous University of Nuevo León with the project PAICyT-UANL (CT1525-21) and by Tecnológico Nacional de México with the projects No. 6691.18-P and 10394.21-P.
Data Availability
The dataset used in the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This article does not contain experiments with humans or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Melissa Fontes Landell
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pedro Aguilar-Zárate, Email: pedro.aguilar@tecvalles.mx.
Guillermo Cristian G. Martínez-Ávila, Email: guillermo.mtzavl@uanl.edu.mx
References
- 1.Horak I, Engelbrecht G, van Rensburg PJJ, Claassens S. Microbial metabolomics: essential definitions and the importance of cultivation conditions for utilizing Bacillus species as bionematicides. J Appl Microbiol. 2019;127:326–343. doi: 10.1111/jam.14218. [DOI] [PubMed] [Google Scholar]
- 2.Bracarense AAP, Takahashi JA. Modulation of antimicrobial metabolites production by the fungus Aspergillus parasiticus. Brazilian J Microbiol. 2014;45:313–321. doi: 10.1590/S1517-83822014000100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz B, Chávez A, Forero A, et al. Production of microbial secondary metabolites: regulation by the carbon source. Crit Rev Microbiol. 2010;36:146–167. doi: 10.3109/10408410903489576. [DOI] [PubMed] [Google Scholar]
- 4.Keller NP. Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol. 2019;17:167–180. doi: 10.1038/s41579-018-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias BC, Said S, de Albuquerque S, Pupo MT. The influence of culture conditions on the biosynthesis of secondary metabolites by Penicillium verrucosum Dierck. Microbiol Res. 2006;161:273–280. doi: 10.1016/j.micres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Ávila-Hernández JG, Carrillo-Inungaray ML, De la Cruz Quiroz R, et al. Beauveria bassiana secondary metabolites: a review inside their production systems, biosynthesis, and bioactivities. Mex J Biotechnol. 2020;5:1–33. doi: 10.29267/mxjb.2020.5.4.1. [DOI] [Google Scholar]
- 7.Aoki T, O’Donnell K, Geiser DM. Systematics of key phytopathogenic Fusarium species: current status and future challenges. J Gen Plant Pathol. 2014;80:189–201. doi: 10.1007/s10327-014-0509-3. [DOI] [Google Scholar]
- 8.Montoya-Martínez AC, Rodríguez-Alvarado G, Fernández-Pavía SP, et al. Design and validation of a robust multiplex polymerase chain reaction assay for MAT idiomorph within the Fusarium fujikuroi species complex. Mycologia. 2019;111:772–781. doi: 10.1080/00275514.2019.1649956. [DOI] [PubMed] [Google Scholar]
- 9.Jurgenson JE, Zeller KA, Leslie JF. Expanded genetic map of Gibberella moniliformis (Fusarium verticillioides) Appl Environ Microbiol. 2002;68:1972–1979. doi: 10.1128/AEM.68.4.1972-1979.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Hove F, Waalwijk C, Logrieco A, et al. Gibberella musae (Fusarium musae) sp. nov., a recently discovered species from banana is sister to F. verticillioides. Mycologia. 2011;103:570–585. doi: 10.3852/10-038. [DOI] [PubMed] [Google Scholar]
- 11.Adame-García J, Rodriguez-Guerra R, Iglesias-Andreu LG, et al. Molecular identification and pathogenic variation of fusarium species isolated from Vanilla planifolia in Papantla Mexico. Bot Sci. 2015;93:669–678. doi: 10.17129/botsci.142. [DOI] [Google Scholar]
- 12.Ibrahim NF, Mohd MH, Mohamed Nor NMI, Zakaria L. Characterization of Fusarium spp. associated with pineapple fruit rot and leaf spot in Peninsular Malaysia. J Phytopathol. 2017;165:718–726. doi: 10.1111/jph.12611. [DOI] [Google Scholar]
- 13.Alberto RT. Pathological response and biochemical changes in Allium cepa L. (bulb onions) infected with anthracnose-twister disease. Plant Pathol Quar. 2014;4:23–31. doi: 10.5943/ppq/4/1/4. [DOI] [Google Scholar]
- 14.Martínez-Bolaños M, Téliz-Ortiz D, Mora-Aguilera A, et al. Antracnosis (Colletotrichum gloeosporioides Penz.) del fruto de litchi (Litchi chinensis Soon.)en Oaxaca. México Rev Mex Fitopatol. 2015;33:140–155. [Google Scholar]
- 15.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baranyi J, Roberts TA. A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol. 1994;23:277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 17.Costa LCB, Pinto JEBP, Bertolucci SKV, et al. In vitro antifungal activity of Ocimum selloi essential oil and methylchavicol against phytopathogenic fungi. Rev Ciência Agronômica. 2015;46:428–435. [Google Scholar]
- 18.Aguilar-Zárate P, Wong-Paz JE, Michel M, et al. Characterisation of pomegranate-husk polyphenols and semi-preparative fractionation of punicalagin. Phytochem Anal. 2017;28:433–438. doi: 10.1002/pca.2691. [DOI] [PubMed] [Google Scholar]
- 19.Gibson DM, Donzelli BGG, Krasnoff SB, Keyhani NO. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat Prod Rep. 2014;31:1287–1305. doi: 10.1039/C4NP00054D. [DOI] [PubMed] [Google Scholar]
- 20.Davis R, John P (2018) Application of Taguchi-based design of experiments for industrial chemical processes. In: Silva V (ed) Statistical Approaches With Emphasis on Design of Experiments Applied to Chemical Processes. IntechOpen, Rijeka, pp 137–155
- 21.Manzoor A, Qazi JI, Haq I, ul, , et al. Significantly enhanced biomass production of a novel bio-therapeutic strain Lactobacillus plantarum (AS-14) by developing low cost media cultivation strategy. J Biol Eng. 2017;11:17. doi: 10.1186/s13036-017-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chenthamarakshan A, Parambayil N, Miziriya N, et al. Optimization of laccase production from Marasmiellus palmivorus LA1 by Taguchi method of Design of experiments. BMC Biotechnol. 2017;17:12. doi: 10.1186/s12896-017-0333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shehata AN, El Aty AAA. Optimization of Process parameters by statistical experimental designs for the production of naringinase enzyme by marine fungi. Int J Chem Eng. 2014;2014:1–10. doi: 10.1155/2014/273523. [DOI] [Google Scholar]
- 24.Aguilar-Zarate P, Cruz-Hernandez MA, Montañez JC, et al (2014) Enhancement of tannase production by Lactobacillus plantarum CIR1: validation in gas-lift bioreactor. Bioprocess BiosystEng 3710.1007/s00449-014-1208-3 [DOI] [PubMed]
- 25.EL-Moslamy SH, Elkady MF, Rezk AH, Abdel-Fattah YR. Applying Taguchi design and large-scale strategy for mycosynthesis of nano-silver from endophytic Trichoderma harzianum SYA.F4 and its application against phytopathogens. Sci Rep. 2017;7:45297. doi: 10.1038/srep45297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velhal SG, Latpate RV, Kulkami SD, Jaybhaye RG. Taguchi design for parameter optimization of size-controlled synthesis of silver nanoparticles. Int J Emerg Technol Comput Appl Sci. 2015;12:144–149. [Google Scholar]
- 27.Katata-Seru L, Lebepe TC, Aremu OS, Bahadur I. Application of Taguchi method to optimize garlic essential oil nanoemulsions. J Mol Liq. 2017;244:279–284. doi: 10.1016/j.molliq.2017.09.007. [DOI] [Google Scholar]
- 28.Dutta D, Das MD. Effect of C/N ratio and microelements on nutrient dynamics and cell morphology in submerged fermentation of Aspergillus giganteus MTCC 8408 using Taguchi DOE. 3 Biotech. 2017;7:34. doi: 10.1007/s13205-017-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petlamul W, Prasertsan P. Medium optimization for production of Beauveria bassiana BNBCRC spores from biohydrogen effluent of palm oil mill using Taguchi design. Int J Biosci Biochem Bioinforma. 2014;4:106–109. [Google Scholar]
- 30.Gunasekaran S, Poorniammal R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. African J Biotechnol. 2008;7:1894–1898. doi: 10.5897/AJB2008.000-5037. [DOI] [Google Scholar]
- 31.Venkatachalam M, Shum-Chéong-Sing A, Dufossé L, Fouillaud M. Statistical optimization of the physico-chemical parameters for pigment production in submerged fermentation of Talaromyces albobiverticillius 30548. Microorganisms. 2020;8:711. doi: 10.3390/microorganisms8050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkata Dasu V, Panda T, Chidambaram M. Determination of significant parameters for improved griseofulvin production in a batch bioreactor by Taguchi’s method. Process Biochem. 2003;38:877–880. doi: 10.1016/S0032-9592(02)00068-7. [DOI] [Google Scholar]
- 33.Mitrović IŽ, Grahovac JA, Dodić JM, et al (2017) Effect of agitation rate on the production of antifungal metabolites by Streptomyces hygroscopicus in a lab-scale bioreactor. Acta Period Technol 231–24410.2298/APT1748231M
- 34.Ortiz-Urquiza A, Keyhani NO (2016) Molecular genetics of Beauveria bassiana infection of insects. In: Lovett B, St Leger R (eds) Genetics and Molecular Biology of Entomopathogenic Fungi. Academic Press, pp 165–249 [DOI] [PubMed]
- 35.Mascarin GM, Jackson MA, Kobori NN, et al. Liquid culture fermentation for rapid production of desiccation tolerant blastospores of Beauveria bassiana and Isaria fumosorosea strains. J Invertebr Pathol. 2015;127:11–20. doi: 10.1016/j.jip.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Tudzynski B. Nitrogen regulation of fungal secondary metabolism in fungi. Front Microbiol. 2014;5:1–15. doi: 10.3389/fmicb.2014.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vázquez-Sánchez AY, Aguilar-Zárate P, Muñiz-Márquez DB, et al. Effect of ultrasound treatment on the extraction of antioxidants from Ardisia compressa Kunth fruits and identification of phytochemicals by HPLC-ESI-MS. Heliyon. 2019;5:e03058. doi: 10.1016/j.heliyon.2019.e03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amin GA, Youssef NA, Bazaid S, Saleh WD. Assessment of insecticidal activity of red pigment produced by the fungus Beauveria bassiana. World J Microbiol Biotechnol. 2010;26:2263–2268. doi: 10.1007/s11274-010-0416-5. [DOI] [Google Scholar]
- 39.Strasser H, Vey A, Butt TM. Are there any risks in using entomopathogenic fungi for pest control, with particular feference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci Technol. 2000;10:717–735. doi: 10.1080/09583150020011690. [DOI] [Google Scholar]
- 40.Jeffs LB, Khachatourians GG. Toxic properties of Beauveria pigments on erythrocyte membranes. Toxicon. 1997;35:1351–1356. doi: 10.1016/S0041-0101(97)00025-1. [DOI] [PubMed] [Google Scholar]
- 41.Meazza G, Dayan FE, Wedge DE. Activity of quinones on colletotrichum species. J Agric Food Chem. 2003;51:3824–3828. doi: 10.1021/jf0343229. [DOI] [PubMed] [Google Scholar]
- 42.Feng P, Shang Y, Cen K, Wang C. Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc Natl Acad Sci U S A. 2015;112:11365–11370. doi: 10.1073/pnas.1503200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagaoka T, Nakata K, Kouno K, Ando T. Antifungal activity of oosporein from an antagonistic fungus against Phytophthora infestans. Z Naturforsch C. 2004;59:302–304. doi: 10.1515/znc-2004-3-432. [DOI] [PubMed] [Google Scholar]
- 44.Mao B, Huang C, Yang G, et al. Separation and determination of the bioactivity of oosporein from Chaetomium cupreum. African J Biotechnol. 2010;9:5955–5961. doi: 10.5897/AJB09.1992. [DOI] [Google Scholar]
- 45.Alurappa R, Bojegowda MRM, Kumar V, et al. Characterisation and bioactivity of oosporein produced by endophytic fungus Cochliobolus kusanoi isolated from Nerium oleander L. Nat Prod Res. 2014;28:2217–2220. doi: 10.1080/14786419.2014.924933. [DOI] [PubMed] [Google Scholar]
- 46.Strasser H, Abendstein D, Stuppner H, Butt TM. Monitoring the distribution of secondary metabolites produced by the entomogenous fungus Beauveria brongniartii with particular reference to oosporein. Mycol Res. 2000;104:1227–1233. doi: 10.1017/S0953756200002963. [DOI] [Google Scholar]
- 47.da Costa Souza PN, Grigoletto TLB, de Moraes LAB, et al. Production and chemical characterization of pigments in filamentous fungi. Microbiology. 2016;162:12–22. doi: 10.1099/mic.0.000168. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen KF, Smedsgaard J. Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography–UV–mass spectrometry methodology. J Chromatogr A. 2003;1002:111–136. doi: 10.1016/S0021-9673(03)00490-4. [DOI] [PubMed] [Google Scholar]
- 49.Ortiz-Urquiza A, Keyhani NO. Stress response signaling and virulence: insights from entomopathogenic fungi. Curr Genet. 2015;61:239–249. doi: 10.1007/s00294-014-0439-9. [DOI] [PubMed] [Google Scholar]
- 50.Ramesha A, Venkataramana M, Nirmaladevi D, et al. Cytotoxic effects of oosporein isolated from endophytic fungus Cochliobolus kusanoi. Front Microbiol. 2015;6:870. doi: 10.3389/fmicb.2015.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He G, Yan J, Wu X-Y, et al. Oosporein from Tremella fuciformis. Acta Crystallogr Sect E Struct Rep Online. 2012;68:o1231. doi: 10.1107/S1600536812012950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Hu Q, Weng Q. Secondary metabolites (SMs) of Isaria cicadae and Isaria tenuipes. RSC Adv. 2019;9:172–184. doi: 10.1039/C8RA09039D. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used in the current study are available from the corresponding author on reasonable request.