Abstract
Iron-overaccumulating mutants were investigated with respect to changes in epidermal cell patterning and root reductase activity in response to iron starvation. In all mutants under investigation, ferric chelate reductase activity was up-regulated both in the presence and absence of iron in the growth medium. The induction of transfer cells in the rhizodermis appeared to be iron regulated in the pea (Pisum sativum L. cv Dippes Gelbe Viktoria and cv Sparkle) mutants bronze and degenerated leaflets, but not in roots of the tomato (Lycopersicon esculentum Mill. cv Bonner Beste) mutant chloronerva, suggesting that in chloronerva iron cannot be recognized by putative sensor proteins. Experiments with split-root plants supports the hypothesis that Fe(III) chelate reductase is regulated by a shoot-borne signal molecule, communicating the iron status of the shoot to the roots. In contrast, the formation of transfer cells was dependent on the local concentration of iron, implying that this shoot signal does not affect their formation. Different repression curves of the two responses imply that the induction of transfer cells occurs after the enhancement of electron transfer across the plasma membrane rather than being causally linked. Similar to transfer cells, the formation of extra root hairs in the Arabidopsis mutant man1 was regulated by the iron concentration of the growth medium and was unaffected by interorgan signaling.
The root epidermis forms the interface between a plant and its environment and serves many important functions such as defense against invasion by pathogens and regulation of the exchange of materials between root cells and the rhizosphere. Root epidermal cells can differentiate into hair or non-hair cells, often in a well-defined, predictable pattern. This binary choice is controlled by the activity of genes that have been identified as negative regulators of hair cell development (Galway et al., 1994; Masucci et al., 1996; Wada et al., 1997). The plant hormones ethylene and auxin are required for normal root hair elongation and promote root hair development by acting after the cell fate speciation genes (Tanimoto et al., 1995; Masucci and Schiefelbein, 1996). In addition, developmental programs, positional cues, and environmental stimuli can modulate cell fate speciation.
The number and length of root hairs are important determinants for the below-ground surface of a plant and thus for the uptake of water and nutrients. Exploration of a great soil volume is of particular importance for the uptake of immobile nutrients such as phosphate and iron. Low bioavailability of both phosphate and iron causes an enlargement of root hairs and an increase in number, thereby enhancing the effective root length (Bates and Lynch, 1996; Landsberg, 1996; Schmidt et al., 2000). A third possibility of cell fate speciation in the root epidermis is the formation of transfer cells. These cells are characterized by a relatively high cytoplasm to vacuole ratio, a high number of cisternae of rough endoplasmic reticulum, numerous mitochondria, densely packed internal membranes, and extensive, often labyrinth-like ingrowths of secondary wall material leading to an increased surface of the plasma membrane (Gunning and Pate, 1969; Pate and Gunning, 1972). Transfer cells have been reported to occur in various tissues such as dermal cells of developing cotyledons (McDonald et al., 1996), reproductive tissues (Briggs, 1995), vascular system of stems (Gunning et al., 1970), the host-parasite interface (Heide-Jørgensen and Kuijt, 1993), the sporophyte haustorium (Renault et al., 1989), and minor veins of leaves (Wimmers and Turgeon, 1991; Bouché-Pillon et al., 1994). Under ordinary conditions transfer cells are not formed in roots but are induced in a number of species by iron shortage (Landsberg, 1982; Römheld and Kramer, 1983; Schmidt and Bartels, 1996). Wall ingrowths were not observed in plants with thin roots forming extensive root hairs like Arabidopsis, suggesting that in such species the formation of transfer cells and extra root hairs represents an alternative strategy to increase the absorptive area under iron-deficient conditions (Schmidt et al., 2000). The regulatory signals for transfer cell differentiation are currently unknown.
Iron is a component of proteins required for crucial cellular processes and performs numerous essential functions including respiration and cell division. In strategy I species, the uptake of iron is supported by reactions aimed at scavenging low amounts of iron from the environment such as acidification of the rhizosphere and reduction of external Fe(III) chelates (Guerinot and Yi, 1994; Marschner and Römheld, 1994; Schmidt, 1999). Because iron can exhibit significant toxicity inside the cell due to the formation of hydroxyl radicals in the Fenton/Haber-Weiss reactions, cells have had to evolve signaling pathways through which the acquisition of iron is regulated. In contrast with vertebrates, where iron homeostasis is controlled by the interaction between “iron regulatory proteins” and “iron-responsive elements” that regulate the stability and translatability of iron-responsive mRNAs (Eisenstein and Blemings, 1998; Aisen et al., 1999), the bases of the molecular control of iron levels in plants are largely unknown. Proteins that sense iron concentrations in plant cells have not been identified up to now, although their existence was proposed more than a decade ago (Bienfait, 1988). In higher plants the situation is complicated by the fact that beside intracellular iron concentrations, shoot-to-root communication appears to be important for the regulation of iron uptake (Grusak, 1995; Grusak and Pezeshgi, 1996; Schmidt et al., 1996).
Although the function of root hairs and transfer cells in iron uptake is the matter of some debate, the regulation of their induction appears to be associated with the maintenance of iron homeostasis. The question of whether all components involved in the acquisition of iron are subject to common regulation is important for designing strategies aimed at identifying proteins that can sense and respond to iron. In the current study, we investigated changes in Fe(III) reduction activity and epidermal cell patterning in a number of mutants with deregulated iron uptake. It is shown that different mechanisms are involved in regulating acclimations to low iron availability.
RESULTS
Iron-Overaccumulating Mutants Differ in Their Pattern of Transfer Cell Formation
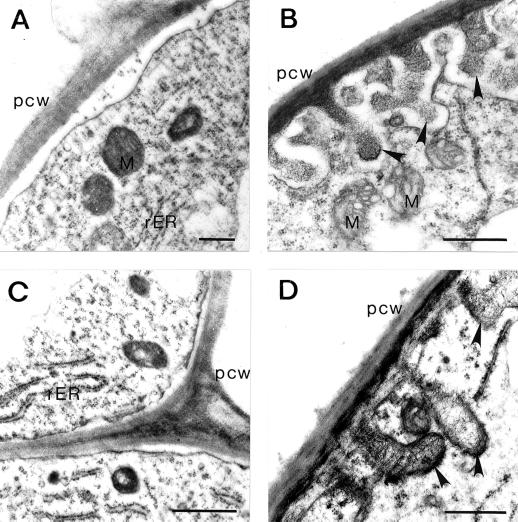
Root Fe(III) chelate reduction activity and frequency of transfer cells in the rhizodermis were determined in iron-overaccumulating mutants grown either with or without iron in the nutrient medium. The nonallelic pea (Pisum sativum L. cv Dippes Gelbe Viktoria and cv Sparkle) mutants degenerated leaflets (dgl) and bronze (brz) are characterized by an extremely high concentration of iron in both roots and shoots when grown at levels that are adequate for their parent genotypes (Grusak et al., 1990; Kneen et al., 1990). Both mutants show reduced growth and necrotic spots on the basal leaves due to excessive iron accumulation (Gottschalk, 1987; Becker et al., 1998). Similar to dgl and brz, the nicotianamine auxotroph chloronerva (chln) mutant of tomato (Lycopersicon esculentum Mill. cv Bonner Beste) also exhibits constitutive iron deficiency responses despite high iron concentrations in both roots and shoots (Becker et al., 1995). Due to a restricted symplasmic iron transport this mutant displays intercostal chlorosis of young leaves (apparent iron deficiency syndrome). Application of nicotianamine rescues the chln phenotype (Scholz et al., 1992). The ultrastructure of epidermal cells from pea and tomato roots is shown in Figure 1. In wild-type roots, transfer cells were formed almost exclusively under −Fe conditions. Although the wall ingrowths formed in pea roots are mainly of the papillate type, labyrinth-like protuberances were observed in tomato roots. In transfer cells of both species deposition of ingrowths are polarized to the outer periclinal walls. When compared with epidermal cells formed under ordinary conditions, the cytoplasm of transfer cells appears to be dense and is characterized by numerous mitochondria and rough endoplasmic reticulum (Fig. 1). The morphology of transfer cells in mutant roots mirrored that of the wild types (data not shown).
Figure 1.
Effect of iron status on the development of epidermis cell in roots of wild-type pea and tomato roots. Epidermal cell of iron-sufficient (A) and iron-deficient (B) tomato roots. Epidermal cell of iron-sufficient (C) and iron-deficient (D) pea roots. Note the wall membrane apparatus with wall ingrowths (denoted by arrowheads) in iron-deficient cells. M, Mitochondria; pcw, peripheral cell wall; rER, rough endoplasmatic reticulum. Bar = 500 nm.
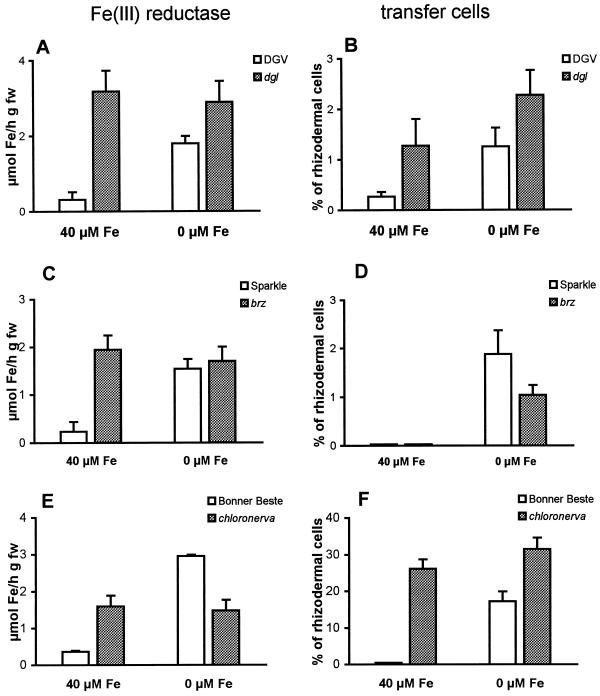
In contrast with pea cv Dippes Gelbe Viktoria and cv Sparkle, in which Fe(III) reductase was repressed by the presence of external iron, the roots of dgl and brz exhibited consistently higher Fe(III) reduction rates independent of the iron concentration of the growth medium (Fig. 2, A and C). Transfer cell frequency in wild-type roots paralleled reduction activity. The number of transfer cells in the rhizodermis of iron-free grown plants was about 5-fold higher relative to those of roots from iron-sufficient plants (Fig. 2B). Similar to its wild type, the formation of transfer cells was repressed by external iron in dgl roots by about 50%. As evidenced by the Wilcoxon‘s test, this difference was statistically significant (P = 0.05). Transfer cell formation in roots of the brz mutant followed a similar pattern. Under iron-sufficient conditions almost no induction of this cell type occurred in roots of the mutant and of the wild-type pea cv Sparkle (Fig. 2D).
Figure 2.
Effect of iron regime on ferric chelate reductase activity (A, C, and E) and transfer cell formation (B, D, and F) in the pea mutant dgl and its wild-type cv Dippes Gelbe Viktoria (A and B), the pea mutant brz and its wild-type Sparkle (C and D), and the tomato mutant chln and its wild-type cv Bonner Beste (E and F). Determinations were made 6 d after applying treatments. Data are the means from three independent experiments with n = 5. Vertical bars represent sd.
The pattern of Fe(III) reduction activity in chln roots was similar to that of the pea mutants. As expected, repression of Fe(III) reduction by external iron was only noted in the wild type, whereas the mutant displays up-regulated reductase activity both in the absence and in the presence of iron (Fig. 2E). The transfer cell frequency in iron-deficient tomato roots was markedly higher than that of pea roots grown under iron-deficient conditions. The highest percentage of transfer cells was observed in chln roots; repression of the formation of wall ingrowths by the presence of iron in the growth medium was only noted in roots of its wild type (Fig. 2F).
Transfer Cell Development and Reductase Activity Are Differently Affected by External Iron
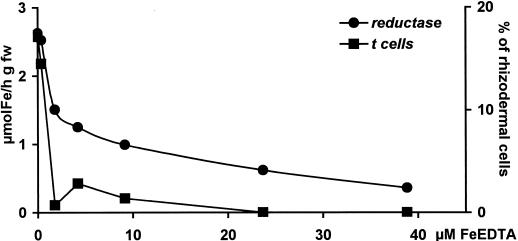
If it is assumed that the development of transfer cells and the induction of root reduction activity are controlled by a shared regulatory system, a parallel repression of the responses by increasing iron concentrations is to be expected. To test this assumption, both reactions were examined at various concentrations of external iron in the growth medium. To allow for a correlation of the amplitude of the responses with the concentration of external iron under consideration of changes in the activity of soluble iron species, such as displacement of Fe3+ from EDTA as the ligand-held ion by divalent metal ions, the concentration of ferric iron bound to EDTA was calculated using the Geochem PC software program (Parker et al., 1995). We chose roots from the tomato wild type as the experimental system because the percentage of transfer cells is sufficient to recognize and quantify even small differences in their number as affected by the external iron concentrations. The results are shown in Figure 3. Although the formation of wall ingrowths was almost completely repressed at 1.68 μm FeEDTA, the reduction activity decreased more gradually with increasing iron concentrations, exhibiting typical saturation kinetics.
Figure 3.
Root reduction activity and transfer cell frequency of wild-type tomato roots as a function of FeEDTA concentration in the nutrient solution. The concentration of FeEDTA was calculated using the Geochem PC software program. Data are from a representative experiment with n = 15. Determinations were made 6 d after applying treatments.
Differences between transfer cell formation and root reduction activity in response to iron in the external medium were also observed in experiments with localized iron supply. In these experiments plants were grown with a divided root system (split-root plants) in which iron was supplied to only one-half of the roots, whereas the other portion was grown in iron-free nutrient solution. As shown previously for Plantago lanceolata roots (Schmidt et al., 1996), the root halves responded differently with respect to the Fe(III) reduction rates. Enhanced reduction activity was determined in the iron-supplied split roots, whereas iron-free grown roots exhibited lower rates relative to uniformly iron-deprived control plants. The pattern of transfer cell formation was apparently different from that observed for the physiological responses. Transfer cell frequency was dependent on the iron concentration, being markedly increased in roots grown in iron-free medium (Table I).
Table I.
Fe(III) reduction activity and transfer cell frequency in split-root tomato wild-type plants
| Root Treatment | Fe(III) Reduction | Transfer Cells |
|---|---|---|
| Iron-supplied split roots | 1.63 ± 0.26 | 6.3 ± 1.2 |
| Iron-deficient split roots | 0.56 ± 0.30 | 28.1 ± 3.2 |
The reduction activity and the no. of transfer cells was determined 4 d after onset of the treatments. Reduction activity is expressed as μmol g−1 fresh wt h−1 ± sd, n = 5; transfer cell frequency is given as a percentage of the total rhizodermal cells ± sd.
The Formation of Extra Root Hairs Is Controlled by the Local Iron Level
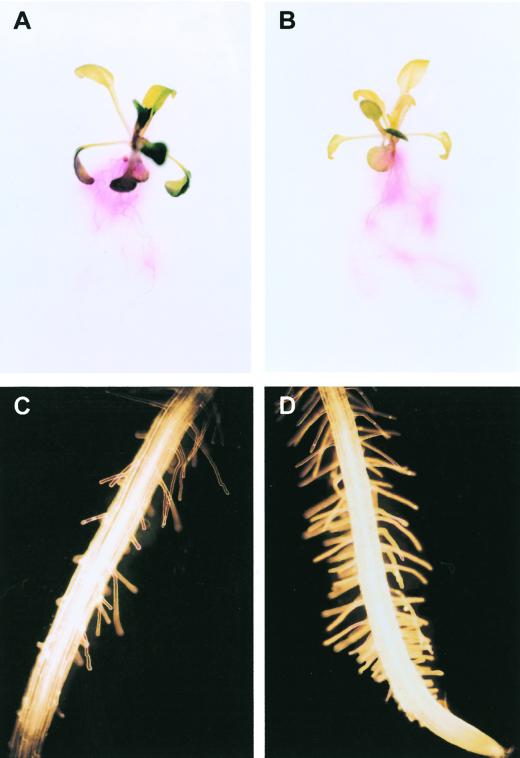
The differential regulation of transfer cell formation and root reductase activity raises the question of whether all morphological responses to iron deficiency, e.g. the formation of transfer cells and the development of extra root hairs, are under the same regulatory control. To test this hypothesis, we used the man1 mutant of Arabidopsis, which was originally identified as a manganese accumulator (Delhaize, 1996). man1 has recently been shown to be allelic to frd3, a mutant constitutively expressing Fe(III) reductase (M.L. Guerinot, personal communication; Eide et al., 1996). As evidenced by the stains for ferric reduction shown in Figure 4, constitutively up-regulated Fe(III) reductase activity in man1 roots was also apparent under the growth conditions of the present study. However, analogous to the formation of transfer cells, the induction of extra root hairs was repressed by adequate iron in the growth medium, suggesting similar components in the regulatory pathways of the two responses (Fig. 4).
Figure 4.
Visualization of ferric reduction activity and iron deficiency-induced alterations in root epidermal cells of iron-sufficient (A and C) and iron-deficient (B and D) roots of the Arabidopsis mutant man1. The resulting Fe(II) is trapped by FerroZine to produce a red product.
DISCUSSION
Root-mediated reduction of ferric chelates is a common feature of so-called strategy I plants (Römheld and Marschner, 1986). This process is catalyzed by an NADH-linked Fe(III) reductase embedded in the plasma membrane, acting upstream from an iron uptake system (Moog and Brüggemann, 1994). Suboptimal iron availability and enhanced shoot demand increase iron uptake rates of root cells (Schmidt, 1999, and references therein). Genes encoding a putative ferric chelate reductase have recently been identified in Arabidopsis. One of these genes, FRO2, is expressed in iron-deficient roots and is coregulated with IRT1, a transporter for ferrous iron (Eide et al., 1996; Robinson et al., 1999). Alterations in root morphology, such as the formation of root hairs and the development of transfer cells in the rhizodermis, are also regulated by iron availability and both sets of responses have been suggested to be under the same regulatory control (Landsberg, 1986; Bienfait, 1988). However, a common regulation has been questioned by several lines of evidence. Moog et al. (1995) showed that the time course of root hair formation differed from that of Fe(III) reductase activity during iron deficiency in Arabidopsis. Differences in the timing of the responses to iron deprivation have also been observed in pea roots with respect to rhizosphere acidification and root Fe(III) reductase stimulation (Grusak and Pezeshgi, 1996). We have recently demonstrated that hormones are involved in iron deficiency-induced formation of extra root hairs but not in the induction of enhanced Fe(III) reduction activity in Arabidopsis, suggesting dissimilar regulatory pathways of the responses (Schmidt et al., 2000).
A separate regulation of Fe(III) chelate reduction and transfer cell formation can be inferred from the results of the present study. As shown previously by others (Grusak et al., 1990; Grusak and Pezeshgi, 1996), both pea mutants displayed up-regulated reductase activity when grown either with or without iron in the growth medium, whereas the respective control wild types exhibited a normal regulation of Fe(III) reduction (Fig. 2). The induction of transfer cells is apparently not affected by these two mutations. In both wild-type and mutant roots the development of wall ingrowths was repressed by the presence of iron, suggesting that their formation might be dependent on the cytosolic iron concentration of the root cells. This is obviously not the case in chln roots of tomato in which neither response was affected by the iron regime. This apparent inconsistency in results may be due to the lack of nicotianamine in chln that might be necessary for transport and/or binding of iron to proteins involved in sensing intracellular iron concentrations. Restricted intracellular transport of iron is evidenced by accumulation and precipitation of iron in leaf and root cells of chln (Becker at al., 1995; Liu et al., 1998).
Although an autonomous root response to iron stress has been demonstrated with roots grown isolated from the shoots (Bienfait et al., 1987), substantial evidence suggests that the leaves can modulate the rate of iron uptake. Based on whole-plant experiments a transmissible signal that conveys information of the shoot’s iron status to the root was proposed to be involved in the regulation of iron reduction (e.g. Grusak and Pezeshgi, 1996; Schmidt et al., 1996). Reciprocal grafting experiments with the pea mutants used in the present study and their respective wild types have shown that the control of the Fe(III) reductase is localized in the shoot (Grusak and Pezeshgi, 1996). Thus, a putative iron sensor affecting the root ferric chelate reductase activity via such a phloem-mobile signal should be localized in the leaves. As evidenced by the repression of wall ingrowths formation in iron-sufficient roots of the pea mutants, this signal does not affect the formation of transfer cells (Fig. 2).
Although the phenotype of the chln mutation was recently shown to be caused by the destruction of the nicotianamine synthase-encoding gene (Herbik et al., 1999), the molecular basis for the brz and dgl phenotype has not yet been elucidated. In contrast with chln, which is completely devoid of nicotianamine, in both pea mutants nicotianamine levels are significantly enhanced relative to the parent genotypes (A. Pich, personal communication). This suggests that the intracellular transport of iron in dgl and brz is not affected and that the basis of these mutations might be a defect somewhere upstream from the synthesis of the signal molecule.
The existence of a signal that affects Fe(III) reductase activity, but not the formation of transfer cells, is evidenced by the split-root experiments (Table I). The iron-supplied split roots morphologically exhibited a phenotype characteristic of an iron-sufficient root, whereas these roots displayed an iron-deficient-like Fe(III) reductase response. From the increase in Fe(III) reduction activity by the iron-supplied root half it can be deduced that the reduction rates are determined by the shoot. The decreased reduction rates of the iron-free split roots is puzzling, but has also been observed in other species (Schmidt et al., 1996). Whatever the cause for the down-regulated Fe(III) reductase in those roots might be, the number of transfer cells is similar to that in undivided iron-deficient control roots, suggesting that their induction is regulated independently of the Fe(III) reductase activity. This assumption is further evidenced by the different repression curves of the two reactions. It appears that transfer cells are induced at a much more severe level of iron deficiency stress than the increase in root Fe(III) reductase activity and are regulated in an all-or-nothing manner, whereas reduction rates decreased in a typical saturation curve (Fig. 3). Induction of morphological changes after the physiological responses to iron deficiency was also observed with respect to the formation of extranumerary root hairs in tomato. In experiments with chelator-buffered nutrient solution, Chaney et al. (1992) found that root reduction activity was increased at levels of iron sufficient to avoid chlorosis, whereas root hair density was increased only in plants with severe chlorosis symptoms. Thus, it appears that morphological responses are induced when the physiological mechanisms are not sufficient to acquire adequate iron levels.
The experiments with the man1 mutant suggest that, similar to transfer cells, root hairs are regulated by the local iron concentration and are not affected by the putative shoot signal. The physiological basis for the chlorotic phenotype of man1 is not entirely clear. The mutant was identified as a manganese overaccumulator, and the high concentrations of manganese might be the cause of the chlorotic phenotype (González et al., 1998). However, growing the plants with a 10-fold lower manganese concentration did not cause any differences in reduction activity and leaf chlorosis (data not shown), making other explanations more likely. The lowered iron concentration in its leaves suggests that man1 is defective in a transporter for iron within the plant. It is alternatively possible that the mutation affects a gene involved in sensing intracellular iron levels. Although the primary cause of the mutation cannot be deduced from the present data, the formation of root hairs is not affected and is thus independent of other responses to iron deficiency.
In conclusion, we have shown in the present study that different regulatory pathways exist with respect to inter- and intra-organ regulation of the responses to iron shortage. The differential regulation of morphological and physiological reactions implies that the search for sensor(s) participating in the regulation of iron homeostasis should not be restricted to a single protein. Identification of the components involved in recognizing and responding to iron will provide the basis for the molecular analysis of iron homeostasis in plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The pea (Pisum sativum) mutants brz and dgl and their parent genotypes cv Sparkle and cv Dippes Gelbe Viktoria were kindly provided by Michael A. Grusak (Children's Nutrition Research Center, Houston). The chln mutant of tomato (Lycopersicon esculentum Mill.) and its wild-type cv Bonner Beste were obtained from Udo W. Stephan (Institute of Plant Genetics and Crop Research, Gatersleben, Germany). The Arabidopsis Col–0 ecotype and the man1–1 mutant were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). All mutants have been described elsewhere.
Arabidopsis plants were grown in a growth chamber on an agar medium as described by Estelle and Somerville (1987). The seeds were surface sterilized by immersing them in 5% (v/v) NaOCl for 5 min and 96% (v/v) ethanol for 5 min followed by four rinses in sterile distilled water. The medium was composed of KNO3 (5 mm), MgSO4 (2 mm), Ca(NO3)2 (2 mm), K2PO4 (2.5 mm), H3BO3 (70 μm), MnCl2 (14 μm), ZnSO4 (1 μm), CuSO4 (0.5 μm), NaCl (10 μm), and Na2MoO4 (0.2 μm) and solidified with 0.5% (w/v) agar. Suc (43 mm) and 4.7 mm MES [2-(N-morpholino)ethanesulfonic acid] were included and the pH was adjusted to 6.0. Seeds were placed on petri dishes containing agar medium and kept for 3 d at 4°C in the dark before the plates were transferred to a growth chamber and grown at 21°C in continuous light (150 μmol m−2 s−1, TL lamps, Philips, Eindhoven, The Netherlands; relative humidity 70%). After 7 d, plants were grown for an additional 4 d either with 40 μm FeEDTA or without iron in the presence of 100 μm 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine sulfonate (FerroZine).
Seedlings of tomato cv Bonner Beste and its mutant chln, pea cv Dippes Gelbe Viktoria and its mutant dgl, and pea cv Sparkle and its mutant brz were grown under controlled conditions in a greenhouse (16 h, 25°C/8 h, 18°C day/night regime; photon flux density of approximately 300 μmol m−2 s−1; relative humidity 50%). Tomato plants were cultivated hydroponically in 11-l tanks (40 plants/tank) containing continuously aerated nutrient solution with the following composition: KNO3 (3 mm), MgSO4 (0.5 mm), CaCl2 (1.5 mm), K2SO4 (1.5 mm), NaH2PO4 (0.5 mm), H3BO3 (25 μm), MnSO4 (1 μm), ZnSO4 (0.5 μm), (NH4)6Mo7O24 (0.05 μm), and CuSO4 (0.3 μm), either without iron or with 40 μm FeEDTA. In some experiments the concentration of FeEDTA was varied as indicated. The nutrient solution was replaced every 7 d and the pH was adjusted to 6.0 with KOH. Plants were harvested 34 d after sowing. Pea seedlings were grown in a similar nutrient solution in the presence of either 40 μm FeEDTA or in the absence of iron. Seeds were germinated for 4 d with tap water in the dark. Iron was added on d 5 after transferring the plants to the nutrient solution. The pH of the nutrient solution was adjusted to 5.5 with KOH every 2nd d. Nutrient solutions were replaced every 7 d. Plants were harvested on d 18.
Fe(III) Chelate Reductase
Spatial localization of Fe(III) reductase was determined by embedding the roots of seedlings in an agar (0.7% [w/v]) medium containing 0.5 mm CaSO4, 0.5 mm FerroZine, and 0.5 mm FeEDTA for 20 min. For electron microscopical analysis, stained root segments were cut off the gel with a razor blade and used for the determination of transfer cell frequency after washing in 0.5 mm CaSO4. Quantitative determination of root reduction activity was performed as described previously (Schmidt, 1994). Reduction activity was measured by following the changes in A562 (absorbance at 562 nm). Reduction rates were calculated using an extinction coefficient of 25,200 m−1 cm−1.
Microscopy
Analysis of root hair patterns was performed by light microscopy in dark field. Photomicrographs were recorded on 100 negative film (Agfa, Lever Kusen, Germany).
Electron Microscopy
Roots were cut into approximately 1-cm-long segments and subsequently washed in 0.5 mm CaSO4. The segments were fixed over night in 0.1 m potassium phosphate buffer (pH 7.4) containing 0.5% (w/v) glutaraldehyde and 1.5% (w/v) paraformaldehyde. After being rinsed three times in 0.1 m potassium phosphate buffer (pH 7.4), the tissue was dehydrated through a graded ethanol series of 20% (v/v) and 40% (v/v) and postfixed in 0.25% (w/v) osmium tetroxide for 2 h in 40% (v/v) ethanol at 4°C. Root segments were washed again in 40% (v/v) ethanol (three times for 10 min) and treated in a solution of 0.3% (w/v) uranyl acetate in 40% (v/v) ethanol for 2 h at 4°C. The material was washed again two times in 40% (v/v) ethanol (10 min each) and once in 50% (v/v) ethanol for 10 min. The samples were then dehydrated in an ethanol series of 75% (v/v) and 90% (v/v) and two times at 100% (v/v) for 30 min each, infiltrated with London Resin White (London Resin Co. Ltd., London), and polymerized at 50°C for 24 h in vacuo. Ultrathin sections were cut with an Ultracut E microtome (Reichert, Vienna, Austria) and stained with uranyl acetate and lead citrate. Sections used for electron microscopy were examined in an electron microscope (EM 902A, Zeiss, Jena, Germany).
For estimating transfer cell frequency, an average of about 800 epidermal cells from 20 root segments per growth type (from eight plants on average) was analyzed.
ACKNOWLEDGMENTS
We thank Michael A. Grusak (Children's Nutrition Research Center), Udo W. Stephan (Institute of Plant Genetics and Crop Research), and the Arabidopsis Biological Resource Center for kindly providing the mutants used in this work. We also thank Professor Wolfgang Eber (University of Oldenburg) who allowed us to use the microscopes in his laboratory.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft.
LITERATURE CITED
- Aisen P, Wessling-Resnick M, Leibold EA. Iron metabolism. Curr Opin Chem Biol. 1999;3:200–206. doi: 10.1016/S1367-5931(99)80033-7. [DOI] [PubMed] [Google Scholar]
- Bates TR, Lynch JP. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996;19:529–538. [Google Scholar]
- Becker R, Fritz E, Manteuffel R. Subcellular localization and characterization of excessive iron in the nicotianamine-less tomato mutant chloronerva. Plant Physiol. 1995;108:269–275. doi: 10.1104/pp.108.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Manteuffel R, Neumann D, Scholz G. Excessive iron accumulation in the pea mutants dgl and brz: subcellular localization of iron and ferritin. Planta. 1998;207:217–223. [Google Scholar]
- Bienfait HF. Proteins under the control of the gene for Fe efficiency in tomato. Plant Physiol. 1988;88:785–787. doi: 10.1104/pp.88.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienfait HF, de Weger LA, Kramer D. Control of the development of iron-efficiency reactions in potato as a response to iron deficiency is located in the roots. Plant Physiol. 1987;83:244–247. doi: 10.1104/pp.83.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché-Pillon S, Fleurat-Lessard P, Serrano R, Bonnemain JL. Asymmetric distribution of the plasma-membrane H+-ATPase in embryos of Vicia faba L. with special reference to transfer cells. Planta. 1994;193:392–397. [Google Scholar]
- Briggs CL. The initiation, development and removal of embryo sac wall ingrowths in the developing seeds of Solanum nigrum L.: an ultrastructural study. Ann Bot. 1995;76:429–439. [Google Scholar]
- Chaney RL, Chen Y, Green CE, Holden MJ, Bell PF, Luster DG, Angle JS. Root hairs on chlorotic tomatoes are an effect of chlorosis rather than part of the adaptive Fe-stress-response. J Plant Nutr. 1992;15:1857–1875. [Google Scholar]
- Delhaize E. A metal-accumulator mutant of Arabidopsis. Plant Physiol. 1996;111:849–855. doi: 10.1104/pp.111.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D, Broderius M, Feit J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein RS, Blemings KP. Iron regulatory proteins, iron responsive elements and iron homeostasis. J Nutr. 1998;128:2295–2298. doi: 10.1093/jn/128.12.2295. [DOI] [PubMed] [Google Scholar]
- Estelle MA, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166:740–754. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- González A, Steffen KL, Lynch JP. Light and excess manganese. Plant Physiol. 1998;118:493–504. doi: 10.1104/pp.118.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk WG. Improvement of the selection value of gene dgl through recombination. Pisum Newsl. 1987;19:9–11. [Google Scholar]
- Grusak MA. Whole-root iron(III)-reductase activity throughout the life cycle of iron-grown Pisum sativum L. (Fabaceae): relevance to the iron nutrition of developing seeds. Planta. 1995;197:111–117. [Google Scholar]
- Grusak MA, Pezeshgi S. Shoot-to-root signal transmission regulates root Fe(III) reductase activity in the dgl mutant of pea. Plant Physiol. 1996;110:329–334. doi: 10.1104/pp.110.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusak MA, Welch RM, Kochian LV. Physiological characterization of a single-gene mutant of Pisum sativum exhibiting excess iron accumulation: I. Root iron reduction and iron uptake. Plant Physiol. 1990;93:976–981. doi: 10.1104/pp.93.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiol. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES, Pate JS. “Transfer cells”: plant cells with wall ingrowths, specialized in relation to short distance transport of solutes: their occurrence, structure, and development. Protoplasma. 1969;68:107–133. [Google Scholar]
- Gunning BES, Pate JS, Green LW. Transfer cells in the vascular system of stems: taxonomy, association with nodes, and structure. Protoplasma. 1970;71:147–171. [Google Scholar]
- Heide-Jørgensen HS, Kuijt J. Epidermal derivatives as xylem elements and transfer cells: a study of the host-parasite interface in two species of Triphysaria (scrophulariaceae) Protoplasma. 1993;174:173–183. [Google Scholar]
- Herbik A, Koch G, Mock HP, Dushkov D, Czihal A, Thielmann J, Stephan UW, Bäumlein H. Isolation, characterization and cDNA cloning of nicotianamine synthase from barley: a key enzyme for iron homeostasis in plants. Eur J Biochem. 1999;265:231–239. doi: 10.1046/j.1432-1327.1999.00717.x. [DOI] [PubMed] [Google Scholar]
- Kneen BE, LaRue TA, Welch RM, Weeden NF. Pleiotropic effects of brz: a mutation in Pisum sativum (L.) cv “Sparkle” conditioning decreased nodulation and increased iron uptake and leaf necrosis. Plant Physiol. 1990;93:717–722. doi: 10.1104/pp.93.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg EC. Transfer cell formation in the root epidermis: a prerequisite for Fe-efficiency? J Plant Nutr. 1982;5:415–432. [Google Scholar]
- Landsberg EC. Function of rhizodermal transfer cells in the Fe stress response mechanism of Capsicum annuum L. Plant Physiol. 1986;82:511–517. doi: 10.1104/pp.82.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg EC. Hormonal regulation of iron-stress response in sunflower roots: a morphological and cytological investigation. Protoplasma. 1996;194:69–80. [Google Scholar]
- Liu DH, Adler K, Stephan UW. Iron-containing particles accumulate in organelles and vacuoles of leaf and root cells in the nicotianamine-free tomato chloronerva. Protoplasma. 1998;201:213–220. [Google Scholar]
- Marschner H, Römheld V. Strategies of plants for acquisition of iron. Plant Soil. 1994;168:261–274. [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks DM, Schiefelbein JW. The homeobox gene GLABRA2 is required for position dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell. 1996;8:1505–1517. doi: 10.1105/tpc.8.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R, Fieuw S, Patric JW. Sugar uptake by the dermal transfer cells of developing cotyledons of Vicia faba L. Planta. 1996;198:502–509. doi: 10.1007/BF00262635. [DOI] [PubMed] [Google Scholar]
- Moog PR, Brüggemann W. Iron reductase systems on the plant plasma membrane: a review. Plant Soil. 1994;165:241–260. [Google Scholar]
- Moog PR, van der Kooij TAW, Brüggemann W, Schiefelbein JW, Kuiper PJC. Responses to iron deficiency in Arabidopsis thaliana: the turbo iron reductase does not depend on the formation of root hairs and transfer cells. Planta. 1995;195:505–513. doi: 10.1007/BF00195707. [DOI] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL. Geochem-PC: a chemical speciation program for IBM-compatibles. In: Loeppert RH, Schwab AP, Goldberg S, editors. Chemical Equilibrium and Reaction Models. Madison, WI: Soil Science Society of America; 1995. pp. 253–269. [Google Scholar]
- Pate JS, Gunning BES. Transfer cells. Annu Rev Plant Physiol. 1972;23:173–196. [Google Scholar]
- Renault S, Despeghel-Caussin C, Bonnemain J-L, Delrot S. The proton electrochemical transmembrane gradients generated by the transfer cells of the haustorium of Polytrichum formosum and their use in the uptake of amino acids. Plant Physiol. 1989;90:913–920. doi: 10.1104/pp.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Conolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Römheld V, Kramer D. Relationship between proton efflux and rhizodermal transfer cells induced by iron deficiency. Z Pflanzenphysiol. 1983;113:73–83. [Google Scholar]
- Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986;80:175–180. doi: 10.1104/pp.80.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. Efects of various inhibitors on in vivo reduction by Plantago lanceolata L. roots. Plant Soil. 1994;165:207–212. [Google Scholar]
- Schmidt W. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol. 1999;141:1–26. [Google Scholar]
- Schmidt W, Bartels M. Formation of root epidermal transfer cells in Plantago. Plant Physiol. 1996;110:217–225. doi: 10.1104/pp.110.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Boomgaarden B, Ahrens V. Reduction of root iron in Plantago lanceolata during recovery from Fe deficiency. Physiol Plant. 1996;98:587–593. [Google Scholar]
- Schmidt W, Tittel J, Schikora A. Role of hormones in the induction of Fe deficiency responses in Arabidopsis roots. Plant Physiol. 2000;122:1109–1118. doi: 10.1104/pp.122.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz G, Becker R, Pich A, Stephan UW. Nicotianamine: a common constituent of strategies I and II of iron acquisition by plants: a review. J Plant Nutr. 1992;15:1647–1665. [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J. 1995;8:943–948. doi: 10.1046/j.1365-313x.1995.8060943.x. [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science. 1997;277:1113–1116. doi: 10.1126/science.277.5329.1113. [DOI] [PubMed] [Google Scholar]
- Wimmers LE, Turgeon R. Transfer cells and solute uptake in minor veins of Pisum sativum leaves. Planta. 1991;186:2–12. doi: 10.1007/BF00201491. [DOI] [PubMed] [Google Scholar]