Abstract
We investigated the antibacterial activity of the antimicrobial peptides h-Lf1-11, MSI-78, LL-37, fengycin 2B, and magainin-2. The minimum inhibitory concentration (MIC) was determined by microdilution technique according to CLSI (M07-A9, 2012) against Escherichia coli, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, carbapenem-resistant Klebsiella pneumoniae, and Acinetobacter baumannii. The MSI-78 showed potent bactericidal activity with MIC range of 1.25–40 mg/L against all bacterial strains. The h-Lf1-11, magainin-2, and LL-37 exhibited moderate activity (MIC range of 40–160, 80–160, and 40–160 mg/L, respectively) while the fengycin 2B did not show significant activity against all bacterial strains tested. These results revealed that MSI-78, h-Lf1-11, magainin-2, and LL-37 have great potential as antibacterial agents and their activity deserves to be more explored in further studies for the treatment of antibiotic-resistant bacteria.
Keywords: Antimicrobial peptides, Pexiganan, Antibiotic resistance, MSI-78, Antibacterial activity
Introduction
Bacterial resistance to antibiotics is a growing problem, present in all countries and which is being discussed worldwide [1, 2]. In Brazil, according to data from Brazilian Health Regulatory Agency (ANVISA), about 25% of registered infections are caused by multi-resistant microorganisms, those that become insusceptible to the action of available antibiotics [2, 3].
The antibiotic resistance crisis has been attributed to several facts, including the indiscriminate use of this type of medicine, as well as the difficulties faced by the pharmaceutical industry in the development of new drugs. In this scenario, the antimicrobial peptides (AMPs) have been tested as alternatives to conventional antibiotics [4].
AMPs are low molecular mass molecules with inhibitory activity against bacteria, viruses, and fungi [5–7]. They belong to a diverse and abundant group of molecules that are produced by cells, both in plants and in animals, as the first line of defense against microorganism attacks. They are grouped according to their intrinsic antimicrobial activity [4, 8].
A common feature of these peptides is the presence of (4, 6, or 8) cysteines, interconnected by disulfide bonds, that have high stability [9]. Its composition of amino acids, amphipathicity, cationic charge, and size cause these peptides to readily enter lipid membranes killing the target microorganism [8, 10, 11].
It has been shown that the molecular basis of the mechanisms of action by which the AMPs action against the pathogen is mainly the differences in membrane fluidity and lipid composition that interfere in the association of peptides antimicrobials to the target membranes, which may lead to their rupture [12]. A positive aspect of the use of peptides when compared to antibiotics is the interaction of AMPs with the bacterial cell membranes that occurs through the charge neutralization and shortly afterwards penetration, reducing the possibility of bacterial drug resistance. In the present study, we investigate the in vitro antimicrobial activity of different AMPs against antibiotic-resistant and sensitive bacterial strains.
Materials and methods
Antimicrobial peptides
The peptides used in this study were purchased from the Chinese company GL Biochem Ltd. (Shanghai, China) in powder form. Five different AMPs were acquired and evaluated for antimicrobial activity: h-LF1-11, MSI-78 LL-37, fengycin 2B, and magainin-2. These AMPs were selected because they have already demonstrated antimicrobial activities against different microorganisms, including bacteria, fungi, and virus [5–7]. To perform the susceptibility tests, these compounds were solubilized in sterile ultrapure water and stored at − 20° C under dark conditions per the manufacturer’s instructions. At the time of use, the working solutions were prepared in Mueller Hinton broth (MHB) (Merck, Brazil), and the concentration range tested was 0.008 to 160.00 mg /L for each of the AMP. Ampicillin (Merck, Brazil) (0.78 to 100 mg/L) was utilized as quality control on the tests.
Microorganisms
Bacterial strains recovered from diverse clinical specimens, belong to our private collection (Mycological Research Laboratory of the Federal University of Santa Maria, Brazil), were evaluated: Escherichia coli (n = 5), methicillin-resistant Staphylococcus aureus (n = 10), Pseudomonas aeruginosa (n = 3), carbapenem-resistant Klebsiella pneumoniae (n = 3), Acinetobacter baumannii (n = 2). A control strain of S. aureus ATCC 29,213 was included as quality control. All strains were identified by the fully automated microbial identification system VITEK® 2 Compact (BioMérieux, Brazil). They were stored in 10% of glycerol solution at − 80 °C and were subcultured in Mueller Hinton agar (MHA) for 24 h at 37° C for the susceptibility tests.
Antimicrobial activity tests
For the evaluation of AMP activity, the minimum inhibitory concentration (MIC) was determined by broth microdilution according to the methodology described in document M07-A9 of the Clinical and Laboratory Standards Institute (CLSI, 2012) [13], supplemented with the protocol suggested by Wiegand et al. (2008) [14] for test antimicrobial peptides, using polypropylene microtiter plates. Bacterial suspensions were standardized from a 24-h culture grown on MHA. Some colonies were then collected and resuspended in sterile PBS (phosphate-saline buffer) until they reached turbidity of the 0.5 McFarland standard tube (~ 1.0 × 108 CFU / mL), spectrophotometer reading at 625 nm. Then, a 1:100 dilution in MHB was performed to obtain a suspension of 1.0 × 106 CFU / mL, which was used in the assays.
For microplate preparation, 50 μL of each dilution of the AMPs was distributed. Also, 50 μL of the bacterial suspensions was distributed in each well, except in the sterility control. The final bacterial concentration on the plate was 1.0 × 105 CFU / mL. Sterility and growth controls will be performed on all tests. The plates were incubated at 37° C for 16 to 20 h. The readings were performed on a microtiter plate reader (620 nm) after 30 min of incubation with 50 μL of 0.1% TTC (2,3,5-triphenyltetrazolium chloride) (Merck, Brazil). The MIC was considered the concentration resulting in 90% growth inhibition compared to the positive control.
To determine the bactericidal activity, a 10-μL aliquot was collected from the wells where there was no detectable growth in the microdilution plates and seeded in Petri dishes containing MHA. MBC (minimum bactericidal concentration) was considered in which there was no bacterial growth after 24 h at 37 °C of incubation. All experiments were performed in triplicates to guarantee test reproducibility.
Statistical analyses
The statistical analysis was performed using BioEstat 5.3 (Mamirauá Institute, Brazil) using one-way ANOVA with post hoc Tukey to compare the MIC means of different AMPs and microorganisms tested. The differences between the MIC means were considered significant if p ≤ 0.05. For results above 160 mg / L, to facilitate statistical analysis, a concentration of 320 mg / L was assigned.
Results
The antibacterial activity of all AMPs is reported in Table 1. MSI-78 differed statistically from all other AMPs presenting the smallest MICs (MIC range of 1.25–40 mg/L), the magainin-2, h-LF1-11, and LL-37 exhibited moderate activity (MIC range of 40–160, 80–160, and 40–160 mg/L, respectively) and did not differ in antibacterial activity, while the fengycin 2B did not inhibit bacterial growth even at the highest concentrations tested and differed significantly from all other AMPs (Table 1, Fig. 1).
Table 1.
Minimal inhibitory (MIC) (and bactericidal, MBC) concentration geometric mean (mg/L) of antimicrobial peptides h-LF1-11, MSI-78, LL-37, fengycin 2B, and magainin-2 against gram-positive and gram-negative pathogenic bacteria
| Bacterial strains | h-LF1-11 | MSI-78 | LL-37 | Fengycin 2B | Magainin-2 |
|---|---|---|---|---|---|
| E. coli 01 |
160 (> 160) |
5 (10) |
80 (80) |
> 160 (> 160) |
80 (> 160) |
| E. coli 02 |
80 (> 160) |
2,5 (2,5) |
80 (80) |
> 160 (> 160) |
160 (> 160) |
| E. coli 05 |
160 (> 160) |
5 (5) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| E coli 06 |
160 (> 160) |
10 (10) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| E. coli 09 |
80 (> 160) |
2,5 (5) |
80 (80) |
> 160 (> 160) |
80 (> 160) |
| MIC mean |
121.25 (> 160) |
4.35 (5.74) |
105.56 (138.28) |
> 160 (> 160) |
121.25 (> 160) |
| S. aureus ATCC 29,213 |
160 (> 160) |
20 (20) |
80 (80) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R1 |
160 (> 160) |
40 (40) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R2 |
160 (> 160) |
40 (80) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R3 |
160 (> 160) |
2,5 (5) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R4 |
160 (> 160) |
40 (80) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R5 |
160 (> 160) |
40 (80) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R6 |
160 (> 160) |
10 (20) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R7 |
160 (> 160) |
10 (20 |
80 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R8 |
160 (> 160) |
40 (80) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R9 |
160 (> 160) |
20 (20) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| S. aureus R10 |
160 (> 160) |
40 (80) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| MIC mean |
160 (> 160) |
21.30 (35.26) |
141.05 (282.11) |
> 160 (> 160) |
160 (> 160) |
| P. aeruginosa 01 |
160 (> 160) |
2,5 (10) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| P. aeruginosa 02 |
160 (> 160) |
5 (10) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| P. aeruginosa 03 |
160 (> 160) |
5 (10) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| MIC mean |
160 (> 160) |
3.96 (3.96) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| A. baumannii 01 |
40 (> 160) |
5 (10) |
160 (> 160) |
> 160 (> 160) |
40 (> 160) |
| A. baumannii 02 |
40 (> 160) |
5 (10) |
160 (> 160) |
> 160 (> 160) |
80 (> 160) |
| MIC mean |
40 (> 160) |
5 (10) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| K. pneumoniae 01 |
160 (> 160) |
5 (5) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| K. pneumoniae 02 |
160 (> 160) |
1,25 (1,25) |
80 (> 160) |
> 160 (> 160) |
160 (> 160) |
| K. pneumoniae 03 |
160 (> 160) |
1,25 (1,25) |
160 (> 160) |
> 160 (> 160) |
160 (> 160) |
| MIC mean |
160 (> 160) |
1.98 (1.98) |
126.99 (> 160) |
> 160 (> 160) |
160 (> 160) |
MIC mean, geometric mean of minimal inhibitory concentration of all strains; E. coli, Escherichia coli; S. aureus, Staphylococcus aureus – methicillin resistant; P. aeruginosa, Pseudomonas aeruginosa; A. baumannii, Acinetobacter baumannii; K. pneumoniae, Klebsiella pneumoniae -carbapenem resistant
Fig. 1.
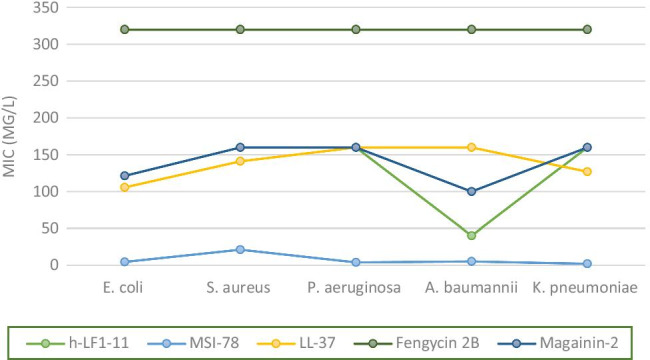
Minimum inhibitory concentration (MIC) means of antimicrobial peptides (h-LF1-11, MSI-78, LL-37, fengycin 2B, and magainin-2) compounds against gram-negative and gram-positive bacteria (mg/L). E. coli, Escherichia coli; S. aureus, Staphylococcus aureus – methicillin resistant; P. aeruginosa, Pseudomonas aeruginosa; A. baumannii, Acinetobacter baumannii; K. pneumoniae, Klebsiella pneumoniae -carbapenem resistant
The AMP MSI-78 peptide stood out for its pronounced activity against carbapenem-resistant K. pneumoniae strains (MIC and MBC geometric mean (GM) = 1.98 mg / L). This AMP also showed excellent activity against the other gram-negative bacteria tested, such as P. aeruginosa (MIC and MBC(GM) = 3.96 mg / L), E. coli (MIC(GM) = 4.35; MBC(GM) = 5.74 mg / L), and A. baumannii (MIC(GM) = 5; MBC(GM) = 10 mg/L), while against S. aureus the MSI-78 was shown to be less active than for gram-negative bacteria (MIC(GM) = 21.30; MBC(GM) = 35.26 mg / L), although it remains the most significant AMP activity against all bacterial strains evaluated in this study (Table 1, Fig. 1).
The antibacterial activities of magainin-2 and h-Lf1-11 were similar; both showed MICs ranging from 80 to 160 for most of the bacteria evaluated and showed more pronounced activity against A. baumannii (h-Lf1-11 showed MICs of 40 mg / L for both strains tested, while magainin-2 showed MICs of 40 and 80 mg / L). LL-37, despite also presenting MIC averages like the two AMPs mentioned above, did not show to be more active against A. baumannii compared to the other bacteria tested (Table 1, Fig. 1).
When evaluating the bactericidal activity of AMPs, it was observed that MSI-78, which obtained the most significant activity in inhibiting bacterial growth, was also capable of causing the death of the tested bacterial strains at concentrations equal to or up to two times higher than those that inhibited growth. The AMPs LL-37 and h-Lf1-11 also showed bactericidal activity, however, most of them at the highest tested concentration of 160 mg / L while magainin-2 and fengycin 2B showed no bactericidal activity (Table 1, Fig. 2).
Fig. 2.
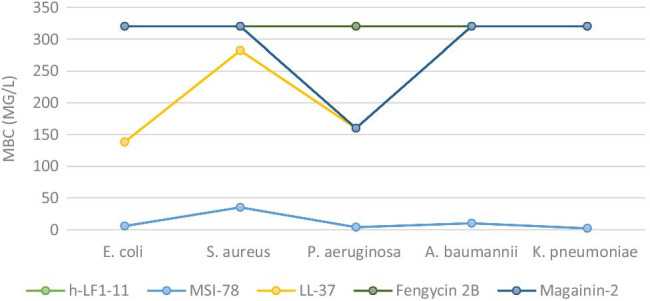
Minimum bactericidal concentration (MBC) mean of antimicrobial peptides (h-LF1-11, MSI-38, LL-37, fengycin 2B, and magainin-2) compounds against gram-negative and gram-positive bacteria (mg/L). E. coli, Escherichia coli; S. aureus, Staphylococcus aureus – methicillin resistant; P. aeruginosa, Pseudomonas aeruginosa; A. baumannii, Acinetobacter baumannii; K. pneumoniae, Klebsiella pneumoniae -carbapenem resistant
Discussion
Peptides with potent antimicrobial activity are part of the first line of defense against pathogens from a wide range of species, including vertebrates and invertebrates. These compounds are extracted from different types of plants, animals, insects, and many others that have been synthesized to obtain a more pronounced antimicrobial activity and lower cytotoxicity. In recent years, studies on its structure, function, and antimicrobial activity have increased and shown that these agents have great potential for clinical use [4].
Magainins are a group of peptides with broad-spectrum antimicrobial activity isolated from African clawed frog (Xenopus laevis) skin. Magainin-2, an α-helical peptide, has been found to form a toroidal pore in the cell membranes. Its presence in the frog’s skin suggests that this peptide acts as the first line of defense against pathogens; however, it has already been observed that magainin-2 acts together with other AMPs derived from frog’s skin, as magainin-1, caerulein-precursor fragment (CPF), and peptide glycine-leucine-amide (PGLa) having a synergistic effect against microorganisms [15, 16].
In this study, we consider that magainin-2 had moderate antimicrobial activity and had no bactericidal effect, which could be explained by the need for joint action with other AMPs in the same family to obtain a maximum antimicrobial effect. Previous studies report magainin-2 MICs ranging from 32 to 128 mg / L for S. aureus and P. aeruginosa, and 1–4 mg / L for E. coli, results that correspond to those obtained here, although the MICs found for E. coli are slightly higher [17].
The MSI-78, also named pexiganan, is a synthetic analog of maganin-2. Currently, a topical formulation of pexiganan (0.8%) is in clinical trials for the treatment of infection associated with diabetic foot ulcers (ClinicalTrials.gov registration numbers NCT01594762/NCT01590758). This AMP was found to have broad-spectrum antimicrobial activity, and to increase the antimicrobial activity of magainin-2 some researchers have made modifications to the molecule, such as Gly to Ala substitution, which was made to increase the stability of the α-helical structure; other systematic single amino acid substitutions were performed, affording the MSI-78 with enhanced antimicrobial activity [18]. It meets the results obtained in this study, in which magainin-2 showed less or no activity against the tested bacteria; however, MSI-78 showed excellent activity against all of them.
The bactericidal action of MSI-78 is thought to result from irreversible membrane-disruptive damage. Due to their effect on the bacterial membrane, AMPs have fast action and infrequent resistance development [19]. The potential toxicity of MSI-78 has been established by measuring the hemolytic activity that showed at least 250 mg/L is necessary to induce 100% hemolysis in human red blood cells [20], which guarantees its safety profile since the highest concentration that killed all bacteria tested was 80 mg / L.
We also highlight the great action of MSI-78 against the strains of K. pneumoniae carbapenem-resistant; these Enterobacterales constitute a serious threat to public health that has emerged in several countries causing several types of life-threatening infections, including pneumonia, urinary tract infections, bloodstream, wound, or surgical site infections, and is associated with high mortality rates [21]; thus, the finding of possible treatment alternatives is very welcome.
Likewise, the bactericidal activity against A. baumannii also deserves to be highlighted; this Gram-negative bacterium is frequently a multidrug-resistant agent that causes complicated infections, mainly in immunosuppressed patients on intensive care units [22]. In a previous study, it was demonstrated that MSI-78, in addition to causing the death of A. baumannii planktonic cells (MIC = 4 mg / L), is also capable of inhibiting biofilms formed by this pathogen in higher concentrations (256 mg / L) [23].
LL-37 belongs to the cathelicidin family of peptides; it is a cationic, amphipathic α-helical peptide that has a 37 amino acids sequence [24]. It is produced by different defense cells such as neutrophils, macrophages, and natural killer lymphocytes, as well as skin and intestine cells. The LL-37 has already shown to have an important role not only in the direct destruction of microorganisms interacting with the phospholipids of bacterial membranes resulting in segregation of its hydrophobic residues but also in the modulation of inflammation [25].
Although other authors have already described the broad antimicrobial action of LL-37 [26, 27], in this study this AMP did not show pronounced activity against the tested bacteria, inhibiting the growth of E. coli, K. pneumoniae, and S. aureus at concentrations close to 100 mg / L. It is noteworthy that the highest concentration tested in this study was 160 mg / L, and that MICs described in other studies reach 224 mg /L [26] indicating that a higher concentration of LL-37 may be required at the site of action for it to exert its antimicrobial activity directly.
On the other hand, the LL-37 has been successfully used to treat autoimmune disorders such as psoriasis and atopic dermatitis, indicating that they may be more effective as an immunomodulator than directly acting against microorganisms like bacteria. It has been shown that LL-37 modulates the TLR function, it can be considered an anti-inflammatory effect, and LL-37 also modulates type I IFN responses and regulates the cell death through inhibition of the caspase-3 and caspase-8 and the apoptosis in neutrophils [28].
The h-Lf1-11 is a human lactoferrin-derived peptide. It has also an α-helical conformation and hydrophobic and cationic characteristics. The h-Lf1-11 increases the production of cytokines and chemokines by monocytes in response to stimulation by microorganisms [29]. This AMP has been shown to have antibacterial and antifungal action, being able to inhibit the growth of multidrug-resistant strains of A. baumannii [30] and methicillin-resistant S. aureus [31], as well as Candida species strains [32]. The antimicrobial activity has been attributed to the two first arginines at the N-terminus of human lactoferrin [33].
In accordance with the study of Dijkshoorn (2005) [30], the most pronounced effect of h-Lf1-11 on bacterial inhibition was against A. baumannii (MIC = 40 mg / L), whereas against strains of methicillin-resistant S. aureus and other bacteria evaluated, h-Lf1-11 inhibited growth only at the highest concentration (160 mg/L). The h-LF1-11 despite inhibiting growth showed no bactericidal activity (> 160 mg /L). It is speculated that the antimicrobial action mechanism of this AMP is mitochondrial damage, with the extracellular ATP being essential but not enough for the microbicidal activity [32].
Fengycin 2B is a biologically active lipopeptide produced by several Bacillus subtilis strains. The structure is composed of a β-hydroxy fatty acid linked to a peptide part comprising 10 amino acids, where 8 of them are organized in a cyclic structure [34]. Like most natural antimicrobial peptides, fengycin likely acts by making the plasma membrane of the target cell more permeable. The molecular mechanism underlying this membrane perturbation is not yet fully understood [35]. Besides, this lipopeptide is known to develop antifungal activity against filamentous fungi; it showed no antibacterial activity.
The mechanism of action against filamentous fungi is based on the interaction of fengycin 2B with specific components of fungal membrane, as ergosterol and phospholipids, leading to their destabilization [36]. We can speculate that there is possibly no affinity between the fengycin 2B molecule and the components of the bacterial membrane, which would explain the lack of activity.
Conclusions
In conclusion, the MSI-78 has been shown to exhibit broad-spectrum antibacterial activity and acts with a bactericidal mechanism against which the likelihood of the development of resistance may be low. AMPs LL-37, magainin-2, and h-Lf1-11 have also been shown to inhibit bacterial growth at higher doses, which suggests that these AMPs deserve further evaluation considering their toxicity. Finally, likely fengycin 2 B is not useful as an antibacterial agent. Our study has limitations that warrant further study, mainly due to the limited number of bacteria strains. Further studies evaluating a large number of isolates, on action mechanism, toxicity, and murine infections models, should be made to consider these compounds as candidates for antibacterial agents.
Acknowledgements
Denardi LB gives thanks as a Post-Doctoral fellow of the CNPq (PDJ -150752/2018-0).
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq; process 406170/2018–5).
Declarations
Ethic approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Jorge Luiz Mello Sampaio
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lushniak BD. Antibiotic resistance: a public health crisis. Public Health Rep. 2014;129:314–316. doi: 10.1177/003335491412900402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant, and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2011;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.ANVISA: Patient Safety and Quality in Health Services Bulletin nº 14, 2016. Accessed in https://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/item/boletim-de-seguranca-do-paciente-e-qualidade-em-servicos-de-saude-n-13-avaliacao-dos-indicadores-nacionais-das-infeccoes-relacionadas-a-assistencia-a-saude-iras-e-resistencia-microbiana-do-ano-de-2015?category_id=25
- 4.Lei J, Sun L, Huang S, et al. The antimicrobial peptides and their potential clinical applications. Am J Transl Res. 2019;11:3919–3931. [PMC free article] [PubMed] [Google Scholar]
- 5.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 6.Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals (Basel) 2013;6:1543–1575. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirski T, Niemcewicz M, Bartoszcze M, Gryko R, Michalski A. Utilization of peptides against microbial infections - a review. Ann Agric Environ Med. 2017;25:205–210. doi: 10.26444/aaem/74471. [DOI] [PubMed] [Google Scholar]
- 8.Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 9.Nawrot R, Barylski J, Nowicki G. Plant antimicrobial peptides. Folia Microbiol (Praha) 2014;59:181–196. doi: 10.1007/s12223-013-0280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 11.Izadpanah A, Gallo RL. Antimicrobial peptides. J Am Acad Dermatol. 2005;52:381–390. doi: 10.1016/j.jaad.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Feder R, Nehushtai R, Mor A. Affinity driven molecular transfer from erythrocyte membrane to target cells. Peptides. 2001;22:1683–1690. doi: 10.1016/s0196-9781(01)00504-6. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute (CLSI) (2012) Methods for dilution antimicrobial susceptibility tests f or bacteria that grow aerobically; approved standard–Ninth Edition
- 14.Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 15.McMillan KAM, Coombs MRP. Review: Examining the natural role of amphibian antimicrobial peptide magainin. Molecules. 2020;20(25):5436. doi: 10.3390/molecules25225436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aisenbrey C, Amaro M, Pospíšil P, Hof M, Bechinger B. Highly synergistic antimicrobial activity of magainin 2 and PGLa peptides is rooted in the formation of supramolecular complexes with lipids. Sci Rep. 2020;15(10):11652. doi: 10.1038/s41598-020-68416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacometti A, Cirioni O, Barchiesi F, et al. In vitro susceptibility tests for cationic peptides: comparison of broth microdilution methods for bacteria that grow aerobically. Antimicrob Agents Chemother. 2000;44:1694–1696. doi: 10.1128/aac.44.6.1694-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottler LM, Ramamoorthy A. Structure, membrane orientation, mechanism, and function of pexiganan-a highly potent antimicrobial peptide designed from magainin. Biochim Biophys Acta. 2009;1788:1680–1686. doi: 10.1016/j.bbamem.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clifton LA, Skoda MW, LeBrun AP, et al. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir. 2015;31:404–412. doi: 10.1021/la504407v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eren T, Som A, Rennie JR, et al. Antibacterial and hemolytic activities of quaternary pyridinium functionalized polynorbornenes. Macromol Chem Phy. 2008;209:516–524. doi: 10.1002/macp.200700418. [DOI] [Google Scholar]
- 21.Spagnolo AM, Orlando P, Panatto D, Perdelli F, Cristina ML. An overview of carbapenem-resistant Klebsiella pneumoniae: epidemiology and control measures. Rev Med Microbiol. 2014;25:7–14. doi: 10.1097/MRM.0b013e328365c51e. [DOI] [Google Scholar]
- 22.Vázquez-López R, Solano-Gálvez SG, Juárez Vignon-Whaley JJ, et al. Acinetobacter baumannii resistance: a real challenge for clinicians. Antibiotics (Basel) 2020;23(9):205. doi: 10.3390/antibiotics9040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaśkiewicz M, Neubauer D, Kazor K, et al. Antimicrobial activity of selected antimicrobial peptides against planktonic culture and biofilm of Acinetobacter baumannii. Probiotics Antimicrob Prot. 2019;11:317–324. doi: 10.1007/s12602-018-9444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 25.Bucki R, Janmey PA. Interaction of the gelsolin-derived antibacterial PBP 10 peptide with lipid bilayers and cell membranes. Antimicrob Agents Chemother. 2006;50:2932–2940. doi: 10.1128/AAC.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leszczyńska K, Namiot D, Byfield FJ, et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J Antimicrob Chemother. 2013;68:610–618. doi: 10.1093/jac/dks434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duplantier AJ, van Hoek ML. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front Immunol. 2013;4:143. doi: 10.3389/fimmu.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol. 2013;191:4895–4901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Does AM, Bogaards SJ, Jonk L, et al. The human lactoferrin-derived peptide hLF1-11 primesmonocytes for an enhanced TLR-mediated immune response. Biometals. 2010;23:493–550. doi: 10.1007/s10534-010-9322-4. [DOI] [PubMed] [Google Scholar]
- 30.Dijkshoorn L, Brouwer CPJM, Bogaards SJP, et al. The synthetic n-terminal peptide of human lactoferrin, hLF(1–11), is highly effective against experimental infection caused by multidrug- resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2004;48:4919–4921. doi: 10.1007/s10534-010-9322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stallmann HP, Faber C, Bronckers AL, et al. Histatin and lactoferrin derived peptides: antimicrobial properties and effects on mammalian cells. Peptides. 2005;26:2355–2359. doi: 10.1007/s10534-010-9322-4. [DOI] [PubMed] [Google Scholar]
- 32.Lupetti A, Paulusma-Annema A, Welling MM. Synergistic activity of the N-terminal peptide of human lactoferrin and fluconazole against Candida species. Antimicrob Agents Chemother. 2003;47:262–267. doi: 10.1128/aac.47.1.262-267.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupetti A, Paulusma-Annema A, Welling MM, et al. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob Agents Chemother. 2000;44:3257–3263. doi: 10.1128/aac.44.12.3257-3263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanittanakom N, Loeffler W, Koch U, Jung G. Fengycin—a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot. 1986;39:888–901. doi: 10.7164/antibiotics.39.888. [DOI] [PubMed] [Google Scholar]
- 35.Deleu M, Paquot M, Nylander T. Fengycin interaction with lipid monolayers at the air-aqueous interface—implications for the effect of fengycin on biological membranes. J Colloid Interface Sci. 2005;283:358–365. doi: 10.1016/j.jcis.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 36.Tang Q, Bie X, Lu Z, et al. Effects of fengycin from Bacillus subtilis fmbj on apoptosis and necrosis in Rhizopus stolonifera. J Microbiol. 2014;52:675–680. doi: 10.1007/s12275-014-3605-3. [DOI] [PubMed] [Google Scholar]


