Abstract
Introduction
Onychomycosis is a chronic fungal infection with increasing incidence and the global prevalence is estimated to be 5.5%. The aim of our study was to perceive objectively severity of onychomycosis by calculating Scoring Clinical Index for Onychomycosis and to correlate this index with accurate laboratory diagnosis in our patients.
Materials and methods
The study population comprised of 417 patients with laboratory confirmed onychomycosis. For each patient, we recorded basic demographic information, site of infection, the most affected nail with onychomycosis, clinical presentation, and type of onychomycosis. The evaluation of the disease severity was based on Scoring Clinical Index for Onychomycosis which was calculated for every patient separately. Mycological identification was done by microscopy and fungal culture.
Results
The majority of patients had distal and lateral subungual onychomycosis (95.44%) that was localized on big toe (62.59%), with female to male ratio 1.24:1. Male patients had significantly more nails affected with onychomycosis compared with female patients (p = 0.011), while female had significantly more often onychomycosis on fingernails 2–5 (p < 0.05), and they reported significantly more often pain (p < 0.05) and esthetic problems (p < 0.05). Mean Scoring Clinical Index for Onychomycosis was 16.76. Dermatophytes were most frequently isolated (91.85%). In patients with onychomycosis caused by dermatophytes, Scoring Clinical Index for Onychomycosis had significantly higher values (p = 0.032).
Conclusion
Comprehensive understanding of disease characteristics will allow introduction of individualized treatment plan for each patient, based on proper fungal identification and standardized method of evaluating disease severity, which could help the patient achieve a complete cure.
Keywords: Onychomycosis, SCIO index, Laboratory diagnosis, Dermatophytes
Introduction
Onychomycosis is a chronic fungal infection that can be caused by dermatophytes, yeasts, and non-dermatophyte molds [1]. Disease can involve any component of the nail apparatus, which gradually destroys the nail plate, and can serve as a chronic reservoir for fungal infections elsewhere in the body [2]. The worldwide incidence of onychomycosis is increasing and the global prevalence is estimated to be 5.5% [3].
The most common etiological agents of onychomycoses in general population are dermatophytes (80–90%) of the genus Trichophyton with the T. rubrum as the most frequent of them. Yeasts accounts for 2–11% of onychomycoses cases with predominance of Candida albicans [4]. Non-dermatophyte molds are found in 2–10%, and are more frequently recognized in warmer climates [5].
At the beginning, nails affected by onychomycosis are asymptomatic but with disease progression they can cause local pain, difficulties in fitting shoes, and also social embarrassment with negative impact on patient quality of life [6]. While onychomycosis is the most common nail disorder seen in clinical practice and accounts for 50% of nail diseases, other nail conditions may mimic the clinical presentation of onychomycosis including nail trauma, bacterial infections, psoriasis, lichen planus, and malignant neoplasms [7]. Therefore, clinical diagnosis of onychomycosis always requires laboratory confirmation in order to avoid misdiagnosis and treatment delay [8]. Furthermore, determination of fungal viability and the identification of the causative agent of onychomycosis are necessary pretreatment information as the efficacy of systemic and local therapies can be species dependent [3].
The treatment approach for onychomycosis can be determined according to clinical form of onychomycosis by calculating the Scoring Clinical Index for Onychomycosis (SCIO) which enables evaluation of disease severity. Based on the clinical form of disease, depth of the nail involvement, and degree of hyperkeratosis Clinical Index Component is first determined. In addition to Clinical Index Component, SCIO has growth component that is based on the location of the onychomycosis (fingernail or toenail, digit number) and the age of the patient. The growth component reflects approximately the time needed for complete outgrowth of the target nail indicating the amount of required therapy [9]. Calculation of SCIO enables comparison of the severity of onychomycosis between nails despite differences in patients’ clinical presentation and demographics. This mathematical tool takes into account key factors for development of onychomycosis and uses 7-grade system to present the severity of onychomycosis as a composite score from 1 to 30.
The aim of our study was to determine causative agents of onychomycosis and to perceive objectively severity of onychomycosis by calculating SCIO index in patients. In addition, we wanted to correlate SCIO index with accurate laboratory diagnosis in our patients.
Materials and methods
Study population
A total of 841 patients clinically suspected for onychomycosis were referred to the Institute of Microbiology and Immunology, Faculty of Medicine, University of Belgrade from November 2016 until February 2019. The study population included 417 patients with laboratory confirmed onychomycosis. We excluded 22 patients who had used topical or systemic antifungal drugs within the previous 6 months.
All subjects were informed about the aims of the study and were eligible to participate if they provided informed consent. The study was approved by the Institutional Ethics Committee. For each patient, we recorded basic demographic information (age, gender), site of infection (toenails or fingernails), the most affected nail with onychomycosis (big toenail, thumbnail and/or toenails 2–5, fingernails 2–5), and clinical type of onychomycosis. Regarding clinical type all patients were classified in three groups: patients with distal and lateral subungual onychomycosis (DLSO), proximal subungual onychomycosis (PSO), and superficial white onychomycosis (SWO). For each patient, clinical presentation of affected nails was recorded including degree of nail involvement (< 1/3, 1/3–2/3, > 2/3 nail unit involved), degree of hyperkeratosis (absent or < 1 mm, 1–2 mm, > 2 mm), number of abnormal-appearing nails, as well as data relating to nail deformation and presence of pain and esthetic problems. The evaluation of the disease severity was based on SCIO onycho-index which was calculated for every patient separately by SCIO electronic calculator available at http://www.onychoindex.com. Based on calculated SCIO index, patients were divided in three groups regarding disease severity: patients with SCIO index value 1.00–6.00, 06.01–16.00, and 16.01–30.00 as candidates for local, systemic, and combinational therapy, respectively [9].
Sampling
Nail samples were obtained by scrapings/clippings depending on clinical type of onychomycosis. The sample collection was taken separately from each nail, and the material was collected from the most proximal part of the affected nail. First, the nail area was thoroughly cleaned with 70% alcohol. Then, for DLSO, the abnormal nail was clipped proximally to the nail bed and the underside of the nail plate was scraped with a curette. For PSO, samples were taken by scraping around the area of the lunula; and for SWO, the white spots on the nail were scraped. Samples obtained by clipping were cut into smaller pieces before subsequent examination. All samples were collected in sterile containers and labeled for further identification.
Laboratory procedures
Each nail sample was divided into two parts: the first part was used for direct microscopy and the second for fungal culture. Two types of direct microscopic preparations (DMP) were examined: first with 15% potassium hydroxide (KOH) observed under a light microscope and second with fluorescent dye Blankophor (BP) and observed under a fluorescent microscope, both under 10 × and 40 × magnification. The second part of each specimen was cultured on the two mycological media: Sabouraud’s dextrose agar (SDA) (HiMedia, Mumbai, India) and dermatophyte test medium (DTM) (Liofilchem, Roseto degli Abruzzi, Italy). Plates were then incubated for 3 weeks at 28 °C and 37 °C and checked periodically for fungal growth. If fungal growth was observed, the causative agent was identified by colony morphology and microscopic characteristics [10]. Plates with no fungal growth even after 4 weeks of incubation were considered negative.
Cultures yielding dermatophytes were considered positive, whereas, cultures with growth of non-dermatophyte molds or yeast were considered positive, only if the same organism grew on the second culture obtained from the new sample, from the same patient. This procedure was done to reduce the potential of contamination, as those fungi can be present in the environment and in different parts of the human body without causing infection. The identification of dermatophytes and non-dermatophyte molds was performed by macroscopic and microscopic morphological examination of colonies, and by biochemical methods, if required. Yeasts isolates were identified by growth on CHROM agar (HiMedia, Mumbai, India) and with the use of the API 20C AUX Commercial System (BioMerieux, Marcy-L’Etoile, France).
Statistical analysis
A descriptive analysis was used to detail the main characteristics of the study population. Statistical analyses were performed using chi-square and Fisher’s test. The statistical software used was SPSS v15 (SPSS Inc., Chicago, Illinois, USA). P-value ˂0.05 was considered to be statistically significant.
Results
A total of 417 patients with laboratory confirmed onychomycosis were included in study, 186 (44.6%) were male (mean age 57.6 years) and 231 (55.4%) were female patients (mean age 55 years). Female to male ratio in patients with laboratory confirmed onychomycosis was 1.24:1. The most common clinical form was DLSO found in 95.44% patients with laboratory confirmed onychomycosis.
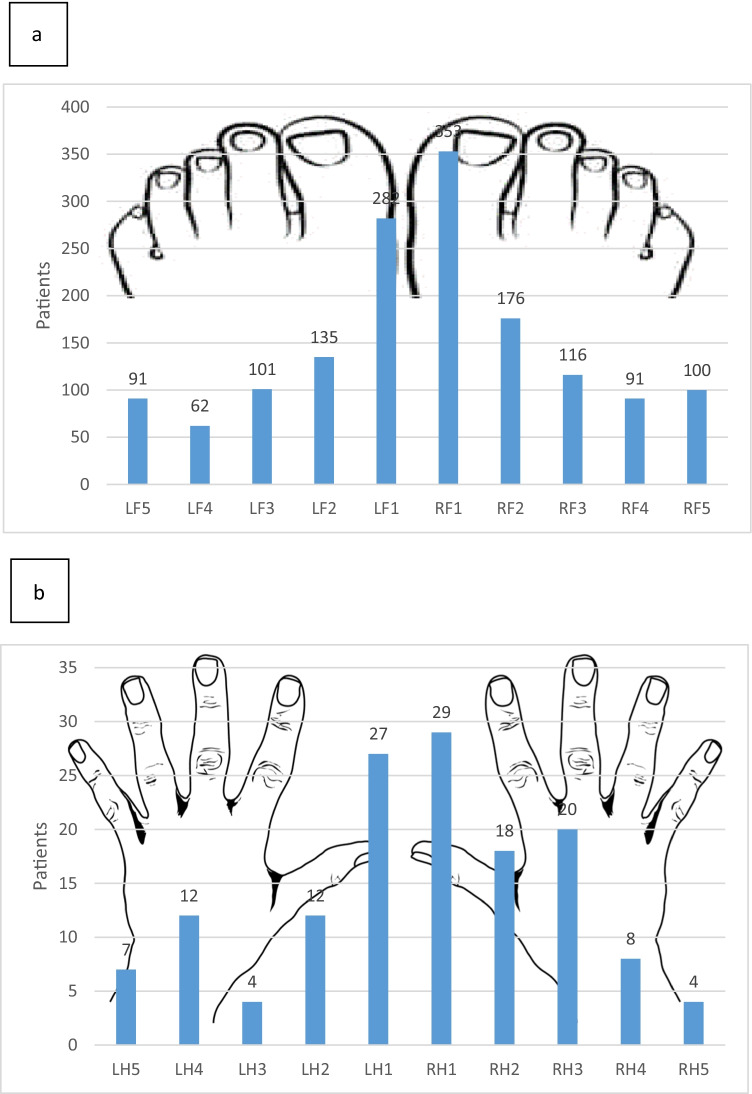
In 375 patients, onychomycosis was localized on toenails, 29 had only onychomycosis on fingernails, while 7 patients had onychomycosis on both localizations. Majority of patients presents with onychomycosis on two nails and on just one nail with 100 and 77 affected patients, respectively. The number of involved nails ranged from one to 20. The most affected nail was big toenail found in 261 (62.59%) patients with onychomycosis (Fig. 1).
Fig. 1.
Localization of toenail (a) and fingernail (b) onychomycosis in 417 patients
Male patients had significantly more nails affected with onychomycosis compared with female patients (p = 0.011). Female patients had significantly more often onychomycosis on fingernails 2–5 (p < 0.05), and they reported significantly more often pain (p < 0.05) and esthetic problems (p < 0.05) associated with onychomycosis compared to male patients. There were no differences in age, SCIO index values, localization of onychomycosis, clinical type of onychomycosis, and presence of nail deformity among male and female patients with onychomycosis (Table 1).
Table 1.
Distribution of different patients’ characteristics by sex in 417 patients with onychomycosis
| Characteristics | Male, n = 186 No. (%) |
Female, n = 231 No. (%) |
p value* |
|---|---|---|---|
| Age | 0.552 | ||
| < 20 | 4 (2.2) | 8 (3.5) | |
| 20–40 | 29 (15.6) | 46 (19.9) | |
| 41–60 | 82 (44.1) | 95 (41.1) | |
| > 60 | 71 (38.2) | 82 (35.5) | |
| SCIO index | 0.808 | ||
| 1.00–6.00 | 40 (21.5) | 45 (19.5) | |
| 6.01–16.00 | 81 (43.5) | 99 (42.9) | |
| 16.01–30.00 | 65 (34.9) | 87 (37.7) | |
| Localization of OM | 0.667 | ||
| Toenail | 166 (89.2) | 209 (90.5) | |
| Fingernail | 15 (8.1) | 14 (6.1) | |
| Both | 5 (2.7) | 8 (3.5) | |
| Clinical type of OM | 0.119 | ||
| DLSO | 174 (93.5) | 224 (97.0) | |
| SWO | 7 (3.8%) | 2 (0.9) | |
| PSO | 5 (2.7) | 5 (2.2) | |
| The most affected nail | 0.000 | ||
| Big toenail | 136 | 125 | |
| Thumb and/or toenails 2–5 | 7 | 3 | |
| Fingernails 2–5 | 43 | 103 | |
| Number of involved nails | 0.011 | ||
| 1–2 | 66 (35.5) | 111 (48.1) | |
| 3–9 | 100 (53.8) | 108 (46.8) | |
| > 9 | 20 (10.8) | 12 (5.2) | |
| Pain | 0.000 | ||
| Yes | 35 (18.8) | 128 (44.6) | |
| No | 151 (81.2) | 126 (55.4) | |
| Esthetic problem | 0.000 | ||
| Yes | 95 (51.1) | 198 (85.7) | |
| No | 91 (48.9) | 33 (14.3) | |
| Nail deformity | 0.394 | ||
| Yes | 124 (66.7) | 164 (71.0) | |
| No | 62 (33.3) | 67 (29.0) |
SCIO, Scoring Clinical Index for Onychomycosis; OM, onychomycosis; DLSO, distal and lateral subungual onychomycosis; SWO, superficial white onychomycosis; PSO, proximal subungual onychomycosis
*According to chi-square or Fisher’s tests
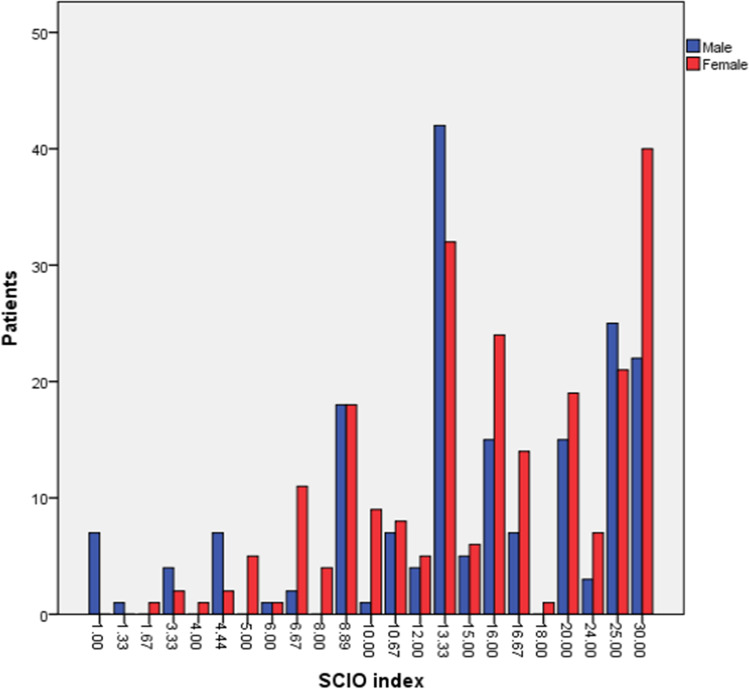
Mean SCIO index value in patients with onychomycosis was 16.76 ± 7.80. Majority of patients, 74 (17.7%) of them had SCIO index value 13.33, followed by 62 (14.80%) patients with SCIO index value 30.00 (Fig. 2).
Fig. 2.
Distribution of SCIO index values among male and female patients with onychomycosis
According to SCIO index values, 180/417 (43.16%) patients were indicated for systemic antifungal therapy, followed by 152 (36.45%) patients indicated for combination therapy. In 89 (21.34%) patients, SCIO index valued 12.00–16.00, which corresponds to the application of systemic therapy using therapeutic protocols recommended for fingernails. In 82 (19.66%) patients, nail changes had SCIO index values of 20.01–30.00, which is an indication for chemical nail avulsion followed by systemic antifungal therapy (Table 2).
Table 2.
Distribution of 417 patients with laboratory confirmed onychomycosis by treatment approach according to SCIO index values
| SCIO | Treatment approach | Patients no. (%) |
|---|---|---|
| Topical therapy | 85 (20.38) | |
| 1.00–3.00 | Topical treatment: remove (cut or scrape off) affected marginal parts of the nail. Use topical antifungals until healthy nail re-grows | 28 (6.71) |
| 3.01–6.00 | Topical treatment with lower success, which often depends on growth rate. Systemic therapy recommended in slower-growing nails or proximal onychomycosis type | 57 (13.67) |
| Systemic therapy | 180 (43.16) | |
| 6.01–9.00 | Systemic therapy. Use scheme proposed for fingernails (e.g., itraconazole: 2 pulses of 200 mg bid) | 39 (9.35) |
| 9.01–12.00 | Systemic therapy. Use scheme proposed for toenails (e.g., itraconazole: 3 pulses of 200 mg bid) | 52 (12.47) |
| 12.01–16.00 | Systemic therapy. Use scheme proposed for fingernails with any antifungal (e.g., 4–5 pulses of itraconazole, 200 mg bid) | 89 (21.34) |
| Combination therapy | 152 (36.45) | |
| 16.01–20.00 | Combination therapy (systemic antifungal + topical measures). Adequate keratolytic treatment recommended | 70 (16.79) |
| 20.01–30.00 | Consider nail avulsion (e.g., with urea paste), continue with systemic therapy | 82 (19.66) |
SCIO, Scoring Clinical Index for Onychomycosis
Dermatophytes were most frequently isolated—91.85% (383/417), followed by yeasts 5.27% (22/417), and non-dermatophyte molds 2.88% (12/417). Among dermatophytes, the most frequent species were Trichophyton rubrum isolated in 337/383 (87.99%), followed by Trichophyton interdigitale in 28/383 (7.31%). Candida albicans was the most frequent among yeast isolated in 16/22 (72.73%), while Aspergillus fumigatus was the most common among non-dermatophyte molds isolated in 4/12 (33.33%). Mean SCIO index value was highest in onychomycosis caused by dermatophytes, followed by non-dermatophyte molds, and yeast with values 17.37, 10.75, and 8.66, respectively. In patients with onychomycosis caused by dermatophytes, SCIO index had significantly higher values (p = 0.032) compared with patients who had onychomycosis caused by yeasts and non-dermatophyte molds (Table 3).
Table 3.
Distribution of SCIO index values by causative fungi
| Causative fungi | SCIO index | ||||
|---|---|---|---|---|---|
| 1.00–6.00 | 6.01–16.00 | 16.01–30.00 | p value* | ||
| Dermatophytes (n = 383) | 73 (85.9%) | 163 (90.6%) | 147 (96.7%) | ||
| Mean SCIO: 17.35 | |||||
| SCIO range: 1–30 | |||||
| Trichophyton rubrum | 69 | 140 | 128 | ||
| Trichophyton interdigitale | 3 | 15 | 10 | ||
| Trichophyton violaceum | 0 | 1 | 1 | ||
| Trichophyton tonsurans | 1 | 4 | 1 | ||
| Trichophyton verrucosum | 0 | 1 | 2 | ||
| Epidermophyton floccosum | 0 | 2 | 5 | 0.032 | |
| Yeasts (n = 22) | 9 (10.6%) | 10 (5.6%) | 3 (2.0%) | ||
| Mean SCIO: 8.66 | |||||
| SCIO range: 1–24 | |||||
| Candida albicans | 6 | 8 | 2 | ||
| Candida parapsilosis | 2 | 2 | 0 | ||
| Candida krusei | 1 | 0 | 1 | ||
| NDM (n = 12) | 3 (3.5%) | 7 (3.9%) | 2 (1.3%) | ||
| Mean SCIO: 10.75 | |||||
| SCIO range: 3.33–20 | |||||
| Aspergillus fumigatus | 2 | 2 | 0 | ||
| Aspergillus flavus | 0 | 1 | 1 | ||
| Aspergillus terreus | 0 | 1 | 0 | ||
| Aspergillus niger | 1 | 1 | 0 | ||
| Scopulariopsis brevicaulis | 0 | 0 | 1 | ||
| Fusarium oxysporum | 0 | 1 | 0 | ||
| Alternaria alternata | 0 | 1 | 0 | ||
SCIO, Scoring Clinical Index for Onychomycosis; NDM, non-dermatophyte molds
*According to chi-square test
Discussion
In our study, female patients were more commonly affected with onychomycosis than male, which is in agreement with some reports [11]. This may be as a result that female patients generally consulted more frequently for onychomycosis and are involved in situations that can cause nail trauma such as repeated pedicure and manicure, frequent housework, and use of detergents [12]. In our study, female patients significantly more often had fingernail onychomycosis, and reported pain and esthetics problems compared to male patients. This is in agreement with some studies that reported that women are more likely to suffer from fingernail onychomycosis [13, 14]. Related to this is the traditional role of women in doing household chores [15]. Manual washing of clothes and dishes and other activities that involve long contact of the nail with water leads to weakening of protective mechanisms and facilitates the penetration of infectious agents to the nail [13]. On the other hand, several studies concluded that males are more frequently infected than females due to the fact that males are more exposed to nail trauma and wearing occlusive footwear [12, 16]. Also, men are more likely to engage in sports activities which further increases their risk. This all contributes to the more frequent occurrence of toenail onychomycosis in men [17].
According to our results, the most common localization of onychomycosis was on the big toe, which is in line with the results of most studies [18, 19]. This observation was expected because of the slow growth of the nail which facilitates the fungal invasion [20]. Namely, the big toe has the largest surface area of all toenails and suffers the most pressure while wearing shoes [21]. Nail plate thickening is more common in the elderly as a result of changes in the nails that occur due to aging as well as due to repeated traumas of the nail plate that inevitably occur during life [22].
Our study finds that DLSO is predominant clinical type which is in concordance with majority of studies [12, 16] as well as with our previous report [23]. In early infection, the nail plate may appear normal, but with disease progression subungual hyperkeratosis and onycholysis can occur [24].
In our study for each patient, we calculated SCIO index in order to classify severity of onychomycosis. Majority of our patients had SCIO index value 13.33 and similar results are reported from recent study [25]. In the study conducted among psychiatric patients, mean SCIO index value was found to be 15.8 [26]. Higher baseline values are found in study conducted among diabetic patients with onychomycosis in which mean SCIO index value was 18.1 [27], as well as in study by Shimoyama et al. among male subjects [28]. Calculation of SCIO index was found to be useful also in efficiency monitoring of topical treatment in patients with severe to very severe onychomycosis [29].
We used the first scoring system proposed by Sergeev [9], which was developed to help physicians to choose appropriate antifungal therapy by providing an objective measurement of disease severity. Afterwards, additional grading systems appeared in literature, but currently there is no standardized system which will be consensually used for clinical trial inclusion criteria, prediction of therapeutic outcome, and as clinician guidance in treatment choice [30]. This is the only system that takes into account rate of nail growth indicating the amount of required therapy, and optimal treatment approach. In our study, 180/417 (43.16%) patients were indicated for systemic antifungal therapy, 152 (36.45%) patients for combination therapy, while 85 required local treatment. Calculation of SCIO index enables choosing optimal antifungal strategy for each patient in order to minimize adverse effects of inappropriate antifungal treatment. Baran et al. introduced second scoring system based on 10 criteria: extent of nail involvement, diffuse nail plate thickening, liner streaks, onycholysis, location, paronychia, melanonychia, patients’ age, predisposing factors, as well as causative organism. This system enables prediction of treatment response but lacks in defining mild, moderate, and severe nail involvement [31]. Carney et al. introduced Onychomycosis Severity Index which takes into account percentage of nail plate involvement, proximity of disease to the matrix, occurrence of dermatophytomas, and degree of subungual hyperkeratosis [32]. This system does not take into account patient’s immune status, the causative organism, rate of nail growth, and interobserver variability may result since it was developed by analyzing photographs of diseased nails.
Onychomycosis is increasingly being recognized as a common problem in dermatological practice [6]. In our study, the leading causes of onychomycosis were fungi from the group of dermatophytes (91.85%), which is in line with epidemiological data from most countries in northern and central Europe [17, 33, 34], as well as with our previous findings where T. rubrum was the most frequent causative agent [35, 36]. Also, in the northern parts of Greece as well as Turkey, the predominance of dermatophytes as causative agents of onychomycosis has been observed [37, 38]. This epidemiological situation is mainly present in countries where the localization of onychomycosis is more often detected on the toenails than on the fingernails [39]. In our study, toenails were 12 times more likely to be affected by the infectious process than fingernails (373:29).
According to our findings, SCIO index values were significantly higher in patients with onychomycosis caused by dermatophytes (p = 0.032). Furthermore, mean SCIO index values were highest in onychomycosis caused by dermatophytes (17.35), compared to yeasts (8.66), and non-dermatophyte molds (10.75). In our study, only patients with dermatophyte onychomycosis had SCIO index greater than 25. Ghannoum et al. [40] showed that people with nail plate thickening are as much as 4 times more likely to develop onychomycosis. Nail plate thickening very often occurs after nail trauma. In men, it mainly occurs as a consequence of sports activities (frequent nail trauma and onychomycosis in football players), while in women it is more often associated with wearing tight shoes due to following fashion trends [17]. In patients with high SCIO values, a decrease in cure rate is recorded, so appropriate diagnosis and identification of causing factor is mandatory for proper treatment [3].
In a smaller percentage of our patients, onychomycosis was caused by yeast (5.27%), mostly on fingernails. According to most studies, Candida spp. are most often isolated from the group of yeasts as causative agents of onychomycosis, and among them C. albicans still predominates among other species of this fungal genus. Candida spp. can be present on the skin around the nail and can be secondarily transmitted to the nail tissue with disease progression [41, 42].
In our study, non-dermatophyte molds were identified in a smaller number of patients (2.88%). This epidemiological situation, when it comes to non-dermatophyte molds in our study, is consistent with the results from the USA and most European countries [33, 40, 43, 44]. Non-dermatophyte onychomycosis has been associated with a poor prognosis possibly due to the difficulty in laboratory diagnosis, since they may be innocuous contaminants of the feet and nails or common laboratory contaminants [45].
The finding of yeasts and non-dermatophyte molds should always be carefully interpreted in accordance with the clinical presentation [46]. Unlike dermatophytes that belong to strictly pathogenic fungi, yeasts and non-dermatophyte molds are opportunistic fungi, which can often be present on human tissues and in the human environment without causing disease. Therefore, it is extremely important for accurate diagnosis to correctly interpret the findings of these fungi [47]. However, considering that the existence of fungal elements was also observed in our patients in direct microscopic preparations, we believe that there was no accidental contamination of the sample or contamination during the performance of laboratory diagnostic procedures. Of course, there is a theoretical possibility that yeasts and non-dermatophyte molds, which otherwise grow faster than dermatophytes, have outgrown any dermatophytes present in the nail sample, since species identification cannot be done based on a direct microscopic preparation.
However, the clinical presentation is not specific enough for certain types of fungi, and even many other diseases can cause changes in the nails that mimic onychomycosis [7]. Furthermore, exostoses, warts, onychomatricoma can also present similarly, as well as utilization of exogenous substances such as nail polish or self-tanning creams, chemotherapeutic agents, tetracycline derivatives, and oral retinoids [48]. Therefore, laboratory confirmation represents a necessary step in the diagnosis of onychomycosis because it enables the determination of the causative agent. Proper and reliable laboratory diagnosis of this disease is a prerequisite for targeted and rational use of antifungals, which reduces the number of side effects and treatment costs [49]. Adapting this approach by making a proper laboratory diagnosis before initiating treatment will avoid the consequences of treating inappropriately or missing the diagnosis of other nail disorders and their associated morbidity [3].
Onychomycosis is there to remain a disease of significance and despite the therapeutic innovations many patients fail to achieve a complete cure, and may have high relapse rates [49]. Comprehensive understanding of disease characteristics will allow introduction of individualized treatment plan for each patient, based on proper fungal identification and standardized method of evaluating disease severity, which could help the patient achieve a complete cure.
Funding
This work was supported by the Ministry of Education, Science, and Technological Development of Serbia (grant number: 175042).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghannoum M, Mukherjee P, Isham N, Markinson B, Rosso JD, Leal L. Examining the importance of laboratory and diagnostic testing when treating and diagnosing onychomycosis. Int J Dermatol. 2018;57:131–138. doi: 10.1111/ijd.13690. [DOI] [PubMed] [Google Scholar]
- 2.Banik A, Durairaj E, Lyngdoh WV, Khyriem AB, Sabhapandit D. Clinico-aetiologic profile of onychomycoses in a tertiary care centre in northeast India. Trop Doct. 2018;48:136–142. doi: 10.1177/0049475517735979. [DOI] [PubMed] [Google Scholar]
- 3.Gupta AK, Versteeg SG, Shear NH. Onychomycosis in the 21st century: an update on diagnosis, epidemiology, and treatment. J Cutan Med Surg. 2017;21:525–539. doi: 10.1177/1203475417716362. [DOI] [PubMed] [Google Scholar]
- 4.Westerberg DP, Voyack MJ. Onychomycosis: current trends in diagnosis and treatment. Am Fam Physician. 2013;88:762–770. [PubMed] [Google Scholar]
- 5.Gupta AK, Stec N, Summerbell RC, Shear NH, Piguet V, Tosti A, Piraccini BM. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020;34:1972–1990. doi: 10.1111/jdv.16394. [DOI] [PubMed] [Google Scholar]
- 6.Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835–851. doi: 10.1016/j.jaad.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 7.Eba M, Njunda AL, Mouliom RN, Kwenti ET, Fuh AN, Nchanji GT, Atashili J. Onychomycosis in diabetic patients in Fako Division of Cameroon: prevalence, causative agents, associated factors and antifungal sensitivity patterns. BMC Res Notes. 2016;9:494. doi: 10.1186/s13104-016-2302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piraccini BM, Alessandrini A. Onychomycosis: a review. J Fungi (Basel) 2015;1:30–43. doi: 10.3390/jof1010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sergeev AY, Gupta AK, Sergeev YV. The Scoring Clinical Index for Onychomycosis (SCIO index) Skin Therapy Lett. 2002;7(Suppl 1):6–7. [PubMed] [Google Scholar]
- 10.Hoog GS, Guarro J, Gene J, Figueras MJ (2000) Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Universitat Rovira i Virgili
- 11.Seck MC, Ndiaye D, Diongue K, et al. Mycological profile of onychomycosis in Dakar (Senegal) J Mycol Med. 2014;24:124–128. doi: 10.1016/j.mycmed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Toukabri N, Corpologno S, Bougnoux ME, El Euch D, Sadfi-Zouaoui N, Simonetti G. In vitro biofilms and antifungal susceptibility of dermatophyte and non-dermatophyte moulds involved in foot mycosis. Mycoses. 2018;61:79–87. doi: 10.1111/myc.12706. [DOI] [PubMed] [Google Scholar]
- 13.Bramono K, Budimulja U. Epidemiology of onychomycosis in Indonesia: data obtained from three individual studies. Jpn J Med Mycol. 2005;46:171–176. doi: 10.3314/jjmm.46.171. [DOI] [PubMed] [Google Scholar]
- 14.Gelotar P, Vachhani S, Patel B, Makwana N. The prevalence of fungi in fingernail onychomycosis. J Clin Diagn Res. 2013;7:250–252. doi: 10.7860/JCDR/2013/5257.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadeganipour M, Mohammadi R. Causative agents of onychomycosis: a 7-year study. J Clin Lab Anal. 2016;30:1013–1020. doi: 10.1002/jcla.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta M, Sharma NL, Kanga AK, Mahajan VK, Tegta GR. Onychomycosis: clinico-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007;73:389–392. doi: 10.4103/0378-6323.37055. [DOI] [PubMed] [Google Scholar]
- 17.Tchernev G, Penev PK, Nenoff P, et al. Onychomycosis: modern diagnostic and treatment approaches. Wien Med Wochenschr. 2013;163:1–12. doi: 10.1007/s10354-012-0139-3. [DOI] [PubMed] [Google Scholar]
- 18.Nazar JR, Gerosa PE, Díaz OA. Onychomycoses: epidemiology, causative agents and assessment of diagnostic laboratory methods. Rev Argent Microbiol. 2012;44:21–25. doi: 10.1590/S0325-75412012000100005. [DOI] [PubMed] [Google Scholar]
- 19.Papini M, Piraccini BM, Difonzo E, Brunoro A. Epidemiology of onychomycosis in Italy: prevalence data and risk factor identification. Mycoses. 2015;58:659–664. doi: 10.1111/myc.12396. [DOI] [PubMed] [Google Scholar]
- 20.Yaemsiri S, Hou N, Slining MM, He K. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420–423. doi: 10.1111/j.1468-3083.2009.03426.x. [DOI] [PubMed] [Google Scholar]
- 21.Puri N, Kaur T. A study of nail changes in various dermatosis in Punjab, India. Our Dermatol Online. 2012;3:164–170. [Google Scholar]
- 22.Kaur R, Kashyap B, Bhalla P. Onychomycosis - epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108–116. doi: 10.4103/0255-0857.40522. [DOI] [PubMed] [Google Scholar]
- 23.Dubljanin E, Dzamic A, Mitrovic S, Arsic Arsenijevic V, Colovic CI. Onychomycosis: clinical findings, etiological agents and evaluation of laboratory methods. Arch Biol Sci. 2014;66:587–594. [Google Scholar]
- 24.Zaikovska O, Pilmane M, Kisis J. Morphopathological aspects of healthy nails and nails affected by onychomycosis. Mycoses. 2014;57:531–536. doi: 10.1111/myc.12191. [DOI] [PubMed] [Google Scholar]
- 25.Pajaziti L, Vasili E. Treatment of onychomycosis - a clinical study. Med Arch. 2015;69:173–176. doi: 10.5455/medarh.2015.69.173-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai M, Suzuki T, Hiruma M, Ikeda S. A retrospective cohort study of tinea pedis and tinea unguium in inpatients in a psychiatric hospital. Med Mycol J. 2014;55:E35–41. doi: 10.3314/mmj.55.e35. [DOI] [PubMed] [Google Scholar]
- 27.Sumikawa M, Egawa T, Honda I, Yamamoto Y, Sumikawa Y, Kubota M. Effects of foot care intervention including nail drilling combined with topical antifungal application in diabetic patients with onychomycosis. J Dermatol. 2007;34:456–464. doi: 10.1111/j.1346-8138.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 28.Shimoyama H, Kuwano Y, Sei Y. Retrospective survey of treatment outcomes of efinaconazole 10% solution and luliconazole 5% solution for onychomycosis in our facility. Med Mycol J. 2019;60:95–100. doi: 10.3314/mmj.19-00009. [DOI] [PubMed] [Google Scholar]
- 29.Parekh M, Ramaiah G, Pashilkar P, Ramanujam R, Johnston P, Ilag LL. A pilot single centre, double blind, placebo controlled, randomized, parallel study of Calmagen® dermaceutical cream and lotion for the topical treatment of tinea and onychomycosis. BMC Complement Altern Med. 2017;17:464. doi: 10.1186/s12906-017-1970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification. J Am Acad Dermatol. 2011;65:1219–1227. doi: 10.1016/j.jaad.2010.09.730. [DOI] [PubMed] [Google Scholar]
- 31.Baran R, Hay RJ, Garduno JI. Review of antifungal therapy and the severity index for assessing onychomycosis: part I. J Dermatolog Treat. 2008;19:72–81. doi: 10.1080/09546630701243418. [DOI] [PubMed] [Google Scholar]
- 32.Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147:1277–1282. doi: 10.1001/archdermatol.2011.267. [DOI] [PubMed] [Google Scholar]
- 33.Drakensjö IT, Chryssanthou E. Epidemiology of dermatophyte infections in Stockholm, Sweden: a retrospective study from 2005–2009. Med Mycol. 2011;49:484–488. doi: 10.3109/13693786.2010.540045. [DOI] [PubMed] [Google Scholar]
- 34.Svejgaard EL, Nilsson J. Onychomycois in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47:131–135. doi: 10.1111/j.1439-0507.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 35.Dubljanin E, ColovicCalovski I, Vujcic I, et al. Clinical evaluation of a T.rubrum-specific polymerase chain reaction and pandermatophyte polymerase chain reaction in the diagnosis of suspected onychomycosis in 183 Serbian patients. Br J Dermatol. 2014;171:1593–5. doi: 10.1111/bjd.13168. [DOI] [PubMed] [Google Scholar]
- 36.Dubljanin E, Dzamic A, Vujcic I, et al. Epidemiology of onychomycosis in Serbia: a laboratory-based survey and risk factor identification. Mycoses. 2017;60:25–32. doi: 10.1111/myc.12537. [DOI] [PubMed] [Google Scholar]
- 37.Koussidou T, Devliotou-Panagiotidou D, Karakatsanis G, Minas A, Mourellou O. Onychomycosis in Nothern Greece during 1994–1998. Mycoses. 2000;45:29–37. [PubMed] [Google Scholar]
- 38.Yenisehirli G, Bulut Y, Sezer E, Gunday E. Onychomycosis infections in the Middle Black Sea Region, Turkey. Int J Dermatol. 2009;48:956–959. doi: 10.1111/j.1365-4632.2009.04126.x. [DOI] [PubMed] [Google Scholar]
- 39.Gupta AK, Jain HC, Lynde CW, Macdonald P, Cooper EA, Summerbell RC. Prevalence and epidemiology of onychomycosis in patients visiting physician officies: a multicenter Canadian survey of 15000 patients. J Am Acad Dermatol. 2000;43:244–248. doi: 10.1067/mjd.2000.104794. [DOI] [PubMed] [Google Scholar]
- 40.Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641–648. doi: 10.1067/mjd.2000.107754. [DOI] [PubMed] [Google Scholar]
- 41.Maraki S, Nioti E, Mantadakis E, Tselentis Y. A 7-year survey of dermatophytosis in Crete, Greece. Mycoses. 2007;50:481–484. doi: 10.1111/j.1439-0507.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 42.Pontes ZB, Lima Ede O, Oliveira NM, Dos Santos JP, Ramos AL, Carvalho MF. Onychomycosis in João Pessoa City, Brazil. Rev Argent Microbiol. 2002;34:95–99. [PubMed] [Google Scholar]
- 43.Dolenc-Voljč M. Dermatophyte infections in the Ljubljana region, Slovenia, 1995–2002. Mycoses. 2005;48:181–186. doi: 10.1111/j.1439-0507.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 44.Romano C, Gianni C, Difonzo EM. Retrospective study of onychomycosis in Italy: 1985–2000. Mycoses. 2005;2005(48):42–44. doi: 10.1111/j.1439-0507.2004.01066.x. [DOI] [PubMed] [Google Scholar]
- 45.Gupta AK, Drummond-Main C, Cooper EA, Brintnell W, Piraccini BM, Tosti A. Systematic review of nondermatophyte mold onychomycosis: diagnosis, clinical types, epidemiology, and treatment. J Am Acad Dermatol. 2012;66:494–502. doi: 10.1016/j.jaad.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 46.Shemer A, Davidovici B, Grunwald MH, Trau H, Amichai B. New criteria for the laboratory diagnosis of nondermatophyte moulds in onychomycosis. Br J Dermatol. 2009;160:37–39. doi: 10.1111/j.1365-2133.2008.08805.x. [DOI] [PubMed] [Google Scholar]
- 47.Diongue K, Diallo MA, Badiane AS, et al. Nondermatophytic and noncandidal fungi isolated in Le Dantec University hospital of Dakar in 2014: Epidemiological, clinical and mycological study. J Mycol Med. 2015;25:181–190. doi: 10.1016/j.mycmed.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Lipner S, Scher RK. Onychomycosis: current and future therapies. Cutis. 2014;93:60–63. [PubMed] [Google Scholar]
- 49.Piaraccini BM, Sisti A, Tosti A. Long-term follow-up of toenail onychomycosis caused by dermatophytes after successful treatment with systemic antifungal agents. J Am Acad Dermatol. 2010;23:411–414. doi: 10.1016/j.jaad.2009.04.062. [DOI] [PubMed] [Google Scholar]