Abstract
Milk is an incredibly healthy food world-wide. However, the ‘lactase deficient’ individuals cannot digest milk’s carbohydrate lactose. A large part of the world population is depriving of highly beneficial milk proteins like casein, lactoalbumin, lactoglobulin, etc. due to lactose intolerance. Production of functional foods and bioactive peptides from milk with natural antioxidants and the addition of probiotics could be the best alternative to extend the use of milk functionalities. Among different probiotics, the lactic acid bacteria (LAB) like Lactobacillus delbrueckii sub sp. bulgaricus, Streptococcus thermophilus and some species of Bifidobacteria and their metabolites (paraprobiotics and postbiotics) have been given more preference to add in milk-derived functional foods. These species are generally considered as heat-tolerant, highly proteolytic, and peptidolytic towards milk proteins and they liberate smaller molecules of bioactive peptides during fermentation and other processes that stimulate the enzyme lactase to help people in digestion of milk carbohydrate lactose. Moreover, the incorporation of natural antioxidants in yoghurt and other dairy products prevents the rancidity of milk fat. The level of bioactive peptides produced in milk-derived functional foods can be determined by capillary zone electrophoresis, mass spectrometry, fractionation, and other modern assessment techniques. Commercial production of functional probiotic products with bioactive peptides could significantly contribute to reduce milk spoilage, enhance health benefits as well as the growth of the agro-processing industry.
Keywords: Milk, Fermented dairy products, Bioactive peptides, Probiotics, Natural antioxidants, Lactic acid bacteria
Introduction
Bioactive peptides are generally defined as short chains of amino acid monomers that are mostly connected with peptide bonds and occurred biologically and have beneficial effects on human health (El-Fattah et al. 2018; Ovando et al. 2018). These are mainly small molecules of proteins and synthesized in the cells as large molecules of pre-pro-peptides that are further broken down and modified in the cells to form active compounds. These biologically active compounds usually function as signaling molecules and play vital roles in biological functions as well as pathogenesis. The bioactive peptides positively contribute to the functions and/or states of our body and thus beneficially affect our health (Muro Urista et al. 2011). In general, the milk proteins especially casein, as well as dairy products including various cheeses and yoghurts have been used as the raw materials for the production, and isolation of small molecular peptides with many effective applications. Besides, the leading cheese production by-product called milk-whey-waste also shows a prominent role as the source of protein for the isolation of biologically effective peptides having different potential industrial implementations.
Milk proteins provide several nutritional, functional, and biological activities. Most of the health-promoting foods have been found to contain milk as potential ingredients because it consists of many proteins that accelerate the biological properties of milk. From milk proteins, many physiologically active peptides can be derived that are usually inactive in the larger molecules of parent proteins but can be released during (1) digestion of milk in gastrointestinal tracts, (2) fermentation of milk with proteolytic starter cultures, or (3) hydrolysis of the milk with proteolytic enzymes (Korhonen and Pihlanto 2006).
Natural antioxidants and bioactive components derived from different food sources have significant roles in maintaining the body functions including prevention of different degenerative diseases like cancer, diabetes, cardiovascular diseases, inflammations, blood pressure, aging, and so on (Caleja et al. 2016; Feng et al. 2019; Kamal et al. 2019a, b). In recent years, there is a growing interest in the use of bioactive compounds in different functional foods, which help to boost antioxidant and health beneficial effects on the human body. Extracts of tropical fruits with medicinal and therapeutic importance have been incorporated in many food items as antioxidant supplements (Córdova-Ramos et al. 2018; Gao et al. 2018). Moreover, these compounds are known to have high oxidative inhibitory capacity due to their potent ability to scavenge free radicals.
Over the past few years, functional foods have remarkably gained consumers’ preferences due to their various potential health benefits attributed to the presence of probiotics. According to the WHO and FAO, probiotics are ‘live microorganisms when administered in adequate amounts confer a health benefit to the host’. However, in recent years, new terminologies are added to the probiotic definition such as ‘paraprobiotics’ (dead/inactive cells of probiotics), ‘postbiotics’ (healthful metabolites of probiotics), and ‘psychobiotic’ (microbial neuroactive metabolites affecting psychological/psychosomatic processes) (Zendeboodi et al. 2020). Researches have also claimed that dead cells (intact or ruptured) and derivatives could also demonstrate significant impacts on human health.
Yoghurt is one kind of functional food produced by lactic acid fermentation mainly by the Streptococcus and Lactobacillus species (Abdel-Hamid et al. 2020; Kennas et al. 2020). It has different health promotional activities including improvement of gut microbiota activity, reduction of lactose absorption and intolerance by improving gastrointestinal functionalities, improvement of lipid digestibility, immune system, and antimicrobial activities (Demirci et al. 2017; Córdova-Ramos et al. 2018; El-Fattah et al. 2018; Jaster et al. 2018; Feng et al. 2019; Khaledabad et al. 2019). These health beneficial properties of yoghurt can be increased, depending on the addition of probiotic microorganisms, prebiotic ingredients, and bioactive peptides, and so on. Although milk is reported to have considerable amounts of polyphenols, different issues such as pasteurization and bacterial actions to milk protein lower the number of polyphenolic compounds and natural antioxidants (Aliakbarian et al. 2015). Fortification of yoghurt with bioactive compounds and natural antioxidants seems to be a suitable food model to satisfy consumer interest in original yoghurt nutrients, beneficial effects of starter cultures, and health benefits of added antioxidants (Baba et al. 2018). Agro-processing industries generate a substantial amount of phenolic-rich by-products, which could be a potential natural source of antioxidants. Over the last few years, numerous researches have been conducted on bioactive peptides and their production from different products of plant, animal, and marine origin (O’Sullivan et al. 2016; El-Fattah et al. 2018; Ovando et al. 2018). However, the detailed information on yoghurt fortification with probiotics, natural antioxidants, and bioactive peptides isolated from dairy-based products is still limited. Moreover, it is imperative to evaluate the current research progress on yoghurt fortification and peptides production and their characterization to identify patterns, trends, and research gaps. Therefore, this review is aimed to different aspects of functional foods, bioactive peptides, and fortification of yoghurt with probiotics and bioactive compounds derived from different food matrices.
Background rationale
Functional foods
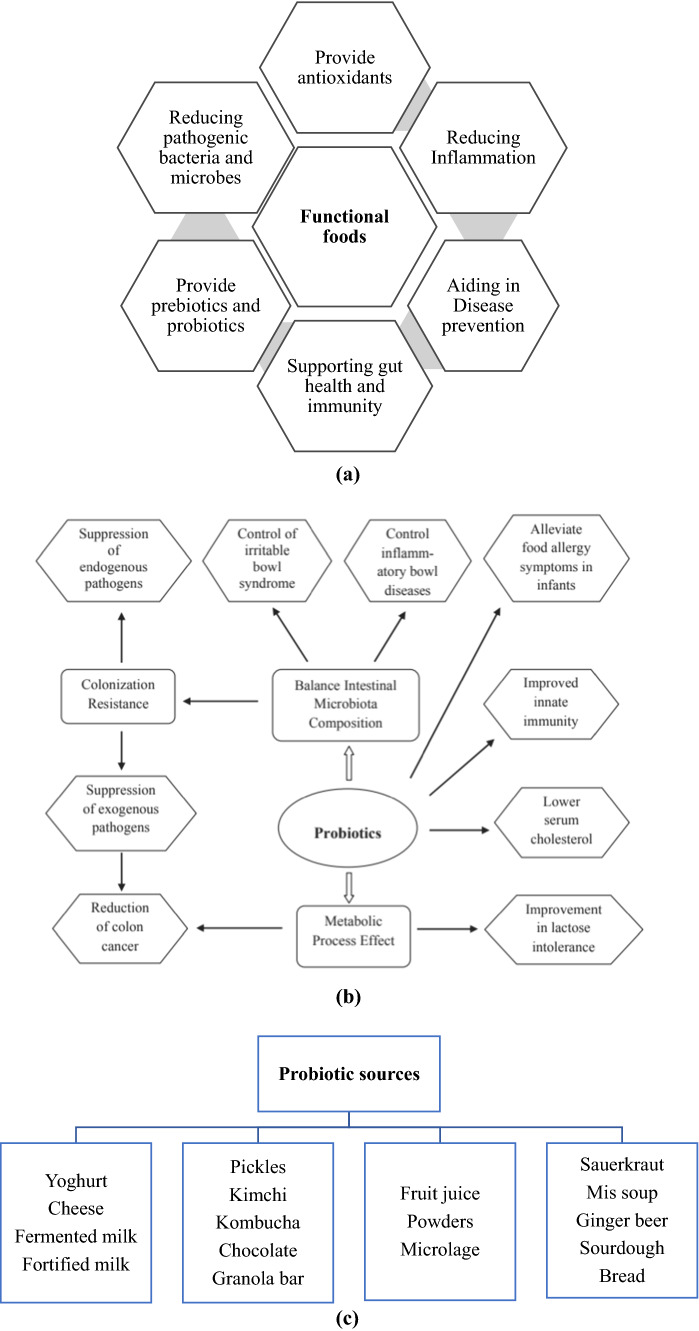
In the current days, the importance of food has shifted from providing essential nutrients for sustaining life and growth to preventing or indeed curing various forms of diseases. Furthermore, the recent technological advancement, population lifestyle changes, and socio-economic trends throughout the world indicate the need for foods with increased health benefits (Tadesse and Emire 2020). These are the key determining and driving forces for the growth of current development and production of functional foods in the global market. The global market of functional foods is expected to cross $192 billion by 2020 (Brown et al. 2015; Konstantinidi and Koutelidakis 2019). Over the last few decades, many definitions have been proposed for functional foods by different scientists and researchers; however, till now there is no commonly accepted definition. Functional foods are intended to be consumed as a part of normal diet and they contain biologically active components that reduce the risk of major diseases (El-Fattah et al. 2018). Hsieh and Ofori (2010) mentioned functional food as ‘foods similar in appearance to conventional foods that are consumed as part of a normal diet and have demonstrated physiological benefits and/or reduce the risk of chronic diseases beyond basic nutritional functions.’ The wholesome foods that have been fortified, enriched, and/or enhanced to provide additional health benefits beyond the provision of different essential nutrients (e.g., vitamins and minerals) are generally considered functional foods (Vicentini et al., 2016). Functional foods can also be naturally occurring foods and are usually consumed at an efficacious level as a part of varied diets regularly. Food can be called a ‘functional food’ when it consists of a food component (whether a nutrient or not) that positively affects one or more predetermined functions in the human body (Karelakis et al. 2019). It can also include foods from which a potentially harmful component has been removed by technological means. It implies that a consumer can be informed about the health benefits of foods that include particular formulations, carrying specific information that would have been otherwise unknown (Kaur et al. 2017). Different names of functional foods like design foods, pharma foods, and nutraceuticals have been proposed over the years based on ingredients that have been incorporated to prevent and treat specific diseases. Likewise, Pariza et al. (1999) recently proposed a new definition for functional food: “a manufactured food for which scientifically valid health claims can be made”. The author also illustrated the word “manufactured” as products of human’s interference (like altering through genetic engineering) as well as conventional food processing practices together with three potential processing techniques of functional foods such as (1) from another food a particular functional ingredient has been added to known foods, (2) the functional ingredients that are new to the food supply have been added to known foods, and (3) addition of one or more functional ingredients to process entirely new foods. A functional food has some beneficial effects on health, most of which have been summarized in Fig. 1a. According to Tidona et al (2009), functional foods are designed to boost human health, more specifically intended for weight management (prevention of obesity), natural defenses (boosting of immunity), bone calcification (prevention of osteoporosis), digestion (prevention of intestinal disorders), cardiovascular health (prevention of heart diseases by lowering the cholesterol level or blood pressure). To combine the beneficial effects of milk, plant extract and probiotic microorganisms, Hadjimbei et al. (2019) recently developed a functional goat’s milk yoghurt using Pistacia atlantica resin extracts and Saccharomyces boulardii. Results proved that Pistacia extracts improved the viability of lactic acid bacteria with retention of functional fatty acids, bioactive compounds, and sensory attributes during the shelf life of yoghurts. Similar findings were also claimed by El-Shafei et al. (2019) for yoghurt supplemented with quinoa extracts.
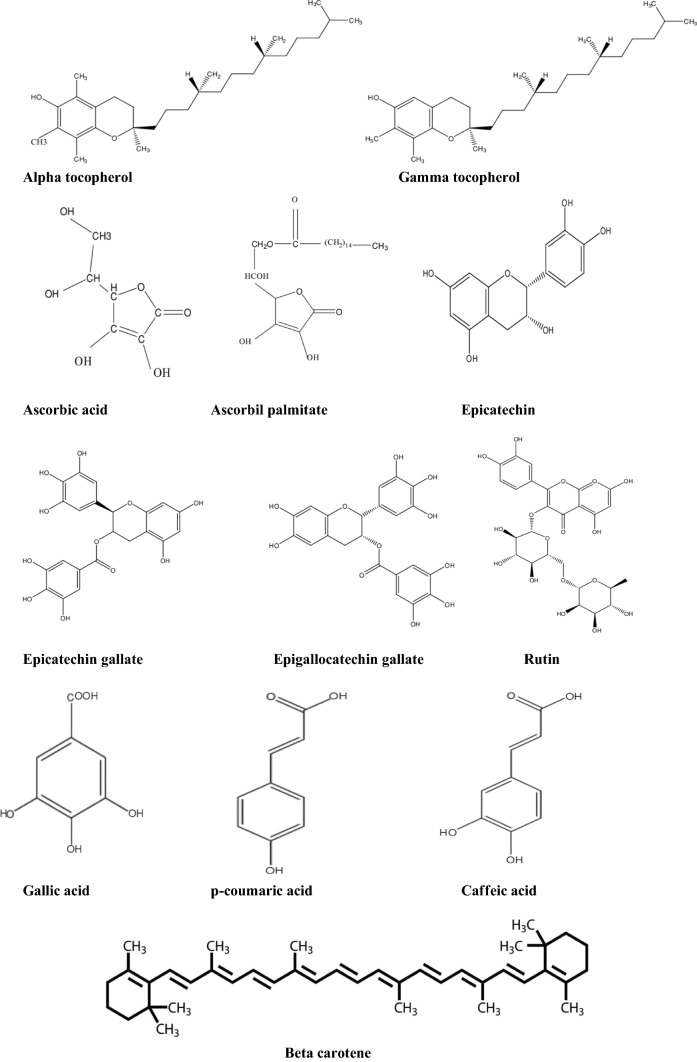
Fig. 2.
Chemical structures of major antioxidants naturally occurring in plants and herbs
Fig. 1.
Functional and probiotic foods: health benefits of a functional foods (Levy 2019) and b probiotics (Tripathi and Giri 2014); and c natural sources of probiotic (Kerry et al. 2018a, b)
Fermented milk products
The dairy food products which are often termed as fermented milk products, cultured dairy products, cultured dairy foods, or cultured milk products have been industrially produced through the fermentation of milk sugar (lactose) with various LAB like Lactobacillus spp., Leuconostoc spp. and Lactococcus spp. The overall production processes that can transform the liquid milk into various fermented dairy products involves the addition of lactic-acid-producing microbial strains such as LAB and/or yeasts which ingest the milk’s lactose to digest and release lactic acid as wastes in the milk and thereby reduce the pH of milk i.e., raise the acidity in the milk. The rise of milk acidity simultaneously accelerates the curdling of milk which is an essential prerequisite for the ultimate production of different fermented dairy products such as yoghurt, cheese, kefir, sour cream, and so on (Kerry et al. 2018a, b). The amount and types of composition in different mammals vary from species to species but most of the dairy products are produced using cow’s milk. Cow’s milk mainly consists of water with approximately 4.8% lactose, 3.2% protein, 3.7% fat, 0.19% non-protein nitrogen, and 0.7% ash. The major proteins found in milk are caseins, whey proteins, and immunoglobulins among which caseins comprise of about 80% of proteins. Saxelin et al. (2003) categorized the milk products into three groups like ‘basic products’, ‘value-added products’, and ‘functional dairy products’. The basic products usually comprise of milk, fermented milk, cheeses, ice cream, etc. The value-added products are usually produced by changing the milk composition, e.g., low-lactose or lactose-free products, hypoallergenic formulae with hydrolyzed protein for milk hypertensive infants, milk products enriched with Ca, vitamins, etc. While the functional dairy products are based on milk enriched with functional components and probiotic bacteria. In recent years, different bioactive compounds are being added to milk and milk-based products to increase their functionality and biological activities in the human body.
Probiotics and probiotic foods
Probiotic bacteria are those viable strains having health beneficial effects through the improvement of microbial balance in the gastrointestinal tract when consumed in a suitable dose. When these live probiotic bacterial strains are used in a food product as a raw material in adequate amounts, the food is then called as probiotic food. The probiotic bacteria are usually added to the probiotic foods in an appropriate concentration to supply the usual nutrients. In terms to define the word ‘probiotics’, FAO/WHO directory has expressed- “probiotics are live microorganisms which when administered in adequate amounts confer a health benefit on the host”. According to the proposed definition by FAO/WHO, probiotics must be alive and abundant once ingested. Probiotic bacteria have several beneficial effects (Zendeboodi et al. 2020) such as: (a) enhancing the nutritional value of food products, (b) controlling and reducing the serum cholesterol, (c) improving the immune system, (d) preventing gut infections and suppressing antibiotic-associated diarrhea, (e) reducing lactose intolerance symptoms, (f) reduction of colon cancer risk, and (g) improving the digestion of gliadin against celiac in gluten-containing foods which are dependent on the type of probiotic strain (Gareau et al. 2010; Oelschlaeger 2010). Concerning these functionalities, people are interested in consuming products containing probiotics. Probiotic strains that are generally used in the production of probiotic foods are summarized in Table 1.
Table 1.
Probiotic bacteria used in yoghurt production
| Probiotic bacteria genera | Species | References |
|---|---|---|
| Lactobacillus | L. plantarum, L. paracasei, L. acidophilus, L. casei, L. rhamnosus, L. crispatus, L. gasseri, L. reuteri, L. bulgaricus | Dixit et al. (2016) |
| Bacillus | B. coagulans, B. subtilis, B. laterosporus | Nguyen et al. (2016) |
| Lactococcus | L. lactis, L. reuteri, L. rhamnosus, L. casei, L. acidophilus, L. curvatus, L. plantarum | Eid et al. (2016) |
| Streptococcus | S. sanguis, S. oralis, S. mitis, S. thermophilus, S. salivarius | Arora et al. (2013) |
| Enterococcus | E. faecium | Onyenweaku et al. (2016) |
| Bifidobacterium | B. longum, B. catenulatum, B. breve, B. animalis, B. Bifidum | Westermann et al. (2016) |
| Saccharomyces | S. boulardii | Chen et al. (2013) |
Potential probiotic isolates help to maintain and improve health and are suitable for the development of functional probiotic products (Roobab et al. 2020). Among various commonly used probiotic bacteria, only those species belonging to genera Lactobacillus and Bifidobacterium have been recognized as beneficial for human health (Dixit et al. 2016; Westermann et al. 2016). Probiotics are microbes that can protect their hosts by preventing different diseases and the well-known species include Lactobacillus acidophilus that has been accounted for many probiotic food products such as yoghurts, acidophilus milk, and different food supplements (Eid et al. 2016). The antibiotics can often decimate the useful bacteria in the intestines whereas the probiotic bacteria can inhibit this unexpected destruction of these beneficial microbial strains in gastrointestinal tracts (Tripathi and Giri 2014). Therefore, the combined intake of probiotic foods and antibiotics would be more beneficial to prevent diarrhea associated with antibiotics (Agamennone et al. 2019). Probiotics can play an important role in the fermentation processes. Besides, there are some probiotic bacteria (e.g., LAB) that are frequently used as a dietary source by many beneficial live microorganisms in intestines, and thus improve the properties of useful indigenous microbiota and ultimately promote beneficial effects of those microbial strains in the host body (Khaledabad et al. 2019). Ingestion of probiotics usually induces the reduction of serum cholesterol, alleviation of lactose intolerance, reduction of indigestion, and other intestinal disorders (Tripathi and Giri 2014). It also reduces cancer risk and resistance to enteric pathogens and increases antihypertensive effects. The health beneficial activities of probiotics have been incorporated in Fig. 1b.
Traditionally, probiotics have been added to yoghurt and currently, more than 70 products containing Lactobacilli or Bifidobacteria are being produced worldwide including sour cream, buttermilk, frozen desserts, probiotic drinks, etc. (Fig. 1c) (Kerry et al. 2018a, b). The incorporation of these microorganisms into dairy products induces unique flavor profiles and texture. The use of a specific organism in a specific product is based on its probiotic and health-promoting effects. Bifidobacterium bifidum and Lactobacillus reuteri have been regarded as the most important probiotic bacteria for human health. Some probiotic effects attributed to these bacteria including improvement in lactose utilization, prevention of diarrhea, colon cancer, hypercholesterolemia, improvement of vitamin synthesis, calcium absorption, and also showed anticarcinogenic activity (Lucatto et al. 2019; Sani et al. 2020). The therapeutic value of any probiotic food typically depends on the viability of these bacteria in the probiotic products. There are different opinions about the number of probiotics that should be viable in a functional food product to perform their probiotic action. Tamime et al. (1995) suggested that generally the dairy products should contain ≥ 106 CFU of viable probiotic bacteria per milliliter or gram of probiotic products to be effective at the time of consumption and should be consumed regularly while the International Dairy Federation (IDF) expressed that a minimum of 107 probiotic bacterial cells should be alive per gram of the probiotic products at the time of consumption. Moreover, some literature claimed that the microorganisms must remain viable in concentrations from 106 to 109 CFU/g or mL (Sarkar 2013) during the processing, storage, and even digestion by the consumer to show their probiotic effects.
In recent years, new terminologies have been proposed by scientists/researchers such as ‘paraprobiotics’ and ‘postbiotics’ to indicate those biologically active compounds that are not able to clarify the term prebiotics, probiotics, and symbiotics or their biological functions against specific diseases (Zendeboodi et al. 2020; Barros et al. 2020). These terms have been arisen after being the use of non-viable bacterial cells, microbial parts, or cell byproducts at appropriate doses either in a soluble or non-soluble form that shows health beneficial effects and bioactivities. Paraprobiotics are the inactivated or ghost probiotics i.e., non-viable microbial cells or cellular extracts when administered in adequate amounts deliberate health beneficial effects (Barros et al. 2020). There are some microbial metabolites such as enzymes, proteins, peptides, polysaccharides, organic acids or lipids and components like lipoteichoic and teichoic acids, peptidoglycans, cell-surface proteins, and polysaccharides, etc. have systemic positive effects in the host body (Ale et al. 2019; Aguilar-Toala et al. 2018). These compounds are known as the ‘postbiotics’ (Zendeboodi et al. 2020). Therefore, the beneficial effects of probiotic cells and their derivatives can be covered by using the new terminology ‘paraprobiotics’ and ‘postbiotics’. Considering the research findings and functionalities, Zendeboodi et al. (2020) have proposed a new definition of probiotics as ‘viable or inviable microbial cell (vegetative or spore; intact or ruptured) that is potentially healthful to the host’.
Moreover, considering the targeted diseases remedial capacities of particular probiotic products, several sub-definition of probiotic has been emerged in the recent literature (Martín and Langella 2019). For instance, when a probiotic product targets health benefit of patients suffering from psychiatric illness is termed as psychobiotics (Dinan et al. 2013), immunobiotics when the health improvement is targeting the mucosal immune level (Clancy 2003), probioceuticals/probiotaceuticals when probiotic derived factors, such as reuterin from Lactobacillus reuteri (Howarth 2010), biogenic when products made by or of life forms including secretions and metabolites, and pharmabiotics when bacterial cells of human origin, or their products, with a proven pharmacological role in health or disease (Martín and Langella 2019). These new approaches have boosted the probiotic area during the last few years with direct impacts on consumers and general public.
Recently, the bioactivities of probiotics and probiotic foods have been reviewed by Chugh and Kamal-Eldin (2020) and they mentioned the ability of probiotic bacteria to produce a wide range of metabolites with health benefits to humans. Literature supported that different bioactive compounds such as bacteriocins, metabolic enzymes, amino acids and peptides, short-chain fatty acids, vitamins, antioxidants, anti-inflammatory, and immunomodulating agents, and exopolysaccharides are produced by the probiotic bacteria (Zendeboodi et al. 2020; Barros et al. 2020; Chugh and Kamal-Eldin 2020). Collectively, these molecules enhance the physiological function of the gut and improve the health of consumers by different mechanisms of actions such as preventing pathogen adhesion or colonization, production of metabolites, modulation of the immune system, scavenging free radical reactions, maintaining the cholesterol level, enhancing the synthesis and bioavailability of dietary nutrients and regulating the enzyme actions (Chugh and Kamal-Eldin 2020; Gaisawat et al. 2019; Lopetuso et al. 2019; Kerry et al. 2018a, b; Rowland et al. 2018).
Natural antioxidant-rich compounds and their utilization in yoghurt
Antioxidants are a heterogeneous family of chemical substances that significantly inhibit or delay the oxidation process. Food antioxidants neutralize or inhibit the formation of free radicals and hence reduce their ability to damage host’s cells by interrupting the spread of free radicals. It prevents food products from autoxidation and rancidity. Common food antioxidants are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate, gallic acid (GA), rutin, tocopherols, and so on. On the other hand, the natural antioxidants are those anti-oxidative compounds that are naturally extracted from different plant products including fruits, vegetables, and spices foods (Xu et al. 2017). The use of these natural antioxidants (AOX) in fermented milk products is recommended to maintain a favorable sensory profile until consumption. Besides, such natural antioxidants may also contribute to increasing the health-promoting potential of milk fat globule membrane (MFGM) containing functional foods (Grazyna et al. 2017). Milk contains many particles of colloidal especially fat globules and protein micelles (casein). Fat globules are unique to milk and a major structural element of cells. Each fat globule of milk comprised of a central core containing mostly triglycerides (TG) is enveloped in a layer of surface-material known as the milk fat globule membrane (MFGM) (El-Salam and El-Shibiny 2020; Guerra et al. 2014). Structurally, MFGM is a trilayer (size: 10–50 nm) biological membrane composed mainly of polar lipid and proteins derived from the endoplasmic reticulum, and a bilayer membrane derived from the apical plasma membrane of the mammary epithelial cells that surrounds fat globules when they are secreted (El-Salam and El-Shibiny 2020; Guerra et al. 2014). The structural integrity of MFGM is subjected to change due to several factors such as milk harvesting and handling, physiological factors of animals, physical, mechanical and environmental issues etc. (El-Salam and El-Shibiny, 2020). The MFGM has a wide range of nutritional and health benefits. It is evidenced that MFGM shows stabilizing and emulsifying properties. Moreover, studies showed that MFGM has a beneficial effect on brain development and function, physical performance, intestinal integrity, and gut microbiota; reduces obesity and inflammation, and ameliorates rotavirus and several other infectious diseases (Hintze et al. 2011; Hernell et al. 2016; Ortega-Anaya and Jimenez-Flores 2019;). These properties of MFGM enables the manufacturers to develop MFGM based functional dairy ingredients. Previous studies reported that MFGM contains several numbers of polypeptides (> 50, ranging from 10 to 300 kDa) which are more similar to the protein polypeptides found in the HMGM of human milk (Spitsberg 2005).
Natural antioxidants are generally categorized as phenolic compounds including flavonoids and phenolic acids, vitamins and volatile compounds. There are some common natural antioxidants (Table 2) that are commercially added to several foods include tocopherols, ascorbic acid, soybean products, oat products, components of crude vegetable oils, amino acids, peptides, herbs and spices (Jiang et al. 2014; Aliakbarian et al. 2015; Periche et al. 2015; Ramirez et al. 2015; Remini et al. 2015; Zaccari et al. 2015).
Table 2.
Bioactive compounds from natural sources
| Antioxidant | Natural sources | References |
|---|---|---|
| Vitamin C | Broccoli, orange juice, green chilli, aonla | Remini et al. (2015), Kamal et al. (2019a) and Mondal et al. (2017) |
| Carotenoids and β-carotene | Grains, orange juice, peppermint, spearmint, red carrots | Wibowo et al. (2015) and Zaccari et al. (2015) |
| Lycopene | Tomatoes | Choi et al. (2014) |
| Carotenoids–xanthophyll | Spinach, Zea mays, Carrot | Pasaporte et al. (2014) |
| Polyphenols | Grains, potato, yam, cocoa bean, green tea | Aliakbarian et al. (2015), Ahmed et al. (2019) and Kamal et al. (2020) |
| Flavonoids | Stevia rebaudiana leaves, Berries, honey | Jiang et al. (2014), Periche et al. (2015), Kamal et al. (2019b) |
| Flavanols and flavonols | Vitis vinifera grape berry skins, green tea | Alcalde-Eon et al. (2014) and Bae et al. (2015) |
| Anthocyanin’s | Berries, red cabbage, mulberry | Ramirez et al. (2015) and Xie et al. (2019) |
| Tannins | Mango kernels, pomegranates, strawberries, walnuts | Álvarez-Fernández et al. (2014) and Martins et al. (2014) |
The plant-derived antioxidants are mainly the extracts of grape seed, sage, thyme, rice bran, basil extract, ginger, plum concentrates, aloe vera, mustard, tea catechins, rosemary, etc. And the polyphenolic compounds having two or more phenolic hydroxyl groups are the most active and effective components among these natural antioxidants (Xu et al. 2017). Mango (Mangifera indica L.) can be considered as a good source of dietary antioxidants such as ascorbic acid, carotenoids, and phenolic compounds. β-Carotene is the most abundant carotenoid in several cultivars of mangoes. Besides, mango peel, a major by-product obtained during the processing of mango products, exhibits good antioxidant activity and may serve as a potential source of phenolic with anticancer activity (Kim et al. 2010).
Food fortification is one of the most important processes for the improvement of the nutrient quality and quantity in food. It can be a very cost-effective public health intervention. Due to the high consumption rate of dairy products such as yoghurt, fortification of these products will efficiently decrease or prevent diseases related to nutritional deficiencies. Over the past few years, different food products rich in bioactive compounds and natural antioxidants have been used in yoghurt because of their health beneficial effect. Table 3 represents some examples of natural antioxidants used in milk-based products such as yoghurt, yoghurt drinks, and beverages, dahi, etc. Recently a study conducted by Karaca et al. (2019) where freeze-dried persimmon and apple powder was incorporated into yoghurt, which enhanced the probiotic activities, increased the minerals and fiber with acceptable sensory properties. Studies reported that fortification of yoghurt with moringa extract boosts the fermentation by promoting the growth of LAB, improves the textural and bioactive properties (Zhang et al. 2019). It was also stated in their study that the incorporation of moringa can increase the radical scavenging activity by 40% in a dose-dependent manner up to 21 days. Jrad et al. (2019) identified 15 phenolic acids in Greek yoghurt fortified with date powder. They also claimed that Greek yoghurt fortified with date powder exhibited the highest DPPH radical scavenging activity, Fe2+ chelating capacity, and Fe3+ reducing power with physical, textural, and microbiological stability. The incorporation of cryoconcentrated strawberry pulp (15% and 30%) in the yoghurt resulted in a product with threefold more anthocyanin content and antioxidant activity with enhanced nutritional properties (Jaster et al. 2018). Antioxidative properties (DPPH and ABTS radical scavenging activity) of yoghurt was found to increase with the addition of rice bran (Demirci et al. 2017). A study was conducted by Tang et al. (2019) to search for the potential of cinnamon residues in boosting the antioxidant activity of yoghurt. In this study, extracts and hydrolysates of cinnamon barks, twigs, and leaves were incorporated into yogurt. Results of in-vitro digestion indicated that yogurt prepared by these extracts and hydrolysates drastically increases the antioxidant activity with considerable amounts of phenolic and flavonoids content which might be influenced by the complex of protein–phenolic interactions.
Table 3.
Fortification of yoghurt and yoghurt like products with natural antioxidant rich compounds
| Source of antioxidants | Product type | Milk source | Concentration of antioxidants used | References |
|---|---|---|---|---|
| Green tea | Yoghurt | Bovine milk | 2% of two types green tea (from Japan and Malaysia) | Amirdivani and Baba (2014) |
| Olive and grape pomace | Fermented milk | Skim milk | Olive pomace: 0.73 mg CAE/mL and grape marc: 2.09 mg CAE/mL | Aliakbarian et al. (2015) |
| Grape pomace | Fermented milk | Goat milk |
Grape pomace extract: 2% Whole grape juice: 15% and 17% |
dos Santos et al. (2017) |
| Matricaria recutita L. and Foeniculum vulgare Mill. decoctions | Yoghurt | UHT milk | 40 mg/100 g each | Caleja et al. (2016) |
| Seaweed extracts | Yoghurt | Whole milk | 0.25 and 0.5% | O’Sullivan et al. (2016) |
| Flaxseed powder | Yoghurt | Whole cow milk | 0, 1, 3, and 5% | Ardabilchi Marand et al. (2020) |
| Walnut and flaxseed oil emulsions | Yoghurt | Skimmed milk powder | 2% | Baba et al. (2018) |
| Jumbo squid powder | Yoghurt | UHT milk | 0, 1, 3, 5, 7 or 10 g/100 Ml | Córdova-Ramos et al. (2018) |
| Strawberry pulp | Yoghurt | Pasteurized milk | 15 and 30% | Jaster et al. (2018) |
| Cinnamon residues | Yoghurt | Skim milk | 10% (cinnamon bark, twigs residue, leaves residue) | Tang et al. (2019) |
| Stevia | Yoghurt | Pasteurized milk | 0, 0.25 and 0.5% | de Carvalho et al. (2019) |
| Date powder | Dromedary Greek yogurt | Dromedary cream and dromedary milk | 10% powder | Jrad et al. (2019) |
| Siraitia grosvenorii fruit extract | Probiotic yoghurt | Buffalo milk | 0, 0.5, 1 and 2% | Abdel-Hamid et al. (2020) |
| Rice bran | Yoghurt | 0, 1, 2, and 3% | Demirci et al. (2017) | |
| Moringa extracts | Yoghurt | Skimmed milk powder | 0.05, 0.1 and 0.2% methanolic extract | Zhang et al. (2019) |
| Gnaphalium affine Hao-Xiang | Yoghurt | Skim milk powder | 0.2, 0,.4, 0.6, 0.8, and 1% | Gao et al. (2018) |
| Jujube pulp | Yoghurt | Goat milk | 0, 3, 6, and 9 g/100 g | Feng et al. (2019) |
| Orange fibre | Yoghurt | Cow milk | 0.5, 1, 1.5 and 2% | Erkaya-Kotan (2020) |
| Pomegranate peel and honey | Yoghurt | Whole milk powder |
Pomegranate peel: 0, 2.5, 5 and 10% Honey: 0, 2.5, and 5% |
Kennas et al. (2020) |
| Persimmon and apple powders | Yoghurt | Cow milk | 1% | Karaca et al. (2019) |
| Wood apple powder | Yoghurt | Whole cow milk | 4, 6, and 8% | Parvin et al. (2019) |
Stevia is one of the antioxidant-rich plants, which possesses various pharmaceuticals applications. Recently, de Carvalho et al. (2019) utilized freeze-dried stevia extract (0.25% and 0.50%) in yoghurt. It was observed that the yoghurt matrix preserved the total phenolic content, antioxidant capacity and total solids during storage. However, the addition of 0.50% stevia extracts exerted a buffering effect on the system. After simulated digestion, the total phenolic content and antioxidant activity were found to increase in the fortified yoghurts compared to the undigested parts.
Pomegranate is widely known for its bioactive compounds and antioxidant properties with therapeutic applications. These fruit extracts have been used in many food applications to boost nutritional quality. Pan et al. (2019) recently used pomegranate juice powder (PJP) (1–5%) as a sugar replacer in fermentation matrix during yoghurt production, and studied the physicochemical, structural, and antioxidant properties. Results depicted that higher level of PJP enhanced the yoghurts’ sensory properties, firmer texture, total phenolic and stronger antioxidant properties. Therefore, it is observed that different plant parts including pulp, bark, leaves extracts are very promising to be applied in yoghurt fortification process which could enhance the bioactive compounds, textural properties, antioxidant stability with better sensory properties.
Production and characterization of bioactive peptides
Peptides represent a quite heterogeneous class of compounds and their characteristics are strongly influenced by the amino acid composition and chain length. Bioactive peptides are small molecules with 2–20 amino acids linked by covalent bonds and have many protective properties and bio-functionality (Korhonen and Pihlanto 2007; Mora et al. 2018). Several peptide groups and their bioactivities are shown in Table 4 and Fig. 3a.
Table 4.
Major peptide groups and their bioactivities (Meisel 2004)
| Bioactive peptide group | Protein precursor | Bioactivity | Systems affected |
|---|---|---|---|
| Casomorphins | β- and α-casein | Opioid agonists, ACE-inhibitory, immunomodulatory | Nervous, cardiovascular immune |
| α-Lactorphin | α-Lactalbumin (α-La) | Opioid agonists, ACE-inhibitory | Nervous, cardiovascular |
| β-Lactorphin | β-Lactoglobulin (β-Lg) | Opioid agonists, ACE-inhibitory, smooth muscle contraction (Ileum) | Nervous, cardiovascular, digestive |
| Lactoferroxins | Lactoferrin | Opioid antagonists | Nervous |
| Casoxins | κ-Casein | Opioid antagonists, ACE-inhibitory, some smooth muscle contraction | Nervous, cardiovascular, digestive |
| Casokinins | β- and α-casein | Antihypertensive, immunomodulatory, cytomodulatory | Immune, cardiovascular |
| Casoplatelins | κ-casein, transferrin | Antithrombiotic | Cardiovascular |
| Immunopeptides | β- and α-casein | Immunomodulatory | Immune |
| Phosphopeptides | β- and α-casein | Mineral carriers | Digestive |
| Lactoferricin | Lactoferrin | Antimicrobial, immunomodulatory | Immune, digestive |
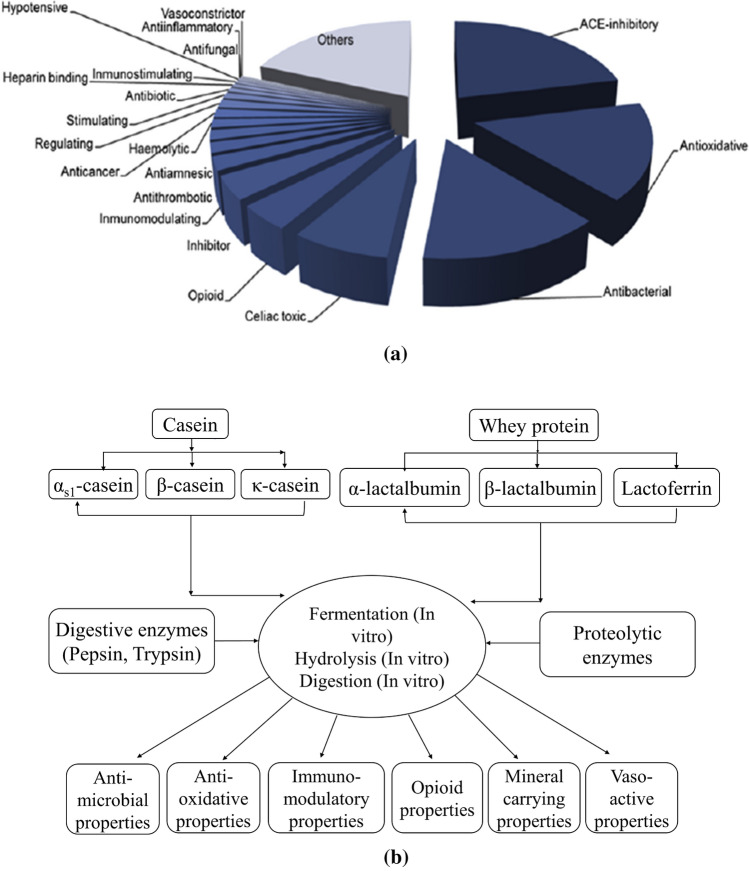
Fig. 3.
a bioactivities of peptides (Mora et al. 2018) and b formation of bioactive compound from major milk proteins (Korhonen and Pihlanto 2007)
In the present days, the demand for functional foods has increased world-wide because of the development of processing and production technologies with people's lifestyle and awareness about the importance of bioactive peptides as health-promoting ingredients (Tadesse and Emire 2020). Bioactive peptides cover several functions based on their sequences and chain lengths. Some bioactivities (Fig. 3a) of peptides have been included in the gastrointestinal system such as the anti-obesity and satiety peptides, the cardiovascular system such as antihypertensive, antithrombotic, antioxidative, hypocholesterolemic peptides and angiotensin-converting enzyme (ACE) inhibitor peptides, the immune system such as antimicrobial, cytomodulatory and immune-modulatory peptides, and the nervous system such as opioid peptides (Mora et al. 2018; Mada et al. 2020).
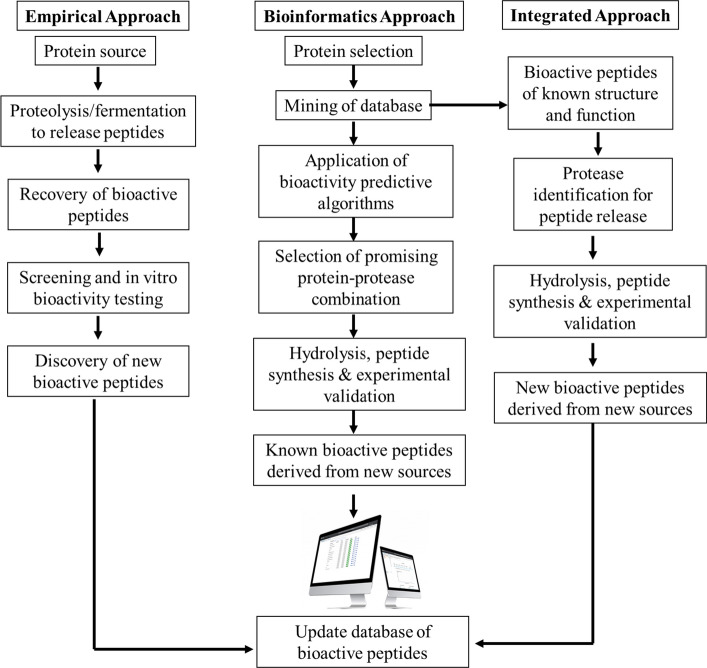
In the last few years, functional food-derived bioactive peptides are gaining interest from researchers due to their antidiabetic and safety issues that regulate sugar absorption and insulin level in the body. ACE inhibitor peptides play a crucial role in the regulation of blood pressure i.e., hypertension by enhancing the conversion of angiotensin I to the potent vasoconstrictor angiotensin II as well as inactivates the vasodilator bradykinin (Daliri et al. 2018). Peptides sequences (e.g., Ile-Pro-Pro and Val-Pro-Pro, ArgTyr-Leu-Gly-Tyr, Ala-Tyr-Phe-Tyr-Pro-Glu-Lue, and Tyr-Gln-Lys-Phe-Pro-Gln-Tyr) found in dairy products such as sour milk, whey hydrolysates, cheese, and some fermented products showed the strongest ACE inhibitor activities (Sultan et al. 2018). Over the last few decades, different degenerative diseases such as hypertension, cancer, cardiovascular diseases, inflammations, diabetes mellitus, neurodegenerative diseases, etc. have been arisen due to the action of free radicals, such as singlet oxygen (1O2), peroxyl radical (RCOO·), hydroxyl radical (·OH), superoxide anion (O2·−), hydrogen peroxide (H2O2), and peroxynitrite (ONOO−) mediated lipid oxidation and oxidative stress (Sah et al. 2016; Sultan et al. 2018; Daliri et al. 2018; Mada et al. 2020). Interestingly, different antioxidative peptides have been discovered that can mitigate the pathophysiological effects of these free radicals. The antioxidant or free radical scavenging activity of bioactive peptides from different products can be evaluated in many ways such as DPPH· and ABTS· scavenging activity, ferric reducing power, nitric oxide scavenging activity, metal chelating activity, and so on. Several antioxidative peptide sequences such as Ser-Asp-Arg-Asp-Leu-Leu-Gly-Pro-Asp-Glu-Glu-Gln-Tyr-Leu-Pro-Lys from hydrolyzed protein of Pennisetum glaucum with digestive enzymes (Agrawal et al. 2016); Glu-Ser-Thr-Val-Pro-Glu-Arg-Thr-HisPro-Ala-Cys-Pro-Asp-Phe-Asn from hydrolysis of hoki frame protein (Kim et al., 2007); Asp-His-Thr-Lys-Glu, Phe-Phe-Glu-Phe-His and Met-Pro-Asp-AlaHis-Leu (Liu et al. (2015); Ala-Glu-GluArg-Tyr-Pro and Asp-Glu-Asp-Thr-Gln-Ala-Met-Pro from hydrolyzed chicken egg (Nimalaratne et al., 2015); and Asp-Cys-Gly-Tyr and Asn-Tyr-Asp-Glu-Tyr (Fan et al., 2012) showed the strongest oxygen and free radical scavenging activities. However, the amino acid composition, chain length, and sequence of the protein significantly determine the existence of antioxidant peptides with required bioactivities (Tejano et al., 2019; Sultan et al. 2016). Furthermore, opioid peptides function as neuro-hormones and neurotransmitters involved in stress reactions, nociception control, sedation, breathing tone, depression, hypotension, appetite, gastrointestinal digestion, and other physiological actions (Daliri et al. 2018). Another diverse group of peptides is the antimicrobial peptides that work as the first line of defense of the host against different microorganisms e.g., bacteria (Listeria, Salmonella, Escherichia, Staphylococcus and Helicobacter), yeasts, fungi, viruses, and even cancer cells. Different literatures mentioned that milk lactoferrin and casein derivatives, α-lactalbumin and β-lactoglobulin, etc. showed the strongest antimicrobial activities (Sah et al. 2016; Daliri et al. 2018; Sultan et al. 2018). However, the functionalities of the antimicrobial peptides are governed by the membrane permeability, charge affinity, cellular structures, and composition, and mostly the hydrophobicity that may enhance its antimicrobial activity against targeted microorganisms (Sah et al. 2016). These biological functionalities of peptides depend on several factors including, sources of proteins, types of enzymes, pretreatment of protein sources (hydrostatic pressure, ultrasound, microwave, pulse electric field), substrate/enzyme ratio, operational conditions like time, temperature, pH, degree of hydrolysis, peptide structure, types of amino acids, molecular weights and so on (Bhandari et al. 2020). Most of the bioactive peptides are obtained from animal sources like dairy-based products, basically from milk, meats, and egg proteins. Plant sources include soy, oat, pulses (chickpea, beans, peas, and lentils), canola, wheat, flaxseed, and hemp seed (Chakrabarti et al. 2018). Furthermore, proteins from marine sources e.g., fish, squid, salmon, sea urchin, oyster, seahorse, and snow crab have also been used (Moller et al. 2008). Bioactive peptides from diverse protein sources are generally produced in three different ways (Sultan et al. 2018). These methodologies are (i) hydrolysis by enzymes from plants or microorganisms (ii) fermentation with LAB (especially proteolytic cultures) (iii) breakdown by digestive enzymes. The combination of these methodologies has been found to be beneficial in the production of bioactive peptides (Korhonen and Pihlanto 2006). A brief of these methods has been illustrated in Fig. 3b. Peptide production through hydrolysis involves the breakdown of parent proteins by enzymes like pepsin, trypsin, alkalase, thermolysin, pancreatin, etc. (Ferreira et al. 2007). This method is considered to be more suitable as compared to microbial fermentation as it is easy to adapt, short reaction time, and predictable (Bhandari et al. 2020). The bio-functionality and peptide sequence depend on the types of enzymes used. In the fermentation process, different probiotic microorganisms especially LAB, yeasts, molds, and fungi are used to hydrolyze the proteins using the enzymes produced by the microbes themselves (Sultan et al. 2018). Also, the combination of different microbes i.e., co-cultures can be used to promote the hydrolysis processes. In this process, microbial strains, fermentation time, and protein sources play important roles in determining the degree of fermentation (Chakrabarti et al. 2018). Protein breakdown by digestive enzymes occurs during the digestion of protein foods in the gastrointestinal tracts due to the action of HCl and pepsin enzymes and sometimes due to the action of gut microbial flora (Moller et al. 2008; Sultan et al. 2018). In a review, Daliri et al. (2018) accumulated and described the major approaches in bioactive peptide discovery and classified them as empirical, bioinformatics, and integrated systems. These approaches have been summarized in Fig. 4.
Fig. 4.
Approaches towards the discovering of bioactive peptides.
Adapted from Daliri et al. (2018)
Several promising technologies have emerged over the few years that can reduce the processing time and costs with an improved yield of bioactive peptides. Among such technologies, the microwave, ultrasound-assisted, high hydrostatic pressure, pulsed electric field, and subcritical water processing have been raised as the effective and potential on the production of antioxidative peptides to reduce the drawbacks of conventional production techniques (Dong et al. 2019; Gohi et al. 2019; Wen et al. 2019; Yang et al. 2017; Tadesse and Emire 2020).
Modern science has developed several techniques to characterize bioactive peptides derived from different products. These techniques include two-dimensional gel electrophoresis, reverse-phase high-performance liquid chromatography, and matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) which have been used to investigate the degradation of milk proteins by different enzymes (Manso et al. 2005;). High-performance liquid chromatography (HPLC) in conjunction with tandem mass spectrometry has been used to identify biologically active peptides by milk fermentation particularly, Angiotensin-I-converting enzyme (ACE) inhibitory peptides (Hernández-Ledesma et al. 2004). Also, confocal microscopy and transmission electron microscopy has been used to examine the mode of action of antimicrobial milk peptides (Van Der Kraan et al. 2004). Nuclear magnetic resonance (NMR) is used to determine the structure of organic molecules and biomolecules in solution. NMR could be used to characterize bioactive peptides. Few studies have been undertaken to examine the structure of bioactive peptides derived from milk; however, natural bioactive peptides have been characterized by this technique as well as some of the milk proteins.
Recently, Agyei et al. (2019) reviewed structure-informed detection and quantification of peptides in food and biological fluids where they mentioned the capillary zone electrophoresis and chromatographic techniques. These techniques are mainly applied for identifying the physical or chemical stabilities, or the bioanalysis of peptides at the highest degree of their sensitivity and amino acid sequences. On the other hand, MS-based techniques have been gaining popularity among researchers in the structure-informed identification and quantification of peptides due to their sensitivity, specificity, and high-throughput analytical capabilities guaranteeing reproducibility (Dallas et al. 2015). Besides, simple ultrafiltration with a 5-kDa MWCO followed by nano-UPLC analysis coupled to high-resolution MS/MS plays an important role by improvements in the analytical instrumentation that allowed to directly examine 17 short peptide sequences without any chemical derivatization (O’Keeffe and Fitzgerald 2015; Capriotti et al. 2016). Solid-phase peptide synthesis process involves four stages: anchoring, de-protection, coupling reaction, and cleavage (Howl 2005). In vivo and in vitro peptide synthesis allows the investigation of the bioactivity and potentially the mechanism of a purified peptide for diversified applications.
Although bioactive peptides have gained a remarkable place in the recent research trims, many issues have been emerged to ensure their safety and toxicity. For instance, intestinal wall disruption, erythrocytes, and lymphocytes toxicity, free radical production, enzymopathic and immunopathic tissue damage, and cytotoxicity are the prime concerns in the biological system. Consumption of such peptides may lead to various health complications (Bhandari et al. 2020). Therefore, it is necessary to ensure the toxicity level of a peptide to be used in the functional food formulations and also for therapeutic purposes.
Conclusion and the future dimension of researches
Bioactive functional foods have occupied a promising place in the modern food markets owing to their roles against oxidative stress and different bio-physiological disorders. Yoghurt is consumed as a functional food due to its high nutritional contents, and its health benefits can be further boosted by adding probiotic bacterial strains and bioactive compounds. Besides, yoghurt fortification with natural compounds will enhance the antioxidative properties thus it could help to utilize the natural resources. Therefore, the fortification of yoghurt with bioactive compounds could be an interesting and potential research aspect in current situations. Besides, by developing a project and/or installation of commercial industries for the production of bioactive peptides from fermented milk products, the spoilage of milk can be reduced to a greater extent, and a new industry in agricultural farming can also be planned. An integrated initiative might provide employment opportunities to a wide range of people that can contribute to the GDP as well as the national socio-economic development of a country. Although the bioactive peptide has the potential to be used in the production of functional foods with health-promoting properties, safety issues should be confirmed first. Moreover, an in-depth research in this arena is needed to evaluate the health-promoting effects and the bioavailability of functional foods-originated bioactive peptides in human subjects to expose their molecular mechanistic interaction.
Authors' contribution
M. A. Ali: Conceived idea, draft preliminary version and collection of literatures. M. M. Kamal: Conceptualization, collection of literatures, writing the original draft and final version of the manuscript. M. H. Rahman: Revision and proofreading of final version. M. N. Siddiqui: Revising, provided critical feedback and helped shape the manuscript and proofreading. M. A. Haque: Revision and reshaping of the manuscript. K. K. Saha: Revision and reshaping of the manuscript. M. A. Rahman: Planning, writing the draft, overall supervision.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Md. Aslam Ali and Md. Mostafa Kamal have contributed equally to this work and are joint first author.
References
- Abdel-Hamid M, Romeih E, Huang Z, et al. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2020;303:125400. doi: 10.1016/j.foodchem.2019.125400. [DOI] [PubMed] [Google Scholar]
- Agamennone V, Krul CAM, Rijkers G, Kort R. A practical guide for probiotics applied to the case of antibiotic-associated diarrhea in the Netherlands. Pharm Weekbl. 2019;154:17–24. doi: 10.1186/s12876-018-0831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal H, Joshi R, Gupta M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016;204:365–372. doi: 10.1016/j.foodchem.2016.02.127. [DOI] [PubMed] [Google Scholar]
- Aguilar-Toala JE, Garcia-Varela R, Garcia HS, Mata-Haro V, Gonzalez-Cordova AF, Vallejo-Cordoba B, Hernandez-Mendoza A. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol. 2018;75:105–114. [Google Scholar]
- Agyei D, Pan S, Acquah C, Bekhit AEDA, Danquah MK. Structure-informed detection and quantification of peptides in food and biological fluids. J Food Biochem. 2019;43:e12482. doi: 10.1111/jfbc.12482. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Jiang GH, Park JS, et al. Effects of ultrasonication, agitation and stirring extraction techniques on the physicochemical properties, health-promoting phytochemicals and structure of cold-brewed coffee. J Sci Food Agric. 2019;99:290–301. doi: 10.1002/jsfa.9186. [DOI] [PubMed] [Google Scholar]
- Alcalde-Eon C, García-Estévez I, Martín-Baz A, et al. Anthocyanin and flavonol profiles of Vitis vinifera L. cv Rufete grapes. Biochem Syst Ecol. 2014;53:76–80. [Google Scholar]
- Ale EC, Batistela VA, Olivar GC, Ferrado JB, Sadiq S, Ahmed HI, Reinheimer JA, Vera-Candioti L, Laws AP, Binetti AG. Statistical optimisation of the exopolysaccharide production by Lactobacillus fermentum Lf2 and analysis of its chemical composition. Int J Dairy Technol. 2019;70:1–12. doi: 10.1111/1471-0307.12639. [DOI] [Google Scholar]
- Aliakbarian B, Casale M, Paini M, et al. Production of a novel fermented milk fortified with natural antioxidants and its analysis by NIR spectroscopy. LWT Food Sci Technol. 2015;62:376–383. [Google Scholar]
- Álvarez-Fernández MA, Hornedo-Ortega R, Cerezo AB, et al. Effects of the strawberry (Fragaria ananassa) purée elaboration process on non-anthocyanin phenolic composition and antioxidant activity. Food Chem. 2014;164:104–112. doi: 10.1016/j.foodchem.2014.04.116. [DOI] [PubMed] [Google Scholar]
- Amirdivani S, Baba ASH. Green tea yogurt: major phenolic compounds and microbial growth. J Food Sci Technol. 2014;52:4652–4660. doi: 10.1007/s13197-014-1670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardabilchi Marand M, Amjadi S, Ardabilchi Marand M, et al. Fortification of yogurt with flaxseed powder and evaluation of its fatty acid profile, physicochemical, antioxidant, and sensory properties. Powder Technol. 2020;359:76–84. [Google Scholar]
- Arora T, Singh S, Sharma RK. Probiotics: interaction with gut microbiome and antiobesity potential. Nutrition. 2013;29:591–596. doi: 10.1016/j.nut.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Baba WN, Jan K, Punoo HA, et al. Techno-functional properties of yoghurts fortified with walnut and flaxseed oil emulsions in guar gum. LWT Food Sci Technol. 2018;92:242–249. [Google Scholar]
- Bae IK, Ham HM, Jeong MH, et al. Simultaneous determination of 15 phenolic compounds and caffeine in teas and mate using RP-HPLC/UV detection: method development and optimization of extraction process. Food Chem. 2015;172:469–475. doi: 10.1016/j.foodchem.2014.09.050. [DOI] [PubMed] [Google Scholar]
- Barros CP, Guimaraes JT, Esmerino EA, Duarte MCKH, Silva MC, Silva R, Ferreira BM, Sant’Ana AS, Freitas MQ, da Cruz AG. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr Opin Food Sci. 2020;32:1–8. [Google Scholar]
- Bhandari D, Rafiq S, Gat Y, et al. A review on bioactive peptides: physiological functions, bioavailability and safety. Int J Pept Res Ther. 2020;26:139–150. [Google Scholar]
- Brown L, Poudyal H, Panchal SK. Functional foods as potential therapeutic options for metabolic syndrome. Obes Rev. 2015;16:914–941. doi: 10.1111/obr.12313. [DOI] [PubMed] [Google Scholar]
- Caleja C, Barros L, Antonio AL, et al. Fortification of yogurts with different antioxidant preservatives: a comparative study between natural and synthetic additives. Food Chem. 2016;210:262–268. doi: 10.1016/j.foodchem.2016.04.114. [DOI] [PubMed] [Google Scholar]
- Capriotti AL, Cavaliere C, Piovesana S, Samperi R, Laganà A. Recent trends in the analysis of bioactive peptides in milk and dairy products. Anal Bioanal Chem. 2016;408:2677–2685. doi: 10.1007/s00216-016-9303-8. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Guha S, Majumder K. Food-derived bioactive peptides in human health: challenges and opportunities. Nutrients. 2018;10:1–17. doi: 10.3390/nu10111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang G, Song JH, et al. Probiotic yeast inhibits VEGFR signaling and angiogenesis in intestinal inflammation. PLoS ONE. 2013;8:1–7. doi: 10.1371/journal.pone.0064227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim DS, Kozukue N, et al. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. J Food Compos Anal. 2014;34:115–127. [Google Scholar]
- Chugh B, Kamal-Eldin A. Bioactive compounds produced by probiotics in food products. Curr Opin Food Sci. 2020;32:76–82. [Google Scholar]
- Clancy R. Immunobiotics and the probiotic evolution. FEMS Immunol Med Microbiol. 2003;38:9–12. doi: 10.1016/S0928-8244(03)00147-0. [DOI] [PubMed] [Google Scholar]
- Córdova-Ramos JS, Gonzales-Barron U, Cerrón-Mallqui LM. Physicochemical and sensory properties of yogurt as affected by the incorporation of jumbo squid (Dosidicus gigas) powder. LWT - Food Sci Technol. 2018;93:506–510. [Google Scholar]
- Daliri EBM, Lee BH, Oh DH. Current trends and perspectives of bioactive peptides. Crit Rev Food Sci Nutr. 2018;58:2273–2284. doi: 10.1080/10408398.2017.1319795. [DOI] [PubMed] [Google Scholar]
- Dallas DC, Guerrero A, Parker EA, Robinson RC, Gan J, German JB, Lebrilla CB. Current peptidomics: applications, purification, identification, quantification, and functional analysis. Proteomics. 2015;15(5–6):1026–1038. doi: 10.1002/pmic.201400310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho MW, Arriola NDA, Pinto SS, et al. Stevia-fortified yoghurt: stability, antioxidant activity and in vitro digestion behaviour. Int J Dairy Technol. 2019;72:57–64. [Google Scholar]
- Demirci T, Aktaş K, Sözeri D, et al. Rice bran improve probiotic viability in yoghurt and provide added antioxidative benefits. J Funct Foods. 2017;36:396–403. [Google Scholar]
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Dixit Y, Wagle A, Vakil B. Patents in the field of probiotics, prebiotics, symbiotic: a review. J Food Microbiol Saf Hyg. 2016;01:1–13. [Google Scholar]
- Dong X, Li J, Jiang G, Li H, Zhao M, Jiang Y. Effects of combined high pressure and enzymatic treatments on physicochemical and antioxidant properties of peanut proteins. Food Sci Nutr. 2019;7(4):1417–1425. doi: 10.1002/fsn3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos KMO, de Oliveira IC, Lopes MAC, et al. Addition of grape pomace extract to probiotic fermented goat milk: the effect on phenolic content, probiotic viability and sensory acceptability. J Sci Food Agric. 2017;97:1108–1115. doi: 10.1002/jsfa.7836. [DOI] [PubMed] [Google Scholar]
- Eid R, Jakee EJ, Rashidy A, et al. Potential antimicrobial activities of probiotic lactobacillus strains isolated from raw milk. J Probiotics Heal. 2016;04:2–9. [Google Scholar]
- El-Fattah AA, Sakr S, El-Dieb S, Elkashef H. Developing functional yogurt rich in bioactive peptides and gamma-aminobutyric acid related to cardiovascular health. LWT Food Sci Technol. 2018;98:390–397. [Google Scholar]
- El-Salam MHABD, El-Shibiny S. Milk fat globule membrane: an overview with particular emphasis on its nutritional and health benefits. Int J Dairy Technol. 2020 doi: 10.1111/1471-0307.12730. [DOI] [Google Scholar]
- El-Shafei SMS, Sakr SS, Abou-Soliman NHI. The impact of supplementing goats' milk with quinoa extract on some properties of yoghurt. Int J Dairy Technol. 2019 doi: 10.1111/1471-0307.12628. [DOI] [Google Scholar]
- Erkaya-Kotan T. In vitro angiotensin converting enzyme (ACE)-inhibitory and antioxidant activity of probiotic yogurt incorporated with orange fibre during storage. J Food Sci Technol. 2020;57:2343–2353. doi: 10.1007/s13197-020-04272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, He J, Zhuang Y, Sun L. Purification and identification of antioxidant peptides from enzymatic hydrolysates of Tilapia (Oreochromis niloticus) frame protein. Molecules. 2012;17(11):12836–12850. doi: 10.3390/molecules171112836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Wang B, Zhao A, et al. Quality characteristics and antioxidant activities of goat milk yogurt with added jujube pulp. Food Chem. 2019;277:238–245. doi: 10.1016/j.foodchem.2018.10.104. [DOI] [PubMed] [Google Scholar]
- Ferreira IMPLVO, Pinho O, Mota MV, et al. Preparation of ingredients containing an ACE-inhibitory peptide by tryptic hydrolysis of whey protein concentrates. Int Dairy J. 2007;17:481–487. [Google Scholar]
- Gaisawat MB, Iskandar MM, MacPherson CW, Tompkins TA, Kubow S. Probiotic supplementation is associated with increased antioxidant capacity and copper chelation in C. difficile-infected fecal water. Nutrients. 2019;11:2007. doi: 10.3390/nu11092007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HX, Yu ZL, He Q, et al. A potentially functional yogurt co-fermentation with Gnaphalium affine. LWT Food Sci Technol. 2018;91:423–430. [Google Scholar]
- Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastro Hepat. 2010;2010(7):503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohi BFCA, Du J, Zeng H-Y, Cao X, Zou KM. Microwave pretreatment and enzymolysis optimization of the Lotus seed protein. Bioengineering. 2019;6(2):1–13. doi: 10.3390/bioengineering6020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazyna C, Hanna C, Adam A, Magdalena BM. Natural antioxidants in milk and dairy products. Int J Dairy Technol. 2017;70:1–14. [Google Scholar]
- Guerra E, Gori A, Cevoli C, Losi G, Caboni MF. Lipid fraction of creams collected in the Parmigiano-Reggiano cheese production area in response to extruded linseed supplementation of dairy cows’ diets: GC-FID and FT-MIR evaluation. Int J Dairy Technol. 2014;67(4):510–520. [Google Scholar]
- Hadjimbei E, Botsaris G, Goulas V, Alexandri E, Gekas V, Gerothanassis IP. Functional stability of goats' milk yoghurt supplemented with Pistacia atlantica resin extracts and Saccharomyces boulardii. Int J Dairy Technol. 2019 doi: 10.1111/1471-0307.12629. [DOI] [Google Scholar]
- Hernández-Ledesma B, Amigo L, Ramos M, Recio I. Application of high-performance liquid chromatography-tandem mass spectrometry to the identification of biologically active peptides produced by milk fermentation and simulated gastrointestinal digestion. J Chromatogr A. 2004;1049:107–114. [PubMed] [Google Scholar]
- Hernell O, Timby N, Domellof M, Lonnerdal B. Clinical benefits of milk fat globule membranes for infants and children. J Ped. 2016;173S:60–65. doi: 10.1016/j.jpeds.2016.02.077. [DOI] [PubMed] [Google Scholar]
- Hintze KJ, Dallin S, Burtenshaw I, Ward RE (2011) Nutraceutical properties of milk fat globular membrane. In: Elnashar M (ed) Biotechnology and biopolymers, 2004–2013 InTech Open Access Company
- Howarth GS. Probiotic-derived factors: probiotaceuticals? J Nutr. 2010;140:229–230. doi: 10.3945/jn.109.118844. [DOI] [PubMed] [Google Scholar]
- Howl J. Peptide synthesis and applications. First: Human Press; 2005. [Google Scholar]
- Hsieh YHP, Ofori JA (2010) Advances in biotechnology for the production of functional foods. In: Bagchi D, Lau FC, Ghosh DK (eds) Biotechnology in functional foods and nutraceuticals. CRC Press, Boca Raton
- Jaster H, Arend GD, Rezzadori K, et al. Enhancement of antioxidant activity and physicochemical properties of yogurt enriched with concentrated strawberry pulp obtained by block freeze concentration. Food Res Int. 2018;104:119–125. doi: 10.1016/j.foodres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Jiang J, Yuan X, Wang T, et al. Antioxidative and cardioprotective effects of total flavonoids extracted from Dracocephalum moldavica L. against acute ischemia/reperfusion-induced myocardial injury in isolated rat heart. Cardiovasc Toxicol. 2014;14:74–82. doi: 10.1007/s12012-013-9221-3. [DOI] [PubMed] [Google Scholar]
- Jrad Z, Oussaief O, Bouhemda T, et al. Potential effects of ultrafiltration process and date powder on textural, sensory, bacterial viability, antioxidant properties and phenolic profile of dromedary Greek yogurt. Int J Food Sci Technol. 2019;54:854–861. [Google Scholar]
- Kamal MM, Ali MR, Rahman MM, et al. Effects of processing techniques on drying characteristics, physicochemical properties and functional compounds of green and red chilli (Capsicum annum L.) powder. J Food Sci Technol. 2019;56:3185–3194. doi: 10.1007/s13197-019-03733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal MM, Rashid MHU, Mondal SC, et al. Physicochemical and microbiological characteristics of honey obtained through sugar feeding of bees. J Food Sci Technol. 2019;56:2267–2277. doi: 10.1007/s13197-019-03714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal MM, Ali MR, Shishir MRI, Mondal SC. Thin-layer drying kinetics of yam slices, physicochemical, and functional attributes of yam flour. J Food Process Eng. 2020;43:e13448. [Google Scholar]
- Karaca OB, Saydam İB, Güven M. Physical, chemical, and sensory attributes of low-fat, full-fat, and fat-free probiotic set yogurts fortified with fiber-rich persimmon and apple powders. J Food Process Preserv. 2019;43:1–13. [Google Scholar]
- Karelakis C, Zevgitis P, Galanopoulos K, Mattas K. Consumer trends and attitudes to functional foods. J Int Food Agrib Market. 2019 doi: 10.1080/08974438.2019.1599760. [DOI] [Google Scholar]
- Kaur A, Scarborough P, Rayner M. A systematic review, and meta-analyses, of the impact of health-related claims on dietary choices. Int J Behavioral Nutr Phys Act. 2017;14(1):93. doi: 10.1186/s12966-017-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennas A, Amellal-Chibane H, Kessal F, Halladj F. Effect of pomegranate peel and honey fortification on physicochemical, physical, microbiological and antioxidant properties of yoghurt powder. J Saudi Soc Agric Sci. 2020;19:99–108. [Google Scholar]
- Kerry RG, Patra JK, Gouda S, et al. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26:927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry RG, Patra JK, Gouda S, Park Y, Shin HS, Das G. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26:927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaledabad MA, Ghasempour Z, Kia EM, Bari MR, Zarrin R. Probiotic yoghurt functionalised with microalgae and Zedo gum: chemical, microbiological, rheological and sensory characteristics. Int J Dairy Technol. 2019 doi: 10.1111/1471-0307.12625. [DOI] [Google Scholar]
- Kim SY, Je JY, Kim SK. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J Nutr Biochem. 2007;18(1):31–38. doi: 10.1016/j.jnutbio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Kim H, Moon JY, Kim H, et al. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010;121:429–436. [Google Scholar]
- Konstantinidi M, Koutelidakis AE. Functional foods and bioactive compounds: a review of its possible role on weight management and obesity’s metabolic consequences. Medicines. 2019;6(94):1–24. doi: 10.3390/medicines6030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen H, Pihlanto A. Bioactive peptides: production and functionality. Int Dairy J. 2006;16:945–960. [Google Scholar]
- Korhonen H, Pihlanto A (2007) Bioactive peptides from food proteins. In: Food products manufacturing, pp 5–37
- Levy J (2019) Functional foods benefits and how to add them to your diet. In: Dr. Axe. https://draxe.com/nutrition/functional-foods-benefits/. Accessed 14 Aug 2020
- Liu J, Jin Y, Lin S, Jones GS, Chen F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015;175:258–266. doi: 10.1016/j.foodchem.2014.11.142. [DOI] [PubMed] [Google Scholar]
- Lopetuso LR, Giorgio ME, Saviano A, Scaldaferri F, Gasbarrini A, Cammarota G. Bacteriocins and bacteriophages: therapeutic weapons for gastrointestinal diseases. Int J Mol Sci. 2019;20:183. doi: 10.3390/ijms20010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucatto JN, Silva-Buzanello RAD, De Mendonca SNTG, Lazarotto TC, Sanchez JL, Bona E, Drunkler DA. Performance of different microbial cultures in potentially probiotic and prebiotic yoghurts from cow and goat milks. Int J Dairy Technol. 2019;70:1–13. doi: 10.1111/1471-0307.12655. [DOI] [Google Scholar]
- Mada SB, Ugwu CP, Abarshi MM. Health promoting effects of food-derived bioactive peptides: a review. Int J Peptide Res Therap. 2020;26:831–848. [Google Scholar]
- Manso MA, Léonil J, Jan G, Gagnaire V. Application of proteomics to the characterisation of milk and dairy products. Int Dairy J. 2005;15:845–855. [Google Scholar]
- Martín R, Langella P. Emerging health concepts in the probiotics field: streamlining the definitions. Front Microbiol. 2019;10:1–5. doi: 10.3389/fmicb.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A, Barros L, Carvalho AM, et al. Phenolic extracts of Rubus ulmifolius Schott flowers: characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014;5:1091–1100. doi: 10.1039/c3fo60721f. [DOI] [PubMed] [Google Scholar]
- Meisel H. Multifunctional peptides encrypted in milk proteins. BioFactors. 2004;21:55–61. doi: 10.1002/biof.552210111. [DOI] [PubMed] [Google Scholar]
- Moller NP, Scholz-Ahrens KE, et al. Bioactive peptides and proteins from foods: indication for health effects. Eur J Nutr. 2008;47:171–182. doi: 10.1007/s00394-008-0710-2. [DOI] [PubMed] [Google Scholar]
- Mondal SC, Kamal MM, Mumin MIA, Hosain MM, Ali MR. Effect of sucrose on the physicochemical properties, organoleptic qualities and shelf-life stability of aonla (Emblica officinalis) candy. IOSR J Environ Sci Toxicol Food Technol. 2017;11:85–94. [Google Scholar]
- Mora L, Aristoy MC, Toldrá F (2018) Bioactive peptides. Encycl Food Chem 381–389 [DOI] [PubMed]
- Muro Urista C, Álvarez Fernández R, Riera Rodriguez F, et al. Review: production and functionality of active peptides from milk. Food Sci Technol Int. 2011;17:293–317. doi: 10.1177/1082013211398801. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Truong DH, Kouhoundé S, et al. Biochemical engineering approaches for increasing viability and functionality of probiotic bacteria. Int J Mol Sci. 2016 doi: 10.3390/ijms17060867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimalaratne C, Bandara N, Wu J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015;188:467–472. doi: 10.1016/j.foodchem.2015.05.014. [DOI] [PubMed] [Google Scholar]
- O’Keeffe MB, FitzGerald RJ. Identification of short peptide sequences in complex milk protein hydrolysates. Food Chem. 2015;184:140–146. doi: 10.1016/j.foodchem.2015.03.077. [DOI] [PubMed] [Google Scholar]
- O’Sullivan AM, O’Grady MN, O’Callaghan YC, et al. Seaweed extracts as potential functional ingredients in yogurt. Innov Food Sci Emerg Technol. 2016;37:293–299. [Google Scholar]
- Oelschlaeger TA. Mechanisms of probiotic actions—a review. Int J Med Microbiol. 2010;2010(300):57–62. doi: 10.1016/j.ijmm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Onyenweaku F, Obeagu EI, Ifediora AC, Nwandikor UU. of innovative and applied research article health benefits of probiotics. Int J Innov Appl Res. 2016;4:21–30. [Google Scholar]
- Ortega-Anaya J, Jimenez-Flores R. The relevance of bovine milk phospholipids in human nutrition—evidence of the effect on infant gut and brain development. J Dairy Sci. 2019;102:2738–2748. doi: 10.3168/jds.2018-15342. [DOI] [PubMed] [Google Scholar]
- Ovando CA, de Carvalho JC, de Melo V, Pereira G, et al. Functional properties and health benefits of bioactive peptides derived from spirulina: a review. Food Rev Int. 2018;34:34–51. [Google Scholar]
- Pan LH, Liu F, Luo SZ, Luo JP. Pomegranate juice powder as sugar replacer enhanced quality and function of set yogurts: structure, rheological property, antioxidant activity and in vitro bioaccessibility. LWT Food Sci Technol. 2019;115:108479. [Google Scholar]
- Pariza MW, Park Y, Cook ME. Conjugated linoleic acid and the control of cancer and obesity. Toxicol Sci. 1999;52:107–110. doi: 10.1093/toxsci/52.suppl_1.107. [DOI] [PubMed] [Google Scholar]
- Parvin I, Haque MA, Akter F, et al. Preparation of low calorie and shelf-life extended yogurt by mixing wood apple powder in the formulation. J Food Process Preserv. 2019;43:1–12. [Google Scholar]
- Pasaporte MS, Rabaya FJR, Toleco MM, Flores DM. Xanthophyll content of selected vegetables commonly consumed in the Philippines and the effect of boiling. Food Chem. 2014;158:35–40. doi: 10.1016/j.foodchem.2014.02.090. [DOI] [PubMed] [Google Scholar]
- Periche A, Castelló ML, Heredia A, Escriche I. Influence of drying method on steviol glycosides and antioxidants in Stevia rebaudiana leaves. Food Chem. 2015;172:1–6. doi: 10.1016/j.foodchem.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Ramirez JE, Zambrano R, Sepúlveda B, et al. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC-HR-ESI-ToF-MS. Food Chem. 2015;176:106–114. doi: 10.1016/j.foodchem.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Remini H, Mertz C, Belbahi A, et al. Degradation kinetic modelling of ascorbic acid and colour intensity in pasteurised blood orange juice during storage. Food Chem. 2015;173:665–673. doi: 10.1016/j.foodchem.2014.10.069. [DOI] [PubMed] [Google Scholar]
- Roobab U, Batool Z, Manzoor MF, Shabbir MA, Khan MR, Aadil RM. Sources, formulations, advanced delivery and health benefits of probiotics. Curr Opin Food Sci. 2020;32:17–28. [Google Scholar]
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Antioxidative and antibacterial peptides derived from bovine milk proteins. Crit Rev Food Sci Nutr. 2016 doi: 10.1080/10408398.2016.1217825. [DOI] [PubMed] [Google Scholar]
- Sani IK, Khaledabad MA, Pirsa S, Kia EM. Physico-chemical, organoleptic, antioxidative and release characteristics of flavoured yoghurt enriched with microencapsulated Melissa officinalis essential oil. Int J Dairy Technol. 2020 doi: 10.1111/1471-0307.12691. [DOI] [Google Scholar]
- Sarkar S. Microbiological considerations for probiotic supplemented foods. Int J Microbiol Adv Immunol. 2013;1:1–7. [Google Scholar]
- Saxelin M, Korpela R, Mäyrä-Mäkinen A (2003) Functional dairy products. Dairy Process Improv Qual 229–245
- Spitsberg VL. Invited review: bovine milk fat globule membrane as a potential nutraceutical. J Dairy Sci. 2005;88:2289–2294. doi: 10.3168/jds.S0022-0302(05)72906-4. [DOI] [PubMed] [Google Scholar]
- Sultan S, Huma N, Butt MS, et al. Therapeutic potential of dairy bioactive peptides: a contemporary perspective. Crit Rev Food Sci Nutr. 2018;58:105–115. doi: 10.1080/10408398.2015.1136590. [DOI] [PubMed] [Google Scholar]
- Tadesse SA, Emire SA. Production and processing of antioxidant bioactive peptides: a driving force for the functional food market. Heliyon. 2020;6:e04765. doi: 10.1016/j.heliyon.2020.e04765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamime BAY, Marshall VME, Robinson RK. Microbiological and technological aspects of milks fermented by bifidobacteria. J Dairy Res. 1995;62:151–187. doi: 10.1017/s002202990003377x. [DOI] [PubMed] [Google Scholar]
- Tang P, Hao E, Deng J, et al. Boost anti-oxidant activity of yogurt with extract and hydrolysate of cinnamon residues. Chinese Herb Med. 2019;11:417–422. [Google Scholar]
- Tejano LA, Peralta JP, Yap EES, Panjaitan FCA. Prediction of bioactive peptides from Chlorella sorokiniana proteins using proteomic techniques in combination with bioinformatics analyses. Int J Mol Sci. 2019;20(1786):1–16. doi: 10.3390/ijms20071786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidona F, Criscione A, Guastella AM, Zuccaro A, Bordonaro S, Marletta D. Bioactive peptides in dairy products. Ital J Anim Sci. 2009;8(3):315–340. [Google Scholar]
- Tripathi MK, Giri SK. Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods. 2014;9:225–241. [Google Scholar]
- Van Der Kraan MIA, Groenink J, Nazmi K, et al. Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides. 2004;25:177–183. doi: 10.1016/j.peptides.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Vicentini A, Liberatore L, Mastrocola D. Functional foods: trends and development of the global market. Ital J Food Sci. 2016;28:228–351. [Google Scholar]
- Wen C, Zhang J, Zhou J, Cai M, Duan Y. Antioxidant activity of arrowhead protein hydrolysates produced by a novel multi-frequency S-type ultrasound-assisted enzymolysis. Nat Prod Res. 2019;34:3000–3004. doi: 10.1080/14786419.2019.1601192. [DOI] [PubMed] [Google Scholar]
- Westermann C, Gleinser M, Corr SC, Riedel CU. A critical evaluation of bifidobacterial adhesion to the host tissue. Front Microbiol. 2016;7:1–8. doi: 10.3389/fmicb.2016.01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibowo S, Vervoort L, Tomic J, et al. Colour and carotenoid changes of pasteurized orange juice during storage. Food Chem. 2015;171:330–340. doi: 10.1016/j.foodchem.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Xie J, Xu Y, Shishir MRI, et al. Green extraction of mulberry anthocyanin with improved stability using β-cyclodextrin. J Sci Food Agric. 2019;99:2494–2503. doi: 10.1002/jsfa.9459. [DOI] [PubMed] [Google Scholar]
- Xu D, Li Y, Meng X, et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Mol Sci. 2017;18:1–32. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Li X, Lin S, Zhang Z, Chen F. Identification of novel peptides from 3 to 10 kDa Pine Nut (Pinus koraiensis) meal protein, with an exploration of the relationship between their antioxidant activities and secondary structure. Food Chem. 2017;219:311–320. doi: 10.1016/j.foodchem.2016.09.163. [DOI] [PubMed] [Google Scholar]
- Zaccari F, Cabrera MC, Ramos A, Saadoun A. In vitro bioaccessibility of β-carotene, Ca, Mg and Zn in landrace carrots (Daucus carota L.) Food Chem. 2015;166:365–371. doi: 10.1016/j.foodchem.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Zendeboodi F, Khorshidian N, Mortazavian AM, da Cruz AG. Probiotic: conceptualization from a new approach. Curr Opin Food Sci. 2020;32:103–123. [Google Scholar]
- Zhang T, Jeong CH, Cheng WN, et al. Moringa extract enhances the fermentative, textural, and bioactive properties of yogurt. LWT Food Sci Technol. 2019;101:276–284. [Google Scholar]