Abstract
The production of 3-indoleacetic acid (IAA) by plant growth-promoting bacteria (PGPR) stimulates root development and plant growth. In addition, morphological changes such as an increased root ramification and root hair production improves nutrient absorption and biomass accumulation. The objective of this work was to evaluate the effect of IAA-producing strains on rice in an advanced stage of its vegetative cycle. Rice was inoculated with Gluconacetobacter diazotrophicus PAL 5 and its lao- mutant, deficient in auxin production, Azospirillum baldaniorum Sp 245, and Escherichia coli DH10b. Both the mutant and wild-type G. diazotrophicus stimulated root elongation, area, volume, and diameter. However, the lao- mutant strain was the only one capable of increasing the number of roots. In turn, inoculation with A. baldaniorum had no significant effect on plant development. The inoculation with E. coli led to changes in root volume, area, and diameter, and a response that may be related to the stress caused by its presence. We conclude that the inoculation with G. diazotrophicus stimulates the root system’s growth independently of their IAA production ability, suggesting that a metabolite other than IAA is responsible for this effect at advanced stages of the rice’s vegetative cycle.
Keywords: Oryza sativa, G. diazotrophicus PAL 5, Gluconacetobacter diazotrophicus lao-, Auxin
Introduction
Plant roots release various organic substances and molecular signals that attract microbial populations, especially those capable of metabolizing root exudates and proliferating in the rhizosphere [1]. Root metabolism is closely associated with these microorganisms [2] and, among them, there are several plant growth-promoting rhizobacteria (PGPR) that benefit plant growth and development.
One of the most commonly used mechanisms to explain the beneficial effects of PGPR on plants is the production of phytohormones, such as auxin [3]. This hormone controls physiological processes in plants, such as cell growth and division, tissue differentiation, and responses to light and gravity [4]. Indole-3-acetic acid (IAA) is the most prevalent and essential auxin for plants [3]. Inoculation studies support the hypothesis that the synthesis of IAA by PGPRs increases plant rooting. The consequent root growth allows plants to explore the soil better, thus benefiting the absorption of water and nutrients. Furthermore, IAA may significantly increase root exudation, which increases root colonization by PGPR, improving the inoculation effect [3, 4].
Among these microorganisms are Gluconacetobacter diazotrophicus [5] and Azospirillum sp. [6], which are nitrogen-fixing and phytohormone-producing bacteria [7]. Although initially isolated from sugarcane plants grown in Brazil [8], G. diazotrophicus colonizes other species endophytically, such as rice [9]. In fact, rice has been used as a plant model to study interactions with G diazotrophicus since it is easy to manipulate and cultivate in the laboratory [10–12]. Furthermore, rice inoculation experiments with variety IAC4440 and the low IAA-producing G. diazotrophicus lao- strain demonstrated the importance of auxin in altering root architecture. Under these conditions, root length has been stimulated by the wild-type strain if compared to lao-inoculated plants [13].
Nevertheless, these evaluations were made within an initial period of 3 and 7 days [13]. Similarly, the inoculation of Azospirillum baldaniorum in Arabidopsis increased the number of root hairs and lateral roots compared to the inoculation of a mutant strain deficient in auxin biosynthesis. This effect has been evaluated only seven days after inoculation [14]. It is possible to infer that the evaluation time is a restrictive factor to observe the inoculation’s actual effectiveness since the PGPR effect may not be expressed throughout the plant cycle. In fact, a study has shown that the population of G. diazotrophicus has decreased throughout the commercial cycle of different sugarcane genotypes [15]. Possibly, the manifestation of growth promotion by G. diazotrophicus was more accentuated in the initial stages.
Through these observations, the hypothesis of our work is that bacterial auxin may have an expressive effect beyond the initial stages of interaction between the plant and bacteria. To test this hypothesis, we evaluated whether IAA-producing bacteria affects root morphology in a more advanced stage of rice’s vegetative cycle. Azospirillum baldaniorum Sp 245 [14], G. diazotrophicus PAL 5, and its lao- IAA-deficient production mutant strain [13]were used as model organisms, allowing us to access to the effect of this hormone on root development.
Material and methods
Strains of bacteria and cultivation conditions
The bacterial strains used in the experiment were Gluconacetobacter diazotrophicus PAL 5 (= BR11281), which is the type strain of its species [16]; Gluconacetobacter diazotrophicus lao-, which is derived from PAL 5 and deficient in the production of IAA due to a mutation in the L-amino acid oxidase (lao) gene [13]; Azospirillum baldaniorum Sp 245 (= BR11005), formerly classified as A. brasilense [17], isolated from wheat, and an IAA producer [18, 19]; and Escherichia coli DH10b, a strain used for cloning in molecular biology assays [20]. E. coli was added as a negative control because it is not a classic PGPR. G. diazotrophicus strain PAL 5 and lao-inocula were prepared in liquid DYGS medium at 30 ºC for 48 h [21], with a final concentration greater than 108 CFU.mL−1. Additionally, the medium used for lao- received kanamycin at a concentration of 200 µg mL−1. E. coli DH10b was grown in LB medium [22], at 37 ºC for 24 h, and the A. baldaniorum Sp 245 inoculum was prepared in DYGS medium for 48 h at 28ºC.
Rice experiment in nutrient solution
The experiment was carried out in a completely randomized design, with five treatments and six replicates. The treatments consisted of inoculation with G. diazotrophicus PAL 5, G. diazotrophicus lao-, A. baldaniorum Sp 245, E. coli DH10b, and a control without inoculation.
The Piauí variety, which has been used as a model for plant nutrition [23–27] and microbiology studies [28], was chosen for experimentation. The seeds were initially disinfected in 2% sodium hypochlorite, for 30 min, under orbital agitation. Then, the seeds were washed ten times in distilled water and allowed to germinate on wet gauze. Ten days after germination (DAG), the seedlings were transferred to 700-mL pots filled with Hoagland’s solution [29] modified at ½ ionic strength. Each pot received four seedlings. Three days later, the seedlings were inoculated with 5 mL of each strain according to their respective treatments. The inocula were applied to the roots any time the nutrient solution was renewed. The plants were cultivated in a growth chamber under a 12 h/12 h (light/dark) photoperiod at 25 °C and sampled 21 DAG.
The following variables were measured: shoot and root’s fresh and dry weights, root length (mm), surface area (mm2), average diameter (mm), and volume (mm3), and the number of root tips. The root variables were measured with the software WinRHIZO Arabidopsis coupled to an image scanner Epson Expression 11000XL LA2400, using the method described by [30, 31]. The rice roots were evenly distributed under a layer of water in a transparent tray (20 cm wide and 30 cm long). Subsequently, the images were digitized, and the mentioned variables were calculated.
Data analysis
Data normality and homoscedasticity of the variances were verified by the Shapiro–Wilk and Bartlett tests, respectively. Outliers were identified based on the boxplot analysis. One outlier was identified per treatment and removed. Thus, five replicates were used for subsequent statistical analyses. Each of the measured variables were subjected to an analysis of variance (ANOVA) with the F test (p < 0.05), and the means were compared to the control by the t-test (p < 0.05). The data was also submitted to principal component analysis (PCA) on the correlation matrix. All analyzes were performed using the R software [32].
Results
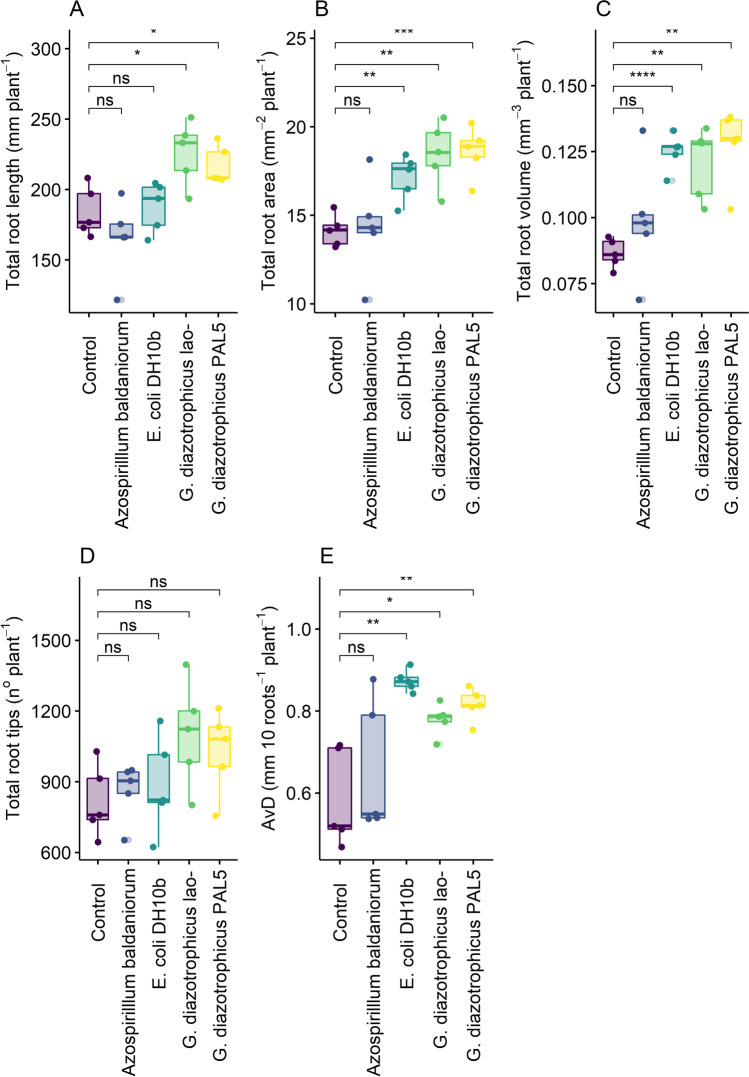
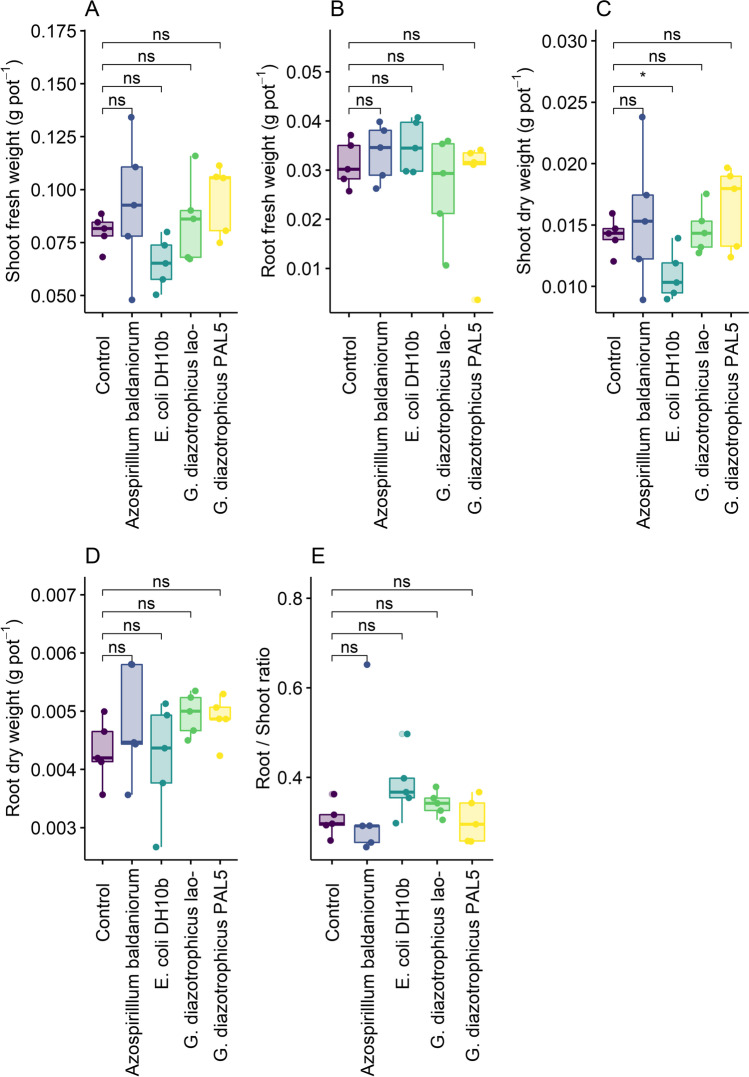
The plants inoculated with either G. diazotrophicus lao- or PAL 5 showed significantly greater root length (Fig. 1A), surface area (Fig. 1B), volume (Fig. 1C), and average diameter (Fig. 1E) compared to the uninoculated plants (Fig. 2). In addition, lao- also stimulated an increase in the number of root tips (Fig. 1d), pointing out to the existence of an IAA-independent mechanism stimulating root growth. The E. coli DH10b inoculation stimulated an increase in root diameter, volume, and surface area (Fig. 1E, C, B), indicating that rice plants can respond to the stimulation of a non-PGPR species. Finally, none of the root morphological variables were affected by A. baldaniorum Sp 245 inoculation (Figs. 1 and 2). No differences were observed among all treatments for root and shoot biomasses and root:shoot ratio (Fig. 2A–E).
Fig. 1.
Length (a), surface area (b), volume (c), number of tips (d), and average diameter (AvD) (e) of roots of rice plants inoculated with A. baldaniorum, E. coli DH10b, G. diazotrophicus lao- ( mutant deficient in the production of IAA) and G. diazotrophicus PAL 5 (IAA-producing wild strain). “Control” stands for the uninoculated treatment. T-test (*p < 0.05; **p < 0.01; ***p < 0.001;****p < 0.0001) for the means of effects between the control and different treatments, ns = not significant
Fig. 2.
Shoot (a) and root (b) fresh weight, shoot (c) and root (d) dry weight, and root/shoot ratio (e) of rice plants inoculated A. baldaniorum, E. coli DH10b, G. diazotrophicus lao- (mutant deficient in the production of IAA) and G. diazotrophicus PAL 5 (IAA-producing wild strain). “Control” stands for the uninoculated treatment. T-test (*p < 0.05; **p < 0.01; ***p < 0.001;**** p < 0.0001) for the means of effects between the control and different treatments, ns = not significant
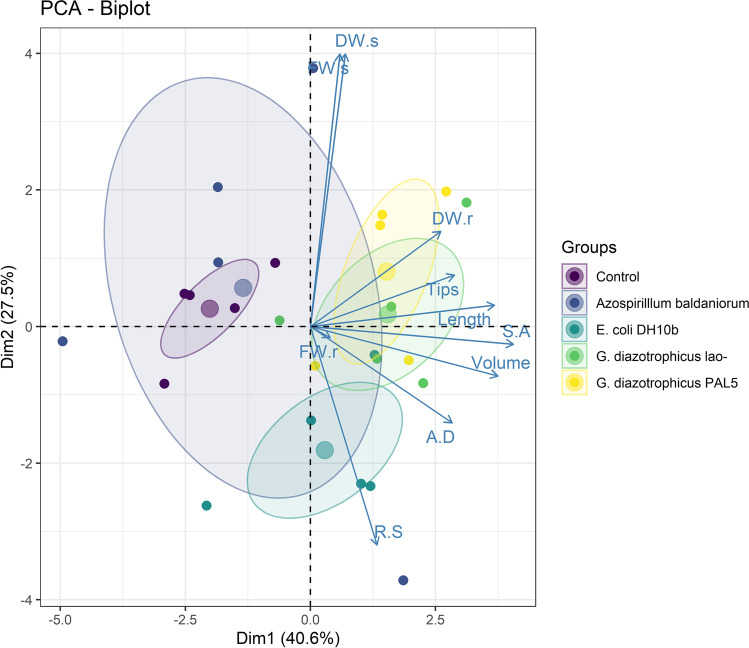
The principal component analysis (PCA) explained 68.1% of the total data variability in the first two components (Fig. 3). The first component explained 40.6% of the variation and represents a combination of root growth variables (root length, volume, diameter, and area, number of tips, and root dry weight), which had the highest weight in this component. The control was negatively correlated to these variables while the inoculations with G. diazotrophicus PAL 5 and lao- were positively correlated (Fig. 3). The second component explained 27.5% of the variation and represented a contrast between the shoot biomass and root: shoot ratio. E. coli DH10b inoculation was negatively correlated with shoot wet and dry weights, but positively correlated with the root:shoot ratio (RS), showing that the plants invested proportionally in root than in shoot growth. Finally, the plant response to A. baldaniorum inoculation was highly variable, as indicated by the high dispersion of the points in the PCA. In general, this treatment showed lower root growth than the inoculation of the other two PGPR.
Fig. 3.
Principal component analysis carried out on rice growth variables. Shoot (FWs) and root (FWr) fresh weight; shoot (DWs) and root (DWr) dry weight; number of roots (tips); root length (length); root surface area (SA); root volume (volume); average root diameter (AD); root/shoot ratio (RS). The circles with a larger diameter represent the centroids for each of the treatments
Discussion
Microorganisms have marked effects on plant establishment in the environment, influencing root growth and development and improving nutrient uptake [33]. These effects are relevant at the beginning of a plant’s growth cycle since they can give plants an advantage to establish in the field. Nevertheless, plants can also benefit from microbial stimulation throughout their growth cycle, which may be relevant for their metabolism, resistance to biotic and abiotic stresses, and improved crop productivity. In the present work, we evaluated the effects of some of these microorganisms during advanced stages of rice’s vegetative growth. We also took advantage of an IAA production-deficient G. diazotrophicus strain to evaluate the role of this hormone.
PGPR, such as Gluconacetobacter and Azospirillum, are associated with plants and positively impact their growth [34]. This stimulatory effect may be related to IAA production, and it has been demonstrated for the first days after root inoculation [13, 14]. Nevertheless, in the present work, both G. diazotrophicus wild-type and mutant strains similarly stimulated rice root growth, indicating that auxins’ production was not the primary factor promoting root growth at the advanced stage of rice’s vegetative cycle. In fact, lao- was the only strain positively affecting the number of lateral rice roots (p < 0.1) compared to the uninoculated treatment (Fig. 1D). This result contrasts with data from Rodrigues et al. [13], who observed a positive effect of G. diazotrophicus PAL 5 three and seven days after inoculation and related this result to auxin production. This finding suggests that the auxin produced by the wild-type strain has an initial effect if we think mainly of this hormone’s function as a signaling molecule among plant bacteria [35]. However, it may no longer be essential for root development at an advanced growth stage.
The genus Azospirillum has been reported to stimulate the growth of different crops, and for this reason, it has been used in agricultural inoculants [36, 37]. Despite Azospirillum’s ability to fix nitrogen, this process has not been considered as its primary plant-growth promotion mechanism since it does not provide significant N amounts for the crops. Therefore, their phytostimulatory effect has been considered the main factor responsible for plant growth promotion [36]. Species of this genus colonize cereals, including rice [38–40]. Unexpectedly, no changes in root morphology were observed upon A. baldaniorum Sp 245 inoculation. A possible explanation may be that the Piauí variety is unresponsive to inoculation with the tested strain. The genetic differences between cultivars are relevant factors and determine, at least in part, the plant response to inoculation [41]. Another possibility may be that environmental conditions affected the plant-bacterial interaction. In vitro tests showed that the concentration of indoles produced by Azospirillum changes in the presence of specific N sources [42]. The presence of these sources also decreased cell multiplication. Therefore, we cannot rule out that N might have affected the stability of the Sp 245 strain. Besides, if we assume that the auxin production is involved in the plant-bacterium interaction [4], it is plausible to suggest that variations on its levels as influenced by the environment affect the plant-bacterium communication and, consequently, bacterial colonization.
E. coli has been used here as a neutral control since it has not been recognized as PGPR. E. coli is generally studied as a human pathogen [43], but the E. coli DH10b strain is non-pathogenic and commonly used in molecular biology procedures [20]. Even so, some lineages have been reported as adapted to the soil and able to colonize plant roots [44]. Unexpectedly, the inoculation of E. coli DH10b affected the root diameter, leading to a greater volume and area (Fig. 2). The phytostimulatory effect of clinical E. coli has been reported primarily by Walker and collaborators [45]. These authors observed that E. coli K-12 increased the maize’s root system, and that this increase was higher than the increase observed upon Azospirillum inoculation. Although inoculation with E. coli DH10b altered the rice’s root system, plant growth was limited (Fig. 1A, C). E. coli DH10b-inoculated plants had lower shoot biomass and higher root/shoot ratio, indicating root thickening at the expense of shoot growth (Fig. 3). We can assume that this strain potentially stressed the root cells, which can compromise the transport of water, nutrients, and, consequently, shoot growth. However, further studies are needed to confirm this hypothesis since the statistical analysis have not detect a significant inoculation effect on shoot growth. These results show that plants can respond to the inoculation of microorganisms that are not PGPR. This effect may have been enhanced by the axenic conditions and the high bacterial cell concentration. It remains to be seen whether the same effects would be observed under natural soil conditions.
Conclusions
The inoculation with G. diazotrophicus stimulates the root system independently of their 3-indoleacetic acid production ability, suggesting that a metabolite other than 3-indoleacetic acid is responsible for this effect at advanced stages of the rice’s vegetative cycle. A. baldaniorum Sp 245 was not effective with rice cultivar Piauí, suggesting a possible genotypic effect. E. coli DH10b inoculation promoted root thickening, indicating a possible stress and compromising shoot biomass accumulation.
Acknowledgements
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship provided to Maura Santos Reis de Andrade da Silva; and the Brazilian National Council for Scientific and Technological Development (CNPq) for the research fellowship provided to Ederson da Conceição Jesus (project 311796/2019-2). The INCT—Plant-Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq, 465133/2014-4, Fundação Araucária-STI, CAPES) has also been sponsored.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Responsible Editor: Fernando R. Spilki
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Márcia Soares Vidal, Email: marcia.vidal@embrapa.br.
Ederson da Conceição Jesus, Email: ederson.jesus@embrapa.br.
References
- 1.Vacheron J, Desbrosses G, Bouffaud M-L, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4: 10.3389/fpls.2013.00356 [DOI] [PMC free article] [PubMed]
- 2.Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Duca DR, Glick BR. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl Microbiol Biotechnol. 2020;104:8607–8619. doi: 10.1007/s00253-020-10869-5. [DOI] [PubMed] [Google Scholar]
- 4.Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 5.Fuentes-Ramirez LE, Jimenez-Salgado T, Abarca-Ocampo IR, Caballero-Mellado J. Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of México. Plant Soil. 1993;154:145–150. doi: 10.1007/BF00012519. [DOI] [Google Scholar]
- 6.Woodward AW. Auxin: regulation, action, and interaction. Ann Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskin N, Vessey K, Tian L. Research progress and perspectives of nitrogen fixing bacterium, Gluconacetobacter diazotrophicus, in Monocot Plants. Int J Agron. 2014;2014:1–13. doi: 10.1155/2014/208383. [DOI] [Google Scholar]
- 8.Cavalcante VA, Dobereiner J. A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil. 1988;108:23–31. doi: 10.1007/BF02370096. [DOI] [Google Scholar]
- 9.Muthukumarasamy R, Kang UG, Park KD, Jeon W-T, Park CY, Cho YS, Kwon S-W, Song J, Roh D-H, Revathi G (2006) Enumeration, isolation and identification of diazotrophs from Korean wetland rice varieties grown with long-term application of N and compost and their short-term inoculation effect on rice plants. J Appl Microbiol 0:061120055200050-???. 10.1111/j.1365-2672.2006.03157.x [DOI] [PubMed]
- 10.Rouws LFM, Meneses CHSG, Guedes HV, Vidal MS, Baldani JI, Schwab S. Monitoring the colonization of sugarcane and rice plants by the endophytic diazotrophic bacterium Gluconacetobacter diazotrophicus marked with gfp and gusA reporter genes: Gfp marked G. diazotrophicus on rice. Lett Appl Microbiol. 2010;51:325–330. doi: 10.1111/j.1472-765X.2010.02899.x. [DOI] [PubMed] [Google Scholar]
- 11.Meneses CHSG, Rouws LFM, Simões-Araújo JL, Vidal MS, Baldani JI. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte gluconacetobacter diazotrophicus. Mol Plant-Microbe Interactions®. 2011;24:1448–1458. doi: 10.1094/MPMI-05-11-0127. [DOI] [PubMed] [Google Scholar]
- 12.Alquéres S, Meneses C, Rouws L, Rothballer M, Baldani I, Schmid M, Hartmann A. The Bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol Plant-Microbe Interactions®. 2013;26:937–945. doi: 10.1094/MPMI-12-12-0286-R. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues EP, Soares C de P, Galvão PG, Imada EL, Simões-Araújo JL, Rouws LFM, Oliveira ALM de, Vidal MS, Baldani JI (2016) Identification of genes involved in indole-3-acetic acid biosynthesis by gluconacetobacter diazotrophicus PAL5 strain using transposon mutagenesis. Front Microbiol 7: 10.3389/fmicb.2016.01572 [DOI] [PMC free article] [PubMed]
- 14.Spaepen S, Bossuyt S, Engelen K, Marchal K, Vanderleyden J. Phenotypical and molecular responses of A rabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium A zospirillum brasilense. New Phytol. 2014;201:850–861. doi: 10.1111/nph.12590. [DOI] [PubMed] [Google Scholar]
- 15.Reis Junior FBD, Silva LGD, Reis VM, Döbereiner J. Occurrence of diazotrophic bacteria in different cane genotypes. Pesqui Agropecuária Bras. 2000;35:985–994. doi: 10.1590/S0100-204X2000000500016. [DOI] [Google Scholar]
- 16.Gillis M, Kersters K, Hoste B, Janssens D, Kroppenstedt RM, Stephan MP, Teixeira KRS, Dobereiner J, De Ley J. Acetobacter diazotrophicus sp. nov., a nitrogen-fixing acetic acid bacterium associated with sugarcane. Int J Syst Bacteriol. 1989;39:361–364. doi: 10.1099/00207713-39-3-361. [DOI] [Google Scholar]
- 17.dos Santos Ferreira N, Hayashi Sant’Anna F, Massena Reis V, Ambrosini A, GazollaVolpiano C, Rothballer M, Schwab S, Baura VA, Balsanelli E, Pedrosa F de O, Pereira Passaglia LM, Maltempi de Souza E, Hartmann A, Cassan F, Zilli JE. Genome-based reclassification of Azospirillum brasilense Sp245 as the type strain of Azospirillum baldaniorum sp. nov. Int J Syst Evol Microbiol. 2020;70:6203–6212. doi: 10.1099/ijsem.0.004517. [DOI] [PubMed] [Google Scholar]
- 18.Costacurta A, Keijers V, Vanderleyden J. Molecular cloning and sequence analysis of an Azospirilium brasilense indole-3-pyruvate decarboxylase gene. Mol Gen Genet MGG. 1994;243:463–472. doi: 10.1007/BF00280477. [DOI] [PubMed] [Google Scholar]
- 19.Baldani VLD, Baldani JI, Döbereiner J. Effects of Azospirillum inoculation on root infection and nitrogen incorporation in wheat. Can J Microbiol. 1983;29:924–929. doi: 10.1139/m83-148. [DOI] [Google Scholar]
- 20.Grant SG, Jessee J, Bloom FR, Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci. 1990;87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD. The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil. 2014;384:413–431. doi: 10.1007/s11104-014-2186-6. [DOI] [Google Scholar]
- 22.Döbereiner J, Andrade V de O, Baldani VLD Protocolos para preparo de meios de cultura da Embrapa Agrobiologia
- 23.de Sampaio RF, de Souza SR, de Sampaio RF, Fernandes MS. Nitrogen metabolism in rice cultivated under seasonal flush of nitrate. J Plant Nutr. 2004;27:395–409. doi: 10.1081/PLN-120028864. [DOI] [Google Scholar]
- 24.Santos LA, Santos WA, Sperandio MVL, Bucher CA, de Souza SR, Fernandes MS. Nitrate uptake kinetics and metabolic parameters in two rice varieties grown in high and low nitrate. J Plant Nutr. 2011;34:988–1002. doi: 10.1080/01904167.2011.555581. [DOI] [Google Scholar]
- 25.Pereira EG, Ferreira LM, Fernandes EDAC, de lima BR, Santos LA, Fernandes MS. Root morphology and ammonium uptake kinetics in two traditional rice varieties submitted to different doses of ammonium nutrition. J Plant Nutr. 2021;44:2715–2728. doi: 10.1080/01904167.2021.1927088. [DOI] [Google Scholar]
- 26.Coelho CP, Santos LA, Rangel RP, Sperandio MVL, Universidade Federal Rural do Rio de Janeiro, Departamento de Solos, Instituto de Agronomia, Rodovia BR 465 Km 7 Seropédica-RJ 23890–000, Brazil, Bucher CA, Universidade Federal Rural do Rio de Janeiro, Departamento de Fitotecnia, Instituto de Agronomia, Rodovia BR 465 Km 7 Seropédica RJ 23890–000, Brazil, Souza SR de, Universidade Federal Rural do Rio de Janeiro, Departamento de Química, Instituto de Ciências Exatas, Rodovia BR 465 Km 7 Seropédica-RJ 23890–000, Brazil, Fernandes MS, Universidade Federal Rural do Rio de Janeiro, Departamento de Solos, Instituto de Agronomia, Rodovia BR 465 Km 7 Seropédica-RJ 23890–000, Brazil (2016) Rice varieties exhibit different mechanisms for Nitrogen Use Efficiency (NUE). Aust J Crop Sci 10:342–352. 10.21475/ajcs.2016.10.03.p7085
- 27.Sperandio MVL, Santos LA, de Araujo OJL, Braga RP, Coelho CP, de Nogueira E, M, Fernandes MS, de Souza SR, Response of nitrate transporters and PM H+-ATPase expression to nitrogen flush on two upland rice varieties contrasting in nitrate uptake kinetics. Aust J Crop Sci. 2014;8(4):568–576. [Google Scholar]
- 28.Vergara C, Araujo KEC, Alves LS, de Souza SR, Santos LA, Santa-Catarina C, da Silva K, Pereira GMD, Xavier GR, Zilli JÉ. Contribution of dark septate fungi to the nutrient uptake and growth of rice plants. Braz J Microbiol. 2018;49:67–78. doi: 10.1016/j.bjm.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoagland DR, Arnon DI (1950) The Water-Culture Method for Growing Plants without Soil. California Agricultural Experiment Station, Circular-347
- 30.Bauhus J, Messier C. Evaluation of fine root length and diameter measurements obtained using RHIZO image analysis. Agron J. 1999;91:142–147. doi: 10.2134/agronj1999.00021962009100010022x. [DOI] [Google Scholar]
- 31.Bouma TJ, Nielsen KL, Koutstaal B. Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant Soil. 2000;218(2):185–196. doi: 10.1023/A:1014905104017. [DOI] [Google Scholar]
- 32.R Core Team (2021) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
- 33.Mantelin S. Plant growth-promoting bacteria and nitrate availability: impacts on root development and nitrate uptake. J Exp Bot. 2003;55:27–34. doi: 10.1093/jxb/erh010. [DOI] [PubMed] [Google Scholar]
- 34.Michiels K, Vanderleyden J, Van Gool A. Azospirillum — plant root associations: A review. Biol Fertil Soils. 1989;8:356–368. doi: 10.1007/BF00263169. [DOI] [Google Scholar]
- 35.Cassán F, Vanderleyden J, Spaepen S. Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Regul. 2014;33:440–459. doi: 10.1007/s00344-013-9362-4. [DOI] [Google Scholar]
- 36.Cassán F, Diaz-Zorita M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol Biochem. 2016;103:117–130. doi: 10.1016/j.soilbio.2016.08.020. [DOI] [Google Scholar]
- 37.Coniglio A, Mora V, Puente M, Cassán F. Azospirillum as biofertilizer for sustainable agriculture: Azospirillum brasilense AZ39 as a model of PGPR and field traceability. In: Zúñiga-Dávila D, González-Andrés F, Ormeño-Orrillo E, editors. Microbial Probiotics for Agricultural Systems. Cham: Springer International Publishing; 2019. pp. 45–70. [Google Scholar]
- 38.Baldani VLD, Döbereiner J. Host-plant specificity in the infection of cereals with Azospirillum spp. Soil Biol Biochem. 1980;12:433–439. doi: 10.1016/0038-0717(80)90021-8. [DOI] [Google Scholar]
- 39.Baldani VLD, Alvarez MA de B, Baldani JI, Döbereiner J. Establishment of inoculatedAzospirillum spp. in the rhizosphere and in roots of field grown wheat and sorghum. Plant Soil. 1986;90:35–46. doi: 10.1007/BF02277385. [DOI] [Google Scholar]
- 40.Thomas J, Kim HR, Rahmatallah Y, Wiggins G, Yang Q, Singh R, Glazko G, Mukherjee A. RNA-seq reveals differentially expressed genes in rice (Oryza sativa) roots during interactions with plant-growth promoting bacteria. Azospirillum brasilense PLOS ONE. 2019;14:e0217309. doi: 10.1371/journal.pone.0217309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.dos Santos SG, Chaves VA, da Silva RF, Alves GC, Reis VM. Rooting and growth of pre-germinated sugarcane seedlings inoculated with diazotrophic bacteria. Appl Soil Ecol. 2019;133:12–23. doi: 10.1016/j.apsoil.2018.08.015. [DOI] [Google Scholar]
- 42.Radwan TE-SE-D, Mohamed ZK, Reis VM. Effect of inoculation with Azospirillum and Herbaspirillum on production of indolic compounds and growth of wheat and rice seedlings. Pesqui Agropecuária Bras. 2004;39:987–994. doi: 10.1590/S0100-204X2004001000006. [DOI] [Google Scholar]
- 43.Fremaux B, Prigent-Combaret C, Vernozy-Rozand C. Long-term survival of Shiga toxin-producing Escherichia coli in cattle effluents and environment: an updated review. Vet Microbiol. 2008;132:1–18. doi: 10.1016/j.vetmic.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Nautiyal CS, Rehman A, Chauhan PS. Environmental Escherichia coli occur as natural plant growth-promoting soil bacterium. Arch Microbiol. 2010;192:185–193. doi: 10.1007/s00203-010-0544-1. [DOI] [PubMed] [Google Scholar]
- 45.Walker V, Bruto M, Bellvert F, Bally R, Muller D, Prigent-Combaret C, Moënne-Loccoz Y, Comte G. Unexpected Phytostimulatory behavior for Escherichia coli and Agrobacterium tumefaciens model strains. Mol Plant-Microbe Interactions®. 2013;26:495–502. doi: 10.1094/MPMI-12-12-0298-R. [DOI] [PubMed] [Google Scholar]