Abstract
Among the fruits, the apple stands out among the most used for elaboration of processed foods. However, the importance of prebiotics in apple products has never been widely analyzed. Prebiotic is a food component resistant to gastric acidity, digestion by mammalian enzymes and gastrointestinal absorption. But following fermentation in the colon, prebiotics result in specific changes in the composition and / or metabolism of the gastrointestinal microbiota, conferring benefits to the health of the host. Therefore, fortifying apple-based products with additional prebiotics is an important strategy for improving consumer health benefits. In this review, after compiling and analyzing scientific and technological studies focusing on prebiotics in apple products, the following benefits of these prebiotics became evident: (1) reduction of water loss in the food matrix; (2) preservation of bioactive and volatile compounds; (3) texture improvement (thickening) in the food industry; (4) increased shelf-live and (5) increased survival of probiotic bacteria, promoting positive effects on microbiota. In addition, this review shows the benefits of different prebiotics for stability and sensory acceptance of apple processed foods.
Keywords: Malus domestica Borkh., Dietary fibers, Short-chain fatty acids, Inulin, Fructooligosaccharides
Introduction
Currently, consumers buy food more consciously, often opting for those that positively impact their health, such as functional foods. Prebiotics, one of the functional food categories, are resistant to acidic stomach conditions and human enzymes, but are fermented by specific microorganisms in the colon and result in specific changes in the activity and / or composition of the gastrointestinal microbiota, conferring health benefit to the host (Gibson et al. 2017). Thus, prebiotics may exert benefits not only in the colon, but elsewhere, like in the oral cavity, urogenital tract and skin (Singh et al. 2017; Puértolas-Balint and Schroeder 2020).
There are also reports on the effects of prebiotics in preventing or delaying chronic diseases such as cardiovascular diseases with hypercholesterolemia, obesity, osteoporosis, diabetes, gastrointestinal infections and gut inflammation (Slavin 2013; Gibson et al. 2017). Prebiotics resist degradation during transit in the upper gastrointestinal tract due to the lack of digestive enzymes or microorganisms that are able to degrade them. However, as they move to the intestine and reach the colon, the microbiota breaks them down and, in the process, obtains the food needed for their maintenance while producing short-chain fatty acids and small carbohydrate molecules, that act as a source of energy for nearby bacteria (Singla and Chakkaravarthi 2017).
Prebiotics, when combined with probiotics, form “symbiotic” that supply the body with good bacteria as they act as a nutrition source for them (Singla and Chakkaravarthi 2017). The current definition states that probiotics are ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ (WHO/FAO 2002). According to Markowiak and Ślizewska (2017), several studies show the benefits of probiotics to prevent and/or treat cancer, gastrointestinal diseases, immunomodulation disorders, allergy, obesity and type-2 diabetes. Prebiotics distinctively stimulate the components of the intestinal microbiota and serve as additional support for it, in a synbiotic manner (Markowiak and Ślizewska 2017). The technical term "synbiotic", implies that the prebiotic component of food favors the development of specific probiotic microorganisms (Rößle et al. 2010; Markowiak and Ślizewska 2017; Puértolas-Balint and Schroeder 2020). This type of combination improves the survival of probiotic microorganisms in the gastrointestinal tract and ensures better results when compared to the isolated probiotic or prebiotic activity (Markowiak and Ślizewska 2017). And it is now known that diet composition combined with the types of microbial communities present in the intestine, and intestinal antimicrobial programming are key items to maintain symbiosis (Puértolas-Balint and Schroeder 2020).
Prebiotics also promote physiological and biochemical processes, resulting in improved overall health and reduced risk of diseases, especially chronic non communicable diseases (Gibson et al. 2017; Puértolas-Balint and Schroeder 2020). The consumption of prebiotic source foods alleviates constipation and diarrhea, stimulates the production of B vitamins and the immune system, reduces the risk of osteoporosis by increasing calcium absorption, alleviates the symptoms of inflammatory bowel disease, reduces the incidence colon cancer, improves carbohydrate metabolism and reduces the risk of diabetes (Slavin 2013; Gibson et al. 2017). Consumption of foods containing prebiotics can also help to reduce the levels of pathogenic bacteria in the intestine, especially Escherichia coli, Campylobacter jejuni, and Salmonella spp. (Slavin 2013; Singla and Chakkaravarthi 2017; Markowiak-Kopeć and Śliżewska 2020). However, based on recent research results, the strict bifidogenic activity (Bifidobacterium strains) of prebiotics has been challenged with certain prebiotic substrates able to stimulate Eubacterium spp., Roseburia spp., Coprococcus spp., and some other bacteria (Singla and Chakkaravarthi 2017; Markowiak-Kopeć and Śliżewska 2020). This indicates that some prebiotics do not constitute selective growth substrates (Singla and Chakkaravarthi 2017).
The best-known prebiotics are inulin, fructooligosaccharides (FOS), glucoligosaccharides (GuOS), galactooligosaccharides (GaOS), xyloligosaccharides (XOS), maltooligosaccharides (MO), isomaltooligosaccharides (IMO), lactulose, lactosaccharose, raffinose, lactulosucrose, fructans, resistant starch, mannitol, maltodextrin, sorbitol, polydextrose and gum arabic (Singh et al. 2017; Davani-Davari et al. 2019). Inulin, FOS and GaOS are the most common, and are used in different food products, including baby foods (Singh et al. 2017; Singla and Chakkaravarthi 2017). Prebiotics naturally exist in some foods (milk, honey and various foods of plant origin). Nevertheless, they are available in low quantities and / or in foods that are not part of the eating habits of the majority of the population (Singh et al. 2017; Davani-Davari et al. 2019).
Several foods of plant origin, in addition to prebiotic properties, are rich in dietary fiber, vitamins, minerals, phytosterols and antioxidants such as carotenoids and polyphenols. The apple (Malus domestica Borkh.), besides fitting the profile of foods with functional properties, is one of the most popular and consumed fruit in the world. Dietary intervention and epidemiological studies show that frequent apple consumption results in reduced risks of cancer, obesity and cardiovascular disease, among other chronic diseases (Koutsos et al. 2015).
In the USA more than 90% of women and 97% of men do not meet recommended daily intakes for dietary fiber (19 g/d). This aligns with intake patterns where whole grains, fruits and vegetables are under consumed by more than 85% of adults (USDA and USDHHS 2020). Stephen et al. (2017), studying dietary fiber measured as nonstarch polysaccharides (NSP) in plant foods, found no recommendation for the ingestion of specific types of fibers in any country. However, in the Europe Union, approved health claims would suggest that an intake of the naturally occurring β-glucans from oats and barley of 3 g/d, wheat fiber/arabinoxylan of 10 g/d, pectin of 6 g/d, and resistant starch replacing 14% of total starch could be recommended. However, these amounts would largely be difficult to achieve through foods with naturally occurring fiber content.
Thus, fortifying foods with additional dietary fiber is an important strategy for increasing fiber intake while keeping calories at recommended levels (Holscher 2017; Lightowler et al. 2018) and, if the fiber used is a prebiotic, it can also subsidize to increased functional food ingestion. Therefore, well-designed strategies, aimed at disclosing the beneficial effects of these foods and stimulating their consumption, may help reversing the current situation and improve the population health status (Davani-Davari et al. 2019). Thus, in this literature review, based on the results compiled from several studies, we seek a broader understanding of the potential effects of prebiotics, added to apple-derived products on consumer health and on the overall quality of food (sensory, rheological, conservation or other main indices).
Apple (Malus domestica Borkh.) composition, properties and health benefits
The apple was chosen as the subject of this study because it is a popular fruit and widely consumed worldwide. In general, its availability occurs throughout the year, as fresh fruit or as a processed food, with emphasis on drinks such as cider and juices, in addition to concentrates and purees (Koutsos et al. 2015). Furthermore, a large part of the population believes that the consumption of apples is associated with health benefits and increased life expectancy (Koutsos and Lovegrove 2015).
As with other foods such as whole grains and vegetables, the apple can alter the intestinal microbiota. This change is attributed to composition and biological activities, which cause a change in health parameters (Licht et al. 2010; Holscher 2017). In the last decade, great efforts have been made in studies on the relevance of intestinal microbiota for human health, focusing mainly on improvements to the immune system, resistance to infections, and prevention of obesity and cancer (Licht et al. 2010).
The apple is predominantly composed of water and carbohydrates, at concentrations of 85% and 14%, respectively, including fiber and sugar, mainly fructose. Vitamins are also found in their composition (especially vitamins C and E), minerals (predominantly potassium), and polyphenols (Koutsos et al. 2015; Bondonno et al. 2017). The fresh apple contain around 2.21 g 100 g−1 total fiber (Bondonno et al. 2017), making up 2% to 3% of their composition. Of this composition, 70% are insoluble fibers, comprising cellulose and hemicellulose, and the remaining 30% are soluble fibers, mainly pectin (Bondonno et al. 2017). According to Englyst et al. (2013), the apple contains 13.13 g / 100 g dry matter of non-starch polysaccharides (NSPs), consisting of rhamnose, fucose, arabinose, xylose, mannose, galactose and glucose. The NSP present in plant cell walls have a structural function and therefore influence the rate and extent of digestion and absorption of other nutrients. The plant cell-wall NSPs are the principal component in all definitions of dietary fiber. The NSPs, such as the pectins, have interesting emulsifying properties for the food industry and also for health. Pectins are complex and branched not metabolized in the upper gastrointestinal tract (Yapo 2011; Gullón et al. 2013; Davani-Davari et al. 2019). This prebiotic soluble fiber helps maintaining the balance of the intestinal microbiota and can be fermented by bifidobacteria in the colon to produce metabolites with both intestinal and systemic effects in the organism (Jiang et al. 2016).
As for the concentration of polyphenols, higher values are observed in the apple peel, when compared to the pulp. This possibly occurs to protect the fruit against the action of ultraviolet light and pathogens. The polyphenols normally found in apple peel are flavonoids (including catechin and epicatechin, procyanidins, chloridzine, and quercetin-glycoside), hydroxybenzoic acids and hydroxycinnamic acids (including chlorogenic acid). In comparison with the apple pulp, the peel has lower concentrations only for chlorogenic acid (Bondonno et al. 2017).
Due to all the beneficial compounds present in the apple (fiber, pectin, polyphenols, among others), its regular consumption provides considerable health benefits. In addition, foods derived from apples (juice, jam, chips, raisins, etc.) can have prebiotics added to them, further increasing the health benefits for consumers (Koutsos et al. 2015; Koutsos and Lovegrove 2015).
Prebiotics
Prebiotics are currently defined as a substrate that is selectively utilized by host microorganisms conferring a health benefit.. This new definition expands the concept of prebiotics to possibly include substances other than carbohydrates, with numerous health benefits and use in various food categories. According to this definition, a prebiotic: (i) must resist host digestion (e.g.: gastric acidity, hydrolysis by mammalian enzymes and gastrointestinal absorption); (ii) must be fermentable by intestinal microorganisms; and (iii) should selectively stimulate the growth and/or activity of intestinal bacteria associated with health and wellbeing (Gibson et al. 2017). Prebiotics are usually carbohydrates, with stable chemical structures over a wide temperature and pH range. They are resistant to hydrolysis by intestinal enzymes, thus undergoing fermentation by the action of the lower gut microbiota, acting as microbial growth promoters (Slavin 2013; Singh et al. 2017). Colon bacteria ferment these undigested ingredient derived from food, producing metabolites, such as short-chain fatty acids, which have fundamental physiological implications on the body (Singh et al. 2017). These metabolites are produced and absorbed in the large bowel promoting the simultaneous reduction of luminal and fecal pH that inhibit the growth of pathogenic microorganisms. In addition, these metabolites participate in different host signaling mechanisms and bacterial cross-feeding interactions, promoting improved integrity and purpose of the intestinal barrier, in addition to modulating glucose and lipid metabolism, the inflammatory responses, and the immune system (Singh et al. 2017; Markowiak-Kopeć and Śliżewska 2020).
The most common types of microorganisms used as probiotics are lactic acid bacteria and bifidobacteria, although other bacteria and certain yeasts are also used. Various bacteria, such as Akkermansia muciniphila, Faecalibacterium prausnitzii, Eubacterium spp., Roseburia intestinalis and Bacteroides spp. have been isolated from the human gut with growing interest in their probiotic potential. These bacteria offer physiological functions that are not always directly conferred by bifidobacteria or lactobacilli, such as the production of propionate, butyrate and other bioactives. However, converting these bacteria into industrially viable probiotics presents challenges (Cunningham et al. 2021).
Thus, the concept and classification of prebiotics is well defined, but it should be noted that they cannot be confused with dietary fiber. Prebiotics and fibers share many properties, but they are not the same. The main difference between them is that prebiotics are fermented by specific microorganisms, usually a specific strain of certain bacteria, while dietary fibers are fermented by most colon microorganisms (Ouwehand et al. 2005; Markowiak and Ślizewska 2017). Therefore, the prebiotics can be a dietary fiber, but dietary fibers will not always be a prebiotic, because fibers are non-digestible carbohydrates and prebiotics are not always carbohydrates (Markowiak and Ślizewska 2017).
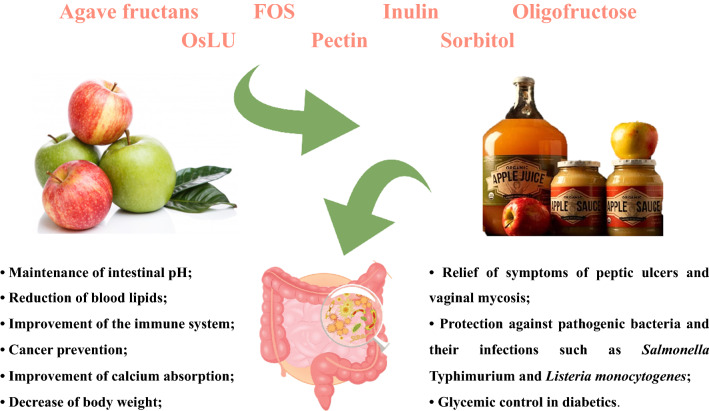
Prebiotics fermentation either directly or indirectly affect the composition and / or activity of the gastrointestinal microbiota, conferring benefits in the proliferation of enterocytes and in the reduction of several risk factor such as intestinal inflammation, colorectal carcinogenesis, colonization by pathogens, and production of nitrogenous metabolites (Markowiak-Kopeć and Śliżewska 2020). Some health benefits promoted by the consumption of apple products with added prebiotics are shown in Fig. 1.
Fig. 1.
Human health benefits of diets including prebiotics, naturally present in apples (e.g., pectin) or added prebiotics (e.g., agave fructans, FOS, inulin, oligofructose, OsLU, pectin, sorbitol) to products derived from apple)
According to Wang (2009), prebiotics can improve sensory acceptability, improving the taste and mouth feel of food products. For prebiotics to serve as functional food ingredients, they must be chemically stable to food industrialization treatments, such as heat, low pH, and Maillard reaction conditions, among others. Wang (2009) also states that the use of inulin and nondigestible oligosaccharides as fiber ingredients is straightforward and often leads to improved taste and texture (Wang 2009).
In order to assess the use of a prebiotic in the formulation of a final product, Pineiro et al. (2008) suggest the following phases: (i) it must have a safe level of prebiotic consumption; (ii) it must be free of contaminants and impurities; and (iii) it must positively alter the microbiota of the host.
There are also indications from the World Gastroenterology Organization on recommended doses of prebiotics for adults, based on clinical experiments: oligofructose (4.0 g, three times daily), lactulose (20.0 g day−1—40.0 g day−1), oligofructose (20.0 g day−1), short-chain fructo-oligosaccharides (5.0 g day−1), galacto-oligosaccharides (3.5 g day−1), and fructooligosaccharide (6.0 g day−1) (Guarner et al. 2017). But, when these doses are not respected, negative effects may occur. When investigated in high doses (e.g.: >20 g day−1), some prebiotics have been shown to have a laxation consequence, increasing stool frequency as well as stool weight. This laxative effect stresses the need for a recommended daily intake and also for the category of prebiotic products to warn against excessive consumption of fortified foods.
Major prebiotics incorporated into apple-derived products
Some carbohydrates, naturally present in plants, are prebiotics. The addition of prebiotics to juices, yogurts and purees, in addition to providing certain sensory characteristics, increases the fiber content and the healthiness of industrialized products (Englyst et al. 2013; Davani-Davari et al. 2019). Consumption of the prebiotics by specific groups within the microbiota (not all strains of lactic acid bacteria / bifidobacteria are probiotics), promotes their growth and metabolic activity. Provision of prebiotics to these select groups of bacteria can also indirectly influence other bacterial groups within the microbiome (Markowiak and Ślizewska 2017; Cunningham et al. 2021). Although, converting probiotics into industrially functional probiotics presents challenges as their requirement for anaerobic conditions and nutrients rich growth media adds cost and complexity. Conversely, converting prebiotics into industrially viable prebiotics appears to be less challenging. However, successful achievements in combining prebiotics with their corresponding probiotics (synbiotic preparations) in industrialized food products are very limited (Cunningham et al. 2021). Listed below are the prebiotics naturally present or currently added to apple-derived food products, aiming at obtaining health benefits, technological improvements and providing an appropriate environment for the maintenance of probiotic microorganisms during the shelf life (Markowiak and Ślizewska 2017).
Pectin
Among other uses, pectins are used as stabilizers in milk and juices, fruit filling for confectionery, and as a gelling agent in fruit-based products (Gullón et al. 2013). Their chemical structure consists in ramified heteropolymers formed by a linear backbone of α (1–4) linked D-galacturonic acid (GalA) units, acetylated and/or methylated (Yapo 2011). Pectins are formed by at least 17 types of monosaccharides, being D-galacturonic acid the predominant one, followed by D-galactose or L-arabinose. They are obtained from citrus rind, apple pomace, sugar beet pulp and several other diverse unconventional sources (Yapo 2011).
Pectins are highly fermentable dietary fibers that have several beneficial health effects (Gullón et al. 2013). These benefits include a desirable fermentation profile in the gut, in vitro anti-cancer properties, cardiovascular protection, as well as antibacterial, anti-inflammatory and antioxidant properties, among others (Markowiak and Ślizewska 2017).
Samout et al. (2016) investigated in vivo the therapeutic outcomes of apple pectin extract against symptoms of obesity (overweight and hyperlipidemia) in male rats divided into four groups and evaluated after seven weeks. They reported that pectin, with prebiotic properties, provided a reduction in the adhesion of pathogenic bacteria (e.g. Salmonella Typhimurium), and increased the adhesion of beneficial bacteria (e.g. Lactobacillus rhamnosus and Bifidobacterium bifidum), to intestinal cells. In addition, prebiotic digestion caused an increase in bacterial groups that carry out the metabolization of insoluble fibers, resulting in metabolites, such as butyric acid, which promote benefits to the colon mucosa by inhibiting inflammation, carcinogenesis and mucosal invasion by enteropathogenesis. They also noticed that polysaccharides with prebiotic properties have the potential to act against oxidative stress and as encapsulation agents to favor probiotic bacteria. Given these results, Samout et al. (2016) concluded that apple-derived pectin has a strong therapeutic effect and potential properties against obesity and may eventually replace synthetic drugs (Table 1).
Table 1.
Prebiotics add in apple products
| Prebiotics | Product | Objective | Results obtained and/or health benefits | References |
|---|---|---|---|---|
| Agave fructans | Dehydrated apple‐based snack | To evaluate the effect of Agave fructans supplementation on short chain fatty acid production in a study with C57BL/6 mice | The dehydrated apple-based snack supplemented with Agave fructans multiplication the production of short-chain fatty acids by increasing the fermentation of prebiotics in the intestine | (González-Herrera et al. 2019) |
| Agave fructans and inulin | Apple leather | To investigate the effect of adding agavins individually and in a mixture with inulin on the microstructural, texture, thermal and sensory properties in an apple leather | Apple leather with agave showed a smoother texture and greater acceptability than those with inulin only. The addition of agavins appears to act as a sweetening enhancer over control | * (García-García et al. 2019) |
| Agave fructans, oligofructose and inulin | Dehydrated apple matrix fruit leather type | To determine the effect of agave fructans, inulin, oligofructose, and mixtures, on the technological and sensory properties of a dehydrated apple matrix fruit leather type | Dehydrated apple matrix fruit leather supplemented with agave fructans had good sensorial acceptability and matched the optimum formulation. Matrices supplemented with agave and oligofructose, were soft and more accept compared to the control. The matrix with inulin demonstrated hardness and low acceptability | * (González-Herrera et al. 2016) |
| FOS | Apple cubes | To determine the effect of temperature, solution concentration and fruit / solution ratio on carbohydrate content in dehydrated apples in FOS preparations obtained from sucrose | Using 50% – 65% w w−1 FOS solutions containing oligosaccharides of temperatures of 40 °C – 70 °C in two–fivefold higher amounts than the amount of the apple, a dehydrated product of a content of fructooligosaccharides of 7% – 9% w w−1 is obtained when the content of dry substance is approximately 30%. An intensification in temperature and amounts of the hypertonic solution intensify the migration of fructooligosaccharides to the fruit tissue, which results in the shortening of the process duration necessary for obtaining the product of a specific content of dry substance, hence the corresponding volume of prebiotic | (Klewicki and Uczciwek 2010) |
| FOS | Apple juice | To evaluate the elimination of patulin by Lactobacillus plantarum ATCC 8014 in artificially contaminated apple juice and its dependence on prebiotic content, citric acid and ascorbic acid | FOS incorporated intensifications the efficiency of patulin removal from apple juice | (Zoghi et al. 2019) |
| FOS | Apple cubes | To compare FOS and sucrose as osmotic agents in the osmotic dehydration of apple cubes cv. Idared | Apple cubes immersed with FOS solution show less diffusion of solute, less water loss, higher temperature and concentration than sucrose solution | (Matusek et al. 2008) |
| FOS | Osmo-dehydrated apple slices | To develop a functional apple cv. Osmo-dehydrated 'Fuji' incorporating FOS | Osmo-dehydrated apples had a high FOS content, as well as acceptable physical, chemical and sensory characteristics | (Egea et al. 2012) |
| FOS and inulin | Apple juice | To explore over 42 days the impact (probiotic strain, inoculum concentration, prebiotic, patulin content, ascorbic acid and citric acid concentration) on the reduction of patulin by probiotic strains in artificially contaminated apple juice | The removal of patulin, 91.23% in 6 weeks, by probiotic bacteria in apple juice depends on the content of fructooligosaccharides present in the product | (Zoghi et al. 2016) |
| FOS | Apple chips | To develop of apple chips using osmotic pretreatment in concentrated cherry or apple juices and mixing of cherry juice and FOS solutions prior to convection drying | Concentrated fruit juices, added with FOS, good osmotic solutions for dehydration and enrichment of dried fruits. The apple chips showed high nutritional and sensory value with good color and sharpness attributes, and high content of polyphenols and acidity | (Kowalska et al. 2018) |
| FOS and inulin | Juices and Jams | To evaluate the conservation capacity of prebiotic compounds after processing and storage of juices and jellies (apple, orange, peach, wild cherry, blueberry, blackberry, whiteberry and Jerusalem artichoke) | The products showed a high content of inulin and oligofructose and a lower content of sucrose than fructose and glucose (reducing sugars). The content of inulin in juices increased with storage by about 23% and that of FOS did not differ | (Davim et al. 2015) |
| Inulin | Apple juice | To optimise lactose, inulin and yeast extract concentration and also culture pH for maximising the growth of a probiotic bacterium, Bifidobacterium animalis subsp. lactis in apple juice | The effects involving inulin, yeast extract and pH on the growth of the bacteria were significant, with 38.5 mg / l being the optimum inulin concentration condition | (Anvari et al. 2014) |
| Inulin | Apple juice | To evaluate apple juice supplementation with probiotic (Lactobacillus rhamnosus GG) and prebiotic (inulin) encapsulated in chitosan-alginate | The inulin addition had a significant positive effect on survival of encapsulated bacteria with an improvement in all sensory characteristics | (Gandomi et al. 2016) |
| Inulin | Apple juice | To obtain inulin syrup from Jerusalem artichoke chips and to evaluate the stability of inulin in apple juice | Dried Jerusalem artichoke concentrate can be used for production of inulin syrup, especially from boiling which guarantees a more concentrated syrup in a short time. Storage (for 10 days at + 4 °C) of apple juice with inulin additive did not cause a considerable loss of carbohydrates or inulin content | (Bekers et al. 2008) |
| Pectin | Apple pectin extract | To investigate the therapeutic effects of apple pectin extract against obesity symptoms such as overweight and hyperlipidemia in male rats rendered obese by a high-calorie diet rich in natural fat | The apple-derived pectin molecules were strong and made anti-obesity effects | * (Samout et al. 2016) |
| Oligofructose | Clarified apple juices | To assess the effect of the addition of oligofructose or sucralose as sugar substitutes and of Lactobacillus paracasei ssp. paracasei as a probiotic culture on the sensory profile and acceptance of clarified apple juice and to determine the influence of the sensory attributes on product acceptance | Oligofructose or sucralose contributed to the increased acceptance (taste and overall impression) of the pure juices, making it similar to that of the sucrose-product | * (Pimentel et al. 2015) |
| Oligofructose and Inulin | Fresh-cut apple wedges | To develop potentially synbiotic fresh-cut apple wedges by applying probiotic bacteria (Lactobacillus rhamnosus GG) and prebiotics in the form of oligofructose and inulin | The alginate coating, with prebiotics, increased nutritional benefits, including polyphenols and apple volatiles and the shelf life of minimally processed apple | * (Rößle et al. 2010) |
| Oligosaccharides derived from lactulose | Oligosaccharides (OsLu) derived from lactulose, milk and apple juice | To characterization of the ingredient containing oligosaccharides derived from lactulose, and to study of the stability of oligosaccharides derived from lactulose during processing of foods with different compositions and pH, namely milk and apple juice | The OsLu are stable in foodstuffs at neutral and acidic pH values and the juice with OsLu were acceptable and similar to those of apple juice with addition of a commercial mixture such as Vivinal® GOS | * (López-Sanz et al. 2015) |
| Sorbitol | Apple cubes | To carry out the osmotic dehydration of apple cubes until reaching the equilibrium values of water loss and sugar gain, using sucrose and sorbitol, and to study the effect of the temperature the mass ratio of sample to solution, and the pressure on the water loss and sugar gain | The use of sorbitol solutions produced osmotically dehydrated apple cubes with lower water activity than the use of sucrose solutions, decreasing their potential deterioration rate | * (Assis et al. 2017) |
*Studies with deepen discussion in the manuscript text
In another in vivo study, Jiang et al. (2016) demonstrated that rats fed a high-fat diet showed evident increases in body weight and also in adipose tissue, in addition to disturbances in the intestinal microbiota, intestinal barrier dysfunction, chronic systemic inflammation, and metabolic endotoxemia. The authors demonstrated that these changes can be reversed and restored to normal levels through food supplementation with apple-derived pectin (Jiang et al. 2016).
In vitro fermentation of fecal slurry with compounds that make up apples, such as apple pectin, or apple extracts, rich in polyphenol compounds, produced short-chain fatty acids, especially butyrate. These constituents of the apple, together with other substances, seem to have synergistic effects, demonstrating that pectin-rich apple products can, provide lipid alterations, delayed glucose response, antioxidant and anti-inflammatory properties, reduced blood pressure and effects on the composition of the microbiota intestinal (Koutsos and Lovegrove 2015).
According to Schwartz et al. (1988), the health effects of pectin go far beyond the in loco benefits to the gut. In one of the pioneer studies with prebiotic supplementation in diabetic (type 2) patients, they observed that individuals who had a low fiber diet (3 g day−1) over a period of 2 weeks, and subsequently, an isocaloric diet supplemented with 20 g day−1 of apple pectin (7 individuals) or α-cellulose (6 individuals), for 4 weeks, had prolonged gastric emptying time, approximately twice after pectin supplementation, and improved glucose tolerance.
Inulin
Inulin consists of fructose units linked to oligosaccharides and polysaccharides, forming structures of (2 → 1) -β-D-fructosyl-fructose. An α-D-glucopyranosyl bond can still be found, joining a terminal glucose unit to a fructan. The physical–chemical properties that determine the applications of inulin are defined by the number of β-D-fructofuranose units and the degree of polymerization of the structure (Borromei et al. 2009).
Inulin can be classified as a prebiotic fiber, because, due to the presence of beta (β) bonds in its structure, it cannot be metabolized by the human gastrointestinal tract, thus promoting an increase in satiety, improving the absorption of minerals such as calcium and magnesium (Borromei et al. 2009; Davani-Davari et al. 2019). Inulin is able to selectively increase the number of bifidobacteria in the intestine because they use this prebiotic as their sole energy source (Gandomi et al. 2016; White and Hekmat 2018). Furthermore, it influences the formation of blood glucose, and reduces the levels of cholesterol and serum lipids. A study by Kolida and Gibson (2007) showed that the ingestion of 5 g to 8 g of inulin per day has a positive effect on the intestine microbiota (Kolida and Gibson 2007).
According to White and Hekmat (2018), fortification with inulin fiber and fermented L. rhamnosus GR1 in apple cider, grape and orange juice is efficient for the development of a probiotic because it has viable counts greater than the minimum requirement (≥ 6 Log UFC mL 1) to be considered probiotic. Anvari et al (2014) observed a significant increase of probiotic growth in apple juice, fermented with Bifidobacterium animalis subsp lactis PTCC 1736, following enrichment with inulin fiber.Silva et al. (2019) evaluated the effects of non-thermal processing using supercritical CO2 on the quality parameters of a new functional beverage obtained from enrichment of clarified apple juice using inulin with an average polymerization degree (≥ 23) under different pressure levels (10 MPa, 15 MPa, and 20 MPa at 35 °C, for 10 min, and 67% CO2 volume ratio) and to conventional thermal treatment (95 °C during 1 min). There was preservation of organic acids, sugars and phenolic compounds present in apple juice with addition of inulin and subjected to supercritical CO2 processing.
Fructooligosaccharides (FOS)
Fructooligosaccharide is a prebiotics and can be obtained from natural sources (for example, inulin) or synthesized from sucrose, by the action of fungal enzymes produced by Aureobasidium sp. or Aspergillus niger (Borromei et al. 2009). Its structure consists of glucose linked by glycosidic bonds, of types β (2–1) or β (2–6), to fructose molecules (between 2 to 60), forming variable FOS, such as 1-kestose (a glucose and two fructose), frutofuranosyl nystose (one glucose and four fructose), among others (Singh et al. 2017).
The digestion of FOS does not occur in the small intestine. It is metabolized to short-chain fatty acids and lactate in the intestinal cecum. The FOS promotes increased absorption of minerals, facilitates the absorption of electrolytes and water, and stimulates the development of beneficial bacteria for the gut microbiota, reducing the incidence of several intestinal diseases, obesity, type-2 diabetes mellitus, among others. It can be used in the food industry in a range of products such as ice cream and yogurt to replace sucrose, as it is less sweet (Flores-Maltos et al. 2016; Singh et al. 2017). Keenan et al. (2011) assessed the stability (30 days at 4 °C) of fructans (inulin and fructooligosaccharide) in apple purees after undergoing pasteurization and high hydrostatic pressure. In this study, the authors found that sensory acceptability improved after the addition of fructans. Furthermore, they suggest that the purees produced met the criteria of consumption (3 g day−1—15 g day−1) for the occurrence of prebiotic effect. Thus, these results demonstrate the possibility of incorporating prebiotics, even in foods treated with high hydrostatic pressure. According to Borromei et al. (2009), inulin when homogenized with water forms a white, paste-like gel that can be used as a fat substitute in foods, and can be used in the production of foods with low caloric value. Zoghi et al. (2016; 2019), aiming the removal of patulin (PAT) from cold-stored apple juice, analyzed the effect of several variables: (a) probiotic strains (Lactobacillus plantarum ATCC 8014 and Lactobacillus acidophilus ATCC 4356), (b) inoculum size, (c) content of prebiotic (fructooligosaccharide and inulin), (d) content of patulin (PAT), (e) concentration of ascorbic acid and (f) concentration of citric acid. They observed that the incorporation of prebiotics in the juice positively affected the viability of probiotics as well as their binding to PAT. They concluded that the presence of FOS (helps the survival of probiotic strains), ascorbic acid (reduces the stability of PAT) and citric acid (increases binding conditions of probiotic and PAT) may lead to efficient toxin removal with a low inoculum size (Zoghi et al. 2016, 2019).
Oligofructose
Oligofructose is a soluble fructan derived from chicory in the same way as inulin (Flores-Maltos et al. 2016; Pol et al. 2018). The main difference is the addition of a hydrolysis step after extraction. It has a sweet taste and can be used to replace sugar (Flores-Maltos et al. 2016; Pol et al. 2018). Oligofructose is neither digested nor absorbed in the small intestine, therefore, it provides less energy when compared to fully digestible carbohydrates (Lightowler et al. 2018). Studies in animals have shown that adding oligofructose to the diet reduces energy intake, preventing body weight gain (González-Herrera et al. 2016; Pol et al. 2018; Franco-Robles et al. 2019). Pimentel et al. (2015) tested the effect of clarified apple juices, with or without oligofructose, with added probiotic culture (Lactobacillus paracasei ssp. paracasei), on physical–chemical characteristics, probiotic viability and acceptability during the refrigerated storage period of the product. They observed that supplementation with oligofructose helped the survival of probiotic cultures. They also observed that apple juice is an appropriate medium for the addition of L. paracasei ssp. paracasei as probiotic. The resulting products were similar in acceptability to pure juice; however, with an acidic flavor, red coloring, and cloudy appearance. Regarding the shelf life of juices enriched with probiotics, reaching the minimum recommended amount, it would be 14 days to 28 days under refrigeration (4 °C).
Oligofructose can be used as a prebiotic and protector of probiotic cultures (L. paracasei ssp paracasei) in apple juices, having no influence on the physicochemical characteristics (pH, titratable acidity, color and turbidity), acceptability, purchase intent and storage stability of the products. Furthermore, it can be considered a sugar substitute, resulting in products with physicochemical characteristics and acceptability similar to products containing added sucrose (Pimentel et al. 2015).
The protective effect on probiotic cultures, increasing their survival and activity during food storage, is due to the fact that oligofructose, in addition to serving as a substrate for probiotic cultures, contributes as a carbon source and protection for bacterial cells against environmental factors such as acidity (Pimentel et al. 2015).
Rößle et al. (2010), studying a synbiotic system, applied a coating of edible alginate plus probiotic bacteria (Lactobacillus rhamnosus GG) and prebiotics (oligofructose and inulin) to apple slices. As a result, they observed reduced loss of bioactive and volatile compounds over 14 days of storage. Synbiotic fresh-cut apple wedges were expected to have higher soluble solids content, but they were significantly higher in apple wedges containing only inulin and oligofructose, not inoculated with probiotic bacteria. The alginate coating, with the function of carrying oligofructose and inulin, increased nutritional benefits and the shelf life of minimally processed apple slices. These coatings not only reduced water loss but also retarded senescence by being selectively gas permeable. Thus, the addition of prebiotics to the alginate coating did not affect the protection of L. rhamnosus, because the probiotic bacteria did not come into direct contact with the prebiotic, as they were part of the coating matrix. Thus, the physical–chemical and sensory properties were slightly influenced by the addition of functional ingredients and, therefore, the products would have similar quality to the apple slices currently available on the market. The alginate coating increased the stability of inherent polyphenols and was able to retain apple volatiles slightly better than uncoated apple wedges (Rößle et al. 2010) (Table 1).
Agave fructans
Agave fructans are reserve carbohydrates found in Agave tequilana Weber plants, with highly branched complex structures [mainly β (2–1) and β (2–6) bonds] with a degree of polymerization ranging from 3 to 30 fructose units (Padilla-Camberos et al. 2018; García-García et al. 2019; Franco-Robles et al. 2019). Although the mode of action in the intestine is little understood, it is known that these carbohydrates are resistant to hydrolysis by human digestive enzymes and are fermented by the colon's microbiota.
In in vivo study the agave fructans showed prebiotic activity, decreasing fat mass, body weight and liver weight in rodents, and displaying steatogenic activity in diabetic and nondiabetic animals (Padilla-Camberos et al. 2018). It appears that this carbohydrate has the ability to decrease body mass index, total body fat percentage and triglyceride levels in obese people, presenting safe levels of intake (Padilla-Camberos et al. 2018; García-García et al. 2019). Franco-Robles et al. (2019) report brain damage reduction and complementary effects of agave fructans in metabolic disorders related to obesity.
García-García et al. (2019) investigated the effect of adding individual fructan agave, and along with inulin, on microstructural, texture, thermal and sensory properties in an apple leather ('Red Delicious'), in two concentrations (6% and 4%), individually and combined. According to the authors, fructan agave showed different behavior in comparison to inulin, with a significant effect on the microstructure at the beginning and after the storage period, showing that both in instrumental measurement and in sensory analysis, leathers added to agave fructans showed reduced values in the sensorial property of hardness, reflecting in a greater acceptability of leathers with fructan agave. When agavins were added, it acted as a sweet flavor enhancer when compared to the control. It was also observed that increases in storage time caused an increase in hardness and decreases in the perception of sweetness and acceptability. The formulation with inulin in an individual way presented the lowest values of acceptability. The final concentration of fructans in apple hides was 10.9% (added 4%) and 15.37% (added 6%)(García-García et al. 2019). These results suggest that the consumption of 35 g of prebiotic apple leather daily would meet the recommendation of prebiotic daily intake of 2 g to 5 g (Volpini-rapina et al. 2012). The concentration of agave fructans increased due to the applied dehydration process. The effect of adding agave fructans to apple slices was positive and significant as a reinforcing and texturing agent and possibly as a flavor enhancer, therefore with different technological properties than inulin.
González-Herrera et al. (2016) evaluated the addition agave inulin, oligofructose and fructans to the matrix of dehydrated 'Red Delicious' apples, in three different concentrations (20, 40 and 60 g.kg−1). The authors verified improvements in the hardness and viscosity in relation to the control, for any of the prebiotics used, but there was a reduction in the acceptability of the product by the consumer. According to them, supplementation with agave fructans to the matrix was effective to improve texture, expanding the potential for applying agave fructans in other processed food matrices.
Oligosaccharides derived from lactulose
Lactulose (4-O-β-D-galactopyranosyl-D-fructose) is a lactose derived carbohydrate, resistant to hydrolysis by enzymes of the small intestine that can reach the proximal colon, where it is selectively fermented by bifidobacteria and lactobacilli, resulting in the production of short-chain fatty acids, hydrogen, and carbon dioxide (Slavin 2013; López-Sanz et al. 2015). However, according to Delgado-Fernandez et al. (2019) this disaccharide cannot reach the distal regions of the colon, where most diseases (e.g. colorectal cancer, colonic polyps, ulcerative colitis, diverticulitis and irritable bowel syndrome) occur. However, it is possible to synthesize lactulose-derived oligosaccharides (OsLu), a group of more slowly fermenting prebiotic, using the transgalactosylation reaction (Delgado-Fernández et al. 2019). Thus, these oligosaccharides are contemplated as developing prebiotics due to their ability to be selectively fermented, especially by Bifidobacterium and Lactobacillus spp. (Samout et al. 2016; Delgado-Fernández et al. 2019) and to exert higher resistance to mammalian digestibility than conventional galactooligosaccharides (Hernandez-Hernandez et al. 2012).
Hernandez-Hernandez et al. (2012), conducting an in vivo study with rats, demonstrated that OsLu is better than GOS, a commercial prebiotic, in regard to gastrointestinal digestion and absorption in the small intestine, and report that it may be due to the β (1 → 4) linkage between galactose and fructose at the reducing end of the molecules. In in vitro study, OsLu presents the bifidogenic effect, for pure lactobacilli and bifidobacteria cultures and in fecal slurries, in addition to having this effect also in animal assays (López-Sanz et al. 2015).
According to López-Sanz et al. (2015) lactulose-derived oligosaccharides (OsLu) are stable in foods with a pH in the range of 3.4 to 6.8 (e.g. fruit juice and milk) during thermal processing and storage. They also claim that these prebiotics are suitable for people with lactose intolerance and for diabetics. According to these authors, the sensory properties of apple juice with OsLu are similar to those of apple juice with the addition of a commercial prebiotic (Vivinal GOS, a galacto-oligosaccharide).
Sorbitol
The polyalcohol sorbitol (C6H14O6) is obtained from catalytic hydrogenation of starch hydrolysate and has a positive impact on the general bowel function by stimulating the growth of probiotics. It is associated with cancer prevention, lowering of LDL-cholesterol, improvement of the immune system and production of bacteriocins against pathogenic bacteria (García et al. 2020).
However, elevated intake of sorbitol can cause symptoms such as diarrhea, abdominal pain, cramps and flatulence as they are not fully used by microorganisms, increasing the water content in the stool (Livesey 2001). Therefore, consuming more than 20 g / day of this polyol is not recommended and an intake of only 7 g – 14 g / day can already cause adverse effects in some individuals (WHO/FAO 2002; Guarner et al. 2017; Singla and Chakkaravarthi 2017).
In a study on osmotic dehydration of apple cubes, until reaching the equilibrium values of water loss and sugar gain, Assis et al. (2017) confirmed the feasibility of sorbitol as an alternative to sucrose as an agent in osmotic dehydration. Sorbitol, in addition to being a prebiotic, is a great substitute for sucrose, with fewer calories, less sweetness and less cariogenic potential than sucrose. However, the benefit in the process may not be justified due to higher material costs.
Apple Prebiotic Action
Apples, among the most consumed fruits in the world, are a rich source of polyphenols and soluble pectin fibers that reach the colon and serve as a substrate for bacterial fermentation, with consequent benefits to human health (Koutsos et al. 2017). According to Wassermann et al. (2019), apples represent a source of direct human exposure to bacterial communities. Using qPCR (16S rRNA gene amplicon), fluorescence in situ hybridization (FISH) and confocal laser scanning microscopy, they observed that the different apple tissues (stem, peel, fruit pulp, seeds, and calyx), were colonized by distinct bacterial communities and that fruit pulp and seeds were bacterial hot spots, while the peel was less colonized. Their results suggest that we consume about 100 million bacterial cells with one apple, many of them with bacterial signatures known for beneficial health-affecting potential (Wassermann et al. 2019). However, the affinity between apple prebiotics and probiotic bacteria naturally present in apple tissues has yet to be demonstrated. In a study to test the feasibility of producing a new probiotic yogurt, Bosnea et al. (2017) evaluated apple cubes, dried raisins and wheat grains (fresh and freeze-dried) as substrate supports to immobilize Lactobacillus casei into yogurt to produce a new probiotic dairy. After 60 days at 4 ºC there was an improvement in the viability of L. casei which was attributed by the authors to the prebiotic character of the apple, raisins and wheat grains. They observed that prebiotics favored the multiplication of probiotic bacteria in the food and provided a protective effect during passage through the gastrointestinal tract. In addition, they also observed that the cells immobilized on the supports proved to be more active than the free cells and benefited from the compounds present on the supports, such as proteins and polysaccharides, to produce a viscous and elastic material (biofilm), providing greater productivity and tolerance to adverse conditions.
Koutsos et al. (2017) studied the effect of three commercial apple varieties (Renetta Canada, Golden Delicious and Pink Lady) on human gut microbiota composition and metabolic activity in vitro, compared to inulin (a prebiotic) and cellulose (poorly fermented). Bacteria of specific interest (e.g., Faecalibacterium prausnitzii and bifidobacteria) were enumerated using quantitative 16S rRNA probes and FISH for detection. The disappearance of apple polyphenols and production of short chain fatty acids (acetate, propionate and butyrate) were monitored throughout fermentation using a targeted LC–MS based metabolomic approach. They concluded that whole apples, with the Renetta Canada variety showing superior performance, beneficially modulate the composition of the intestinal microbiota that degrades soluble fibers and polyphenols, producing short-chain fatty acids and phenolic acids.
Final considerations
Prebiotics have a significant effect on human health, and there are great possibilities for incorporating them into a wide range of common foodstuffs. Their role is played by fermentable carbohydrates, which stimulate, preferentially, the growth of probiotic bacteria, thus enhancing the gastrointestinal and immune systems. However, aiming at the formulation of a wide variety of functional foods, it is essential to further study this class of dietary fibers. With the addition of prebiotics, it is important to assess the viability of probiotic cultures (synbiotic) during processing and storage of the products, as they are available substrates for the metabolism of these microorganisms. Another important factor is whether the overall quality of the product (sensorial, rheology, shelf life or other key indices) present any changes due to the incorporation of prebiotics.
In this review, twenty studies focusing on addition of prebiotics in apple products (snack, leather, cubes, juice, slices, chips, jams and marc) were found. After compiling and analyzing the results of these studies, the following benefits of apple prebiotics became evident: (1) reduction of water loss in the food matrix; (2) preservation of bioactive and volatile compounds; (3) texture improvement (thickening) in processed food; (4) extension of shelf life and (5) contribution to survival of probiotic bacteria, promoting positive effects on consumer health.
The apple, besides being rich in nutrients and fiber, is a functional food that can be considered a prebiotic due to the amount of pectin that is present in it. Therefore, stimulating consumption of fresh apples, as well as products derived from it, and products with added of apple prebiotics, is important in order to maximize the nutritional gains and benefits that these foods provide. However, as it is little known, the benefits provided by prebiotic enriched foods are still poorly known by a large portion of the population.
Finally, although there are few studies, they demonstrate favorable effects of apple prebiotics and / or apple products added with prebiotics, on human health and the overall quality of the food. Thus, in this context, we believe there is a wide scope for further studies on this topic.
Acknowledgments
The authors would like to acknowledge CAPES, from the Ministry of Education (Brazil), for providing scholarships for doctoral students and the PRPPGI/UFPel for complementary financial assistance.
Authors' contributions
Jardel Araujo Ribeiro: Conceptualization, Methodology, Investigation, Writing, Writing—Review & Editing. Elisa dos Santos Pereira: Investigation, Writing, Writing—Review & Editing. Chirle de Oliveira Raphaelli: Investigation, Writing, Writing—Review & Editing. Marjana Radünz: Investigation, Writing, Writing—Review & Editing. Taiane Mota Camargo: Investigation, Writing, Writing—Review & Editing. Fernanda Izabel Garcia da Rocha Concenço: Investigation, Writing, Writing—Review & Editing. Ângela Maria Fiorentini: Review. Rufino Fernando Flores Cantillano: Review. Leonardo Nora: Supervision & Review
Funding
Ministry of Education (Brazil) - CAPES (doctoral scholarships: 00034094067 and 00106998056) and EMBRAPA - CAPES (grant number 02.13.05.003.00.00).
Availability of data and material:
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
There are no conflicts of interest.
Ethical Approval
Not applicable
Consent for publication
Not applicable
Consent for publication
Not applicable
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jardel Araújo Ribeiro, Email: jardelaraujoribeiro@gmail.com.
Elisa dos Santos Pereira, Email: lisaspereira@gmail.com.
Chirle de Oliveira Raphaelli, Email: chirleraphaelli@hotmail.com.
Marjana Radünz, Email: marjanaradunz@gmail.com.
Taiane Mota Camargo, Email: tainecamargo@yahoo.com.br.
Fernanda Izabel Garcia da Rocha Concenço, Email: fernanirocha@yahoo.com.br.
Rufino Fernando Flores Cantillano, Email: fernando.cantillano@embrapa.br.
Ângela Maria Fiorentini, Email: angefiore@gmail.com.
Leonardo Nora, Email: l.nora@me.com.
References
- Anvari M, Khayati G, Rostami S. Optimisation of medium composition for probiotic biomass production using response surface methodology. J Dairy Res. 2014;4:59–64. doi: 10.1017/S0022029913000733. [DOI] [PubMed] [Google Scholar]
- Assis FR, Morais RUIMSC, Morais AMMB (2017) Mathematical modelling of osmotic dehydration kinetics of apple cubes. J Food Process Preserv 43:e12895. 10.1111/jfpp.12895
- Bekers M, Grube M, Upite D, et al. Inulin syrup from dried jerusalem artichoke. Latv Lauksaimn Univ Raksti. 2008;21:116–121. [Google Scholar]
- Bondonno NP, Bondonno CP, Ward NC, et al (2017) The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci Technol. 10.1016/j.tifs.2017.04.012
- Borromei C, Careri M, Cavazza A, et al. Evaluation of fructooligosaccharides and inulins as potentially health benefiting food ingredients by HPAEC-PED and MALDI-TOF MS. Int J ofAnalytical Chem. 2009;2009:1–9. doi: 10.1155/2009/530639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnea LA, Kopsahelis N, Kokkali V, et al. Production of a novel probiotic yogurt by incorporation of L. casei enriched fresh apple pieces, dried raisins and wheat grains. Food Bioprod Process. 2017;102:62–71. doi: 10.1016/j.fbp.2016.11.010. [DOI] [Google Scholar]
- Cunningham M, Azcarate-Peril MA, Barnard A, et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021 doi: 10.1016/j.tim.2021.01.003. [DOI] [PubMed] [Google Scholar]
- Davani-Davari D, Negahdaripour M, Karimzadeh I, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:1–27. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davim S, Andrade S, Oliveira S, et al. Development of fruit jams and juices enriched with fructooligosaccharides. Int J Fruit Sci. 2015;15:100–116. doi: 10.1080/15538362.2014.948749. [DOI] [Google Scholar]
- Delgado-Fernández P, Corzo N, Lizasoain S, et al. Fermentative properties of starter culture during manufacture of kefir with new prebiotics derived from lactulose. Int Dairy J. 2019;93:22–29. doi: 10.1016/j.idairyj.2019.01.014. [DOI] [Google Scholar]
- Egea MB, Borsato D, Sérgio R. Osmo-dehydrated functional product containing fructo- oligosaccharides : physical, chemical and sensorial characteristics. Brazilian Arch Biol Technol. 2012;55:927–936. doi: 10.1590/S1516-89132012000600017. [DOI] [Google Scholar]
- Englyst HN, Hudson GJ, Quigley ME (2013) Carbohydrates | Dietary fiber measured as nonstarch polysaccharides in plant foods. In: Encyclopedia of Analytical Science, 3rd edn. Elsevier Inc. pp 366–371
- Flores-Maltos DA, Mussatto SI, Contreras-Esquivel JC, et al. Biotechnological production and application of fructooligosaccharides. Crit Rev Biotechnol. 2016;36:259–267. doi: 10.3109/07388551.2014.953443. [DOI] [PubMed] [Google Scholar]
- Franco-Robles E, Ramírez-Emiliano J, López MG. Agave fructans and oligofructose decrease oxidative stress in brain regions involved in learning and memory of overweight mice. Nat Prod Res. 2019;33:1527–1530. doi: 10.1080/14786419.2017.1423297. [DOI] [PubMed] [Google Scholar]
- Gandomi H, Abbaszadeh S, Misaghi A, et al. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT—Food Sci Technol. 2016;69:365–371. doi: 10.1016/j.lwt.2016.01.064. [DOI] [Google Scholar]
- García-García AB, Ochoa-Martínez LA, Lara-Ceniceros TE, et al (2019) Changes in the microstructural, textural, thermal and sensory properties of apple leathers containing added agavins and inulin. Food Chem 301:124590. 10.1016/j.foodchem.2019.03.143 [DOI] [PubMed]
- García B, Moreno J, Morales G, Melero JA, Iglesias J. Production of sorbitol via catalytic transfer hydrogenation of Glucose. Applied Sciences. 2020;10(5):1843. doi: 10.3390/app10051843. [DOI] [Google Scholar]
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- González-Herrera S, Rocha N, Simental-Mendía L, et al (2019) Dehydrated apple‐based snack supplemented with Agave fructans exerts prebiotic effect regulating the production of short‐chain fatty acid in mice. J Food Process Preserv e14026. 10.1111/jfpp.14026
- González-Herrera SM, Rutiaga-Quiñones OM, Aguilar CN, et al. Dehydrated apple matrix supplemented with agave fructans, inulin, and oligofructose. LWT—Food Sci Technol. 2016;65:1059–1065. doi: 10.1016/j.lwt.2015.09.037. [DOI] [Google Scholar]
- Guarner F, Sanders ME, Eliakim R, et al (2017) Probiotics and prebiotics. In: World Gastroenterol. Organ. Glob. Guidel. https://www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics/probiotics-and-prebiotics-english. Accessed 19 Aug 2020
- Gullón B, Gómez B, Martínez-Sabajanes M, et al. Pectic oligosaccharides: manufacture and functional properties. Trends Food Sci Technol. 2013;30:153–161. doi: 10.1016/j.tifs.2013.01.006. [DOI] [Google Scholar]
- Hernandez-Hernandez O, Marín-Manzano M, Rubio L, et al. Monomer and linkage type of galacto-oligosaccharides affect their resistance to ileal digestion and prebiotic properties in rats. J Nutr. 2012;142:1232–1239. doi: 10.3945/jn.111.155762. [DOI] [PubMed] [Google Scholar]
- Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Gao X, Wu C, et al. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients. 2016;8:126. doi: 10.3390/nu8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan D, Brunton N, Butler F, et al. Evaluation of thermal and high hydrostatic pressure processed apple purees enriched with prebiotic inclusions. Innov Food Sci Emerg Technol. 2011;12:261–268. doi: 10.1016/j.ifset.2011.04.003. [DOI] [Google Scholar]
- Klewicki R, Uczciwek M. Effect of osmotic dehydration in the carbohydrate mixtures containing fructooligosaccharides on the oligosaccharide content of apples. Acta Aliment. 2010;39:357–367. doi: 10.1556/AAlim.39.2010.3.12. [DOI] [Google Scholar]
- Kolida S, Gibson GR. Prebiotic capacity of inulin-type fructans. J Nutr. 2007;137:2503S–2506S. doi: 10.1093/jn/137.11.2503S. [DOI] [PubMed] [Google Scholar]
- Koutsos A, Lima M, Conterno L, et al (2017) Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 9: 533. 10.3390/nu9060533 [DOI] [PMC free article] [PubMed]
- Koutsos A, Lovegrove J (2015) Chapter 12. An Apple a Day Keeps the Doctor Away—Inter-Relationship Between Apple Consumption, the Gut Microbiota and Cardiometabolic Disease Risk Reduction. In: Diet-Microbe Interactions in the Gut: effects on Human Health and Disease. pp 173–194
- Koutsos A, Tuohy KM, Lovegrove JA. Apples and cardiovascular health–is the gut microbiota a core consideration? Nutrients. 2015;7:3959–3998. doi: 10.3390/nu7063959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska H, Marzec A, Kowalska J, et al. Development of apple chips technology. Heat Mass Transf. 2018;54:3573–3586. doi: 10.1007/s00231-018-2346-y. [DOI] [Google Scholar]
- Licht T, Hansen M, Bergström A, et al. Effects of apples and specific apple components on the cecal environment of conventional rats: Role of apple pectin. BMC Microbiol. 2010;10:13. doi: 10.1186/1471-2180-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightowler H, Thondre S, Holz A, Theis S. Replacement of glycaemic carbohydrates by inulin-type fructans from chicory (oligofructose, inulin) reduces the postprandial blood glucose and insulin response to foods: report of two double-blind, randomized, controlled trials. Eur J Nutr. 2018;57:1259–1268. doi: 10.1007/s00394-017-1409-z. [DOI] [PubMed] [Google Scholar]
- Livesey G (2001) Tolerance of low-digestible carbohydrates: a general view. Br J Nutr 85:S7–S16. 10.1079/BJN2000257 [DOI] [PubMed]
- López-Sanz S, Montilla A, Moreno FJ, Villamiel M. Stability of oligosaccharides derived from lactulose during the processing of milk and apple juice. Food chemistry. 2015;183:64–71. doi: 10.1016/j.foodchem.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Markowiak-Kopeć P, Śliżewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12:1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowiak P, Ślizewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9. 10.3390/nu9091021 [DOI] [PMC free article] [PubMed]
- Matusek A, Czukor B, Merész P. Comparison of sucrose and fructo-oligosaccharides as osmotic agents in apple. Innov Food Sci Emerg Technol. 2008;9:365–373. doi: 10.1016/j.ifset.2007.10.003. [DOI] [Google Scholar]
- Ouwehand A, Derrien M, De Vos W, et al. Prebiotics and other microbial substrates for gut functionality. Curr Opin Biotechnol. 2005;16:212–217. doi: 10.1016/j.copbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Padilla-Camberos E, Barragán-Álvarez CP, Diaz-Martinez NE, et al. Effects of agave fructans (agave tequilana weber var. azul) on body fat and serum lipids in obesity. Plant Foods Hum Nutr. 2018;73:34–39. doi: 10.1007/s11130-018-0654-5. [DOI] [PubMed] [Google Scholar]
- Pimentel TC, Madrona GS, Garcia S, Prudencio SH. Probiotic viability, physicochemical characteristics and acceptability during refrigerated storage of clarified apple juice supplemented with Lactobacillus paracasei ssp. paracasei and oligofructose in different package type. LWT—Food Sci Technol. 2015;63:415–422. doi: 10.1016/j.lwt.2015.03.009. [DOI] [Google Scholar]
- Pol K, de Graaf C, Meyer D, Mars M. The efficacy of daily snack replacement with oligofructose-enriched granola bars in overweight and obese adults: a 12-week randomised controlled trial. Br J Nutr. 2018;119:1076–1086. doi: 10.1017/S0007114518000211. [DOI] [PubMed] [Google Scholar]
- Puértolas-Balint F, Schroeder BO. Does an apple a day also keep the microbes away? the interplay between diet, microbiota, and host defense peptides at the intestinal mucosal barrier. Front Immunol. 2020;11:1–21. doi: 10.3389/fimmu.2020.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rößle C, Brunton N, Gormley RT, et al. Development of potentially synbiotic fresh-cut apple slices. J Funct Foods. 2010;2:245–254. doi: 10.1016/j.jff.2010.09.001. [DOI] [Google Scholar]
- Samout N, Bouzenna H, Dhibi S, et al. Therapeutic effect of apple pectin in obese rats. Biomed Pharmacother. 2016;83:1233–1238. doi: 10.1016/j.biopha.2016.08.038. [DOI] [PubMed] [Google Scholar]
- Schwartz SE, Levine RA, Weinstock RS, et al. Sustained pectin ingestion: effect on gastric emptying and glucose tolerance in non-insulin-dependent diabetic patients. Am J Clin Nutr. 1988;48:1413–1417. doi: 10.1093/ajcn/48.6.1413. [DOI] [PubMed] [Google Scholar]
- Silva EK, Arruda HS, Eberlin MN et al (2019) Effects of supercritical carbon dioxide and thermal treatment on the inulin chemical stability and functional properties of prebiotic-enriched apple juice. Food Res Int 125: 108561.10.1016/j.foodres.2019.108561 [DOI] [PubMed]
- Singh SP, Jadaun JS, Narnoliya LK, Pandey A. Prebiotic oligosaccharides: special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl Biochem Biotechnol. 2017;183:613–635. doi: 10.1007/s12010-017-2605-2. [DOI] [PubMed] [Google Scholar]
- Singla V, Chakkaravarthi S. Applications of prebiotics in food industry: a review. Food Sci Technol Int. 2017;23:649–667. doi: 10.1177/1082013217721769. [DOI] [PubMed] [Google Scholar]
- Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen AM, Champ MM-J, Cloran SJ, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. 2017;30:149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- USDA & USDHHS U& U (2020) Dietary Guidelines for Americans, 2020–2025. In: U.S. Dep. Agric. U.S. Dep. Heal. Hum. Serv. (USDHHS). www.dietaryguidelines.gov. Accessed 15 Feb 2020
- Volpini-rapina LF, Sokei FR, Conti-silva AC. Sensory profile and preference mapping of orange cakes with addition of prebiotics inulin and oligofructose. LWT—Food Sci Technol. 2012;48:37–42. doi: 10.1016/j.lwt.2012.03.008. [DOI] [Google Scholar]
- Wang Y. Prebiotics: present and future in food science and technology. Food Res Int. 2009;42:8–12. doi: 10.1016/j.foodres.2008.09.001. [DOI] [Google Scholar]
- Wassermann B, Müller H, Berg G. An apple a day: which bacteria do we eat with organic and conventional apples? Front Microbiol. 2019;10:1629. doi: 10.3389/fmicb.2019.01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Hekmat S. Development of probiotic fruit juices using lactobacillus rhamnosus gr-1 fortified with short chain and long chain inulin fiber. Fermentation. 2018;4:27. doi: 10.3390/fermentation4020027. [DOI] [Google Scholar]
- WHO/FAO (2002) Guidelines for the Evaluation of Probiotics in Food. In: World Heal. Organ. Food Agric. Organ. United Nations. https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed 1 Apr 2020
- Yapo BM. Pectic substances: From simple pectic polysaccharides to complex pectins—a new hypothetical model. Carbohydr Polym. 2011;86:373–385. doi: 10.1016/j.carbpol.2011.05.065. [DOI] [Google Scholar]
- Zoghi A, Khosravi-Darani K, Sohrabvandi S, Attar H. Patulin removal from synbiotic apple juice using Lactobacillus plantarum ATCC 8014. J Appl Microbiol. 2019;126:1149–1160. doi: 10.1111/jam.14172. [DOI] [PubMed] [Google Scholar]
- Zoghi A, Khosravi-Darani K, Sohrabvandi S, Attar H, Alavi SA. Effect of probiotics on patulin removal from synbiotic apple juice. J Sci Food Agric. 2017;97(8):2601–2609. doi: 10.1002/jsfa.8082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data and material:
Not applicable.
Code availability
Not applicable.