Abstract
Objective
To document socioepidemiological theories used to explain the relationship between socioeconomic disadvantage and multimorbidity.
Design
Scoping review.
Methods
A search strategy was developed and then applied to multiple electronic databases including Medline, Embase, PsychInfo, Web of Science, Scielo, Applied Social Sciences, ERIC, Humanities Index and Sociological Abstracts. After the selection of studies, data were extracted using a data charting plan. The last search was performed on the 28 September 2021. Extracted data included: study design, country, population subgroups, measures of socioeconomic inequality, assessment of multimorbidity and conclusion on the association between socioeconomic variables and multimorbidity. Included studies were further assessed on their use of theory, type of theories used and context of application. Finally, we conducted a meta-narrative synthesis to summarise the results.
Results
A total of 64 studies were included in the review. Of these, 33 papers included theories as explanations for the association between socioeconomic position and multimorbidity. Within this group, 16 explicitly stated those theories and five tested at least one theory. Behavioural theories (health behaviours) were the most frequently used, followed by materialist (access to health resources) and psychosocial (stress pathways) theories. Most studies used theories as post hoc explanations for their findings or for study rationale. Supportive evidence was found for the role of material, behavioural and life course theories in explaining the relationship between social inequalities and multimorbidity.
Conclusion
Given the widely reported social inequalities in multimorbidity and its increasing public health burden, there is a critical gap in evidence on pathways from socioeconomic disadvantage to multimorbidity. Generating evidence of these pathways will guide the development of intervention and public policies to prevent multimorbidity among people living in social disadvantage. Material, behavioural and life course pathways can be targeted to reduce the negative effect of low socioeconomic position on multimorbidity.
Keywords: epidemiology, public health, social medicine
Strengths and limitations of this study.
This is the first scoping review exploring the use of theories to explain the association between socioeconomic position and multimorbidity.
Our review has identified critical gaps in the literature that must be addressed if interventions and public policies are to be designed to reduce socioeconomic inequalities in multimorbidity.
We applied a comprehensive search strategy to identify relevant articles and applied a peer-reviewed robust methodology to assess theories in studies on socioeconomic inequalities in multimorbidity.
Articles that were not in English were excluded from our review. This could have obstructed the inclusion of papers from countries where English is not the main language, therefore limiting the generalisability of our findings.
Introduction
Multimorbidity is a societal challenge and an increasingly recognised public health concern.1–3 It is described as the co-occurrence of two or more chronic conditions in an individual.4 Multimorbidity leads to reduced quality of life, high psychological distress, burden of polypharmacy and managing multiple treatment protocols, and an increased risk of premature death in people.5 There is an emerging threat of increased multimorbidity worldwide, primarily due to population ageing and the epidemiological transition from communicable to non-communicable diseases.6 The COVID-19 pandemic has put a spotlight on multimorbidity as people with existing chronic conditions have suffered a higher risk of its infection, as well as more severe consequences of SARS-CoV-2 infection.7 Furthermore, multiple studies have reported socioeconomic inequalities in multimorbidity within countries regardless of their level of economic development.8–12
A meta-analysis of 24 cross-sectional studies reported that low education compared with high education was associated with 64% higher odds of multimorbidity.13 Another systematic review with 41 studies from high-income countries reported that people with the lowest level of income had 4.4 times higher odds of multimorbidity than those with the highest level of income, while those in most deprived areas had 1.42 times higher odds of multimorbidity than those in the least deprived areas.14 A clear causal relationship between socioeconomic conditions and multimorbidity has also been argued based on empirical evidence;10 however, pathways through which socioeconomic disadvantage leads to multimorbidity are not well studied.4
Theories are used in epidemiology to understand the relationships between exposure to, for example, socioeconomic disadvantage and non-communicable diseases. This is mainly because, as opposed to conceptual frameworks, specific theoretical pathways can be tested using empirical data. Theories provide insight into the mechanisms through which an exposure (eg, socioeconomic position) leads to a health outcome,15 and as such, they are particularly helpful in informing intervention designs. Since the release of the Black Report in 1982,16 several categories of theories have been proposed to explain associations between social inequalities and health outcomes16 17 although in the context of single diseases or health measure. These include:
Behavioural: the behavioural explanation posits that people from different backgrounds behave differently and make health-related choices that are commonly based on their socioeconomic background. As people experience socioeconomic deprivation, they also encounter more barriers to adopting healthy lifestyles. For instance, individual health damaging and promoting behaviours are differentially distributed across the social scale, with more disadvantaged groups more likely to engage in health damaging behaviours such as smoking, and advantaged groups more likely to engage in health-promoting behaviours such as physical activity.18 As a result, poor health outcomes are commonly clustered at the lower end of the socioeconomic scale.17 Behavioural theory can be extended to apply to multimorbidity from a common risk factor approach, as a behavioural risk factor can cause multiple diseases (eg, smoking can cause cancer, asthma and cardiovascular diseases19 20).
Psychosocial: this theory postulates that the emotions that arise due to social inequality can directly affect biological health.17 This can be caused in two ways, either through the practice of health compromising behaviours or through biological changes due to the individual being in a sustained state of stress.17 Hence, the behavioural explanation can be a descendent of psychosocial processes under this explanation. The perceived lack of control and psychosocial stress may lead to adverse health behaviours and may activate neuroendocrine mechanisms, and in doing so, may affect multiple body systems and lead to multimorbidity.
Materialist: the material environment has a significant impact on the health of an individual. Exposure to health risk or health protective factors varies according to socioeconomic position due to differential access to material resources; differences are more evident in non-egalitarian societies. For instance, individuals living in socioeconomic disadvantage are less likely to be able to access information and resources necessary to maintain good health compared with their more advantaged counterparts.17 Socioeconomically deprived individuals are also more likely to be exposed to hazardous working environments.17 The materialist theory proposes these explanations as pathways between socioeconomic deprivation and health inequalities.17 Lack of material resources such as inadequate housing, for example, can lead to multimorbidity by causing depression as well as respiratory illnesses such as asthma.
Social support: this theory holds that positive social support mitigates the detrimental effect of socioeconomic deprivation in health.21 22 Accordingly, strong social networks and good social relationships are linked to good health, and conversely, poor social relations and weak social support networks are deleterious to health. Social support is considered to be a distal determinant of health that may influence health through multiple mechanisms, for example, by reducing stress and providing access to local resources, and in doing so, may prevent both mental and physical multimorbidity.
Social capital: while variously defined, social capital is broadly described as the functioning of social groups through a shared sense of identity, trust, cooperation, reciprocity and shared understandings, norms and values.23 Social capital emphasises that a more unequal distribution in income undermines trust and damages social relationships at a population level. This theory attempts to explain why egalitarian societies tend to be healthier than non-egalitarian societies.24 25 Similar to social support, high social capital is likely to boost health and prevent multimorbidity by reducing stressors and increasing access to shared resources.
In addition to the above-mentioned theories, a life course framework examines the effect of early life socioeconomic exposures on later health outcomes.26 Two models are proposed to explain the life course framework: the accumulation model and the critical periods model. The accumulation model emphasises the cumulative effect of exposure to socioeconomic disadvantage across different stages in life on subsequent increased risk of poor health outcomes.27 The critical periods model focuses on the effect of exposure to factors influencing health during critical periods of development.27 Finally, a neo-liberal framework for health inequalities emphasises the role of political arrangements in leading to socioeconomic inequalities and in turn health inequalities.28
We aim to review the socioepidemiological theories applied to explain the relationship between socioeconomic disadvantage and multimorbidity in the population. Where possible, we examined whether theories applied were tested using robust analytical methods such as mediation analysis.
Methods
We conducted a scoping review to examine epidemiological theories applied to explain the association between socioeconomic disadvantage and multimorbidity29 30 and to map the information available in the current literature. Because the primary purpose of this study was to identify and categorise the theories being used in the existing literature, a scoping review was preferred over a systematic review. We followed the steps of a scoping review as per previously defined guidelines.29 30
Stage I: identifying the research question
Our review question was: ‘How are the socio-epidemiologic theories applied to explain the relationship between socioeconomic disadvantage and multimorbidity?’.
Stage II: identifying relevant studies
We identified search terms and keywords relevant to socioeconomic disadvantage, theoretical pathways and multimorbidity from published systematic reviews13 31 and tailored them to answer our research question. First, a detailed search strategy was developed using keywords and hierarchically defined subject headings. Once the search terms were agreed on, they were adapted for multiple electronic databases including Medline, Embase, PsychInfo (Ovid platform), Web of Science, Scielo, Applied Social Sciences, ERIC, Humanities Index and Sociological Abstracts (see online supplemental appendix 1). The reference lists of all selected articles were screened to identify any additional studies. Search alerts were set up to notify the research team of articles published after 25 May 2018 when literature search was implemented. This search was updated on 11 December 2019 and then on 28 September 2021.
bmjopen-2021-055264supp001.pdf (114.4KB, pdf)
Stage III: study selection
We applied a strict inclusion and exclusion criteria; these are displayed in table 1. We use the term socioeconomic position to reflect socioeconomic status of individuals or groups in the population. Socioeconomic status indicates the position in which an individual or a group is located within the social structure. It can be measured using educational attainment, income, occupation, wealth and area level measures (deprivation, socioeconomic scores). We use the term socioeconomic inequalities in health to indicate the differences in disease levels between people living with different socioeconomic positions. Socioeconomic disadvantage refers to those who have the low socioeconomic position. For inclusion in this review, socioeconomic position could be measured using the following indicators: occupation, income (household or individual), educational attainment, area level socioeconomic deprivation, wealth and social class.17 32
Table 1.
Study selection criteria
| Inclusion criteria | Exclusion criteria |
|
|
We excluded studies on ‘comorbidity’ as such studies are focused on an index condition (eg, diabetes).33 The terms multimorbidity and comorbidity are often used interchangeably as both describe the presence of multiple chronic conditions. However, comorbidity is a disease-centred term that describes the presence of additional conditions associated with an index disease.4 The focus of this review is multimorbidity only. Studies on institutionalised individuals, qualitative research and those written in a language other than English were excluded. A detailed list of inclusion and exclusion criteria can be found in table 1. Abstracts and full-text articles were reviewed for inclusion by LFA using the citation manager EndNote. A second reviewer (AS) cross-checked 10% of these articles.
Stage IV: charting the data
A data charting form was created that included study details (study design, country, population subgroups, measures of socioeconomic inequality, assessment of multimorbidity and conclusion on the association between socioeconomic variables and multimorbidity), use of theory, type of theories and context of application. Use of theory was categorised as inferred by us (reviewers/readers) or explicitly mentioned by the original study authors. It is important to distinguish between the two because the former relies on the reviewers/readers’ subjective judgement (which may not be accurate), while the latter accurately reflects the theoretical reasoning of the original authors. Data charting was performed by LFA and 10% of the studies were cross checked by AS.
Each study was examined for the type of theory (example: psychosocial or material), extent of use (whether used in a post hoc manner or integrated within an analysis) and their context of use (background, methods or discussion section of retrieved paper(s)). We recorded whether theories that were directly mentioned or inferred were consistent with any of the existing socioepidemiological theories. When directly mentioned, types of theories were recorded verbatim. This follows the approach previously applied in a published study examining the application of socioepidemiological theories in studies on the relationship between social inequality and oral health.31
Stage V: collating, summarising and reporting the results
We carried out a narrative synthesis to summarise the results from the retrieved data. Because the objective of this review is to offer a snapshot of the available evidence of theories explaining socioeconomic inequalities in multimorbidity and not on assessing the effect of socioeconomic disadvantage on multimorbidity development, we did not assess the quality of included papers in accordance with the guidelines for conducing scoping reviews.29
Patient and public involvement
No patients were directly involved in this study as this is a review of published studies.
Results
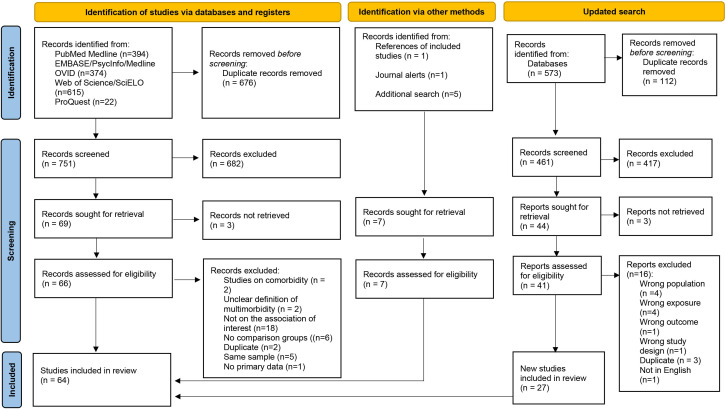
Our initial search led to the identification of 751 unique papers that underwent title and abstract screening. Sixty-nine papers were deemed eligible for full-text review. In addition, two studies were included for full-text review from other sources. Thirty-six studies proceeded to data charting stage after completion of full-text review. Online supplemental appendix 2 displays a list of studies with reasons for exclusion after full-text review. The updated search on 28 September 2021 led to a further screening of 461 titles and abstracts from the 573 newly identified records. After full-text screening of 44 studies, 27 new studies were included in the review. A total of 64 studies were included in this review. A flow chart of this process is shown in figure 1.
Figure 1.
Flow chart of the study selection process.
Summary characteristics of included studies
Twenty studies were from low-income and middle-income countries12 34–52 and the remaining 45 studies were from high-income countries. The majority of articles were conducted among adults and only three study included children.53–55 More than half (n=38/64) were cross-sectional and 26/64 used longitudinal data9 10 42 54 56–76 (table 2).
Table 2.
Summary characteristics of included studies
| Study | Study design | Location | Population focus | Assessment of multimorbidity (presence/ nature/extent/ both) | List of chronic conditions | Measure of socioeconomic disadvantage |
| Andersén et al79 | Cross-sectional | Finland | Adults aged 20–69 years | Presence of multimorbidity. Cut-off of two conditions. |
18 chronic conditions including: respiratory diseases, cardiovascular diseases, diabetes, mental disorders, dyspepsia/reflux disease, chronic kidney failure, sleep apnea, osteoporosis and chronic pain. | Occupation. |
| Calvo et al58 | Longitudinal | USA | Adults aged 60–61 and 70–71 years | Count of chronic conditions. | Eight conditions including high blood pressure, diabetes, cancer, chronic lung disease, heart problems, stroke, mental illness, arthritis or rheumatism. | Retirement sequence. |
| Craig et al41 | Cross-sectional | Jamaica | Individuals aged 15–74 years | Patterns of multimorbidity. | 11 chronic conditions including hypertension, obesity, hypercholesterolemia, diabetes, asthma, arthritis, cardiovascular disease, mental health disorders, COPD, stroke and glaucoma. | Occupational status, education and income level. |
| Head et al62 | Longitudinal | England | Adults aged 18 years and over | Presence of multimorbidity. Cut-off of two and three conditions. |
211 conditions listed elsewhere.62 | Area-level deprivation. |
| Hernández et al77 | Cross-sectional | 4 high income countries | Adults aged 52–85 years | Patterns of multimorbidity. | 10 conditions including cardiovascular diseases, diabetes, arthritis, cancer, lung disease, osteoporosis and psychological disorders. | Education, household income and employment. |
| Hone et al52 | Cross-sectional | Brazil | Individuals of any age | Presence of multimorbidity. Count of chronic conditions. |
53 chronic conditions. | Education. |
| Khanolkar et al64 | Longitudinal | UK | Adults aged 36–69 years | Presence of multimorbidity. Count of chronic conditions. |
18 health conditions including metabolic conditions, cardiovascular diseases, cancer, respiratory disorders, kidney disorders, gastrointestinal disorders, skin disorders, osteoarthritis, rheumatoid arthritis, neurological disorders and mental disorders. | Social class and education. |
| Lee et al67 | Longitudinal | South Korea | Adults aged 45 years and over | Multimorbidity clusters. Cut-off of two conditions. |
9 chronic conditions including: hypertension, diabetes, cancer, chronic lung disease, liver disease, heart disease, cerebrovascular disease, arthritis or rheumatoid arthritis and depression | Education, household income and employment. |
| Moin et al90 | Cross-sectional | Canada | Adults aged 22–95 years | Presence of multimorbidity. Cut-off of two and three conditions. |
18 chronic diseases including cardiovascular diseases, respiratory conditions, diabetes, mental illness, musculoskeletal conditions, renal failure, inflammatory bowel failure and cancers. | Household income and education. |
| Zacarías-Pons et al75 | Longitudinal | Europe | People aged 50 years and older | Latent transition analysis for types of multimorbidity. | Heart attack, hypertension, hypercholesterolaemia, stroke or cerebral vascular disease, diabetes or high blood sugar, chronic lung disease (COPD), cancer, stomach or duodenal ulcer, Parkinson disease, cataracts, dementia, other affective or emotional disorders, rheumatoid arthritis, osteoarthritis and osteoporosis | Education, employment and material deprivation index. |
| Vidyashree et al 48 | Cross-sectional | India | People aged 60 years and above in rural area | Presence of multimorbidity (cut-off unclear). |
Unclear. | Economic dependency. |
| Sharma and Maurya47 | Cross-sectional | India | People aged 60 years and above | Presence and patterns of multimorbidity. Cut-off of two conditions. |
Arthritis, rheumatism or osteoarthritis, cerebral embolism stroke or thrombosis, heart diseases, diabetes, chronic lung disease, asthma, depression, hypertension, Alzheimer’s disease, cancer, dementia, liver or gall bladder illness, osteoporosis, renal or urinary tract infection, cataract, loss of all-natural teeth, accidental injury in the past 1 year, injury due to fall, skin disease and paralysis. | Educational attainment, working status and wealth quintile. |
| Singh et al72 | Longitudinal | Australia | People aged 15 years and above | Presence of multimorbidity. Cut-off of two conditions. |
Arthritis, cancer, type 1 diabetes mellitus, type 2 diabetes mellitus, hypertension, heart disease, asthma, bronchitis or depression. | Financial hardship. |
| Aminisani et al56 | Longitudinal | New Zealand | Adults aged 55–70 years | Presence of multimorbidity. Cut-off of two conditions. |
Nine groups of chronic diseases: cardiovascular diseases, neurological diseases, musculoskeletal conditions, diabetes mellitus, respiratory diseases, chronic liver conditions, cancer and mental disorders. | Education and income. |
| Chamberlain et al83 | Cross-sectional | USA | Adults aged 20 years and over | Presence of multimorbidity. Cut-off of two conditions. |
21 conditions including cardiovascular diseases, metabolic conditions, respiratory diseases, arthritis, osteoporosis, chronic kidney disease, autism spectrum disorder, hepatitis, HIV, depression, dementia, schizophrenia and substance. | Area level deprivation. |
| Costa et al39 | Cross-sectional | Brazil | Adults aged 20–59 years | Presence of multimorbidity. Cut-off of two conditions. |
14 conditions including cardiovascular diseases, metabolic diseases, chronic pulmonary disease, digestive disorders, neurological disorders, cancer, kidney disease and depression. | Economic status and education. |
| Kim et al66 | Longitudinal | South Korea | Adults aged 19 years and over. | Presence of multimorbidity. Cut-off of two conditions. |
28 chronic conditions as listed elsewhere.63 | Household income and education. |
| Møller et al68 | Longitudinal | Denmark | People aged 16 years and over | Multimorbidity patterns or classes (Latent Class Analysis). | 47 diseases. | Education and employment. |
| Odland et al45 | Cross-sectional | Burkina Faso | Adults aged 40 years and older | Presence and patterns of multimorbidity. Cut-off of two conditions. |
11 conditions including: cancer, HIV, chronic respiratory disease, stroke heart disease, hypertension, diabetes, anxiety, depression and dementia/cognitive decline. | Education and wealth. |
| Pati et al46 | Cross-sectional | India | Adults aged 18 years and older | Presence of multimorbidity. Cut-off of two conditions. |
21 chronic conditions. | Poverty level and education. |
| Zhao et al50 | Longitudinal | China | People aged 50 years and older | Presence of multimorbidity. Cut-off of two conditions. |
Diagnosed. | Annual per-capita household consumption. |
| Yildiz et al85 | Cross-sectional | The Netherlands | People aged 18–64 years | Presence of multimorbidity. Cut-off of two and three conditions most prevalent chronic diseases |
List of most prevalent chronic diseases (cardiovascular diseases, psychological disorders, inflammatory conditions and respiratory diseases). | Employment status and education. |
| Wister et al82 | Cross-sectional | Canada | People aged 45–85 years | Presence of multimorbidity. Cut-off of two conditions. |
High blood pressure, osteoarthritis, back problems, cancer, diabetes, heart disease, thyroid dysfunction, lung disease, osteoporosis, urinary incontinence, migraine headaches, irritable bowel syndrome, intestinal and stomach ulcer, glaucoma, peripheral vascular disease, angina, macular degeneration, heart attack, transient ischaemic attack, kidney disease, rheumatoid arthritis, bowel incontinence, stroke, multiple sclerosis, epilepsy, Parkinson’s disease, as well as dementia and Alzheimer’s disease. | Vancouver. |
| Vinjerui84 | Cross-sectional | Norway | People aged 25–100 years | Complex multimorbidity. Three or more diseases involving three or more different body (organ) systems. |
51 chronic conditions including following body systems or types: neoplasms, endocrine/nutritional/metabolic, mental/behavioural, eye/adnexa, ear/mastoid, circulatory system, respiratory system, digestive system, skin/subcutaneous tissue, musculoskeletal/connective tissue and genitourinary systems. | Occupational groups. |
| Ba et al36 | Cross-sectional | Vietnam | Individuals aged 15 years and over | Presence of multimorbidity. Cut-off of two conditions. |
A list of 11 conditions including: cancer, heart and circulatory conditions, chronic joint problems, chronic pulmonary diseases, chronic kidney problems, chronic digestive problems, psychological illness, diabetes, and/or other chronic conditions (such as eye, nose, sore and throat, teeth problems, etc). | Educational level and occupational status. |
| Dugravot et al60 | Longitudinal | UK | Adults aged 35–55 years | Presence of multimorbidity. Cut-off of two and five conditions. |
9 conditions including diabetes, coronary heart disease, stroke, COPD, depression, arthritis, cancer, dementia and Parkinson’s disease. | Occupational position, education. |
| Johnston et al63 | Longitudinal | Scotland | Adults aged 45–51 years | Presence of multimorbidity. Cut-off of two conditions. |
Six conditions. List not provided. | Father’s occupation during childhood. Educational attainment in adulthood. |
| Park et al69 | Longitudinal | South Korea | Adults aged 50 years and older | Presence and patterns of multimorbidity (LCA). Cut-off of two conditions. |
10 chronic diseases: hypertension, dyslipidaemia, stroke, osteoarthritis, tuberculosis, asthma and allergies. | Household income, educational level and occupation. |
| Russell et al55 | Longitudinal | New Zealand | Age 2 years | Presence of multimorbidity. Cut-off of two conditions. |
Asthma requiring medication, eczema requiring medication, a birth condition, epilepsy, permanent hearing problems, vision problems not correctable with glasses and obesity. | Index constructed from maternal education, employment, financial stress, beneficiary status, housing tenure, overcrowding and residential mobility. |
| Seo71 | Longitudinal | South Korea | Working age adults | Presence of multimorbidity. Cut-off of two conditions. |
23 conditions. | Type of employment, income and education. |
| Calderón-Larrañaga et al57 | Longitudinal | Sweden | Adults aged 60 years and over | Presence of multimorbidity was explored as rapid or slow development of multiple chronic conditions. Cut-off of two conditions. |
List not provided. A disease was considered chronic if it had a long and if residual disability remained or life quality was worsened or long period of care, treatment or rehabilitation was needed. | Educational level and occupation. |
| Costa et al40 | Cross-sectional | Brazil | Adults aged 60 years and over | Presence and nature of multimorbidity. Cut-off of two conditions. |
31 conditions: cardiovascular diseases, metabolic conditions, musculoskeletal conditions, incontinence and constipation, neurological diseases, mental disorders, cancer, respiratory diseases and kidney disease. | Educational level and monthly income per capita (National Economic Index). |
| Mondor et al80 | Cross-sectional | Canada | Adults aged 18 years and older | Presence of multimorbidity. Cut-off of two conditions. |
12 chronic conditions including high blood pressure, diabetes, osteoarthritis, rheumatoid arthritis, heart attack, stroke, cancer, chronic lung disease, hip fracture, Parkinson’s disease, Alzheimer’s disease and affective disorders. | Household income and educational level inequalities. |
| Stanley et al3 | Cross-sectional | New Zealand | Adults aged 18 years and older | Presence of multimorbidity. Cut-off of two conditions. |
List of diseases not provided but listed elsewhere.64 | Area-based measure of socioeconomic deprivation |
| Stokes et al91 | Cross-sectional | New Zealand | Pacific and Maori adults aged 35 years and older | Presence of multimorbidity. Cut-off of two conditions. |
31 chronic conditions. | Area based measure of socioeconomic deprivation. |
| Alimohammadian et al35 | Cross-sectional | Iran | Adults aged 40–75 years | Presence of multimorbidity. Cut-off of two conditions. |
A total of nine conditions were explored: cardiovascular disease, diabetes (types I and II), COPD, chronic liver disease, tuberculosis, gastro-oesophageal reflux disease and cancers. | Socioeconomic status. Education. |
| Canizares et al59 | Longitudinal | Canada | Adults aged 20–69 | Presence and extent of multimorbidity. Cut-off of two conditions. |
18 chronic conditions were explored: arthritis, back problems, respiratory conditions, allergies (excluding food allergies), cardiovascular diseases, diabetes, cancer, ulcers, urinary incontinency, dementia, migraine, glaucoma and cataracts. | Education and household income. |
| Hayek et al61 | Longitudinal | Israel | Adults aged 21 years and over | Presence of multimorbidity. Cut-off of two conditions. |
10 conditions were assessed: asthma, arthritis, cancer, diabetes, dyslipidaemia, heart attack, hypertension, migraine, osteoporosis or thyroid disease. | Monthly household income and years of schooling. |
| Katikireddi et al10 | Longitudinal | Scotland | Adults aged 35–75 years | Presence and extent of multimorbidity. Cut-off of two conditions. |
40 conditions. | Area-based deprivation level. |
| Ki et al65 | Longitudinal | South Korea | Adults aged 30 years and over | Presence and extent of multimorbidity. Cut-off of two conditions. |
66 chronic conditions. | Educational attainment, employment status and relative poverty index. |
| Nielsen et al92 | Cross-sectional | 15 European countries | Adults aged 50 years and over | Presence of multimorbidity. Cut-off of two conditions. |
13 chronic conditions: high blood pressure, diabetes, osteoarthritis, rheumatoid arthritis, heart attack, stroke, cancer, chronic lung disease, hip fracture, Parkinson’s disease, Alzheimer’s disease and affective disorders. | Education and household income. |
| Nunes et al12 | Cross-sectional | Brazil | Adults aged 18 years and over | Presence of multimorbidity. Cut-off of two conditions. |
21 chronic conditions including: cardiovascular diseases, respiratory conditions, mental disorders, musculoskeletal conditions, metabolic disorders, arthritis/rheumatism, cancer and kidney problem. | State level of education and wealth quintiles. |
| Puth et al81 | Cross-sectional | Germany | Adults aged 18 years and older | Presence of multimorbidity. Cut-off of two conditions. |
15 chronic diseases: hypertension, coronary heart disease, myocardial infarction, chronic heart failure, stroke, diabetes mellitus, bronchial asthma, any type of cancer, hypercholesterolaemia, chronic bronchitis, chronic liver disease, arthrosis, osteoporosis, arthritis and depression. | Level of education. |
| Congdon93 | Cross-sectional | London, UK | Adults aged between 65 and 75 years | Presence of multimorbidity. Cut-off of two conditions. |
A list of 15 chronic conditions were assessed: cardiovascular diseases, diabetes, asthma, COPD, dementia, depression, serious mental illness (psychosis or bipolar disorder), cancer and chronic kidney disease. | Area-level socioeconomic deprivation. |
| Garin et al43 | Cross-sectional | 9 low to upper middle-income countries | Adults aged 50 years of age | Presence of multimorbidity. Cut-off of two conditions. |
9 conditions explored: arthritis, asthma, cataract, COPD, depression, diabetes, edentulism, hypertension, cognitive impairment, obesity and stroke. | Household income and education. |
| Jackson et al9 | Longitudinal | Australia | Women aged 45–50 years | Multimorbidity patterns (psychosomatic, musculoskeletal, cardiometabolic, cancer and respiratory syndromes). | 23 conditions examined including cardiovascular diseases, musculoskeletal conditions, respiratory diseases, cancer, allergies, mental conditions, diabetes, impaired glucose tolerance and chronic fatigue syndrome. | Education, occupation and income management. |
| Tomasdottir et al73 | Longitudinal | Norway | Adults aged 20–59 years | Presence of multimorbidity. Cut-off of two conditions. |
17 chronic conditions. | Financial hardship (worries). |
| Afshar et al34 | Cross-sectional | 28 low-income to middle-income countries | Adults aged 18 years and over | Presence of multimorbidity. Cut-off of two conditions. |
Seven chronic conditions including: arthritis, angina pectoris, asthma, depression, schizophrenia or psychosis and diabetes. | Level of education. |
| Chung et al38 | Cross-sectional | China | Adults aged 15 years and older | Presence and extent of multimorbidity. Cut-off of two conditions. |
List not provided. | Household income, educational attainment, employment status and type of housing. |
| Diaz et al94 | Cross-sectional | Norway | Immigrants aged 15 years and over | Presence of multimorbidity. Cut-off of two conditions. |
List not provided. | Personal income level. Reason for migration. |
| Prazeres and Santiago,95 | Cross-sectional | Portugal | Adults aged 18 years and older | Extent and presence of multimorbidity. Cut-off of two and three conditions. |
List not provided. | Years of educations, professional status and self-perceived socioeconomic status. |
| Roberts et al96 | Cross-sectional | Canada | Adults aged 20 years and older | Presence and extent of multimorbidity. Cut-off of three or three conditions. |
A list of nine conditions including arthritis, mood disorder and/or anxiety, asthma, diabetes mellitus, heart disease, COPD, cancer, stroke, and Alzheimer’s disease. | Educational level and household income. |
| Banjare and Pradhan37 | Cross-sectional | India | Adults aged over 60 years | Extent of multimorbidity (no morbidity, one morbidity, two morbidities and three or more morbidities). | 23 chronic conditions were assessed: musculoskeletal conditions, cardiovascular disease, respiratory conditions, neurological disorders, severe dental conditions, kidney or renal disorders, depression, liver or gall bladder illness, accidental injury, injury due to fall and skin disease. | Education, state of economic independence, quintiles of wealth, living arrangement and caste. |
| Habib et al44 | Cross-sectional | Lebanon | Palestinian refugees aged between 14 and 87 years old | Presence and extent of multimorbidity. Cut-off of two conditions. |
List not provided. | Educational attainment and wealth index. |
| McLean et al78 | Cross-sectional | Scotland | Adults aged 25 years and over | Presence and pattern of multimorbidity (physical only, mental only and mixed physical and mental multimorbidity). Cut-off of two conditions. |
A list of 35 physical and eight mental conditions were included but not specified on the paper. | Area-based deprivation. |
| Violán et al97 | Cross-sectional | Spain | Adults aged 19 years and older | Presence of multimorbidity. Cut-off of two conditions. |
31 chronic conditions. | Area-level of deprivation. |
| Alaba and Chola51 | Cross-sectional | South Africa | Adults aged 18 years and over | Presence or absence of multimorbidity. Cut-off of two conditions. |
Eight chronic conditions were assessed including tuberculosis, high blood pressure, diabetes or high blood sugar, stroke, asthma and cancer. | Years of schooling, household income, social assistance and employment. |
| Cornish et al54 | Longitudinal | Bristol, UK | Children aged 0–18 years | Presence and extent of multimorbidity. Cut-off of two conditions. |
As listed in the Johns Hopkins University Adjusted Clinical Groups System. | Parent’s educational level. Occupational social class. Housing tenure. Family adversity index during pregnancy. Area socioeconomic deprivation. |
| Demirchyan et al42 | Longitudinal | Armenia | Adults aged 37–90 years | Presence of multimorbidity. Cut-off of two conditions. |
List not provided. | Education, perceived low affordability of healthcare services and perceived living standards. |
| Weiman et al49 | Cross-sectional | South Africa | People aged 15 years and older | Presence of multimorbidity. Cut-off of two conditions. |
List not provided. | Multidimensional poverty index. |
| Agborsangaya et al86 | Cross-sectional | Canada | Adults aged 18 years and over | Presence of multimorbidity. Cut-off oftwo conditions. |
>16 chronic conditions explored, including diabetes, respiratory conditions, cardiovascular diseases, depression or anxiety, chronic pain, arthritis, gastrointestinal tract disease and kidney diseases. | Educational level and annual household income. |
| Barnett et al53 | Cross-sectional | UK | Individuals from all ages | Presence of multimorbidity Cut-off of two conditions. |
40 chronic conditions. | Area-level deprivation. |
| Schäfer et al70 | Longitudinal | Germany | Adults aged 65 years and older | Presence and patterns of multimorbidity (cardiometabolic disorders and anxiety, depression, somatoform disorders and pain) Cut-off of three conditions. |
29 chronic conditions. | Education, autonomy on former occupation and household income. |
| Tucker-Seeley et al74 | Longitudinal | USA | Adults aged 50 years and over | Presence and extent of multimorbidity. Count of chronic conditions. |
Six chronic conditions: cancer, heart disease, lung disease, stroke, diabetes and hypertension. | Childhood financial hardship (yes/no). Average lifetime earnings during young and middle adulthood. Educational attainment as indicator of adult socioeconomic status. |
COPD, chronic obstructive pulmonary disease.
Educational attainment was the preferred measure of socioeconomic position (n=38/64), and 38 studies used multiple measures of socioeconomic position as exposures. The majority of studies (n=51/64) simply documented the presence of multimorbidity, and approximately one-third (n=13/64) additionally examined different patterns of multimorbidity9 40 41 45 47 53 55 67–70 72 75 77 78 (table 2).
Types of theories
Overall, nearly half of studies (33/64) referred to at least one socioepidemiological theory. Therefore, 31 studies can be considered largely atheoretical, without any emphasis on pathways through which socioeconomic disadvantage leads to multimorbidity. In the 33 studies applying a theory, the following theories were referred to: behavioural,10 34 35 37 38 40–42 46 51–53 59 71 72 79–82 materialist38 41 42 45 46 48 50 52 71 72 74 79 82–85 and psychosocial.34 42 51 52 57 72 73 82 84–86 In addition, four studies applied a theoretical construct called ‘sense of coherence’, which indicates an individual’s coping capacity to deal with life and stressful events,87 and is an indicator of self-efficacy and psychosocial well-being (consistent with psychosocial explanations),73 and also encompasses social capital51 and social support,57 which are widely considered as psychosocial assets (table 3). Five studies used a life-course framework.10 55 63 64 74 Collectively, behavioural theory was the most referred to among studies.
Table 3.
Types of theories and context of application
| Study* | Theoretical application | Materialist | Behavioural | Psychosocial | Social capital | Life course | Neoliberal |
| Vidyashree et al 2021†48 | ✓ | ✓ | |||||
| Singh et al 2021†72 | Theory tested | ✓ | ✓ | ✓ | ✓ | ||
| Hone et al 2021†52 | ✓ | ✓ | ✓ | ||||
| Herández et al 2021†77 | ✓ | ||||||
| Khanolkar et al 2021‡64 | ✓ | ||||||
| Craig et al 2021‡41 | ✓ | ✓ | |||||
| Anderén et al 2021†79 | ✓ | ||||||
| Zhao et al 2020†50 | ✓ | ||||||
| Yidiz et al 2020§85 | ✓ | ✓ | ✓ | ||||
| Wister et al 2020§82 | ✓ | ✓ | ✓ | ||||
| Vinjerui et al 2020§84 | ✓ | ✓ | ✓ | ||||
| Chamberlain 2020†83 | ✓ | ||||||
| Odland et al 2020†45 | ✓ | ||||||
| Pati et al 2020†46 | ✓ | ✓ | |||||
| Russell et al 2019†55 | ✓ | ||||||
| Seo 2019†71 | ✓ | ✓ | |||||
| Johnston et al 2019‡63 | Theory tested | ✓ | |||||
| Calderón-Larrañaga et al 2018‡57 | ✓ | ||||||
| Mondor et al 2018‡80 | Theory tested | ✓ | |||||
| Alimohammadian et al 2017‡35 | ✓ | ||||||
| Katikireddi et al 2017‡10 | Theory tested | ✓ | ✓ | ||||
| Tomasdottir et al 2016‡73 | ✓ | ||||||
| Chung et al 2015‡5 | Theory tested | ✓ | ✓ | ||||
| Barnett et al 2012†53 | ✓ | ||||||
| Agborsangaya et al 2012‡86 | ✓ | ||||||
| Tucker-Seeley et al 2011‡74 | ✓ | ✓ | |||||
| Costa et al 2018†40 | ✓ | ||||||
| Canizares et al 2017†59 | ✓ | ||||||
| Puth et al 2017†81 | ✓ | ||||||
| Afshar et al 2015†34 | ✓ | ✓ | |||||
| Banjare and Pradhan 2014†37 | ✓ | ||||||
| Alaba and Chola 2013†51 | ✓ | ✓ | ✓ | ||||
| Demirchyan et al 2013†42 | ✓ | ✓ | ✓ |
*Restricted to studies with identified use of theories.
†Theory was identified and inferred by the reviewers.
‡Specific theory was explicitly mentioned by the authors.
§One or more theories were explicitly mentioned but one or more identified and inferred by the reviewers.
Context of application of theories
Of the papers using theories, 15 explicitly stated those theories,10 35 38 41 57 63 64 72–74 80 82 84–86 and the other 21 studies were inferred to be consistent with a presumed theoretical pathway, based on definitions from existing literature.
Testing the explanatory potential of theories
Only five studies10 38 63 72 80 tested variables consistent with theoretical pathways as mediators between socioeconomic disadvantage and multimorbidity. Applying material theory, Chung et al38 examined perceptions of financial hardship, an indicator of economic deprivation, as a mediator between housing tenure and multimorbidity. They found a small mediation effect (1.41%), indicating that increased financial burden puts private housing residents at a higher risk of suffering multimorbidity when compared with public housing residents.38
Drawing on behavioural theory as well as a life course framework, Katikireddi et al10 quantified mediation by five behavioural risk factors (diet, smoking, physical activity, alcohol and body mass index (BMI)) acting on the association between two socioeconomic measures (area-based deprivation and household income) and multimorbidity over the life course. Their analyses showed that the combination of behavioural factors partially mediated (by 40.8%) the inverse association between area level deprivation and multimorbidity.
The life course framework was applied by Johnston et al63 in their examination of educational attainment during adulthood as a mediator of the association between father’s occupational social class at birth and multimorbidity. Their analyses showed a partial attenuation of the effect of childhood socioeconomic position on multimorbidity by educational attainment. Authors did not report the proportion of effect that was mediated by adult educational attainment.
Mondor et al80 also drew on behavioural theory in their study that quantified the mediation effect of lifestyle factors (physical activity, smoking and BMI) on the association between income inequalities and multimorbidity. Lifestyle factors only explained a small proportion of observed income-related inequalities in multimorbidity. Physical activity explained 10.9% of income inequalities, while smoking and BMI only accounted for 1.8% and 0.4%, respectively.
Finally, Singh et al72 examined social support as a mediator between financial hardship and multimorbidity among Australian adults and found that 30% of the total effect of financial hardship on multimorbidity was transmitted through social support.
Discussion
Summary of findings
Overall, we found limited use of theories to explain the relationship between socioeconomic position and multimorbidity. When used, theories were seldom explicitly mentioned or tested. Among all the potential explanations, behavioural theories were the most frequently used, followed by materialist and then psychosocial theories.
Only five studies tested the explanatory potential of theories and their mediation effect on the association between socioeconomic position and multimorbidity. Although we identified the use of seven different theories, materialist, behavioural, psychosocial and life course theories were the only ones tested. Existing evidence partially support these theories10 38 63 72 80; however, their use was mostly limited to post hoc explanations of findings in the overall literature.
Our findings are consistent with the two major evidence gaps highlighted in the report ‘Multimorbidity: a priority for global health research’.4 First, evidence of the relationship between socioeconomic disadvantage and multimorbidity is largely cross-sectional. This is a limitation of the existing evidence, as temporal ordering between exposure (social disadvantage) and outcome (multimorbidity), a key undisputed criterion of causality,88 is difficult to establish cross-sectionally. Second, there is a paucity of evidence regarding pathways (eg, behavioural, material and psychosocial) between the shared causal factor (exposure to socioeconomic disadvantage) and multiple conditions that co-occur in multimorbidity.4 The lack of evidence precludes policymakers from intervening on causal mechanisms that can prevent or mitigate observed socioeconomic inequalities in multimorbidity.89 Among those studies testing theories, there was a predominance of the application of the behavioural theory. However, the use of contemporary approaches to causal inference, using a counterfactual framework to maximise exchangeability between exposed and unexposed participants, was limited.72 89 Therefore, we cannot rule out bias arising from mediator-outcome confounding, time varying confounding or the presence exposure–outcome interaction. Approaches need to shift towards a more comprehensive examination of pathways to allow policymakers to select interventions with maximum capacity to reduce inequalities. It is also worth noting that given the variations in the relationship of interest according to individual (eg, age) and contextual characteristics (eg, country level of income development), future studies should examine the relevance of theories across different contexts and age groups.
Strengths and limitations
Our study has some strengths and limitations. To our knowledge, this is the first scoping review that explores the use of theories to explain the association between socioeconomic position and multimorbidity in the current literature. We identified numerous gaps in the literature that need to be addressed to improve our understanding of the socioeconomic inequalities in multimorbidity. Our search strategy drew on a wide range of electronic databases, and we used a robust methodology, already piloted and verified in previous work.31 A key limitation is that articles not in English were excluded in our review. Moreover, we did not use any tool to assess the quality of the included studies. This information is already provided by existing reviews.13 14 Lastly, we restricted this review to articles assessing only multimorbidity and excluded those looking at comorbidities. We acknowledge that some authors use both terms interchangeably, therefore papers using the term comorbidity to indicate the presence of multiple independent chronic conditions may be missing from this review.
Conclusion
Our understanding of the pathways between socioeconomic inequalities and multimorbidity is limited and mostly unexplained. Studies often focus on the patterns of distribution of multimorbidity across the population, rather than the mechanisms shaping these distributions. Robust evidence from longitudinal and interventional studies is needed to understand the pathways between socioeconomic disadvantage and multimorbidity. Generating such evidence will guide the development of interventions and public policies to prevent multimorbidity among people living in disadvantage.
Supplementary Material
Footnotes
Contributors: LFA contributed towards the development of search strategy, screening, data extraction and appraisal of included studies and manuscript preparation. AS contributed towards the design, development of search strategy, screening, data extraction and appraisal of included studies and manuscript preparation. EY contributed towards the development of search strategy, data extraction of included studies and manuscript preparation. TK and DC-S contributed towards the development of search strategy and manuscript preparation. SZ contributed towards manuscript preparation. LFA and AS are the guarantors.
Funding: TK is also supported by an Australian Research Council Discovery Early Career Research Award (DE200100607).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1.Xu X, Mishra GD, Dobson AJ, et al. Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: a 20-year cohort study. PLoS Med 2018;15:e1002516. 10.1371/journal.pmed.1002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran J, Norton R, Conrad N, et al. Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: a population-based cohort study. PLoS Med 2018;15:e1002513. 10.1371/journal.pmed.1002513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley J, Semper K, Millar E, et al. Epidemiology of multimorbidity in New Zealand: a cross-sectional study using national-level hospital and pharmaceutical data. BMJ Open 2018;8:e021689. 10.1136/bmjopen-2018-021689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Academy of Medical Sciences . Multimorbidity: a priority for global health research. London: The Academy of Medical Sciences, 2018. [Google Scholar]
- 5.Wallace E, Salisbury C, Guthrie B, et al. Managing patients with multimorbidity in primary care. BMJ 2015;350:h176. 10.1136/bmj.h176 [DOI] [PubMed] [Google Scholar]
- 6.Kingston A, Robinson L, Booth H, et al. Projections of multi-morbidity in the older population in England to 2035: estimates from the population ageing and care simulation (PACSim) model. Age Ageing 2018;47:374–80. 10.1093/ageing/afx201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iaccarino G, Grassi G, Borghi C, et al. Age and multimorbidity predict death among COVID-19 patients. Hypertension 2020;76:366–72. 10.1161/HYPERTENSIONAHA.120.15324 [DOI] [PubMed] [Google Scholar]
- 8.Ataguba JE-O. Inequalities in multimorbidity in South Africa. Int J Equity Health 2013;12:64. 10.1186/1475-9276-12-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson CA, Dobson AJ, Tooth LR, et al. Lifestyle and socioeconomic determinants of multimorbidity patterns among mid-aged women: a longitudinal study. PLoS One 2016;11:e0156804. 10.1371/journal.pone.0156804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katikireddi SV, Skivington K, Leyland AH, et al. The contribution of risk factors to socioeconomic inequalities in multimorbidity across the lifecourse: a longitudinal analysis of the Twenty-07 cohort. BMC Med 2017;15:152. 10.1186/s12916-017-0913-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunna R, San Sebastian M, Stewart Williams J. Measurement and decomposition of socioeconomic inequality in single and multimorbidity in older adults in China and Ghana: results from the who study on global ageing and adult health (SAGE). Int J Equity Health 2017;16:79. 10.1186/s12939-017-0578-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunes BP, Chiavegatto Filho ADP, Pati S, et al. Contextual and individual inequalities of multimorbidity in Brazilian adults: a cross-sectional national-based study. BMJ Open 2017;7:e015885. 10.1136/bmjopen-2017-015885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pathirana TI, Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health 2018;42:186–94. 10.1111/1753-6405.12762 [DOI] [PubMed] [Google Scholar]
- 14.Ingram E, Ledden S, Beardon S, et al. Household and area-level social determinants of multimorbidity: a systematic review. J Epidemiol Community Health 2021;75:232–41. 10.1136/jech-2020-214691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcaya MC, Arcaya AL, Subramanian SV. Inequalities in health: definitions, concepts, and theories. Glob Health Action 2015;8:27106. 10.3402/gha.v8.27106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townsend P, Davidson N, Black DS. Inequalities in health : the Black report. In: Townsend P, Davidson N, Black DS, eds. Great Britain. Working group on inequalities in H. Harmondsworth: Penguin, 1982. [Google Scholar]
- 17.Bartley M. Health inequality : an introduction to theories, concepts, and methods. Cambridge, UK: Polity Press, 2004. [Google Scholar]
- 18.Ball K, Timperio A, Salmon J, et al. Personal, social and environmental determinants of educational inequalities in walking: a multilevel study. J Epidemiol Community Health 2007;61:108–14. 10.1136/jech.2006.048520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation 2015;132:1795–804. 10.1161/CIRCULATIONAHA.115.017926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2017;2:e356–66. 10.1016/S2468-2667(17)30118-4 [DOI] [PubMed] [Google Scholar]
- 21.House JS, Landis KR, Umberson D. Social relationships and health. Science 1988;241:540–5. 10.1126/science.3399889 [DOI] [PubMed] [Google Scholar]
- 22.Shumaker SA, Brownell A. Toward a theory of social support: closing conceptual gaps. J Soc Issues 1984;40:11–36. 10.1111/j.1540-4560.1984.tb01105.x [DOI] [Google Scholar]
- 23.Baum FE, Ziersch AM. Social capital. J Epidemiol Community Health 2003;57:320–3. 10.1136/jech.57.5.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawachi I, Berkman L. Social cohesion, social capital, and health. Social epidemiology 2000;174. [Google Scholar]
- 25.Kawachi I, Kennedy BP. Health and social cohesion: why care about income inequality? BMJ 1997;314:1037–40. 10.1136/bmj.314.7086.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger N. A glossary for social epidemiology. J Epidemiol Community Health 2001;55:693–700. 10.1136/jech.55.10.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawachi I, Subramanian SV, Almeida-Filho N. A glossary for health inequalities. J Epidemiol Community Health 2002;56:647–52. 10.1136/jech.56.9.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coburn D. Income inequality, social cohesion and the health status of populations: the role of neo-liberalism. Soc Sci Med 2000;51:135–46. 10.1016/s0277-9536(99)00445-1 [DOI] [PubMed] [Google Scholar]
- 29.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 31.Singh A, Harford J, Schuch HS, et al. Theoretical basis and explanation for the relationship between area-level social inequalities and population oral health outcomes - A scoping review. SSM Popul Health 2016;2:451–62. 10.1016/j.ssmph.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Psychological Association . Measuring socioeconomic status and subjective social status. public interest Directorate, socioeconomic status office, resources and publication 2018.
- 33.Bonavita V, De Simone R. Towards a definition of comorbidity in the light of clinical complexity. Neurol Sci 2008;29 Suppl 1:S99–102. 10.1007/s10072-008-0898-1 [DOI] [PubMed] [Google Scholar]
- 34.Afshar S, Roderick PJ, Kowal P, et al. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the world health surveys. BMC Public Health 2015;15:776. 10.1186/s12889-015-2008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alimohammadian M, Majidi A, Yaseri M, et al. Multimorbidity as an important issue among women: results of a gender difference investigation in a large population-based cross-sectional study in West Asia. BMJ Open 2017;7:e013548. 10.1136/bmjopen-2016-013548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ba NV, Minh HV, Quang LB, et al. Prevalence and correlates of multimorbidity among adults in border areas of the central highland region of Vietnam, 2017. J Comorb 2019;9:2235042X19853382. 10.1177/2235042X19853382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banjare P, Pradhan J. Socio-Economic inequalities in the prevalence of multi-morbidity among the rural elderly in Bargarh district of Odisha (India). PLoS One 2014;9:e97832. 10.1371/journal.pone.0097832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung RY, Mercer S, Lai FTT, et al. Socioeconomic determinants of multimorbidity: a population-based household survey of Hong Kong Chinese. PLoS One 2015;10:e0140040. 10.1371/journal.pone.0140040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa Ândria Krolow, Bertoldi AD, Fontanella AT, et al. Does socioeconomic inequality occur in the multimorbidity among Brazilian adults? Rev Saude Publica 2020;54:138. 10.11606/s1518-8787.2020054002569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costa CDS, Flores TR, Wendt A, et al. Inequalities in multimorbidity among elderly: a population-based study in a City in southern Brazil. Cad Saude Publica 2018;34:e00040718. 10.1590/0102-311X00040718 [DOI] [PubMed] [Google Scholar]
- 41.Craig LS, Cunningham-Myrie CA, Hotchkiss DR, et al. Social determinants of multimorbidity in Jamaica: application of latent class analysis in a cross-sectional study. BMC Public Health 2021;21:1197. 10.1186/s12889-021-11225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demirchyan A, Khachadourian V, Armenian HK, et al. Short and long term determinants of incident multimorbidity in a cohort of 1988 earthquake survivors in Armenia. Int J Equity Health 2013;12:68. 10.1186/1475-9276-12-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garin N, Koyanagi A, Chatterji S, et al. Global multimorbidity patterns: a cross-sectional, population-based, Multi-Country study. J Gerontol A Biol Sci Med Sci 2016;71:205–14. 10.1093/gerona/glv128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habib RR, Hojeij S, Elzein K, et al. Associations between life conditions and multi-morbidity in marginalized populations: the case of Palestinian refugees. Eur J Public Health 2014;24:727–33. 10.1093/eurpub/cku089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odland ML, Payne C, Witham MD, et al. Epidemiology of multimorbidity in conditions of extreme poverty: a population-based study of older adults in rural Burkina Faso. BMJ Glob Health 2020;5:e002096. 10.1136/bmjgh-2019-002096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pati S, Swain S, Knottnerus JA, et al. Magnitude and determinants of multimorbidity and health care utilization among patients attending public versus private primary care: a cross-sectional study from Odisha, India. Int J Equity Health 2020;19:57. 10.1186/s12939-020-01170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma P, Maurya P. Gender differences in the prevalence and pattern of disease combination of chronic multimorbidity among Indian elderly. Ageing Int 2021;14. 10.1007/s12126-021-09419-9 [DOI] [Google Scholar]
- 48.Vidhyashree MD, Adhilakshmi R, Kamini B. Socio-Economic determinants of multimorbidity among the elderly population in a rural area in Kancheepuram district of Tamil Nadu. Journal of Research in Medical and Dental Science 2021;9:436–41. [Google Scholar]
- 49.Weimann A, Dai D, Oni T. A cross-sectional and spatial analysis of the prevalence of multimorbidity and its association with socioeconomic disadvantage in South Africa: a comparison between 2008 and 2012. Soc Sci Med 2016;163:144–56. 10.1016/j.socscimed.2016.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Atun R, Oldenburg B, et al. Physical multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: an analysis of population-based panel data. Lancet Glob Health 2020;8:e840–9. 10.1016/S2214-109X(20)30127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alaba O, Chola L. The social determinants of multimorbidity in South Africa. Int J Equity Health 2013;12:63. 10.1186/1475-9276-12-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hone T, Stokes J, Trajman A, et al. Racial and socioeconomic disparities in multimorbidity and associated healthcare utilisation and outcomes in Brazil: a cross-sectional analysis of three million individuals. BMC Public Health 2021;21:1287. 10.1186/s12889-021-11328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 54.Cornish RP, Boyd A, Van Staa T, et al. Socio-Economic position and childhood multimorbidity: a study using linkage between the Avon longitudinal study of parents and children and the general practice research database. Int J Equity Health 2013;12:66. 10.1186/1475-9276-12-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russell J, Grant CC, Morton SMB. Multimorbidity in early childhood and socioeconomic disadvantage: findings from a large New Zealand child cohort. Acad Pediatr 2020;20:619–27. 10.1016/j.acap.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 56.Aminisani N, Stephens C, Allen J, et al. Socio-Demographic and lifestyle factors associated with multimorbidity in New Zealand. Epidemiol Health 2020;42:e2020001. 10.4178/epih.e2020001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calderón-Larrañaga A, Santoni G, Wang HX, et al. Rapidly developing multimorbidity and disability in older adults: does social background matter? J Intern Med 2018;283:489–99. 10.1111/joim.12739 [DOI] [PubMed] [Google Scholar]
- 58.Calvo E, Azar A, Shura R, et al. A new path to address multimorbidity? longitudinal analyses of retirement sequences and chronic diseases in old age. J Appl Gerontol 2021:7334648211031038. 10.1177/07334648211031038 [DOI] [PubMed] [Google Scholar]
- 59.Canizares M, Hogg-Johnson S, Gignac MAM, et al. Increasing trajectories of multimorbidity over time: birth cohort differences and the role of changes in obesity and income. J Gerontol B Psychol Sci Soc Sci 2018;73:1303–14. 10.1093/geronb/gbx004 [DOI] [PubMed] [Google Scholar]
- 60.Dugravot A, Fayosse A, Dumurgier J, et al. Social inequalities in multimorbidity, frailty, disability, and transitions to mortality: a 24-year follow-up of the Whitehall II cohort study. Lancet Public Health 2020;5:e42–50. 10.1016/S2468-2667(19)30226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayek S, Ifrah A, Enav T, et al. Prevalence, correlates, and time trends of multiple chronic conditions among Israeli adults: estimates from the Israeli National health interview survey, 2014-2015. Prev Chronic Dis 2017;14:E64. 10.5888/pcd14.170038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Head A, Fleming K, Kypridemos C, et al. Inequalities in incident and prevalent multimorbidity in England, 2004–19: a population-based, descriptive study. The Lancet Healthy Longevity 2021;2:e489–97. 10.1016/S2666-7568(21)00146-X [DOI] [PubMed] [Google Scholar]
- 63.Johnston MC, Black C, Mercer SW, et al. Impact of educational attainment on the association between social class at birth and multimorbidity in middle age in the Aberdeen children of the 1950s cohort study. BMJ Open 2019;9:e024048. 10.1136/bmjopen-2018-024048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khanolkar AR, Chaturvedi N, Kuan V, et al. Socioeconomic inequalities in prevalence and development of multimorbidity across adulthood: a longitudinal analysis of the MRC 1946 national survey of health and development in the UK. PLoS Med 2021;18:e1003775. 10.1371/journal.pmed.1003775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ki M, Lee YH, Kim Y-S, et al. Socioeconomic inequalities in health in the context of multimorbidity: a Korean panel study. PLoS One 2017;12:e0173770. 10.1371/journal.pone.0173770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Keshavjee S, Atun R. Trends, patterns and health consequences of multimorbidity among South Korea adults: analysis of nationally representative survey data 2007-2016. J Glob Health 2020;10:020426. 10.7189/jogh.10.020426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SA, Joo S, Chai HW, et al. Patterns of multimorbidity trajectories and their correlates among Korean older adults. Age Ageing 2021;50:1336–41. 10.1093/ageing/afab002 [DOI] [PubMed] [Google Scholar]
- 68.Møller SP, Laursen B, Johannesen CK, et al. Patterns of multimorbidity and demographic profile of latent classes in a Danish population-A register-based study. PLoS One 2020;15:e0237375. 10.1371/journal.pone.0237375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park B, Lee HA, Park H. Use of latent class analysis to identify multimorbidity patterns and associated factors in Korean adults aged 50 years and older. PLoS One 2019;14:e0216259. 10.1371/journal.pone.0216259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schäfer I, Hansen H, Schön G, et al. The influence of age, gender and socio-economic status on multimorbidity patterns in primary care. first results from the multicare cohort study. BMC Health Serv Res 2012;12:89. 10.1186/1472-6963-12-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seo S. Multimorbidity development in working people. Int J Environ Res Public Health 2019;16:4749. 10.3390/ijerph16234749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh A, Contreras Suarez D, You E, et al. Role of social support in the relationship between financial hardship and multimorbidity-a causal mediation analysis. Eur J Public Health 2021;31:482–7. 10.1093/eurpub/ckab015 [DOI] [PubMed] [Google Scholar]
- 73.Tomasdottir MO, Sigurdsson JA, Petursson H, et al. Does 'existential unease' predict adult multimorbidity? Analytical cohort study on embodiment based on the Norwegian HUNT population. BMJ Open 2016;6:e012602. 10.1136/bmjopen-2016-012602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tucker-Seeley RD, Li Y, Sorensen G, et al. Lifecourse socioeconomic circumstances and multimorbidity among older adults. BMC Public Health 2011;11:313. 10.1186/1471-2458-11-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zacarías-Pons L, Vilalta-Franch J, Turró-Garriga O, et al. Multimorbidity patterns and their related characteristics in European older adults: a longitudinal perspective. Arch Gerontol Geriatr 2021;95:104428. 10.1016/j.archger.2021.104428 [DOI] [PubMed] [Google Scholar]
- 76.Zou S, Wang Z, Bhura M, et al. Prevalence and associated socioeconomic factors of multimorbidity in ten regions of China: a cross-sectional analysis. The Lancet 2020;396:S12. 10.1016/S0140-6736(20)32432-6 [DOI] [PubMed] [Google Scholar]
- 77.Hernández B, Voll S, Lewis NA, et al. Comparisons of disease cluster patterns, prevalence and health factors in the USA, Canada, England and ireland. BMC Public Health 2021;21:1674. 10.1186/s12889-021-11706-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McLean G, Gunn J, Wyke S, et al. The influence of socioeconomic deprivation on multimorbidity at different ages: a cross-sectional study. Br J Gen Pract 2014;64:e440–7. 10.3399/bjgp14X680545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andersén H, Kankaanranta H, Tuomisto LE, et al. Multimorbidity in Finnish and Swedish speaking Finns; association with daily habits and socioeconomic status - Nordic EpiLung cross-sectional study. Prev Med Rep 2021;22:101338. 10.1016/j.pmedr.2021.101338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mondor L, Cohen D, Khan AI, et al. Income inequalities in multimorbidity prevalence in Ontario, Canada: a decomposition analysis of linked survey and health administrative data. Int J Equity Health 2018;17:90. 10.1186/s12939-018-0800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puth M-T, Weckbecker K, Schmid M, et al. Prevalence of multimorbidity in Germany: impact of age and educational level in a cross-sectional study on 19,294 adults. BMC Public Health 2017;17:826. 10.1186/s12889-017-4833-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wister A, Rosenkrantz L, Shashank A, et al. Multimorbidity and socioeconomic deprivation among older adults: a cross-sectional analysis in five Canadian cities using the CLSA. Journal of Aging and Environment 2020;34:435–54. 10.1080/26892618.2020.1734138 [DOI] [Google Scholar]
- 83.Chamberlain AM, Finney Rutten LJ, Wilson PM, et al. Correction to: neighborhood socioeconomic disadvantage is associated with multimorbidity in a geographically-defined community. BMC Public Health 2020;20:1412. 10.1186/s12889-020-09527-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vinjerui KH, Bjerkeset O, Bjorngaard JH, et al. Socioeconomic inequalities in the prevalence of complex multimorbidity in a Norwegian population: findings from the cross-sectional HUNT study. BMJ Open 2020;10:e036851. 10.1136/bmjopen-2020-036851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yildiz B, Schuring M, Knoef MG, et al. Chronic diseases and multimorbidity among unemployed and employed persons in the Netherlands: a register-based cross-sectional study. BMJ Open 2020;10:e035037. 10.1136/bmjopen-2019-035037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agborsangaya CB, Lau D, Lahtinen M, et al. Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health 2012;12:201. 10.1186/1471-2458-12-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antonovsky A, Sourani T. Family sense of coherence and family adaptation. J Marriage Fam 1988;50:79–92. 10.2307/352429 [DOI] [Google Scholar]
- 88.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. 10.1177/003591576505800503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.VanderWeele TJ. Explanation in causal inference : methods for mediation and interaction. New York: Oxford University Press, 2015. [Google Scholar]
- 90.Moin JS, Glazier RH, Kuluski K, et al. Examine the association between key determinants identified by the chronic disease indicator framework and multimorbidity by rural and urban settings. J Comorb 2021;11:26335565211028157. 10.1177/26335565211028157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stokes T, Azam M, Noble FD. Multimorbidity in Māori and Pacific patients: cross-sectional study in a Dunedin general practice. J Prim Health Care 2018;10:39–43. 10.1071/HC17046 [DOI] [PubMed] [Google Scholar]
- 92.Nielsen CR, Halling A, Andersen-Ranberg K. Disparities in multimorbidity across Europe – Findings from the SHARE Survey. Eur Geriatr Med 2017;8:16–21. 10.1016/j.eurger.2016.11.010 [DOI] [Google Scholar]
- 93.Congdon P. Area variations in multiple morbidity using a life table methodology. Health Serv Outcomes Res Methodol 2016;16:58–74. 10.1007/s10742-015-0142-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diaz E, Poblador-Pou B, Gimeno-Feliu L-A, et al. Multimorbidity and its patterns according to immigrant origin. A nationwide register-based study in Norway. PLoS One 2015;10:e0145233. 10.1371/journal.pone.0145233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prazeres F, Santiago L. Prevalence of multimorbidity in the adult population attending primary care in Portugal: a cross-sectional study. BMJ Open 2015;5:e009287. 10.1136/bmjopen-2015-009287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roberts KC, Rao DP, Bennett TL, et al. Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promot Chronic Dis Prev Can 2015;35:87. 10.24095/hpcdp.35.6.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Violán C, Foguet-Boreu Q, Fernández-Bertolín S, et al. Soft clustering using real-world data for the identification of multimorbidity patterns in an elderly population: cross-sectional study in a Mediterranean population. BMJ Open 2019;9:e029594. 10.1136/bmjopen-2019-029594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055264supp001.pdf (114.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.