Abstract
Background
When vaccines against the novel COVID-19 were available in Senegal, many questions were raised. How long should non-pharmaceutical interventions (NPIs) be maintained during vaccination roll-out? What are the best vaccination strategies?
Methods
In this study, we used an age-structured dynamic mathematical model. This model uses parameters based on SARS-CoV-2 virus, information on different types of NPIs, epidemiological and demographic data, some parameters relating to hospitalisations and vaccination in Senegal.
Results
In all scenarios explored, the model predicts a larger third epidemic wave of COVID-19 in terms of new cases and deaths than the previous waves. In a context of limited vaccine supply, vaccination alone will not be sufficient to control the epidemic, and the continuation of NPIs is necessary to flatten the epidemic curve. Assuming 20% of the population have been vaccinated, the optimal period to relax NPIs would be a few days from the last peak. Regarding the prioritisation of age groups to be vaccinated, the model shows that it is better to vaccinate individuals aged 5–60 years and not just the elderly (over 60 years) and those in high-risk groups. This strategy could be more cost-effective for the government, as it would reduce the high costs associated with hospitalisation. In terms of vaccine distribution, the optimal strategy would be to allocate full dose to the elderly. If vaccine doses are limited, half dose followed by full dose would be sufficient for people under 40 years because whether they receive half or full dose, the reduction in hospitalisations would be similar and their death-to-case ratio is very low.
Conclusions
This study could be presented as a decision support tool to help devise strategies to control the COVID-19 pandemic and help the Ministry of Health to better manage and allocate the available vaccine doses.
Keywords: COVID-19, mathematical modelling, vaccines, control strategies, prevention strategies
Key questions.
What is already known?
Since the beginning of the COVID-19 pandemic, many countries have relied on non-pharmaceutical interventions (NPIs), such as mask wearing and social distancing, to control the spread of the disease.
While NPIs are effective at controlling the spread of COVID-19, they are associated with considerable social and economic harm.
Vaccines against COVID-19 are now available, but it is necessary to determine optimal, context-specific strategies for the rollout of these vaccines, particularly in low-income and middle-income countries (LMICs) such as Senegal, which have a young population and where vaccine supplies are often limited.
What are the new findings?
In a context of limited vaccine supply we found that:
Vaccination alone will not be sufficient to control the spread of COVID-19; therefore, maintaining NPIs will be necessary to flatten the epidemic curve.
Regarding the prioritisation of age groups to be vaccinated, our model shows that it is better to vaccinate the entire population and not only the elderly or individuals in high-risk groups.
In the context of scarcity of vaccines, the best strategy for vaccine delivery would be: first, to give full dose to the elderly and those with comorbidities, then fractionated doses (half dose followed by full dose) for individuals under 40 years.
Key questions.
What do the new findings imply?
A strategy of vaccinating the entire population in an LMIC could be more cost-effective for the government as it would reduce the high costs associated with hospitalisations.
A strategy of giving fractionate doses to individuals aged less than 40 years is advisable because, whether these individuals receive half or full dose, the reduction in hospitalisations would be similar and their death-to-case ratio is very low.
Introduction
Since 31 December 2019, the world has been affected by an unprecedented pandemic caused by the novel COVID-19. As of May 2021, and based on data from the WHO COVID-19 Dashboard, more than 151 million confirmed cases of COVID-19 and over 3 million deaths with the disease had been reported worldwide, with a global death-to-case ratio of 2.09%.1 Compared with the rest of the world, Africa remains the least-affected continent, with 3.3 million confirmed cases and 82 870 deaths.1 In response to the COVID-19 pandemic, many countries around the world have introduced restrictive measures to limit the spread of SARS-CoV-2, the virus that causes COVID-19. African countries were early warned about importations of COVID-19 cases.2 In many sub-Saharan African countries considered as low-income and middle-income countries (LMICs), control measures were introduced very early in the epidemic. As early as 2 January 2020, Ivory Coast implemented enhanced surveillance at airports.3 With the exception of Ethiopian airlines, most of African airlines have suspended flights to China.4 As early as April 2020, Senegal conducted a survey that showed that 72.5% of people were in favour of a 2-week lockdown.5 From March to mid-June 2020, nine sub-Saharan African countries (Ghana, Nigeria, South Africa, Sierra Leone, Sudan, Tanzania, Uganda, Zambia and Zimbabwe) implemented lockdown measures to help inhibit COVID-19 transmission.6 Non-pharmaceutical interventions (NPIs), including restrictions on movement, public gatherings and schools, were also implemented. However, even if NPIs are effective at controlling the spread of COVID-19, they are associated with considerable social and economic harm.7 8
By May 2021, Senegal had experienced two epidemic waves of COVID-19. The first wave, which occurred between the beginning of, March and mid-November 2020 resulted in 15 598 confirmed cases and 328 deaths. The second wave lasted from mid-November 2020 to 2 May 2021 (the date of the most recent update) and resulted in 24 626 confirmed cases and 781 deaths. The Senegalese government introduced restrictive measures as soon as the first coronavirus cases were detected, such as mask wearing, the closure of schools and universities, banning international travel and public gatherings, and imposing curfews (online supplemental material 1, table S3).
bmjgh-2021-007236supp001.pdf (5.6MB, pdf)
Towards the end of 2020, effective vaccines against COVID-19 became available. The need for both a global vaccination programme and global availability of vaccines led WHO to develop two major guidance documents. The first was the Strategic Advisory Group of Experts on Immunisation Values Framework, which aims to ensure that effective COVID-19 vaccines are shared equitably among and within countries.9 The second was a roadmap for prioritising subpopulations for vaccination against COVID-19.10 These documents have been contextualised according to countries’ needs, by taking into account various factors such as the NPIs implemented, the epidemiology of COVID-19 at a local level and the demographic structure of the population.
Regarding vaccination, the objectives set by the Senegalese government are to obtain 6 million doses of vaccine by the end of 2021.11 The availability of vaccines for LMICs is often limited, and they must be obtained via the COVAX assistance programme or by direct purchase.12 13 Direct purchase situations enable rapid access to vaccines, but the costs of such an approach to obtaining vaccines can be significant. In the context of vaccine scarcity, vaccine supply is a major challenge, especially in LMICs. For vaccines’ doses distribution, using fractionated doses could provide a feasible solution that extends limited supplies of vaccines against COVID-19.14 For AstraZeneca vaccine, an initial half dose showed a lower immune response than a full dose while a half dose followed by full dose gave similar postsecond dose immune responses as two full doses.15
In February 2021, Senegal received 25 000 doses of the AstraZeneca vaccine from India, as well as 324 000 doses of the AstraZeneca vaccine and 200 000 doses of the Sinopharm vaccine through the COVAX initiative.16 Senegal began its vaccination campaign by initially targeting healthcare workers and people aged more than 50 years. Then, the vaccination programme was extended to all age groups except children. However, the vaccination programme is faced with a dilemma: to reduce hospitalisation costs by vaccinating the most populous age groups or to minimise vaccination costs by vaccinating the high-risk age groups, individuals at high risk of hospitalisation having comorbidities such as heart disease, diabetes, tuberculosis, obesity, etc to reduce mortality. Identifying these optimal vaccination strategies could help policy-makers make better decisions for disease control.
Here, we specifically adapted a model to the epidemiological context in Senegal and used it to address the following questions. First, what would be the impact of relaxing NPIs with different vaccination scenarios? Second, given a fixed allocation of doses (sufficient for 20% of the population) beyond those for individuals aged more than 60 years (from whom vaccines have already been assigned), which is the best age group to target? Third, in the interests of saving more lives and providing at least partial protection to a greater number of people, how to distribute the first tranche of available vaccines in a short time period?
Methods
Data collection method
Demographic data
Demographic data for Senegal, structured by age, were extracted from the United Nations World Population Prospects 2019.17 The mean household size was obtained from the National Agency for Statistics and Development in Senegal.18 In 2020, the population of Senegal comprised 16 705 608 people, and the median age was 19 years.19
Hospitalisation parameters and epidemiological data
Admissions data for individuals in Senegal who were hospitalised with COVID-19 were obtained through a follow-up cohort of patients in isolation and treatment centres. For more detailed information on this cohort see.20 These data, including the percentage of patients requiring intensive care unit (ICU)/ventilator treatment, their length of stay, and the probability of death of those individuals requiring ICU/ventilator, were derived from a cohort of hospitalised patients for whom their patient information was recorded. The reported daily numbers of new cases and deaths were obtained from the Senegalese Ministry of Health and Social Action (MoH).11
Intervention parameters
The start and end dates for each of the NPIs implemented in Senegal are provided in online supplemental material 1, table S3. The evaluation of NPIs was estimated using data from a Facebook coronavirus survey (online supplemental material 1, figure S2),21 Google Community Mobility Reports,22 Institute of Health Metrics and Evaluation Data,23 and by optimal model fitting to the Senegalese surveillance data obtained during the study period.
Finally, virus-related parameters, including the incubation period, duration of symptomatic infection, duration of immunity, risk of asymptomatic infection, risk of symptomatic infection (having clinical symptoms) and admittance to ICU with or without requiring a ventilator, were based on the values used in the COVID-19 International Modelling (CoMo) Consortium model.24 The CoMo Consortium operates via a participatory modelling approach, which involves in-country experts leading the development of the model to ensure that the local context, including infrastructure, human resources and sociocultural considerations, is fully accounted for.
Mathematical modelling
Model description
We adapted the CoMo model24 to simulate the spread of SARS-CoV-2 in Senegal in the context of NPIs and vaccination strategies. The CoMo model is a dynamic susceptible-exposed-infected-recovered-susceptible model. It is an age-structured model with infected compartments stratified by symptoms, severity and treatment-seeking and access24 (online supplemental material 1, figure S1). A description of the variables used and a list of all parameters included in the full model are given in online supplemental material 1, table S4.
Model calibration
We analysed the evolution of the COVID-19 epidemic in Senegal between 2 March 2020 (the date of the first reported case in the country) and 1 July 2021. The model was calibrated using data from the period 28 January 2021 to 1 July 2021. Various types of NPIs were implemented and their impact assessed, for example, school closures, social distancing, international travel ban, mask wearing (online supplemental material 1, table S3). The CoMo model was adapted to the Senegalese context using daily cases and mortality data, demographic data, information on the different types of NPIs carried out and some parameters relating to hospitalisations and vaccination in Senegal. All data used for simulation in Senegal are presented in online supplemental material 2.
bmjgh-2021-007236supp002.pdf (481.2KB, pdf)
Calibration was performed by first minimising the residual function corresponding to the least squares distance between the simulated and observed cumulative mortality data. Optimality was obtained by varying the parameter related to the amplitude of the NPI to minimise this residual function. The shape of the NPI action curves was defined from the Facebook mask survey data,21 and from the google mobility database for the control measures.22 In a second step, a Bayesian optimisation was performed on the remaining parameters.
Sensitivity analysis
We ran 300 simulations varying the parameters with an SD of 0.1 at a 95% CI to determine their effects on daily cases and cumulative deaths.
Assessment of vaccine impact
The weighted average of the efficacy of the 1.5 million vaccine doses received by June 2021 was used to assess the effectiveness of the vaccine in Senegal. As, it has been shown that, for example, one dose of Astra Zeneca is documented as giving 30.7% protection against the Delta variant compared with two doses that give 67.0%.25 We explored the scenario where a single injection of vaccine reduces its efficacy by half. Therefore, the observed efficacy against disease (eg, in a trial with a disease endpoint) is 86%, while the efficacy against severe disease in patients who have already been infected and are ill is 95%. Efficacy against infection is set at 20%.
Throughout the analysis, we assume that the number of vaccine doses available corresponds to the maximum number of doses guaranteed by the COVAX programme, sufficient to vaccinate 20% of the population, that is, corresponding to 6 697 572 doses, and 525 000 doses obtained via direct purchase and bilateral international assistance. Furthermore, we assume that vaccination of 80% of high-risk individuals, as well as the elderly (aged more than 60 years), required 1 290 068 million doses, beginning at the end of February 2021 and ending on 1 August 2021. The remaining 5 932 503 doses will be provided to the general public. This represents 21% of the total population (which was 16.7 million in 2020) or 22% of those aged 5−60 years.
Assessment of dates for relaxing NPIs
Assuming 20% (corresponding to the 6 million vaccine doses) of the population are vaccinated, we investigated various dates for the relaxation of NPIs during the remainder of 2021: 15 July, 1 August, 1 September, 1 October, 1 November and 1 December.
Vaccine strategy according to age group
Here, we propose the simulation of three vaccination scenarios for the remaining 5 932 503 doses of vaccine.
Strategy 1. We assume that the remaining doses of vaccine are allocated to the 50−60 years age group, representing 70% of this age group. This age group represents the most vulnerable individuals (the 50−60 age group is the second highest age group for COVID-19 mortality after the over-60 age group).
Strategy 2. We assume that the remaining doses of vaccine are allocated to the 5−30 years age group, representing 35% of this age group. This category represents the largest age group in the population (50% of the population).
Strategy 3. We assume that the remaining doses of vaccine are allocated to the 5−60 years age group, representing 22% of this age group.
Cost-effectiveness analysis
We performed a cost-effectiveness analysis by comparing the costs associated with vaccination and hospitalisation in terms of lifetime years of life saved (YLS).
Cost evaluation
In this work, the costs comprise the cost of the vaccine and the cost of hospitalisation.
Equation 1
We assume that the vaccination cost per dose is US$23, of which 75% is spent on vaccine purchase and 25% on the vaccination campaign. The cost of occupying hospital beds is calculated by simulating the number of beds occupied by patients who have COVID-19. Beds are divided into three categories: standard bed (daily cost US$280), intensive care bed (daily cost US$630) and ventilator bed (daily cost US$750). These costs are only for Dakar, and were obtained through the MoH.11
Effectiveness assessment
Effectiveness is measured in terms of years of life gained by vaccination, based on the value of the third quartile of the age distribution of the Senegalese population which is equal to 76 years. We define years of life to death (yld), as:
Equation 2
where I = {15,35,45,55,65,75}, the mean age of the age groups: less than 30, (30, 40), (40, 50), (50, 60), (60, 70) and more than 70 years; Da is the cumulative number of deaths of age a, from 12 April 2021 to 1 July 1 2021; and αa is a discount rate for future life years gained.
YLS are therefore defined as:
Equation 3
Optimisation
To investigate the optimal strategy for vaccination by age group, we formulate the problem as follows:
Let us note that p1 (respectively, p2) is the proportion of individuals vaccinated with half dose (respectively, full doses) for the age group 5−30 years; p3 (respectively, p4) is the proportion of individuals vaccinated with half dose (respectively, full doses) for the age group 30−40 years; and p5 (respectively, p6) is the proportion of individuals vaccinated with full doses for the age group 40−50 years (respectively, for the age group 50−60 years). Let us note that: p1 + p2 + p3 + p4 + p5 + p6=1, V is the number of doses and N is the total population, then:
Equation 4
The last constraint is that we cannot allocate more doses to an age class than its population. Thus, let q1, q2, q3 and q4 be the fractions of the age groups 5−30, 30−40, 40−50 and 50−60 years, respectively. Then, we have, (p1 + p2) ≤ q1, (p3 + p4) ≤ q2, p5 ≤ q3 and p5 ≤ q4.
For each strategy, pi with i {1,…,6}, we calculate the YLS gained and the cost of this strategy.
To find the optimal strategy, that is, the one that maximises the YLS gained while minimising the costs, a simulation matrix based on a Latin square was constructed. The simulation matrix allows us to see the screening factor of {pi with i {1,…,6}} for the number of deaths and for hospital costs. The parameters were optimised using a Bayesian optimisation routine. All parameters are described in online supplemental material 1, table S4.
Results
By 1 July 2021, a total of 43 263 confirmed cases and 1168 deaths due to COVID-19 were recorded in Senegal. Figure 1 shows that a third epidemic wave began in July 2021 and that it is the most important in terms of incidence compared with the previous two waves.
Figure 1.
Epidemic curve for daily cases and deaths in Senegal. The daily cases and deaths correspond to smoothed raw data. The red curve represents daily cases, and the blue curve represents daily deaths. Vertical text indicates the official dates intervention measures were taken by the Senegalese government.
Figure 2 shows that there is an optimal fit to the incidence of cases and cumulative deaths in Senegal. Figure 3 shows that for each date when NPIs are relaxed, the model predicts a new epidemic wave starting thereafter. The NPIs can delay the peak of the incidence curve (figure 3A). Of the various possible dates for the relaxation of NPIs, maintaining NPIs until few days after the last peak (1 August 2021) appears to be the most appropriate, as the peak of the incidence curve is the smallest and the third wave will begin later, in November 2021 (figure 3A); a similar pattern is seen for the cumulative deaths (figure 3B).
Figure 2.
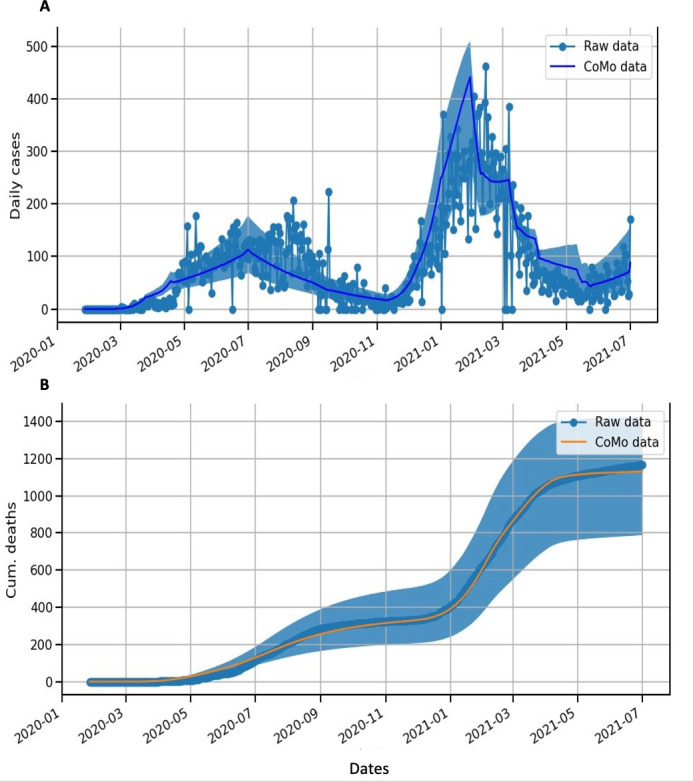
Mathematical model of COVID-19 in Senegal. (A) Blue dots represent confirmed cases. Blue lines and regions represent model estimates of daily cases (median and 95% CIs). (B) Orange lines represent the cumulative number of deaths. Blue lines and regions represent model estimates of cumulative numbers of deaths (median and 95% CIs).
Figure 3.
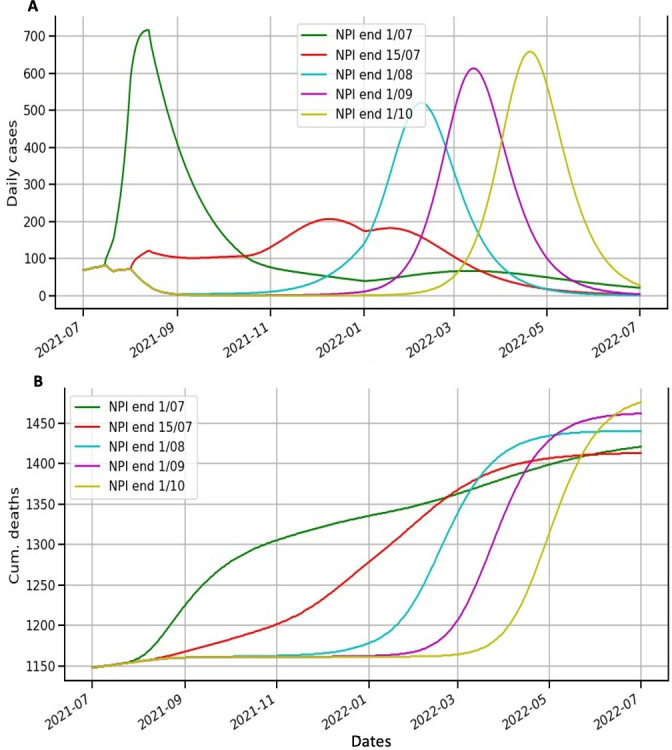
Modelling scenarios of daily cases and cumulative deaths according to different end dates for non-pharmaceutical interventions (NPIs). (A) Lines represent model scenarios for daily cases for each date for the relaxation of NPIs. The green (respectively, red, cyan, purple and yellow) line is for the scenario where NPIs are relaxed on 1 July 2021 (respectively, 15 July, 1 August, 1 September and 1 October 2021). (B) Lines represent model scenarios for the cumulative number of deaths for each date for the relaxation of NPIs. Tthe green (respectively, red, cyan, purple and yellow) line is for the scenario where NPIs are relaxed on 1 July 2021 (respectively, 15 July, 1 August, 1 September and 1 October 2021).
When investigating different vaccination strategies, figure 4 also shows a prediction that the third wave is the most important in terms of incidence and deaths. According to the baseline scenario, the peak in incidence during this new wave could occur in August 2021 (what really happened) (figure 4A). However, if vaccination programmes were implemented using any of the three strategies simulated (vaccinating 22% of 5–60 years, 35% of 5–30 years or 70% of 30–60 years), the peaks in incidence would be reduced (figure 4A). We noticed that vaccinating the oldest age group decreases the number of deaths but is not sufficient to reduce the number of daily cases. In contrast, vaccinating the populous age group decreases the number of daily cases but increases the number of deaths.
Figure 4.
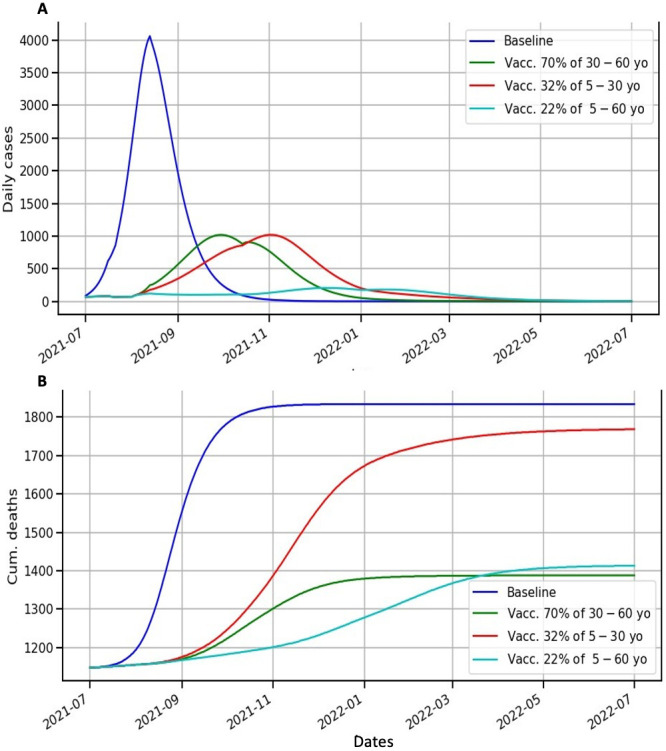
Modelling scenarios of daily cases and cumulative deaths according to different vaccine coverage rates and age groups. (A) Lines represent model scenarios for daily cases for different vaccine coverage rates and age groups. Blue represents the model’s output for the baseline scenario. The green (respectively, red and cyan) line is for the scenario where 70% of 30–60 years (respectively, 32% of 5–30 years and 22% of 5–60 years) are vaccinated. (B) Lines represent model scenarios for the cumulative number of deaths for different vaccine coverage rates and age groups. Blue represents the model’s output for the baseline scenario. The green (respectively, red and cyan) line is for the scenario where 70% of 30–60 years (respectively, 32% of 5–30 years and 22% of 5–60 years) are vaccinated.
In terms of YLS, vaccinating 22% of 5–60 years is also the best option (online supplemental material 1, figure S3). This scenario reduces the number of cases from the baseline scenario by 76% and the number of deaths by 11%. On the other hand, the strategy of vaccinating the 50–60 years age group slightly reduces the number of deaths compared with vaccinating the 5–60 years age group, by 1.8%, but greatly increases the number of cases, which explains why this strategy is 57% less cost-effective than the strategy of vaccinating the entire population.
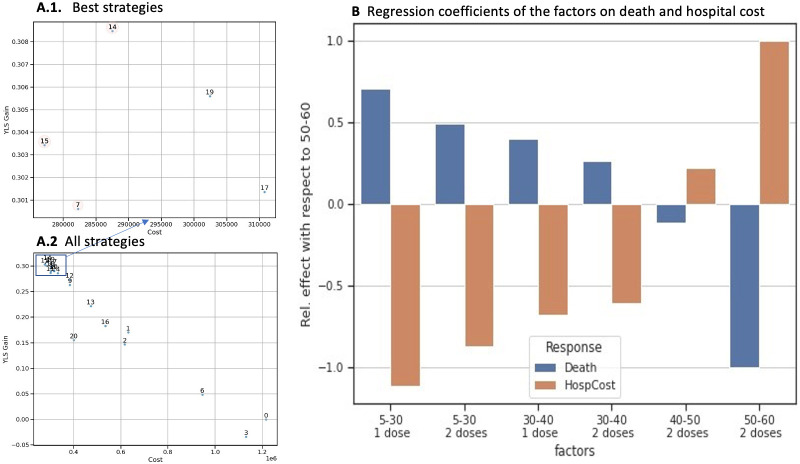
We consider an ‘optimal’ strategy to be a strategy where no other strategy has more YLS gained for a lower cost. The results of our investigation into the optimal allocation of vaccine doses by age group are shown in table 1 (all strategies are shown in online supplemental material 1, table S5). We found that strategies 7, 14 and 15 are optimal. We noticed that strategy 7 differed in that it did not include providing a half dose of vaccine to the 30–40 years age group (table 1). Strategies 14 and 15, on the other hand, are more uniform and differ only in the 40–50 years age group (27% and 53% vaccination coverage for strategy 14 and 15, respectively).
Table 1.
The percentage of vaccination for each age group for the optimal strategies 7, 14 and 15
| Age group (years) | Less than 30 | 30–40 | 40–50 | 50–60 | |||
| No of doses | Half dose | Full dose | Half dose | Full dose | Full dose | Full dose | |
| Proportions | p1/q1 | p2/q1 | p3/q2 | p4/q2 | p5/q3 | p6/q4 | |
| Strategy numbers | 7 | 0.19 | 0.09 | 0 | 0.3 | 0.21 | 0.41 |
| 14 | 0.17 | 0.08 | 0.25 | 0.27 | 0.27 | 0.36 | |
| 15 | 0.14 | 0.07 | 0.21 | 0.23 | 0.53 | 0.31 | |
The percentages are taken from the age group.
In terms of the number of deaths, strategies 7, 14 and 15 are comparable (online supplemental material 1, figure S4), differing at most by 0.8%. The peak in daily deaths for strategy 15 is slightly higher than that seen for strategies 7 and 14 (online supplemental material 1, figure S4A). These differences are accompanied by an increase in hospital costs of 1% for a reduction in deaths of just 0.013%. The comparison between strategies 14 and 15 is more informative. Indeed, strategy 15 results in slightly fewer deaths than strategy 14 (ie, 3%). However, strategy 15 results in more daily cases than strategy 14 (ie, 1.7%). This leads to an increase in hospital costs of 3.6% for a loss in YLS of 3.7%. Moreover, strategy 15 has a low vaccination rate of 53% in the 40–50 years age group (table 1).
In the context of performing a cost–benefit analysis, a screening analysis was conducted to determine the relative importance of various factors. This screening analysis enabled us to see that vaccinating the 40–60 years age group (ie, p5 and p6) has a negative effect on hospital costs but a positive impact on the number of deaths (figure 5B). Vaccinating the under-40-years has a positive effect on hospital costs but a negative effect on the number of deaths. Note that deaths and hospitalisations are more common in the 40–60 years age group (online supplemental material 1, figure S5B).
Figure 5.
Cost-effectiveness analysis and effects on deaths and hospital costs. (A1 and A2) The x-axis represents the hospitalisation cost and the y-axis represents the gain in YLS. (A1) shows a zoomed-in area of the best strategies and (A2) represents all strategies. (B) represents the regression coefficients according to age group and number of vaccine doses. On the x-axis, the factors represent the number of doses allocated according to age group and the y-axis shows the regression coefficient. blue bars represent deaths and orange bars represent hospitalisation costs. YLS, years of life saved.
Discussion
This study could help policy-makers to make more informed choices by providing the details, benefits and risks associated with various vaccination policy options.
NPIs, such as wearing face masks, observing social distancing and restricting travel, have been imposed by several African countries during the COVID-19 pandemic, with some success, significantly reducing the number of cases. However, these measures have come at great financial cost.8 Therefore, maintaining NPIs for as long as possible could have damaging economic consequences. On the other hand, relaxing NPIs too early could have serious epidemiological consequences and lead to the failure of the vaccination plan.
According to our findings, the optimal time to relax NPIs would be a few days from the last peak. This date coincides with the start of the school holidays in Senegal, when all schools and colleges close. The closure of schools and colleges will result in a considerable reduction in SARS-CoV-2 infections in Senegal, as the country’s population is very young (the median age is 19 years). The reduced mobility of children and students, who frequently carry the virus home and infect older people, may explain the reduction in deaths from COVID-19 during this period. To our knowledge, there are no studies in Africa on the effectiveness of school closures on reducing COVID-19 cases. However, studies conducted in the USA by Auger et al,26 have shown that school closures have been shown to be temporally linked to lower COVID- 19 incidence and deaths.
The objectives of a vaccination strategy are to reduce deaths and hospital costs. These two objectives are inextricably linked with the age structure of a population and that population’s acceptance of vaccination. However, for a young population such as that in Senegal, where young people may constitute more than half of the population, these two objectives seem incompatible. In the context of the COVID-19 pandemic, several vaccination strategies have been proposed, such as prioritising healthcare workers, older people or individuals who are at high-risk due to chronic health conditions.16 27 Although young people generally experience milder symptoms of COVID-19, in a younger population their large number means that they account for the bulk of hospital expenditure. Therefore, vaccinating young people, a matter of ongoing debate,28 can help to minimise hospital costs and boost a country’s economy by enabling them to go to work. However, the limitation of all age-dependent models is that they ignore any other non-age-related vulnerability, such as the population at high risk related to chronic health issues.
The current WHO guideline for COVID-19 vaccination is a full dose vaccine. However, in most LMICs, the quantity of vaccine doses accessible is limited, preventing comprehensive vaccination of the entire population within a year. Therefore, vaccinating young people reduces the number of vaccine doses available to the most vulnerable age group, which contributes to an increased number of deaths. These findings are supported by a study conducted in Australia,29 which showed that including younger people in the vaccine priority group can reduce transmission and overall infection rates, but that this benefit is negated by the higher mortality rates seen in older people. Similarly, we found that the immunisation of younger individuals had a negative impact on disease transmission. Indeed, vaccinating the most populous age group provided substantial protection against infection and might postpone the expected epidemic peak, resulting in fewer deaths than vaccinating the oldest age group.30 31
Another option to address this problem is to vaccinate the entire population. Although this strategy requires a larger quantity of vaccine, it is more cost-effective for the government as it would reduce the high cost of hospitalisations. If vaccinating the whole population when vaccine supplies are limited, administering a half dose (that would be followed by full doses) for the 5−30 years age group, may be a useful technique for increasing vaccination rates, particularly in the younger age groups where mortality rates are low.
Regarding the age distribution and mortality of the Senegal population, we have shown that distributing vaccines in half or full doses using scenario number 15 is the optimal approach, as follows: for individuals aged less than 30 years, give half and full dose to 14% and 7% of them, respectively; for individuals aged between 30 and 40 years, give half and full dose to 21% and 23% of them, respectively; for individuals aged between 40 and 50 years, give full to 53% of them; finally, for individuals aged between 50 and 60 years, give full to 31% of them. This result may be even more apparent if the model is used to simulate a more realistic effectiveness value for a half dose.
The results of this study should be treated with caution. First, mathematical modelling can give a thorough understanding of the impact of many parameters, as is typically the case. The model used here, on the other hand, is based on the assumption that epidemiological factors work uniformly across a population. This assumption is valid when there are large numbers of affected individuals, but has limitations when small groups are affected, such as when the number of infected individuals is small, for example, at the beginning of an epidemic, or they are present in limited areas. Models that take into account individual and societal variables are needed in these circumstances.32 Second, few studies have been conducted to establish the efficacy of the vaccines against COVID-19 at a population level, both in terms of infection and transmission efficacy33; therefore, we have assumed that the effectiveness of the vaccine is as proposed by the manufacturer. Third, some parameters used for the model (eg, age-based relative fatality ratio in a well-resourced scenario and the age-stratum-specific number of infections that lead to hospitalisation) are based on studies carried out in LMICs other than Senegal.34 We used these parameters because we assumed a universal response of individuals to the virus. However, it is possible that ‘natural’ resistance or population immunity (due to unknown environmental conditions, for example) have a moderating influence on the transmission of the virus and its resulting mortality. Finally, this model does not take into account the emergence of new strains that may be less effectively controlled by a vaccine. Furthermore, the impact of comorbidities on vulnerable patients is not taken into account.
Conclusion
The CoMo model was appropriately adjusted to reflect daily COVID-19 cases and deaths in Senegal. In all scenarios explored, the model predicted a third epidemic wave that is larger in terms of new cases and deaths than the previous waves. In a context of limited vaccine supply, vaccination alone will not be sufficient to control the epidemic; therefore, maintaining NPIs will be necessary to flatten the epidemic curve. Assuming 20% of the population is vaccinated, the optimal time to relax NPIs would be a few days from the last peak, which coincides with the start of school and college holidays in Senegal. Regarding the prioritisation of age groups to be vaccinated, the model shows that it is better to vaccinate the entire population and not only the elderly and individuals in high-risk groups. The former strategy could be more cost-effective for the Senegal government, as it would reduce the high cost of hospitalisations. Regarding the distribution of vaccine doses, the best strategy is to give full doses to the elderly. If there are not enough doses of vaccine, a fractionated dose could be an alternative for people aged less than 40 years, because regardless of whether they receive half or full dose, the reduction in hospitalisations would be similar and their death-to-case ratio is very low. This study provides a decision support tool to better control the COVID-19 pandemic. It could be applied to other African countries by identifying the best strategies to adopt in each specific context.
Acknowledgments
The authors wish to thank the Senegalese Ministry of Health, the Institut Pasteur de Dakar, and all of their staff. The authors also thank all the members of the REPAIR consortium and the CoMo consortium.
Footnotes
Handling editor: Seye Abimbola
Collaborators: REPAIR Consortium: Mohamed Hamidouche (Departement of Production and Development of Viral Vaccines, Institut Pasteur d'Algérie, Alger, Algeria), Ramatoulaye Hamidou Lazoumar (Epidemiologie Santé-Environnement-Climat, Centre de Recherche Médicale Sanitaire, Niamey, Niger), Jules Brice Tchatchueng (Service d'épidémiologie et de santé publique, Centre Pasteur du Cameroun, Yaounde, Centar, Cameroon), Walid Ben Aribi (Bio-Informatics, Mathematics, Statistics, Institut Pasteur de Tunis, Tunis, Tunisia), Nesrine Ben Yahia (National School of Computer Sciences, University of Manouba, Manouba, Tunisia), Ahmed Nasri (Bio-Informatics, Mathematics, Statistics, Institut Pasteur de Tunis, Tunis, Tunisia; National School of Computer Sciences, University of Manouba, Manouba, Tunisia), Bechir Naffeti (Bio-Informatics, Mathematics, Statistics, Institut Pasteur de Tunis, Tunis, Tunisia), Arsene Brunelle Sandie (Service d'épidémiologie et de santé publique, Centre Pasteur du Cameroun, Yaounde, Centar, Cameroon).
Contributors: MD, SBM and LJW designed and supervised the study. MD and SBM performed the model analysis and simulations. AB, MD, JF, CT and CL provided hospitalisation data and performed epidemiological data management. MD and SBM wrote the initial draft of the manuscript and acted as the guarantor of this study. All authors revised the draft manuscript.
Funding: This study was funded (or cofunded) by the French Ministry for Europe and Foreign Affairs via the project ‘REPAIR Covid-19-Africa’, coordinated by the Pasteur International Network association. The investigators also acknowledge the philanthropic support of donors to the University of Oxford’s COVID-19 Research Response Fund. We also thank Ricardo Águas and all of the CoMo Consortium technical team for orientation and automatisation of the template for the CoMo Shiny App. Medical writing assistance and editorial support was provided by Adam Bodley, according to Good Publication Practice guidelines.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: REPAIR consortium, Mohamed Hamidouche, Ramatoulaye Hamidou Lazoumar, Jules Brice Tchatchueng, Walid Ben Aribi, Nesrine Ben Yahia, Ahmed Nasri, Bechir Naffeti, and Arsene Brunelle Sandie
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The CoMo template containing all necessary data for this analysis is provided as online supplemental material file.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Patient hospitalisation data were obtained from a cohort study and approved by the Senegalese National Ethics Committee for Research in Health (reference number 00000068/MSAS/CNERS/Sec, accessed on 10 April 2020).
References
- 1.World Health Organization . Coronavirus disease (COVID-19) Dashboard. Available: https://covid19.who.int [Accessed 22 Jan 2021].
- 2.Gilbert M, Pullano G, Pinotti F, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet 2020;395:871–7. 10.1016/S0140-6736(20)30411-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus : la Côte d’Ivoire prend des mesures sanitaires l’aéroport d’Abidjan, 2020. Available: https://www.france24.com/fr/20200201-coronavirus-la-c%C3%B4te-d-ivoire-prend-des-mesures-sanitaires-%C3%A0-l-a%C3%A9roport-d-abidjan [Accessed 18 Dec 2021].
- 4.Nkengasong JN, Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. The Lancet 2020;395:841–2. 10.1016/S0140-6736(20)30464-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Nestour A, Mbaye S, Moscoviz L. Enquête téléphonique sur La crise Du Covid Au Sénégal. Center for Global Development, 2020: 25. [Google Scholar]
- 6.Haider N, Osman AY, Gadzekpo A, et al. Lockdown measures in response to COVID-19 in nine sub-Saharan African countries. BMJ Glob Health 2020;5:e003319. 10.1136/bmjgh-2020-003319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OECD . Evaluating the initial impact of COVID-19 containment measures on economic activity. Available: https://www.oecd.org/coronavirus/policy-responses/evaluating-the-initial-impact-of-covid-19-containment-measures-on-economic-activity-b1f6b68b/ [Accessed 8 Dec 2021].
- 8.Chen S, Igan D, Pierri N, et al. Tracking the economic impact of COVID-19 and mitigation policies in Europe and the United States. IMF Working Papers 2020;20. 10.5089/9781513549644.001 [DOI] [Google Scholar]
- 9.World Health Organization . Who SAGE values framework for the allocation and prioritization of COVID-19 vaccination, 14 September 2020. Geneva: World Health Organization, 2020. https://apps.who.int/iris/handle/10665/334299 [Google Scholar]
- 10.Omer S, Faden R, Kochhar S. Who SAGE roadmap for prioritizing uses of COVID-19 vaccines in the context of limited supply. World Health Organization, 2020. [Google Scholar]
- 11.Senegalese Ministry of health. real time Covid-19 tracking in Senegal. Available: https://cartosantesen.maps.arcgis.com/apps/opsdashboard/index.html#/260c7842a77a48c191bf51c8b0a1d3f6 [Accessed 5 Nov 2020].
- 12.92 low- and middle-income economies eligible to get access to COVID-19 vaccines through Gavi COVAX AMC. Available: https://www.gavi.org/news/media-room/92-low-middle-income-economies-eligible-access-covid-19-vaccines-gavi-covax-amc [Accessed 16 Aug 2021].
- 13.COVAX. Available: https://www.who.int/initiatives/act-accelerator/covax [Accessed 16 Aug 2021].
- 14.Cowling BJ, Lim WW, Cobey S. Fractionation of COVID-19 vaccine doses could extend limited supplies and reduce mortality. Nat Med 2021;27:1321–3. 10.1038/s41591-021-01440-4 [DOI] [PubMed] [Google Scholar]
- 15.Interim statement on dose-sparing strategies for COVID-19 vaccines (fractionated vaccine doses). Available: https://www.who.int/news/item/10-08-2021-interim-statement-on-dose-sparing-strategies-for-covid-19-vaccines-(fractionated-vaccine-doses) [Accessed 17 Sep 2021].
- 16.Gavi, World Health Organization, UNICEF . COVAX, 2020. Available: https://www.gavi.org/covax-facility
- 17.United Nations . World Population Prospects - Population Division - United Nations. Available: https://population.un.org/wpp/Download/Standard/Population/ [Accessed 3 Aug 2021].
- 18.Agence Nationale de la Statistique et de la Demograpgie . Rapport définitif RGPHAE-2013, 2013: 20. [Google Scholar]
- 19.Agence Nationale de la Statistique et de la Demographie . Projections des indicateurs démographiques Du Sénégal 2013-2035. Available: https://satisfaction.ansd.sn/ressources/publications/indicateurs/Projections-demographiques-2013-2025.htm [Accessed 17 Aug 2021].
- 20.Taieb F, Mbaye KD, Tall B, et al. Hydroxychloroquine and azithromycin treatment of hospitalized patients infected with SARS-CoV-2 in Senegal from March to October 2020. J Clin Med 2021;10:2954. 10.3390/jcm10132954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Facebook . COVID-19 trends and impact survey. Facebook data for good. Available: https://dataforgood.fb.com/tools/symptomsurvey/ [Accessed 3 Aug 2021].
- 22.Google . COVID-19 community mobility report. COVID-19 community mobility report. Available: https://www.google.com/covid19/mobility?hl=en [Accessed 30 Jul 2021].
- 23.Institute for Health Metrics and Evaluation . COVID-19 projections. Institute for health metrics and evaluation. Available: https://covid19.healthdata.org/ [Accessed 30 Jul 2021].
- 24.Aguas R, White L, Hupert N, et al. Modelling the COVID-19 pandemic in context: an international participatory approach. BMJ Glob Health 2020;5:e003126. 10.1136/bmjgh-2020-003126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585–94. 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA 2020;324:859–70. 10.1001/jama.2020.14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt H, Pathak P, Sönmez T, et al. Covid-19: how to prioritize worse-off populations in allocating safe and effective vaccines. BMJ 2020;371:m3795. 10.1136/bmj.m3795 [DOI] [PubMed] [Google Scholar]
- 28.Wilson C. Is it time to vaccinate children? New Scientist 2021;251:8–9. 10.1016/S0262-4079(21)01223-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacIntyre CR, Costantino V, Trent M. Modelling of COVID-19 vaccination strategies and herd immunity, in scenarios of limited and full vaccine supply in NSW, Australia. Vaccine 2021. 10.1016/j.vaccine.2021.04.042. [Epub ahead of print: 24 Apr 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luangasanatip N, Pan-Ngum W, Prawjaeng J, et al. Optimal vaccine strategy to control COVID-19 pandemic in middle-income countries: modelling case study of Thailand. Research Square. [Google Scholar]
- 31.Gulden TR, Hartnett GS, Vardavas R. Protecting the most vulnerable by vaccinating the most active. Available: https://www.rand.org/pubs/perspectives/PEA1068-1.html [Accessed 30 Jul 2021].
- 32.Railsback SF, Grimm V. Agent-Based and individual-based modeling: a practical introduction. Princeton University Press, 2011. https://www.jstor.org/stable/j.ctt7sns7 [Google Scholar]
- 33.Lipsitch M, Kahn R. Interpreting vaccine efficacy trial results for infection and transmission. Vaccine 2021;39:4082–8. 10.1016/j.vaccine.2021.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008;5:e74. 10.1371/journal.pmed.0050074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-007236supp001.pdf (5.6MB, pdf)
bmjgh-2021-007236supp002.pdf (481.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The CoMo template containing all necessary data for this analysis is provided as online supplemental material file.