Abstract
Purpose
Chronic obstructive pulmonary disease (COPD) affects millions of people worldwide. Obesity is commonly seen concomitantly with COPD. People with COPD have reduced quality of life, reduced physical activity, chronic respiratory symptoms, and may suffer from frequent clinical exacerbations. Liraglutide is a glucagon-like peptide-1 receptor agonist (GLP-1RA) approved for weight loss and treatment of type-2 diabetes mellitus. In addition, liraglutide exerts anti-inflammatory actions by reducing IL-6 and MCP-1 levels. We investigated the effect of liraglutide on pulmonary function in people suffering from obesity and COPD.
Patients and Methods
In this controlled, double-blind trial, 40 people with obesity and COPD from two outpatient clinics were allocated randomly to receive liraglutide (3.0 mg, s.c.) or placebo (s.c.) for 40 weeks. At baseline and after 4, 20, 40, and 44 weeks, participants underwent pulmonary-function tests, 6-min walking test, and replied to a questionnaire regarding the clinical impact of COPD (COPD assessment test (CAT)-score).
Results
Compared with placebo, liraglutide use resulted in significant weight loss, increased forced vital capacity (FVC) and carbon monoxide diffusion capacity, and improved CAT-score. We found no significant changes in forced expiratory volume in one second (FEV1), FEV1/FVC, or 6-min walking distance.
Conclusion
In patients suffering from obesity and COPD, 40 weeks of treatment with liraglutide improved some measures of pulmonary function. Our study suggests that liraglutide at 3.0 mg may be appropriate treatment in patients with obesity and COPD.
Keywords: GLP-1 RA, COPD, obesity, inflammation, spirometry
Introduction
Chronic obstructive pulmonary disease (COPD) affects ~250 million people worldwide and is predicted to be the third leading cause of death in 2020.1,2
The prevalence of COPD increases worldwide. By 2060, 5.4 million deaths are expected due to COPD or related conditions. COPD is characterized by pulmonary inflammation with increased numbers of alveolar macrophages, neutrophils, and T-lymphocytes, as well as increased secretion of proinflammatory markers, such as C-reactive protein (CRP), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1). Pathologic changes in airways, lung parenchyma and pulmonary vasculature result in chronic inflammation and structural changes. The result is airflow limitation and reduced pulmonary capacity.3,4
People with COPD also have reduced quality of life, reduced physical activity, chronic respiratory symptoms, and may suffer from frequent clinical exacerbations.2,4
Obesity is commonly seen concomitantly with COPD. The prevalence of obesity in COPD ranges between 18% and 54%, and about half of all patients with COPD attending pulmonary rehabilitation (PR) programs are overweight or obese.5,6
In end-stage COPD, low body mass index (BMI) is predictive of a poor prognosis. Overweight and mild obesity in COPD are associated with improved survival and a slower decline in lung function, a phenomenon referred to as the “COPD obesity paradox”. It seems, however, plausible that the increased mortality seen with lower bodyweight is related to the disease state and to tobacco smoking rather than a survival benefit of being overweight per se.6
Combined weight loss and exercise training also seem feasible in people with COPD, and results in a clinically relevant weight loss. However, the importance of voluntary weight loss in people with COPD and obesity has not been fully established.7,8
Glucagon-like peptide (GLP)-1 is a gut incretin hormone secreted from enteroendocrine cells after food intake and stimulates insulin secretion in a glucose-dependent manner. In addition, GLP-1 inhibits gastric emptying and induces satiety.9,10 The GLP-1 receptor agonist (GLP-1RA) liraglutide has been approved for weight loss and weight maintenance if given at 3.0 mg (s.c.) once-daily and has been reported to result in a mean weight loss of 5.6 kg in excess of placebo.11
In humans, GLP-1Rs are expressed in several tissues, including lung tissue.12 GLP-1 appears in much higher concentrations within broncho alveolar fluid compared with that in serum, which may indicate a specific function in the lungs. GLP-1RAs also exhibit anti-inflammatory properties that result in reduced concentrations of interleukin-6 and monocyte chemoattractant protein-1 and, in the lungs, GLP-1 promotes vasodilation, surfactant production, and bronchodilation.13,14
The effects of the GLP-1RAs liraglutide and exenatide have been tested in a murine model of COPD. Along with downregulation of expression of proinflammatory cytokines, treatment with GLP-1RAs resulted in marked reduction in measures of COPD-severity and mortality.15 GLP-1RAs might be appropriate for the treatment of people suffering from obesity and COPD because they can reduce bodyweight and reducing systemic inflammation.13–15
We hypothesized that treatment with the GLP-1RA liraglutide may improve lung function in patients suffering from obesity and COPD.
Materials and Methods
Study Overview
This 44-week prospective, randomized, placebo-controlled, double-blind, two-center, parallel-group trial was conducted between February 2018 and March 2020 at the Department of Pulmonary Diseases within Hospital South West Jutland (University Hospital of Southern Denmark, Esbjerg) and the Department of Medicine, Section of Pulmonary Diseases within Lillebaelt Hospital (University Hospital of Southern Denmark, Vejle) in Denmark.
The trial was conducted in accordance with the Declaration of Helsinki 1964 and its later amendments. The study protocol was approved by the Scientific Ethics Committee of the Region of Southern Denmark (S-20170147) and Eudract (2017–003551-32). The study has been registered at clinicaltrials.gov (NCT03466021) and monitored according to Good Clinical Practice by the Good Clinical Practice Unit of Odense University Hospital (Odense, Denmark).
Study Participants
We recruited 40 participants when visiting the outpatient clinic at the participating centers or through newspaper advertisements. We included people with COPD defined as forced expiratory volume in one second relative to forced vital capacity (FEV1/FVC) <70% after maximal bronchodilation in accordance with Global Initiative for Chronic Obstructive Lung Disease guidelines.3
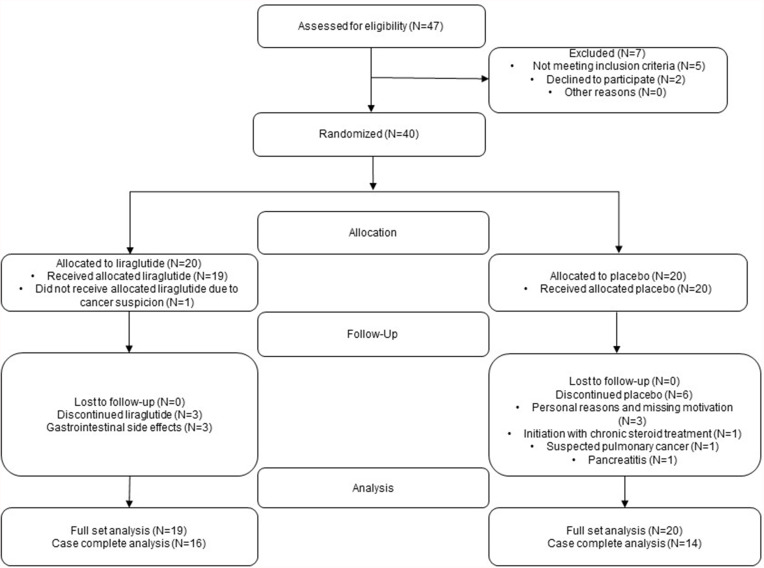
Participants were former smokers with 20 or more pack-year history of smoking and were 40–75 years of age. Liraglutide 3.0 mg is indicated for people with BMI above 30 or above 27kg/m2 and one or more obesity-related diseases. Accordingly, BMI above 27 kg/m2 was defined as inclusion criteria. Exclusion criteria were treatment with systemic corticosteroids; diabetes mellitus of any type; interstitial pulmonary disease; asthma or asthma-COPD Overlap Syndrome (ACOS), severe hepatic, renal, or heart disease; history of pancreatitis; pregnancy or breastfeeding. Medical Research Council dyspnea score (MRC), blood eosinophil levels, number of exacerbations and Charlson Comorbidity Index score (CCI) were retrieved from the patient files. All participants provided written informed consent. Figure 1 shows the flowchart of the trial.
Figure 1.
Flowchart of the trial. Flowchart from screening to data analyses in the liraglutide group and placebo group. Reasons for discontinuation are listed in the boxes.
We randomized participants to once-daily treatment with liraglutide (3 mg, s.c.) or placebo (s.c.) in a 1:1 manner by an electronic device. This randomization method resulted in 20 patients in each group. To reduce gastrointestinal side effects, liraglutide was titrated each week by doses of 0.6 mg per day to reach 3 mg per day or corresponding placebo at week 4, and this dose was maintained until week 40. Common side effects of liraglutide were nausea, vomiting, diarrhea and constipation. If moderate-to-severe nausea or vomiting occurred, we titrated slower with steps of 2 weeks. If continuous severe nausea or vomiting was present, the participant was withdrawn from the trial as indicated in the flowchart.
Participants were assessed at baseline, after 4 weeks of treatment (to investigate the effect of the study drug before expected weight loss), and at 20 weeks (to ensure compliance with study procedures). At 40 weeks, participants were assessed to elucidate the full effect of liraglutide (3 mg) and weight loss. By this time, the study medication was stopped, and 4 weeks later (week 44), participants were reassessed for the effect of weight loss without ongoing liraglutide exposure.
At each visit from randomization to trial termination, participants underwent a physical examination, including measurement of bodyweight and calculation of the waist: hip ratio. We reviewed their medication for changes in pulmonary agents, antibiotics, and corticosteroids. We accepted changes in medication except for initiation of chronic or long-term treatment with corticosteroids, which would lead to termination of study participation, whereas short-term treatment with corticosteroids or antibiotics due to COPD exacerbation were accepted. We ensured compliance and tolerance with the study medication.
Pulmonary-function tests were carried out using a spirometer (ZAN 100; Nspire Health, Longmont, CO, USA) with software GPI 3.00, or a similar device providing measures of FEV1, FEV1%, FVC, and the FEV1/FVC ratio. Body plethysmography with Masterscreen™ PFT Pro (Vyaire, Höchberg, Germany) revealed total lung capacity (TLC), residual volume (RV) and diffusion capacity for carbon monoxide (DLCO). Experienced nurses carried out all measurements.
Blood samples were taken at each visit to exclude the development of severe renal or hepatic insufficiency, anemia, or pancreatitis. We measured levels of sodium, potassium, creatinine, carbamide, hemoglobin, pancreas-amylase and undertook liver-function tests.
The global impact of COPD on health status was assessed by the COPD assessment test (CAT). A 6-min walking test was done to measure physical capacity.
Statistical Analyses
Stata 17 (StataCorp, College Station, TX, USA) was employed for statistical analyses. Summary statistics were calculated for each group. Distributions were examined for each variable, and they were found to have a normal distribution. Comparisons between the intervention group and placebo group at baseline were made using the unpaired t-test. Endpoints were calculated by a random-effect model taking into account the baseline value and adjusting for age, sex, and bodyweight. P < 5% was considered significant.
We evaluated the effect of liraglutide compared with that of placebo at 4, 20, 40, and 44 weeks. The effect of liraglutide compared with that of placebo was evaluated: at 4 weeks of treatment to assess the initial effect of liraglutide: after 40 weeks to evaluate the full effect of liraglutide and the resultant weight loss; after 44 weeks to evaluate a persistent effect after stopping liraglutide treatment.
To assess the impact of the observed weight loss in the liraglutide group, we correlated the change in weight from baseline to week 40 with changes in FEV1, FVC, DLCO, and RV by calculating the Pearson’s correlation coefficient.
Endpoints were evaluated in the full analysis set (intention-to-treat analyses). We also analyzed completed cases, but results are provided only if different from the full-set analysis set.
Results
Of 47 individuals screened, 40 people were found to be eligible for trial participation, and were randomized equally for liraglutide treatment or placebo treatment. In the liraglutide group, one participant was withdrawn after randomization before the first dose due to a suspicion of cancer, while three participants discontinued due to persistent and unacceptable gastrointestinal side effects. Sixteen participants in the liraglutide group and 14 in the placebo group completed the trial.
The baseline characteristics of participants are given in Table 1. MRC, number of exacerbations the last year before inclusion, blood eosinophil levels and comorbidities specified by CCI score are also noted in Table 1. Participants had a mean age of 64.7 years, were predominantly male, and were overweight-to-severely obese. FEV1, FEV1% and FEV1/FCV were slightly higher at baseline in the liraglutide group, whereas all other variables did not differ significantly between groups.
Table 1.
Baseline Characteristics of Anthropometric Measures, Pulmonary Variables, Inflammation Markers and 6-Min Walking Test in the Liraglutide Group and Placebo Group
| Group Characteristics | Liraglutide 3.0 mg (n = 20) | Placebo (n = 20) | p |
|---|---|---|---|
| Age (years) | 64.0 ± 8.4 | 65.3 ± 6.7 | NS |
| Male/female | 13/7 | 11/9 | NS |
| Bodyweight (kg) | 104.4 ± 13.6 | 102.5 ± 18.0 | NS |
| BMI (kg/m2) | 35.1 ± 3.7 | 36.6 ± 5.6 | NS |
| Waist: hip ratio | 1.07 ± 0.08 | 1.04 ± 0.09 | NS |
| FEV1 (L) | 1.8 ± 0.67 | 1.4 ± 0.67 | NS (p = 0.056) |
| FEV1 (%) | 62.8 ± 17.8 | 50.3 ± 20.2 | < 0.05 |
| FEV1/FVC (%) | 57.6 ± 9.4 | 48.2 ± 11.9 | <0.05 |
| FVC (L) | 3.16 ± 1.01 | 2.83 ± 0.99 | NS |
| FVC (%) | 86.4 ± 19.1 | 81.5 ± 19.3 | NS |
| TLC (%) | 106 ± 18 | 114 ± 22 | NS |
| DLCO (%) | 67.3 ± 25.1 | 52.2 ± 24.3 | NS |
| RV (%) | 147 ± 48 | 176 ± 53 | NS |
| MRC score | 2.05 ± 1.08 | 3 ± 1.06 | 0.01 |
| Eosinophils (10E9/L) | 0.19 ± 0.12 | 0.16 ± 0.11 | NS |
| Number of exacerbations the year before inclusion | 0.56 ± 0.70 | 0.77 ± 0.90 | NS |
| CCI | 3.26 ± 1.10 | 3.26 ± 1.05 | NS |
| CAT-score | 15.4 ± 6.1 | 17.8 ± 5.8 | NS |
| 6-min walking distance (m) | 419 ± 91 | 308 ± 138 | <0.01 |
| CRP (mg/L) | 5.3 ± 7.7 | 5.4 ± 4.7 | NS |
| IL-6 (pg/mL) | 5.3 ± 3.2 | 6.4 ± 3.6 | NS |
| MCP-1 (pg/mL) | 274 ± 95 | 281 ± 63 | NS |
Notes: Data are the mean ± SD. Sex is given in numbers. Significant changes between groups are given with p-values.
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CRP, C reactive protein; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; CAT-score, COPD assessment test score; IL-6, Interleukin 6; MCP-1, Monocyte Chemoattractant Protein-1; TLC, total lung capacity; DLCO, diffusion capacity of the lung for carbon monoxide; RV, residual volume; NS, non-significant.
Results for group differences in bodyweight, pulmonary measures, inflammation markers, CAT-score, and 6-min walking test were calculated for all visits and are listed in Table 2. Except for bodyweight, there were no significant differences between liraglutide and placebo at week 4 and week 20 (data not shown). As expected, bodyweight decreased in the liraglutide group compared with that in the placebo group, and reached a maximal difference of 8.4 kg at week 40 with persistence of a 7.1-kg difference 4 weeks later. The difference was significant at all time points. In completed cases, the differences were 8.5 kg and 7.3 kg, respectively.
Table 2.
Average Baseline-Adjusted Group Differences (Liraglutide – Placebo) in Bodyweight, Pulmonary Variables, and 6-Min Walking Distance
| Variable | Study Visit | p | ||||
|---|---|---|---|---|---|---|
| Week 4 | Week 20 | Week 40 | Week 44 | Week 40 | Week 44 | |
| Bodyweight (kg) | −4.9 | −8.2 | −8.4 | −7.1 | <0.01 | <0.01 |
| FEV1 (L) | 0.019 | 0.056 | 0.106 | −0.041 | NS | NS |
| FEV1 (%) | 0.28 | 1.64 | 2.94 | −3.23 | NS | NS |
| FEV1/FVC (%) | −1.3 | −0.9 | −0.5 | −1.4 | NS | NS |
| FVC (L) | 0.06 | 0.18 | 0.33 | 0.04 | <0.01 | NS |
| FVC (%) | 1.67 | 4.96 | 7.69 | −0.00 | <0.05 | NS |
| TLC (%) | −9.74 | −1.02 | 0.97 | −9.13 | NS | <0.05 |
| DLCO (%) | 3.49 | 0.56 | 9.67 | 1.89 | <0.05 | NS |
| RV (%) | −7.83 | −8.55 | −9.62 | −19.81 | NS | <0.05 |
| CAT-score | −1.23 | −0.18 | −3.94 | −0.68 | <0.05 | NS |
| 6-min walking distance (m) | 3.8 | −2.8 | 27.9 | 19.3 | NS | NS |
| CRP (mg/L) | −0.24 | −0.83 | −2.22 | −0.62 | NS | NS |
| IL-6 (pg(mL) | 1.31 | 1.35 | −0.25 | −0.08 | NS | NS |
| MCP-1 (pg/mL) | 4.11 | −16.66 | 6.56 | −30.93 | NS | NS |
Note: p-values are specified for week 40 (effect of liraglutide and weight loss) and week 44 (effect of weight loss after cessation of liraglutide treatment).
At week 40, FVC% increased by 7.69% in the liraglutide group compared with that in the placebo group (p = 0.018). Likewise, FVC best measure (FVCbest) increased by 0.33 L in the liraglutide group compared with that in the placebo group (p = 0.007). The between-group difference in FVCbest and FVC% did not persist until week 44. We found no significant effect of liraglutide upon pulmonary function or pulmonary capacity as measured by FEV1, FEV1% or FEV1/FVC. However, at week 40, FEV1 was 106-mL higher in the liraglutide group than that in the placebo group (p = 0.095).
TLC and RV decreased significantly by 9.13% (p = 0.013) and 19.81% (p = 0.039), respectively, in the liraglutide group compared with that in the placebo group, at week 44 but, at week 40, there was no significant difference. Pulmonary diffusion capacity as measured by DLCO remained stable in the liraglutide group until week 40 but decreased over time in the placebo group, resulting in a significant between-group difference of 9.7% at week 40 (p = 0.012).
There were no significant differences between the treatment groups in CRP, IL-6 or MCP-1 from baseline to any subsequent visits (all p > 0.1).
The CAT-score decreased significantly by 3.94 points at week 40 in the liraglutide group compared with that in the placebo group (p = 0.012), but this effect was no longer present at week 44.
There was no significant difference in the 6-min walking distance at week 40 and week 44. In the complete case group, there was a 47-m improvement in the liraglutide group compared placebo at week 40, but this difference did not reach significance (p = 0.075).
In the liraglutide group, there were no significant correlations between the change in bodyweight from baseline to week 40 or the change in pulmonary variables. Hence, at least some of the observed effects could be ascribed to liraglutide treatment rather than weight loss per se (p > 0.2 for all).
Discussion
This is the first study to evaluate the effect of GLP-1RAs in people suffering from obesity and COPD. We examined the effect of liraglutide (3.0 mg) for 40 weeks on pulmonary measures and the clinical impact of COPD. As anticipated, liraglutide resulted in significant weight loss after 4 weeks of treatment, and this weight loss was maintained throughout the study. In the liraglutide group, compared with placebo, we found an increase in FVC (both in liters and percentage of expected), DLCO, and a reduced clinical impact of COPD as assessed by the CAT-score. We found no significant differences in FEV1, FEV1/FVC, or 6-min walking test.
COPD and obesity are common conditions. Relatively few studies have assessed the effect of lifestyle-induced or surgery-induced weight loss in people with COPD, which may be due to the well-established association between low bodyweight and a poor prognosis of COPD. People undergoing bariatric surgery experience substantial weight loss, and in parallel, they have improved measures of pulmonary function (FEV1, FVC, TLC, DLCO). These effects are present a short time after the surgical procedure and are also seen in the most severely obese group (BMI ≤60 kg/m2).8,16 Resistance exercise training in patients suffering from obesity and COPD results in reduced BMI, alongside better exercise tolerance, health status, and improvement in FVC.7
Liraglutide has been approved for weight-loss therapy and weight maintenance in people with obesity. In addition, some studies have emphasized its anti-inflammatory properties.10,17 COPD is characterized by dysfunctions of the innate immune system. This phenomenon involves decreased expression and downregulation of anti-inflammatory cytokines and abnormal lymphocyte function, both of which are important mechanisms in COPD. There is a link between innate immunity in people with COPD and defect in GLP-1R signaling, and liraglutide-induced activation of GLP-1R signaling decreases the severity of acute exacerbation of COPD, possibly by an increase in interferon-gamma production and by ameliorating T-cell dysfunction.18
We did not find significant reduction in CRP, IL-6 or MCP-1 from baseline to subsequent visit. In previous studies, liraglutide decreased inflammation markers such as IL-6 and MCP-1.13,14 Based on MRC score, blood eosinophils, number of exacerbations and CCI, our study population was less severely affected by their disease, and accordingly a possible reduction in inflammation might be overlooked.
Observational studies support a possible role of GLP-1RA treatment in pulmonary disease. Treatment with GLP-1RAs compared with all other glucose-lowering agents in adults with type-2 diabetes and asthma results in a significantly lower prevalence of asthma exacerbations, and the effect seems to be independent of weight loss following GLP-1RA use.19 Liraglutide may also reduce the blood level of surfactant protein D in people with type-2 diabetes.20 Whether the GLP-1R serves as a possible immunomodulatory target for COPD therapy is not known.
Our protocol was prompted by the results from a study conducted by Viby and co-workers.15 They assessed the effect of GLP-1RAs on pulmonary function in a murine model of COPD. They assessed pulmonary function by non-ventilated unrestrained whole-body plethysmography (Penh) after treatment with a GLP-1RA or an inactive control substance. GLP-1RA-treated mice had a significant and remarkable improvement in survival and lung function compared with that in mice treated with the inactive substance. Penh is considered a measure of bronchoconstriction and, accordingly, corresponds most closely with FEV1 in humans. Penh is correlated with airway responsiveness (but not to airway resistance) and its translational value to human conditions is controversial.15,21–23 We found no significant effect of liraglutide upon FEV1. This observation could have been due to species-specific differences in the response to GLP-1RA or to the method used to assess pulmonary function. In addition, the weight-adjusted liraglutide dose given in the mouse study was much higher than the maximal dose approved for treatment of humans.
We found no effect upon FEV1 nor FEV1, but a significant difference between groups in FVC, at week 40. This was due to improvement in the liraglutide group and deterioration in the placebo group. FEV1 is a better predictor than FVC for all-cause mortality in people with moderate COPD,24 but FEV1 tends to underestimate bronchodilation effects, and FVC may be a superior measure of bronchodilator reversibility in COPD.25 Hence, concomitant treatment with liraglutide might increase the effect of bronchodilation treatment in people with pulmonary disease. Whether a longer duration of GLP-1RA treatment affects air trapping, hyperinflation, and, therefore, FEV1, is not known.26,27
We found a positive effect of liraglutide compared to placebo on TLC and RV at the final study visit 4 weeks after treatment cessation. This difference was not present during liraglutide treatment at week 40, so these results should be interpreted with caution. Bronchodilators reduce hyperinflation and improve FVC by reducing RV, FRV, and TLC. This phenomenon results in increased inspiratory capacity, a parameter linked to improvement in exercise tolerance and dyspnea perception in COPD.28
We measured diffusion capacity by DLCO, which remained constant in the liraglutide group over 44 weeks, but decreased in the placebo group by 14% and 11% at week 40 and week 44, respectively. This decline in the placebo group was higher than expected. Wu et al stated that in people with COPD, DLCO was predicted to decrease by an average of 1.34% per year.29 They did not find BMI to be a predictor for a faster decline, but their study population was much leaner than ours, and the impact of severe obesity as a risk factor for a fast decline in DLCO must be clarified.
We found a significant improvement in the CAT-score during liraglutide treatment compared to that in the placebo group. People with higher CAT-scores are more likely to be admitted to the intensive care unit than those with lower CAT-scores. A reduction in the CAT-score is, therefore, a possible predictor of reduced disease activity.4 A significant difference between the groups at week 40 disappeared at follow-up 4 weeks later, which indicated that the effect was linked directly to the ongoing effect of liraglutide rather than bodyweight because bodyweight remained almost unchanged until week 44.
The significant effect on FVC and DLCO at week 40 was no longer present at week 44. As bodyweight remained almost stable until week 44 this might indicate that the effect is linked to treatment with liraglutide rather than bodyweight alone.
Our study had three main strengths. First, it had a randomized controlled design. Second, we investigated pulmonary-function measures and symptom measures. Third, participants were examined after short-term liraglutide treatment, during liraglutide treatment following weight loss, and shortly after cessation of liraglutide treatment. Hence, it was possible to distinguish between the direct effects of liraglutide and the effects of weight loss per se.
Our study also has limitations. First, we based our calculation of sample size on a dropout rate of 20%, but the actual dropout rate was 25%. This difference may have limited the possibility of finding smaller effect sizes and induced the risk of type-2 statistical errors. Second, a treatment period of 40 weeks may not be sufficient to detect the effects of liraglutide and weight loss. Hence, longer-term studies might be needed to explore the full potential of liraglutide in this study population, especially with regard to COPD exacerbations.
Conclusions
In patients suffering from obesity and COPD, 40 weeks of treatment with the GLP-1RA liraglutide improved FVC, DLCO, and the CAT-score. GLP-1RAs are not approved for COPD treatment, but our study suggests that liraglutide at 3.0 mg may be an appropriate treatment option in patients with obesity and COPD because it appears to target obesity and pulmonary function. Further studies are needed to clarify the full anti-inflammatory effect of liraglutide in people with obesity and COPD.
Acknowledgments
We grateful to the Open Patient data Explorative Network. We are indebted to the Unit for Thrombosis Research, Department of Clinical Biochemistry, in Hospital South West Jutland, University Hospital of Southern Denmark, Esbjerg, Denmark for handling blood samples and biochemical measurements. We are also thankful to the study nurses at Hospital South West Jutland and to Sygehus Lillebaelt for excellent technical assistance. We thank Jeppe Gram (MD, PhD) for invaluable scientific advice.
Funding Statement
Study medication and running costs were provided by Novo Nordisk as a part of the Investigator Sponsored Studies Program. We also had financial support from Karola Jørgensens Forskningsfond, Research Council of Hospital South West Jutland, University Hospital of Southern Denmark, and Region of Southern Denmark.
Abbreviations
ACOS, Asthma-COPD Overlap Syndrome; BMI, Body mass index; CAT-score, COPD assessment test; CCI, Charlson Comorbidity Index; CRP, C reactive protein; COPD, Chronic obstructive pulmonary disease; DLCO, Diffusion capacity for carbon monoxide; FEV1, Forced expiratory volume in one second; FRV, Forced respiratory volume; FVC, Forced vital capacity; FVCbest, Forced vital capacity best measure; FVC%, forced vital capacity, in percentage; GLP-1R, glucagon-like peptide-1 receptor; GLP-1RA, glucagon like peptide-1 receptor agonist; IL-6, Interleukin 6; MCP-1, Monocyte Chemoattractant Protein-1; MRC, medical research council dyspnea score; PR, Pulmonary rehabilitation; RV, residual volume; TLC, total lung capacity.
Data Sharing Statement
- Whether the authors intend to share individual deidentified participant data
- Our permissions do not include sharing of individual data
- What specific data they intend to share
- None
- What other study documents will be made available
- None
- How the data will be accessible
- Data are only accessible to the author group and stored in database and according to GCP-guidelines.
- When and for how long they will be made available
- Data will be stored until 31st December 2022 and will be deleted 31st December 2027.
Disclosure
CBJ serves as a speaker for Novo Nordisk but had no financial interest in the current study. Dr Ayse Dudu Altintas Dogan reports grants from Novo Nordisk, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Afroz N, Gutzwiller FS, Mackay AJ, Naujoks C, Patalano F, Kostikas K. Patient-Reported Outcomes (PROs) in COPD clinical trials: trends and gaps. Int J Chron Obstruct Pulmon Dis. 2020;15:1789–1800. doi: 10.2147/COPD.S235845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1257–1266. doi: 10.1056/NEJMra1900500 [DOI] [PubMed] [Google Scholar]
- 3.Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease (2021 Report). Global initiative for chronic obstructive lung disease; 2021. Available from: https://goldcopd.org/2021-gold-reports/. Accessed February 4, 2022.
- 4.Pinto-Plata V, Toso J, Lee K, et al. Profiling serum biomarkers in patients with COPD: associations with clinical parameters. Thorax. 2007;62(7):595–601. doi: 10.1136/thx.2006.064428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James BD, Jones AV, Trethewey RE, Evans RA. Obesity and metabolic syndrome in COPD: is exercise the answer? Chron Respir Dis. 2018;15(2):173–181. doi: 10.1177/1479972317736294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zewari S, Hadi L, van den Elshout F, Dekhuijzen R, Heijdra Y, Vos P. Obesity in COPD: comorbidities with practical consequences? Copd. 2018;15(5):464–471. doi: 10.1080/15412555.2018.1509951 [DOI] [PubMed] [Google Scholar]
- 7.McDonald VM, Wood LG, Holland AE, Gibson PG. Obesity in COPD: to treat or not to treat? Expert Rev Respir Med. 2017;11(2):81–83. doi: 10.1080/17476348.2017.1267570 [DOI] [PubMed] [Google Scholar]
- 8.Santana AN, Souza R, Martins AP, Macedo F, Rascovski A, Salge JM. The effect of massive weight loss on pulmonary function of morbid obese patients. Respir Med. 2006;100(6):1100–1104. doi: 10.1016/j.rmed.2005.09.021 [DOI] [PubMed] [Google Scholar]
- 9.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. [DOI] [PubMed] [Google Scholar]
- 10.Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21. doi: 10.1111/dom.13129 [DOI] [PubMed] [Google Scholar]
- 11.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 12.Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48(5):736–743. doi: 10.2967/jnumed.106.038679 [DOI] [PubMed] [Google Scholar]
- 13.Brock C, Hansen CS, Karmisholt J, et al. Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy. Br J Clin Pharmacol. 2019;85(11):2512–2523. doi: 10.1111/bcp.14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Huang J, Li J, Mao Q, He J. Effects of liraglutide combined with insulin on oxidative stress and serum MCP-1 and NF-kB levels in Type 2 diabetes. J Coll Physicians Surg Pak. 2019;29(3):218–221. doi: 10.29271/jcpsp.2019.03.218 [DOI] [PubMed] [Google Scholar]
- 15.Viby NE, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154(12):4503–4511. doi: 10.1210/en.2013-1666 [DOI] [PubMed] [Google Scholar]
- 16.Wei YF, Tseng WK, Huang CK, Tai CM, Hsuan CF, Wu HD. Surgically induced weight loss, including reduction in waist circumference, is associated with improved pulmonary function in obese patients. Surg Obes Relat Dis. 2011;7(5):599–604. doi: 10.1016/j.soard.2011.04.221 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DV, Linderholm A, Haczku A, Kenyon N. Glucagon-like peptide 1: a potential anti-inflammatory pathway in obesity-related asthma. Pharmacol Ther. 2017;180:139–143. doi: 10.1016/j.pharmthera.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Yi H, Zhao C, et al. Glucagon-like peptide-1 receptor (GLP-1R) signaling ameliorates dysfunctional immunity in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3191–3202. doi: 10.2147/COPD.S175145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma exacerbations in patients with Type 2 diabetes and asthma on glucagon-like peptide-1 receptor agonists. Am J Respir Crit Care Med. 2021;203(7):831–840. doi: 10.1164/rccm.202004-0993OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carolina LC, Ciudin A, Sánchez E, et al. Liraglutide improves forced vital capacity in individuals with type 2 diabetes: data from the randomized cross-over LIRALUNG Study. Diabetes. 2021;4:540. [DOI] [PubMed] [Google Scholar]
- 21.Frazer DG, Reynolds JS, Jackson MC. Determining when enhanced pause (Penh) is sensitive to changes in specific airway resistance. J Toxicol Environ Health A. 2011;74(5):287–295. doi: 10.1080/15287394.2010.514235 [DOI] [PubMed] [Google Scholar]
- 22.Lundblad LK, Irvin CG, Hantos Z, Sly P, Mitzner W, Bates JH. Penh is not a measure of airway resistance Eur Respir J. 2007;30(4):805. doi: 10.1183/09031936.00091307 [DOI] [PubMed] [Google Scholar]
- 23.Xu WH. Repetitive measurements of enhanced pause (Penh). Respir Physiol Neurobiol. 2015;206:41–44. doi: 10.1016/j.resp.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 24.Bikov A, Lange P, Anderson JA, et al. FEV(1) is a stronger mortality predictor than FVC in patients with moderate COPD and with an increased risk for cardiovascular disease. Int J Chron Obstruct Pulmon Dis. 2020;15:1135–1142. doi: 10.2147/COPD.S242809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Saad H, Préfaut C, Tabka Z, Zbidi A, Hayot M. The forgotten message from gold: FVC is a primary clinical outcome measure of bronchodilator reversibility in COPD. Pulm Pharmacol Ther. 2008;21(5):767–773. doi: 10.1016/j.pupt.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 26.Casanova C, Gonzalez-Dávila E, Martínez-Gonzalez C, et al. Natural course of the diffusing capacity of the lungs for carbon monoxide in COPD: importance of sex. Chest. 2021;160(2):481–490. doi: 10.1016/j.chest.2021.03.069 [DOI] [PubMed] [Google Scholar]
- 27.Vigna M, Aiello M, Bertorelli G, Crisafulli E, Chetta A. Flow and volume response to bronchodilator in patients with COPD. Acta Biomed. 2018;89(3):332–336. doi: 10.23750/abm.v89i3.5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirza S, Clay RD, Koslow MA, Scanlon PD. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488–1502. doi: 10.1016/j.mayocp.2018.05.026 [DOI] [PubMed] [Google Scholar]
- 29.Wu TD, Ejike CO, Wise RA, McCormack MC, Brigham EP. Investigation of the obesity paradox in chronic obstructive pulmonary disease, according to smoking status, in the United States. Am J Epidemiol. 2019;188(11):1977–1983. doi: 10.1093/aje/kwz185 [DOI] [PMC free article] [PubMed] [Google Scholar]