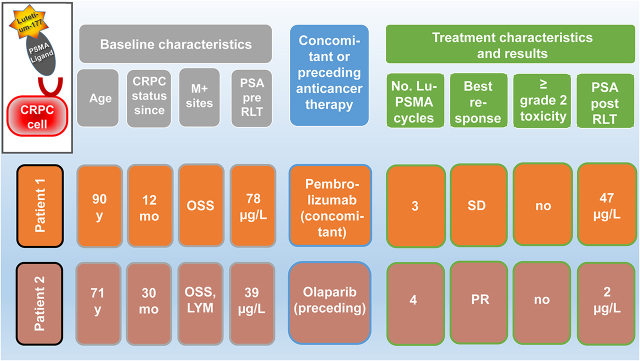
Visual Abstract
Keywords: PSMA, 177Lu, pembrolizumab, olaparib
Abstract
Synergistic effects of immunotherapy with pembrolizumab or drugs targeting DNA damage, such as olaparib, might be used to overcome the limitations of radioligand therapy (RLT) with 177Lu-prostate-specific membrane antigen (PSMA) in metastasized castration-resistant prostate cancer. Here, we present 2 patients receiving such combination or sequential therapies. Methods: RLT was performed at 6- to 8-wk intervals after the patients either exhausted or were considered unfit for all approved conventional treatments. Patient 1 was on pembrolizumab for his squamous cell carcinoma of the skin, whereas patient 2 received RLT sequentially 4 wk after 3 mo of monotherapy with olaparib. Results: Both patients tolerated RLT without any significant hematotoxicity. Patient 2 showed a radiologic and biochemical response, whereas patient 1 achieved prostate-specific antigen stabilization after 3 therapy cycles. Conclusion: These cases indicate that RLT in combination with pembrolizumab or sequentially after olaparib might be well tolerated in single patients.
Prostate-specific membrane antigen (PSMA)–targeting radioligand therapy (RLT) for metastasized castration-resistant prostate cancer (mCRPC) shows good therapeutic efficacy in one third of patients, whereas another approximately one third are nonresponders despite showing intense PSMA expression on PET (1,2). Additional to PSMA heterogeneity, differences in tumor biology and response to internal irradiation can explain this heterogeneity in the therapeutic efficacy of RLT (3). Hence, there is a need for new strategies to augment the therapeutic efficacy of RLT. In a phase II trial, pembrolizumab has shown promising clinical activity in mCRPC (4). Similarly, the PROfound study has shown the efficacy of olaparib in mCRPC with DNA damage response (DDR) gene aberrations (5). RLT together with pembrolizumab or sequentially after olaparib might have synergistic effects; however, there are few data on the tolerability of such combined treatments. Here, we present 2 cases that showed good tolerability to such combination or sequential therapies.
MATERIALS AND METHODS
Patient Characteristics
Two of 69 prostate cancer patients receiving RLT between January 2018 and June 2020 were treated either sequentially or in combination with olaparib or pembrolizumab. The institutional review board (or equivalent) approved this retrospective case series study, and both subjects gave written informed consent. Both patients were in an advanced stage of mCRPC with no conventional therapeutic options left. RLT was offered to the patients after an interdisciplinary tumor board discussed the therapeutic strategy.
Radiopharmaceuticals and Imaging
177Lu was acquired from ITM. Good-manufacturing-practice–grade PSMA-11, PSMA-617, and PSMA Imaging and Therapy (I&T) were acquired from Endocyte/ABX Scintomics (Endocyte through ABX for PSMA-617 and Scintomics for PSMA I&T). Radiolabeling was performed according to the established procedure in a good-manufacturing-practice–certified radiopharmacy laboratory (6). PSMA-ligand PET before and after therapy was performed as previously described (7,8).
RESULTS
Clinical Course of Patient 1
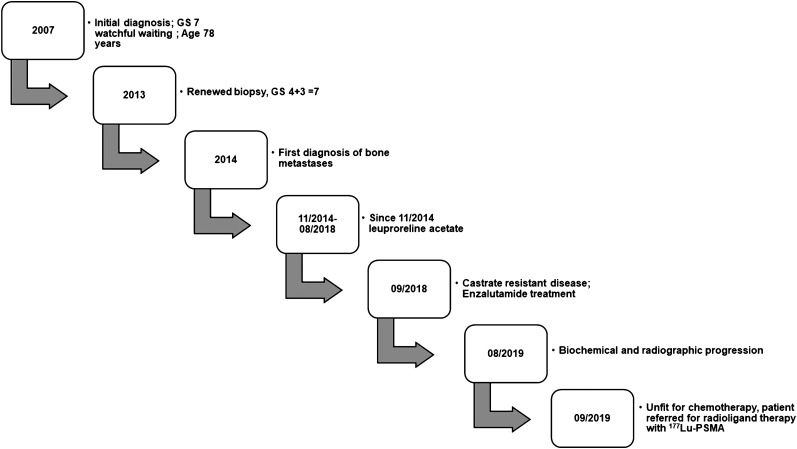
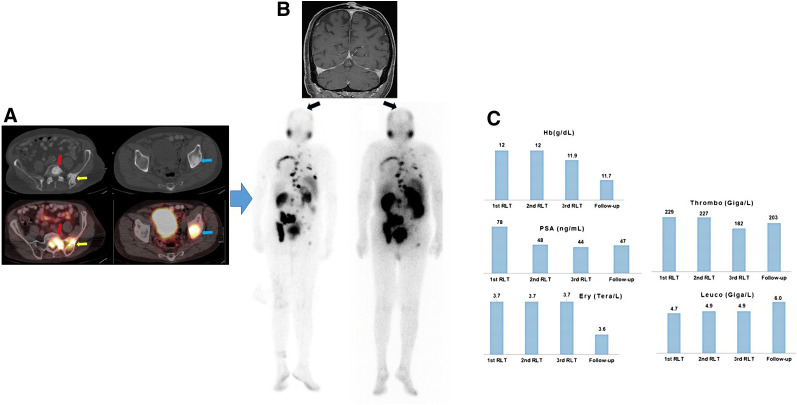
A 90-y-old patient (Fig. 1) was first diagnosed with Gleason score 4 + 3 prostate cancer in 2007. Because of his advanced age he was kept under watchful waiting until 2013, when he underwent a repeated biopsy, which revealed no change. However, because of a rising level of prostate-specific antigen (PSA), in 2014 he underwent bone scanning, which showed bone metastases, resulting in treatment with leuprolide. In August 2018 he developed mCRPC, for which he was treated with enzalutamide. In September 2019, together with the rising PSA level, bone scanning and CT revealed progressive asymptomatic osteoblastic bone lesions. Because of his advanced stage, the patient was considered unfit for chemotherapy, and because his bone metastases had no symptoms, no 223Ra treatment was offered. Hence, the patient underwent PSMA PET/CT, which revealed intense uptake of PSMA in bone lesions. He then was referred for RLT. At the time of RLT, he was receiving 3 weekly 200-mg doses of pembrolizumab for a second malignancy (locally advanced cutaneous squamous cell carcinoma of the forehead region). He was treated with 5 GBq of 177Lu-PSMA per cycle (the first 2 cycles with 177Lu-PSMA 617 and the third cycle with 177Lu-PSMA I&T). The reason for performing 2 cycles with 177Lu-PSMA 617 and one with 177Lu-PSMA I&T was purely logistic and based on the availability of the peptides. His Eastern Cooperative Oncology Group 1 status remained unchanged after the therapy. The hematologic toxicity profile revealed no significant changes in erythrocyte, thrombocyte, hemoglobin, or leukocyte counts (Fig. 2). Renal function remained unchanged. Overall, the posttherapy scan revealed stable to mildly decreasing PSMA uptake in known bone lesions, with no evidence of new foci. The squamous cell carcinoma showed no PSMA uptake in the posttherapy scan. His PSA level declined by 40%.
FIGURE 1.
Clinical course of patient 1.
FIGURE 2.
Patient 1. (A) Axial CT (top) and PET/CT (bottom) images. Intense 18F DCFPyL uptake by osteoblastic bone lesions in first sacral vertebra (red arrow), left iliac bone/iliosacral joint (yellow arrow), and left acetabulum (blue arrow) is seen on PET/CT. (B) Coronal MRI. T1-weighted sequence at start of first RLT shows squamous cell carcinoma in parietal region of skull; this lesion showed no PSMA uptake on posttherapy scans. The 2 images below MR image are posttherapy 177Lu-PSMA scans after first therapy cycle (left) and after third therapy cycle (right). (C) Sequential lab tests showed PSA decline from 78 to 47 ng/mL but no substantial changes in hematologic parameters. Ery = erythrocyte; thrombo = thrombocyte; Hb = hemoglobin; leuco = leukocyte.
Clinical Course of Patient 2
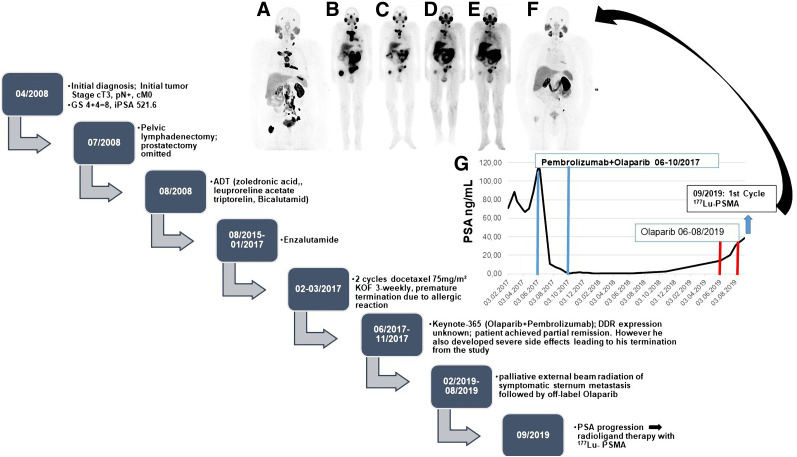
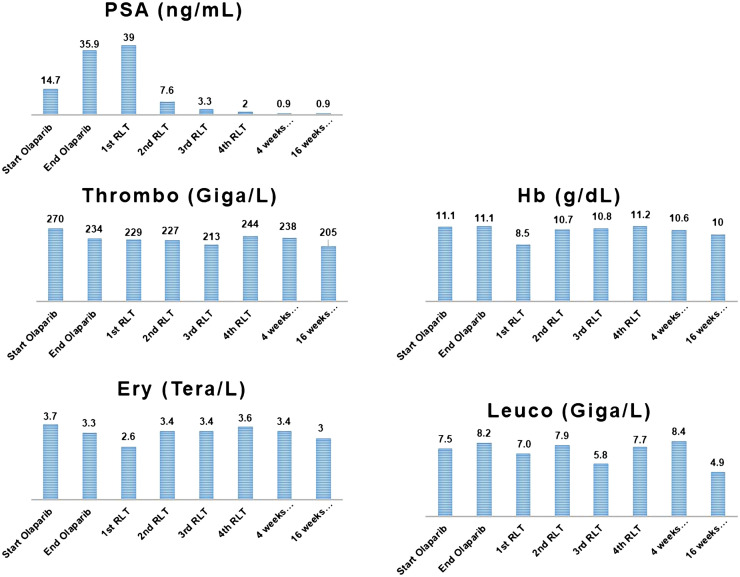
A 71-y-old patient (Fig. 3) was first diagnosed in April 2008 with cT3 pN+ cM0 prostate cancer that had a Gleason score of 4 + 4 (initial PSA level, 521.6 ng/mL). At initial diagnosis, he was 59 y old and was offered pelvic lymphadenectomy and prostatectomy. At surgery, the primary tumor was seen to be inoperable; consequently, only pelvic lymphadenectomy was performed. Afterward, from August 2008 until January 2017, he was treated with goserelin acetate (Zoladex; AstraZeneca), leuprolide acetate (Trenantone; Takeda Pharma), triptorelin (Pamorelin; Debiopharm), and bicalutamide followed by enzalutamide (Astellas Pharma) (mCRPC in August 2015). Unfortunately, the disease progressed biochemically and radiologically in February 2017, leading to treatment with docetaxel, which had to be terminated prematurely because of severe allergic reactions. Thus, the patient was evaluated for further treatment options and was enrolled in the Keynote-365 (olaparib + pembrolizumab) study. Despite showing an excellent biochemical and radiologic response, in November 2017 he had to be withdrawn from the study because of significant pembrolizumab-related adverse events, including immune-related arthritis and hypophysitis. The PSA level and imaging findings remained stable until February 2019, when he developed pain in the sternum. On a CT scan, disease progression was confirmed. For symptom control, his sternal metastasis was locally irradiated, and considering his excellent response in the Keynote-365 trial, olaparib monotherapy was started afterward. Pembrolizumab could not be added, because of known severe side effects. Without responding to the olaparib rechallenge, the patient showed PSA progression in August 2019 and was referred for RLT, which began 4 wk after the last dose of olaparib. As shown in Figure 4, he had an excellent persistent response to RLT even at 16 wk after the fourth RLT. His laboratory parameters showed no significant bone marrow toxicity, and his renal function also remained unchanged from baseline (Fig. 4).
FIGURE 3.
Patient 2. (A–F) PSMA PET/CT maximum-intensity-projection images (A and F) and posttherapy 177Lu-PSMA scintigraphy images (B–E). (G) PSA follow-up of patient 2 starting from February 2017. Chart shows clinical course of disease. GS = Gleason score; ADT = androgen deprivation therapy; iPSA = initial PSA; KOF = body surface area.
FIGURE 4.
Laboratory values of patient 2. Ery = erythrocyte; thrombo = thrombocyte; Hb = hemoglobin; leuco = leukocyte.
DISCUSSION
In this report, we have presented 2 mCRPC patients with good tolerance of 177Lu-PSMA RLT in combination with pembrolizumab or sequentially after olaparib, which in the future might be an interesting option to improve therapeutic efficacy or overcome resistance to RLT.
One reason for an insufficient response to RLT in mCRPC patients might be spatial and temporal heterogeneity of PSMA expression. It is well known that PSMA expression is upregulated in most but not all cases of prostate cancer (9,10). Even intensely PSMA-expressing lesions on PSMA PET often show an insufficient response to 177Lu-PSMA RLT. The level of PSMA expression is only one of many factors influencing responsiveness to RLT. Other factors, such as DNA repair mechanisms or immune phenomena, probably also play a crucial role in resistance to 177Lu-PSMA RLT. One of the proposed strategies to potentiate the efficacy of RLT is by combining or sequencing it with other targeted agents.
Selecting the right drug to combine with RLT is a challenge, considering that all targeted drugs have their own side effects. Interaction between tumor and internal radiation takes place at the intracellular and extracellular levels. For inducing a durable response, sufficient double-strand breakage in DNA is needed (11). There are 2 pathways in which cells react to radiation: homologous recombination and nonhomologous end joining (12). Poly(adenosine diphosphate-ribose)polymerase (PARP) is involved in this repair process. Drugs inhibiting this pathway may increase radiation sensitivity. Recently, olaparib, a PARP inhibitor, was approved for patients with prostate cancer harboring DNA damage repair gene aberrations. That is why combining RLT with a PARP inhibitor appears to be an attractive option. However, a combination may not be without toxicity. In our series, patient 2 was treated sequentially with RLT after olaparib. Because olaparib can be hematotoxic, we allowed a sufficient washout time, treating the patient with RLT 4 wk after the last dose of olaparib. Indeed, the follow-up hematologic parameters showed that the 4-wk washout period did not lead to significant hematotoxicity whereas the RLT efficacy seemed to be maintained. It is difficult to say if the good response seen in this patient was RLT-related or due to the synergistic effects of olaparib.
Although the intracellular interaction with DNA is central to the therapeutic efficacy of RLT, its potential positive effect on tumor immunity makes RLT extremely attractive for combination immunotherapy (13,14). Patient 1 was receiving pembrolizumab because of his cutaneous squamous cell carcinoma. It was unclear whether RLT in combination with immunotherapy would be well tolerated, especially considering the patients’ age of 90 y. Additionally, at the time the patient underwent RLT, there were no data on the optimum dosage of pembrolizumab and RLT required to maintain individual therapeutic efficacy without inducing toxicity. This case showed that RLT with 5 GBq in combination with 3 weekly 200-mg doses of pembrolizumab can be well tolerated. The therapeutic efficacy was adequate, showing a biochemical response with a PSA decline of 40%.
One of the major limitations of this work, apart from the low number of patients, is that we did not have information on the DDR or PD-1 status of the lesions. Olaparib has been shown to increase overall survival and progression-free survival in patients harboring DDR mutations, and because lesions harboring DDR expression show higher PSMA expression, it is important to perform lesion biopsies before such sequential or combination therapies to assess the DDR status. Similarly PD-1 positivity can help in selecting appropriate patients for immunotherapy. However, we do not think these limitations diminish the importance of these 2 cases, as the primary aim of this article is to highlight the fact that sequential olaparib or combined pembrolizumab with RLT is well tolerated. Ongoing prospective phase 1 and 2 studies (ClinicalTrials.gov identifiers NCT03874884 and NCT03805594) will provide more data on RLT in combination with pembrolizumab or olaparib.
CONCLUSION
These 2 cases indicate that RLT in combination with pembrolizumab or sequentially after olaparib might be well tolerated in single patients. Further studies on larger patient populations are needed to assess the safety and efficacy of DNA repair gene–targeting drugs and immunotherapy in combination with RLT.
DISCLOSURE
Vikas Prasad is a scientific advisor for Point Biopharma and has acted as a consultant for ITM. Neil Fleshner is a cofounder of Point Biopharma. Ambros Beer has acted as a consultant for Siemens. Christian Bolenz discloses speaking fees and sponsored research from AstraZeneca. Friedemann Zengerling has an advisory role with Merck Sharp & Dohme. Matthias Eiber received funding from the Deutsche Forschungsgemeinschaft within project B11. Christian Bolenz, Meinrad Beer, Friedemann Zengerling, and Ambros Beer are part of the i2SOUL consortium at Ulm University Hospital, which supported this work. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is it feasible to perform RLT with 177Lu-PSMA on mCRPC patients in combination or sequentially with pembrolizumab or olaparib?
PERTINENT FINDINGS: In single patients, RLT in combination with pembrolizumab or sequentially with olaparib might be well tolerated.
IMPLICATIONS FOR PATIENT CARE: In selected patients with mCRPC, patients can be treated with RLT while receiving pembrolizumab or after receiving olaparib.
REFERENCES
- 1. Ravi Kumar AS, Hofman MS. Mechanistic insights for optimising PSMA radioligand therapy. Clin Cancer Res. 2020;26:2774–2776. [DOI] [PubMed] [Google Scholar]
- 2. Miyahira AK, Pienta KJ, Morris MJ, et al. Meeting report from the Prostate Cancer Foundation PSMA-directed radionuclide scientific working group. Prostate. 2018;78:775–789. [DOI] [PubMed] [Google Scholar]
- 3. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–379. [DOI] [PubMed] [Google Scholar]
- 4. Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–2102. [DOI] [PubMed] [Google Scholar]
- 6. Delker A, Fendler WP, Kratochwil C, et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51. [DOI] [PubMed] [Google Scholar]
- 7. Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging—version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 8. Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paschalis A, Sheehan B, Riisnaes R, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damjanovic J, Janssen JC, Prasad V, et al. 68Ga-PSMA-PET/CT for the evaluation of liver metastases in patients with prostate cancer. Cancer Imaging. 2019;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Driscoll M, Jeggo PA. The role of double-strand break repair: insights from human genetics. Nat Rev Genet. 2006;7:45–54. [DOI] [PubMed] [Google Scholar]
- 12. Haber JE. Partners and pathways repairing a double-strand break. Trends Genet. 2000;16:259–264. [DOI] [PubMed] [Google Scholar]
- 13. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. [DOI] [PubMed] [Google Scholar]
- 14. Wu Y, Pfeifer AK, Myschetzky R, et al. Induction of anti-tumor immune responses by peptide receptor radionuclide therapy with 177Lu-DOTATATE in a murine model of a human neuroendocrine tumor. Diagnostics (Basel). 2013;3:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]