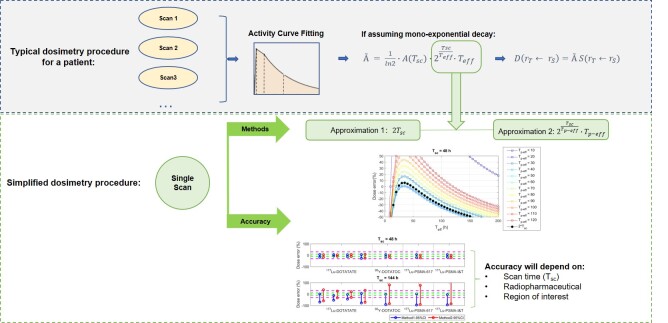
Visual Abstract
Keywords: dosimetry, quantitation, radiopharmaceutical therapy, SPECT, single-time-point
Abstract
Because of challenges in performing routine personalized dosimetry in radiopharmaceutical therapies, interest in single-time-point (STP) dosimetry, particularly using only a single SPECT scan, is on the rise. Meanwhile, there are questions about the reliability of STP dosimetry, with limited independent validations. In the present work, we analyzed 2 STP dosimetry methods and evaluated dose errors for several radiopharmaceuticals based on effective half-life distributions. Methods: We first challenged the common assumption that radiopharmaceutical effective half-lives across the population are gaussian-distributed (i.e., follow a normal distribution). Then, dose accuracy was estimated using 2 STP dosimetry methods for a wide range of potential post injection (p.i.) scan time points for different radiopharmaceuticals applied to neuroendocrine tumors and prostate cancer. The accuracy and limitations of each of the STP methods were discussed. Results: A lognormal distribution was more appropriate for capturing effective half-life distributions. The STP framework was promising for dosimetry of 177Lu-DOTATATE and for kidney dosimetry of different radiopharmaceuticals (errors < 30%). Meanwhile, for some radiopharmaceuticals, STP accuracy was compromised (e.g., in bone marrow and tumors for 177-labeled prostate-specific membrane antigen [PSMA])). The optimal SPECT scanning time for 177Lu-DOTATATE was approximately 72 h p.i., whereas 48 h p.i. was better for 177Lu-PSMA. Conclusion: Simplified STP dosimetry methods may compromise the accuracy of dose estimates, with some exceptions, such as for 177Lu-DOTATATE and for kidney dosimetry in different radiopharmaceuticals. Simplified personalized dosimetry in the clinic continues to be challenging. On the basis of our results, we make suggestions and recommendations for improved personalized dosimetry using simplified imaging schemes.
Image-based personalized dosimetry as applied to radiopharmaceutical therapies allows physicians to limit toxicity to critical organs and, potentially, to predict response to treatment and enable personalized therapy decisions (1). To perform dosimetry with acceptable accuracy, multiple quantitative scans (e.g., SPECT) are often assumed necessary to assess the distribution of the therapeutic compound over time and quantify its biokinetics in organs and tumors. Nevertheless, this task is challenging in a clinical environment because of the requirement that a series of scans be performed over multiple days for each therapy cycle in each patient, followed by complex and time-consuming data processing and calculations (2).
To address this challenge, with the aim of enabling routine dosimetry in the clinic, several simplified approaches have been proposed. One approach is to perform dosimetry estimation using only a single SPECT scan (single-time-point [STP] dosimetry) (3–8).
STP methods have been proposed by Hänscheid et al. (3) and Madsen et al. (4). Both methods assume that clearance of the radiopharmaceutical from a region of interest (ROI) follows a monoexponential decay behavior and has an effective half-life (Teff); therefore, the time-integrated activity à in the ROI can be calculated using the following formula:
| Eq. 1 |
where Tsc is the SPECT scan time point and A(Tsc) is activity in the ROI measured at Tsc. This activity can be determined from the quantitatively reconstructed SPECT image. However, Teff may be difficult to measure, requiring a series of SPECT scans with subsequent data fitting for the ROIs. Furthermore, Teff can vary significantly between individual patients and/or between therapy cycles.
The STP method of Hänscheid et al. (3) proposes that the last component of Equation 1, that is, ⋅Teff, can be approximated by 2Tsc. Their theoretic calculations indicated that if Tsc remains within 0.75–2.5 times the patient-specific Teff, errors in dose estimates would be below 10%. Hänscheid et al. analyzed 29 177Lu-DOTATATE/DOTATOC patient studies to confirm this theoretic finding (3).
On the other hand, the work published by Madsen et al. (4), based on 90Y-DOTATOC data from a clinical trial, used a population mean Teff (Tp-eff) and demonstrated that the accuracy of STP dosimetry for kidneys would be improved by setting Tsc close to or slightly larger than Tp-eff (4). This STP dosimetry framework was verified to have good accuracy by Amato et al. in the treatment of hyperthyroidism with 131I radioiodine (9). Both formulations—that of Hänscheid et al. (method 1) and that of Madsen et al. (method 2)—are presented in Table 1.
TABLE 1.
STP Dosimetry Application Methods
Inspired by these works, we recently investigated the accuracy of STP dosimetry for kidneys using different values of Tsc (4, 24, and 72 h p.i.) for data from 53 neuroendocrine tumors in 39 patients undergoing 177Lu-DOTATATE therapy (6). The impact of the deviation of a particular Teff from Tp-eff on the accuracy of kidney doses was estimated. The most favorable Tsc, that is, the scan that would result in kidney dosimetry errors below 10%, was suggested to be set at times between 1 and 1.5 Tp-eff. However, our previous study was focused solely on kidney doses and on a single specific compound, namely 177Lu-DOTATATE.
For a given patient, the Teff for radiopharmaceutical clearance from tumors usually differs from that from organs at risk (OARs). Compromise is needed to achieve acceptably accurate dose estimates using STP methods, as the optimal Tsc for tumors may be different from that for OARs. These time points would also differ for different radiopharmaceutical compounds.
In the present work, we compared the accuracy of doses estimates using STP methods 1 and 2 for different compounds commonly applied in radiopharmaceutical therapies. We also challenged the common default assumption that Teff has a gaussian (normal) distribution across a population. On the basis of our results, we provide recommendations for dosimetry workflows.
MATERIALS AND METHODS
Because the mean organ-based absorbed dose can be considered nearly proportional to Ã, the dose error (DE) can be estimated from the time-integrated activities (as estimated using either method 1 or 2) as follows:
| Eq. 2 |
where ÃMx represents either à for either method 1 or method 2 (Table 1), and reference à is calculated using Equation 1. We investigated Tsc ranging from 24 to 144 h (days 1–6) after activity injection.
Using these Tsc values, we estimated DEs for generic patient-specific Teff values ranging from 0 to 200 h. Whereas method 1 does not depend on Teff, for method 2 Tp-eff was set between 10 and 120 h to capture a wide range of possibilities. Subsequently, for both tumors and OARs, DEs were investigated using these 2 STP methods for 4 commonly applied radiopharmaceuticals, namely 177Lu-DOTATATE and 90Y-DOTATOC, as used in treatments of neuroendocrine tumors, and 177Lu-labeled prostate-specific membrane antigen (PSMA) compounds (including 177Lu-PSMA-617 and 177Lu-PSMA-I&T), as used for prostate cancer.
To investigate the accuracy of STP dosimetry, we aimed to first obtain 95% confidence intervals (CIs) for the distributions of Teff for a given radiopharmaceutical and organ or target of interest. Table 2 summarizes Teff for different radiopharmaceuticals as available in the literature or from our own data. For most datasets, limited information, such as mean, standard deviation (SD) and ranges for Teff, could be found, with the exception of 5 datasets from our own clinical studies (i.e., studies 1, 4 and 8 in Table 2, for which the individual Teff values for patients were available). For studies 1 and 4, patient data were from the radiopharmaceutical therapy trial NCT02754297, approved by the CHU de Québec–Université Laval institutional Ethics Committee, and all patients provided written consent to participate. Study 8 was based on retrospective and anonymized data, acquired for routine clinical dosimetry, as approved by the Ethics Committee of LMU Munich 20-520, including a waiver of consent.
TABLE 2.
Mean Teff and SD, and Computed 95% CI, of Organs and Lesions for Commonly Applied Radiopharmaceuticals That Were Used in This Investigation
| Agent | Study | Reference | Patients (n) | Organ or target | Median | mean (h) | SD (h) | 95% CI (h) |
|---|---|---|---|---|---|---|---|---|
| 177Lu-DOTATATE | 1 | Hou et al. 2019 (12) | 30 (87) | Kidney* | 46 (30–82) | 47.0 | 11.6 | 28.5–73.6 |
| 2 | Heikkonen et al. 2016 (13) | 24 (24) | Kidney | NA (36–59) | 45.3 | 5.9 | 34.8–57.4 | |
| 3 | Hänscheid et al. 2018 (3)† | 27 (54) | Kidney‡ | 51 (40–68) | 51.0 | 7.0 | 38.8–66.0 | |
| 25 (25) | Liver‡ | 67 (55–117) | 76.5 | 15.5 | 51.4–110.7 | |||
| 27 (27) | Spleen‡ | 68 (52–99) | 68.0 | 11.8 | 49.5–91.6 | |||
| 22 (22) | Tumor‡ | 77 (56–130) | 85.4 | 18.5 | 56.0–125.8 | |||
| 4 | Del Prete et al. 2018 (11) | 158 (1,117) | Kidney* ‡ | 47 (23–159) | 50.8 | 16.9 | 25.6–91.0 | |
| Desy et al. 2020 (14) | 158 (474) | Bone marrow* ‡ | 70 (29–160) | 76.4 | 27.6 | 36.2–142.8 | ||
| 158 (2,166) | Tumor* ‡ | 84 (16–161) | 87.8 | 30.5 | 42.8–160.6 | |||
| 90Y-DOTATOC | 5 | Menda et al. 2018 (15) | 25 (69) | Kidney | NA (25–92) | 37.5 | 12.5 | 19.4–67.2 |
| 177Lu-PSMA-617 | 6 | Kurth et al. 2018 (16) | 25 (25) | Whole body | NA (22–86) | 40.5 | 15.8 | 18.8–79.1 |
| 7 | Sarnelli et al. 2019 (17) | 9 (9) | Parotid gland | 33 (26–61) | 35.4 | 10.6 | 22.2–53.2 | |
| 9 (9) | Kidney | 31 (12–81) | 39.2 | 20.9 | 17.2–76.2 | |||
| 9 (9) | Red marrow | 8 (3–15) | 8.0 | 4.7 | 3.2–16.3 | |||
| 9 (9) | Liver | 25 (13–63) | 33.5 | 20.0 | 13.4–69.1 | |||
| 9 (9) | Whole body | 40 (32–80) | 52.4 | 22.2 | 27.2–90.5 | |||
| 177Lu-PSMA-I&T | 8 | Written communications | 15 (290) | Bone metastases* | 38 (13–191) | 42.6 | 19.1 | 16.9–90.0 |
| 9 | Baum et al. 2016 (18) | 30 (NA) | Bone metastases‡ | 52 (14–149) | 52.0 | 30.0 | 16.2–132.6 | |
| 30 (NA) | Lymph nodemetastases‡ | 43 (25–160) | 43.0 | 32.0 | 9.9–132.7 | |||
| 30 (NA) | Kidney‡ | 33 (19–83) | 33.0 | 14.0 | 14.8–64.9 | |||
| 30 (NA) | Parotid gland‡ | 25 (20–43) | 25.0 | 5.0 | 16.7–36.1 |
Teff of each individual ROI (organ or lesion) was available (i.e., complete listing of Teff for all patients).
Overall dataset (29 patients) was primarily 177Lu-DOTATATE (22 patients) but also included 177Lu-DOTATOC (7 patients).
Teff was published as median and range. For studies 3 and 9, corresponding mean and SD were calculated using method of Hozo et al. 2005 (19). For study 4, we had access to complete listing of Teff.
NA = not applicable.
Data in parentheses are range (for median) or total number of ROIs (for number of patients).
On the basis of a common default assumption that Teff follows a normal distribution, 95% CIs would be set at mean ± 1.96 SD. By contrast, our analysis in this work showed that a lognormal distribution is a more proper assumption. For each of our 5 full datasets with a complete listing of Teff values (studies 1, 4, and 8 in Table 2), the Kolmogorov–Smirnov test was performed to check the null hypothesis that our data or their corresponding log-transformation were normally distributed. Furthermore, distribution fittings were performed and analytic 95% CIs for the Teff per ROI were calculated and compared with the true range of 95% CI data as calculated using the quantile function. Having found that a lognormal distribution is a more appropriate model, we mapped the reported means and SDs from different studies to lognormal distributions with exponent parameters μ and σ (i.e., mean and SD of the natural logarithm) as given in the following equations:
| Eq. 3 |
| Eq. 4 |
Subsequently, from the mapped lognormal distributions, 95% CI ranges were computed. Finally, DEs for the computed ranges (spreads) of Teff were estimated using both method 1 and method 2.
RESULTS
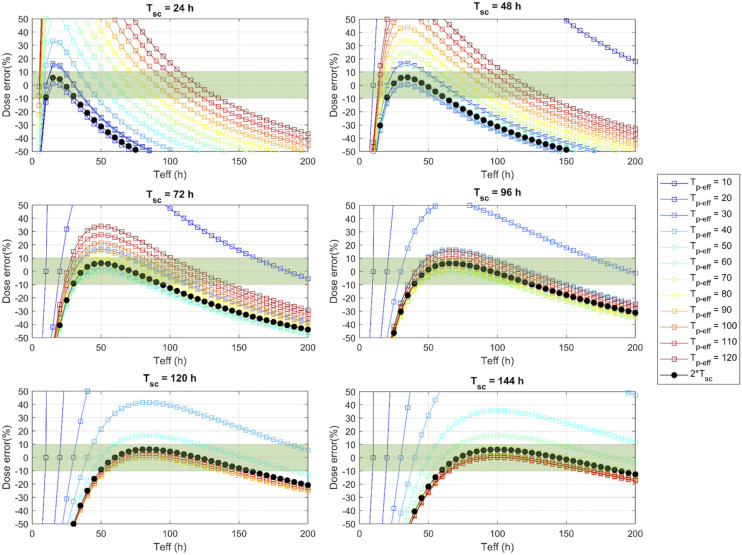
DEs calculated using methods 1 and 2 are presented in Figure 1 for a wide range of Tp-eff and Teff. The DEs within ±10% are highlighted in green. Overall, for both STP methods, the accuracy of dose estimates strongly depended on the spread of patient Teff values and the scan time selected by the users.
FIGURE 1.
DEs (%) resulting from method 1 (black line) and method 2 (with Tp-eff ranging from 10 to 120 h; colored lines) relative to true Teff of radiopharmaceutical washout. DEs within ±10% are highlighted in green.
The Teff distributions were then investigated using the 5 datasets that had a complete listing of Teff values for 177Lu-DOTATATE and 177Lu-PSMA-I&T (studies 1, 4, and 8). Supplemental Table 1 lists the Kolmogorov–Smirnov test results and p-values, demonstrating that most Teff data followed a lognormal distribution (supplemental materials are available at http://jnm.snmjournals.org). Supplemental Figure 1 shows the corresponding histograms with probability density function curves of lognormal distribution fittings, as well as the true and analytically estimated 95% CI ranges based on normal and lognormal distribution assumptions. Supplemental Figure 2 shows the DEs from these 3 different types of ranges (i.e., computed on the basis of the true 95% CI vs. normal and lognormal assumptions), confirming that doses computed from a lognormal distribution were more similar to those from the true 95% CI range, further strengthening the lognormal assumption.
Table 2 shows our computed 95% CI ranges based on a lognormal distribution assumption, using the means and SDs reported in the literature. Had we assumed a normal distribution, the computed 95% CIs of Teff would extend below 0 for some studies. Based on these Teff, the DEs for the investigated radiopharmaceuticals are presented in Figures 2 and 3 for kidney and bone marrow, respectively, which are OARs for the particular radiopharmaceutical therapies investigated, and in Figure 4 for tumors. For 177Lu-DOTATATE, the estimated DEs were relatively low, whereas for some other radiotracers, large errors were possible for both STP dosimetry methods. Additionally, a comparison between the DEs for the published range listed in Table 2 and those for the estimated 95% CI is shown in Supplemental Figure 3.
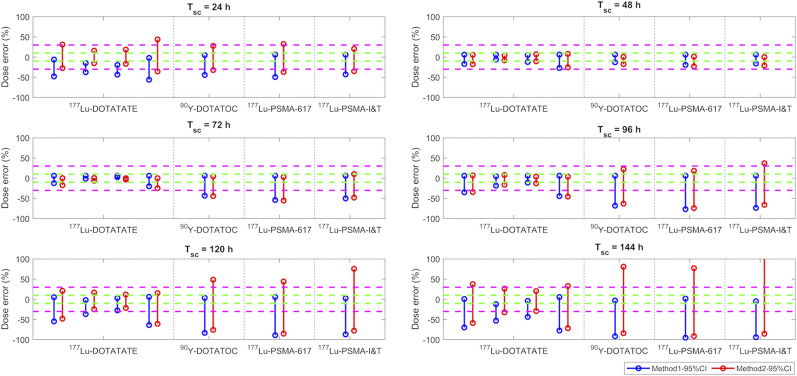
FIGURE 2.
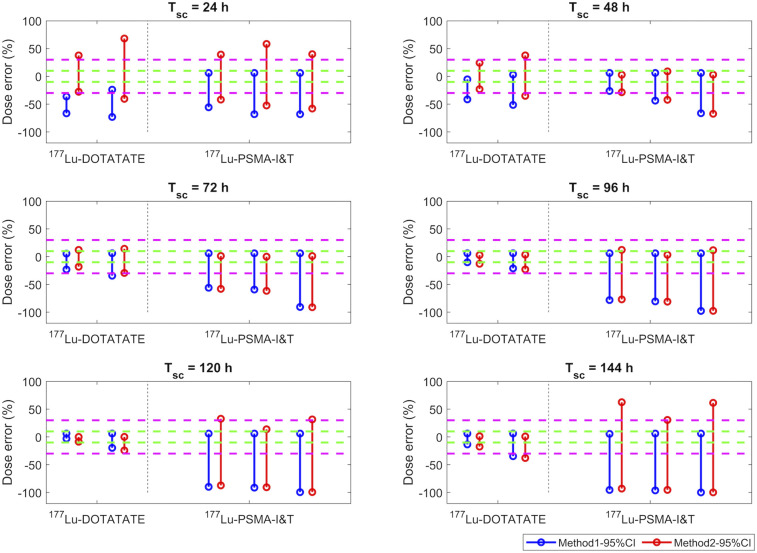
DEs (%) of kidney doses estimated using method 1 (blue) and method 2 (red) when patient Teff is within simulated 95% CI range listed in Table 2. Green and magenta dashed lines indicate ±10% and ±30% of DEs, respectively. Four sets of results shown in 177Lu-DOTATATE column correspond to Teff data from studies 1–4.
FIGURE 3.
DEs (%) of bone marrow doses estimated using method 1 (blue) and method 2 (red) when patient Teff is within simulated 95% CI range from Table 2. Green and magenta dashed lines indicate ±10% and ±30% of DEs, respectively. Two sets of results correspond to data from studies 4 and 7.
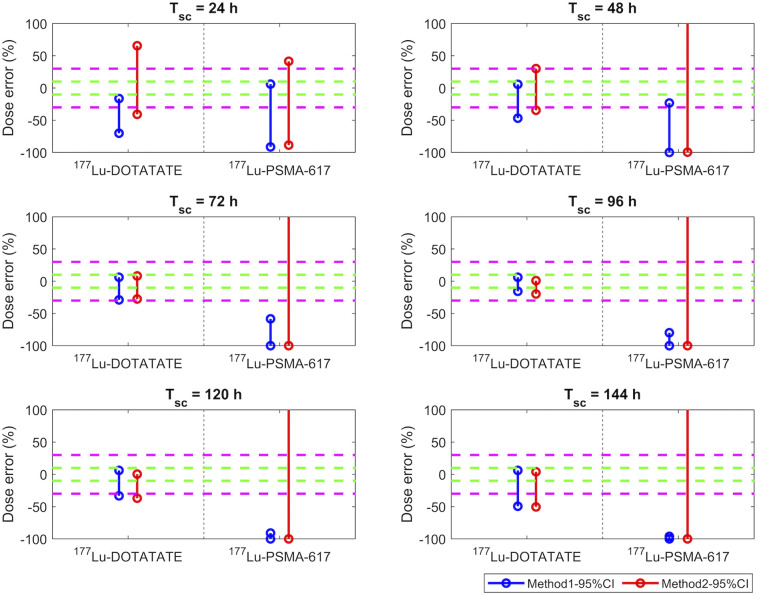
FIGURE 4.
DEs (%) of tumor doses estimated using method 1 (blue) and method 2 (red) when patient Teff is within 95% CI range from Table 2. Green and magenta dashed lines indicate ±10% and ±30% of DEs, respectively. Data in 177Lu-DOTATATE column correspond to studies 3 and 4, whereas data in 177Lu-PSMA-I&T column correspond to bone and lymph node metastasis data from studies 8 and 9.
DISCUSSION
As shown in Figure 1, when Tsc ranged from 0.8 to 1.6 Tp-eff, the DEs from both method 1 and method 2 were similar (differences < 7%). DE was small (<10%) when Tsc was set within 0.75–2.5 Teff for method 1, as is consistent with prior reporting (3). However, for method 2, the accuracy of absorbed dose depended not only on Tsc but also on the deviation of Teff from Tp-eff. Overall, Tsc should preferably be set in the range 1–1.5 Tp-eff in order to achieve a lower DE but allow larger deviations between Teff and Tp-eff. This recommendation agrees with Figure 4 of Zhao et al. (6). For example, if Tp-eff was 47 h (the kidney’s Tp-eff for 177Lu-DOTATATE in study 1; Table 2) and Tsc was set to 72 h, less than a 10% DE could be observed when the deviation between Tp-eff and Teff was within a 95% CI of −38% to 43% if Tsc was set to 72 h. However, when Tsc was set to 24 h p.i., the deviation between Tp-eff and Teff had to stay within a 95% CI of −10% to 10% in order to have a DE of less than 10%.
Figures 2–4 summarize potential DEs based on the analysis of our own or published Teff values for commonly used radiopharmaceuticals. In general, our results suggest that there is a high possibility of dose underestimation when using an STP framework. The extent of this underestimation would depend on the radiopharmaceutical and the considered ROI, particularly the kidneys, the bone marrow, and neuroendocrine tumors.
For the kidneys, similar DEs were obtained from both method 1 and method 2 when Tsc was set to 48–96 h (i.e., days 2–4 p.i.), as shown in Figure 2. However, large differences between methods 1 and 2 were observed for either an early Tsc (24 h p.i.) or a late Tsc (>96 h p.i.). For most of the investigated radiopharmaceuticals, DEs were smallest when Tsc was set to 48 h p.i. However, for 177Lu-DOTATATE, a Tsc of 72 h p.i. was slightly better than a Tsc of 48 h p.i., with a DE of less than 10%. For 177Lu-PSMA-compound therapies, DE could be larger than 20% even at an optimized Tsc.
For bone marrow dosimetry (Fig. 3), DEs were smallest (<30%) when Tsc was set to 72–96 h p.i. for 177Lu-DOTATATE. However, for 177Lu-PSMA-617, STP methods should be used only with great caution, because in most of our investigated scenarios, STP methods largely underestimated the dose, especially method 1.
For neuroendocrine tumor lesions treated with 177Lu-DOTATATE (Fig. 4), similar to bone marrow dosimetry, a Tsc of 72–120 h p.i. resulted in a DE of less than 30%, whereas for prostate cancer lesions treated with 177Lu-PSMA-I&T, DEs (<30%) were lowest for a Tsc of 48 h p.i., as displayed in Figure 4.
Additionally, differences in DEs for the same radiopharmaceutical and ROI were found between different studies, mainly because of differences in the methods used to estimate Teff by different groups, including segmentation method, imaging type, and Tsc, as well as fitting model. For example, the analysis in study 1 was based on small-volume kidney dosimetry, using a 4 -mL region within the kidney for dose estimation, whereas for study 2, the data were from whole-kidney segmentation. As another example, study 8 used 3 SPECT/CT scans, whereas 5 hybrid SPECT/planar scans were used in study 9. However, these differences did not influence our main findings in this work.
The STP dosimetry framework is suitable mostly for dosimetry of 177Lu-DOTATATE and of various radiopharmaceuticals in kidneys. Our results were less reliable for 177Lu-PSMA compounds in bone marrow and lesions. If the results for kidneys, bone marrow, and tumors are combined, SPECT scanning at 72 h p.i. should be considered optimal for 177Lu-DOTATATE whereas earlier time points, such as 48 h p.i., would be better for 177Lu-PSMA therapies, in agreement with findings by Zhao et al. and Jackson et al. (6,7). The results for methods 1 and 2 were equally accurate when the suggested scan time was used.
The potentially large differences in pharmaceutical clearance rate between patients, and the limited data available for our analysis, suggest that the DEs obtained from STP methods could be 30% or more for some therapeutic compounds and targets. More studies or potential modifications of the STP methods are needed. For example, Jackson et al. (7) applied an STP method to predict doses from 177Lu-PSMA-617 therapy using a population of normalized pharmacokinetic curves. However, the accuracy of that method has not been confirmed, and accuracy was represented by the mean absolute error, which could be smaller than the DE format we report here (8). In addition, individual clinical data might be used to narrow the range of Teff around which STP scans could be performed. As an example, we suggest that variations in estimated glomerular filtration rate (eGFR) may indicate varying kidney function, in turn impacting Teff. In fact, we investigated and found a significant correlation between these 2 factors (Supplemental Fig. 4). A similar correlation between kidney dose and eGFR was also found by Del Prete et al. in 2017 (10). Thus, a potentially improved STP framework would use a modified estimated Teff based on such clinical parameters.
Our results show that the accuracy of STP framework depends strongly on patient-specific radiopharmaceutical clearance times, and the large differences between patients suggest that STP methods may not be appropriate for all therapies or radiopharmaceuticals. When STP may be problematic, an alternative practical approach for patient-specific dosimetry may be to use at least 2 time points for imaging and subsequent Teff calculation for the first cycle of treatment to estimate patient-specific pharmaceutical kinetics, specifically patient- and organ-specific Teff . However, the choice of Tsc for the 2 scans is crucial, and one should avoid setting them during the early radiopharmaceutical uptake phase. Afterward, this information could be incorporated into the more generalized form (Eq. 1) to estimate Teff for the following cycles. The accuracy of such an approach was investigated by Del Prete et al. (11), who observed DEs of approximately −0.5%, 15.7%, and −5.6% for kidney, bone marrow, and tumor, respectively, for 177Lu-DOTATATE.
CONCLUSION
We have analyzed the accuracy of 2 STP dosimetry methods for several radiopharmaceutical therapy agents. We found that for some therapeutic compounds, use of these simplified methods may largely compromise the accuracy of dose estimates. Two prominent exceptions are 177Lu-DOTATATE, wherein reasonably small (<30%) DEs can be achieved for an STP framework that scans at approximately 72 h p.i., and kidney dosimetry in general for radiopharmaceuticals investigated in this work. To improve STP dosimetry, we suggest the alternative approach of determining patient-specific biokinetics using 2 or, ideally, more scans during the first treatment cycle and the STP framework for the following cycles. However, this approach needs to be validated, since Teff might change over multiple treatment cycles. Other alternatives to existing STP methods include improved estimation of patient-specific Teff from clinical data, as exemplified in this work using eGFRs for the kidney. Enabling routine personalized dosimetry in the clinic remains challenging given the complex data processing and calculations for high accuracy on the one hand and interest in more feasible methods on the other.
DISCLOSURE
This work was supported by grant 137993 from the National Institutes of Health/Canadian Institutes of Health Research (CIHR) Quantitative Imaging Network, by the German Research Foundation within Research Training Group GRK2274, by a clinical research scholarship from the Fonds de recherche du Québec–Santé, and by CIHR operating grant MOP-142233. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is STP dosimetry feasible in current clinical studies for all radiopharmaceutical therapies?
PERTINENT FINDINGS: The accuracy of STP dose estimation was compromised for some radiopharmaceuticals—for example, 177Lu-PSMA compounds in bone marrow and bone lesions.
IMPLICATIONS FOR PATIENT CARE: We suggest caution and provide guidance for the use of simplified dosimetry protocols in clinical radiopharmaceutical therapy.
REFERENCES
- 1. Stabin MG, Madsen MT, Zaidi H. Personalized dosimetry is a must for appropriate molecular radiotherapy. Med Phys. 2019;46:4713–4716. [DOI] [PubMed] [Google Scholar]
- 2. Ljungberg M, Gleisner KS. Personalized dosimetry for radionuclide therapy using molecular imaging tools. Biomedicines. 2016;4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hänscheid H, Lapa C, Buck AK, Lassmann M, Werner RA. Dose mapping after endoradiotherapy with 177Lu-DOTATATE/DOTATOC by a single measurement after 4 days. J Nucl Med. 2018;59:75–81. [DOI] [PubMed] [Google Scholar]
- 4. Madsen MT, Menda Y, O’Dorisio TM, O’Dorisio MS. Technical note: single time point dose estimate for exponential clearance. Med Phys. 2018;45:2318–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willowson KP, Eslick E, Ryu H, Poon A, Bernard EJ, Bailey DL. Feasibility and accuracy of single time point imaging for renal dosimetry following 177Lu-DOTATATE (‘Lutate’) therapy. EJNMMI Phys. 2018;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao W, Esquinas PL, Frezza A, Hou X, Beauregard JM, Celler A. Accuracy of kidney dosimetry performed using simplified time activity curve modelling methods: a 177Lu-DOTATATE patient study. Phys Med Biol. 2019;64:175006. [DOI] [PubMed] [Google Scholar]
- 7. Jackson PA, Hofman MS, Hicks RJ, Scalzo M, Violet J. Radiation dosimetry in 177Lu-PSMA-617 therapy using a single posttreatment SPECT/CT scan: a novel methodology to generate time- and tissue-specific dose factors. J Nucl Med. 2020;61:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hänscheid H, Lassmann M. Will SPECT/CT cameras soon be able to display absorbed doses? Dosimetry from single activity concentration measurements. J Nucl Med. 2020;61:1028–1029. [DOI] [PubMed] [Google Scholar]
- 9. Amato E, Campennì A, Ruggeri RM, Auditore L, Baldari S. Comment on: “technical note: single time point dose estimate for exponential clearance. Med Phys [Med. Phys. 45(5), 2318-2324 (2018)]. 2019;46:2776–2779. [DOI] [PubMed] [Google Scholar]
- 10. Del Prete M, Buteau FA, Beauregard JM. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: a simulation study. Eur J Nucl Med Mol Imaging. 2017;44:1490–1500. [DOI] [PubMed] [Google Scholar]
- 11. Del Prete M, Arsenault F, Saighi N, et al. Accuracy and reproducibility of simplified QSPECT dosimetry for personalized 177Lu-octreotate PRRT. EJNMMI Phys. 2018;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou X, Zhao W, Beauregard JM, Celler A. Personalized kidney dosimetry in 177Lu-octreotate treatment of neuroendocrine tumours: a comparison of kidney dosimetry estimates based on a whole organ and small volume segmentations. Phys Med Biol. 2019;64:175004. [DOI] [PubMed] [Google Scholar]
- 13. Heikkonen J, Mäenpää H, Hippeläinen E, Reijonen V, Tenhunen M. Effect of calculation method on kidney dosimetry in 177Lu-octreotate treatment. Acta Oncol. 2016;55:1069–1076. [DOI] [PubMed] [Google Scholar]
- 14. Desy A, Bouvet GF, Frezza A, Després P, Beauregard JM. Impact of dead time on quantitative 177Lu-SPECT (QSPECT) and kidney dosimetry during PRRT. EJNMMI Phys. 2020;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Menda Y, Madsen MT, O’Dorisio TM, et al. 90Y-DOTATOC dosimetry-based personalized peptide receptor radionuclide therapy. J Nucl Med. 2018;59:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurth J, Krause BJ, Schwarzenböck SM, Stegger L, Schäfers M, Rahbar K. External radiation exposure, excretion, and effective half-life in 177Lu-PSMA-targeted therapies. EJNMMI Res. 2018;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarnelli A, Belli ML, Di Iorio V, et al. Dosimetry of 177Lu-PSMA-617 after mannitol infusion and glutamate tablet administration: preliminary results of EUDRACT/RSO 2016-002732-32 IRST protocol. Molecules. 2019;24:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. [DOI] [PubMed] [Google Scholar]
- 19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]