Visual Abstract
Keywords: β-adrenergic receptor, chronotropic incompetence, PET, sympathetic nervous system
Abstract
The mechanism of chronotropic incompetence (CTI), which has been associated with autonomic dysfunction, has not been elucidated in patients without heart failure (HF). Methods: Cardiac PET using 11C-CGP12177 was performed to investigate the cardiac β-adrenergic receptor density (β-ARD) in 13 patients with CTI without HF and 6 healthy controls. The maximum number of available specific 11C-CGP12177 binding sites per gram of tissue was calculated in regions of interest using an established graphical method. Results: Peak heart rate was significantly lower in CTI patients than in controls (116.9 ± 11.0 vs. 154.8 ± 14.4 beats/min, P < 0.001). β-ARD of the total myocardium was significantly lower in CTI patients than in controls (4.3 ± 1.7 vs. 7.0 ± 1.7 pmol/mL, P = 0.005). Conclusion: β-adrenergic receptor downregulation was demonstrated in patients with CTI without HF. Decreased β-ARD is a common feature in patients with CTI, with or without HF.
Chronotropic incompetence (CTI), defined as the inability of the heart to increase its rate commensurate with increased activity or demand, results in exercise intolerance that impairs quality of life (1). It is an independent predictor of major adverse cardiovascular events and overall mortality (1). CTI is common in patients with cardiovascular disease, such as sinus node dysfunction (SND) (1) and heart failure (HF) (2). Cardiac autonomic dysfunction has been associated with CTI (3), particularly in patients with HF (2). In contrast, the mechanism underlying CTI has not been elucidated in patients with CTI without HF. To gain insight into the mechanism, we here investigated the cardiac β-adrenergic receptor density (β-ARD) in patients with CTI without HF using cardiac PET, using 11C-(−)4-((S)-3-tert-butylamino-2-hydroxypropoxy)-1,3-dihydrobenzoimidazol-2-one (11C-CGP12177), which is the most appropriate ligand for assessing β-ARD. Our hypothesis was that patients with CTI without HF will have β-ARD levels different from those in healthy volunteers without a history of heart disease.
MATERIALS AND METHODS
Study Subjects
The study was approved by Nagoya City Rehabilitation Center Institutional Review Board (reference number 201815), and all subjects provided written informed consent. The research was performed in accordance with the Declaration of Helsinki.
The case-control study consisted of 13 patients with CTI and 6 healthy volunteers who had no history of heart disease as controls (mean age, 65.7 ± 12.8 y; 63.2% men). Five of 13 patients with CTI had been implanted with a dual-chamber pacemaker due to SND, and none of them required ventricular pacing. Each participant underwent echocardiography immediately before the PET examination. The left ventricular end-diastolic and end-systolic volume indices and left ventricular ejection fraction were measured using the modified Simpson formula. Blood samples for B-type natriuretic peptide and norepinephrine measurements were obtained immediately before the first 11C-CGP infusion.
Patient exclusion criteria were as follows: patients on β-blocker therapy; patients with any history of heart disease, including HF, a left ventricular ejection fraction of less than 50%, or atrioventricular block; or a physical inability to undergo cardiopulmonary exercise testing (CPX).
CTI
All participants underwent symptom-limited CPX by bicycle ergometry, with simultaneous analysis of expired ventilatory gas. Peak oxygen consumption was defined as the highest oxygen consumption value during CPX. Peak heart rate (HR) was defined as HR at peak oxygen consumption. HR recovery, defined as the decrease in HR at 1 min after the cessation of CPX, was also obtained. The age-predicted maximal HR was defined using the Astrand (220 − age) formula (4). HR reserve was determined from the change in HR from rest to peak exercise, divided by the difference between the resting HR and the age-predicted maximal HR (5). In this study, CTI was defined as a measured HR reserve lower than 80% of the reserve calculated with the age-predicted maximal HR.
11C-CGP12177 PET
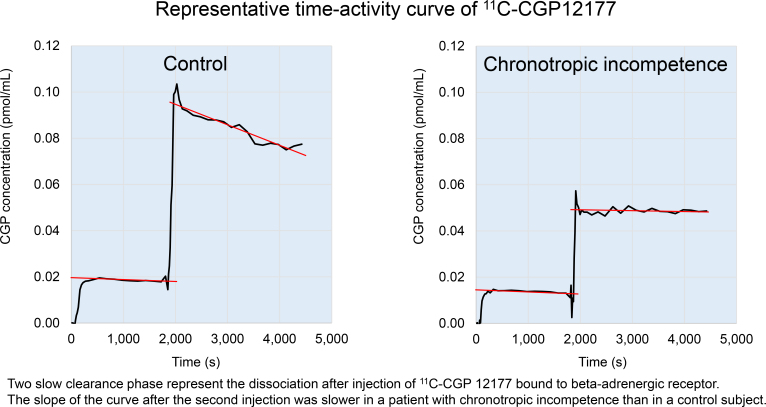
All participants underwent PET examination. After a transmission scan, we obtained dynamic 11C-CGP12177 images according to the modified double-injection protocol. Briefly, during a 75-min dynamic emission scan, the first dose of 11C-CGP12177, with a high specific activity (124.0 ± 2.5 MBq, 2.1 ± 2.6 nmol), was infused intravenously over 2 min. After 30 min, the second dose of 11C-CGP12177, with a low specific activity (234.1 ± 31.2 MBq, 78.4 ± 14.7 nmol), was infused over 2 min. A 54-frame dynamic emission scan was used to measure sequential distributions of the tracer in vivo. During the 30 min after the start of the first infusion, 24 time frames were acquired (8 × 15, 4 × 30, 2 × 60, 2 × 120, and 8 × 150 s). After the second infusion, the scan was completed with 30 additional frames (8 × 15, 4 × 30, 2 × 60, 2 × 120, and 14 × 150 s). All emission sinograms were reconstructed using filtered backprojection with time-of-flight measurements. All data were corrected for dead time, decay, and measured photon attenuation. Cardiac uptake of 11C-CGP12177 was estimated using dedicated software. The total left ventricular myocardium was divided into 17 segments, which were assigned to specific coronary artery territories of the left anterior descending coronary artery, left circumflex coronary artery, and right coronary artery, according to the American Heart Association guidelines. The curve sections that corresponded to the 2 slow clearance phases, which represented the dissociation of 11C-CGP12177 bound to β-adrenergic receptors, were extrapolated back to the start of the infusions. β-ARD was then determined as the maximum number of available specific 11C-CGP12177 binding sites per gram of tissue in the regions of interest, based on the modified equation described by Delforge et al. (6).
Statistical Analysis
Continuous variables are presented as the mean ± SD for normally distributed variables and as the median and interquartile range for nonnormally distributed variables. Categoric variables are summarized as frequencies and percentages. For comparison of the 2 groups, unpaired Student t tests were used for normally distributed continuous variables and Mann–Whitney U tests were used for nonnormally distributed continuous variables. Differences in prevalence between the 2 groups were compared using the χ2 test. Relationships between the 2 parameters were evaluated by univariate regression analysis. Differences with a P value of less than 0.05 were considered statistically significant.
RESULTS
Clinical characteristics did not differ between groups. Peak HR (116.9 ± 11.0 vs. 154.8 ± 14.4 beats/min, P < 0.001), the double product (21,800 ± 4,200 vs. 31,400 ± 4,600 beats/min·mm Hg, P < 0.001), and peak oxygen consumption (15.9 ± 3.7 vs. 23.5 ± 5.5 mL/kg/min, P = 0.003) were significantly lower in patients with CTI than in controls. Peak O2 pulse and HR recovery did not differ between groups (Table 1, Supplemental Table 1, and Supplemental Table 2; supplemental materials are available at http://jnm.snmjournals.org).
TABLE 1.
Comparisons of Clinical Characteristics Between Patients with CTI and Controls
| Characteristic | CTI (n = 13) | Controls (n = 6) | P |
|---|---|---|---|
| Sex | 0.22 | ||
| Male | 7 | 5 | |
| Female | 6 | 1 | |
| Age (y) | 70.4 ± 8.9 | 55.5 ± 14.8 | 0.06 |
| Height (cm) | 160.3 ± 7.1 | 164.6 ± 10.0 | 0.30 |
| Weight (kg) | 58.5 ± 7.0 | 65.5 ± 11.7 | 0.12 |
| Rest HR (beats/min) | 66.7 ± 13.8 | 67.8 ± 12.2 | 0.86 |
| Peak HR (beats/min) | 116.9 ± 11.0 | 154.8 ± 14.4 | <0.001 |
| Peak systolic blood pressure (mm Hg) | 183.5 ± 23.3 | 203.2 ± 17.6 | 0.09 |
| Double product (beats/min⋅mm Hg) | 21,800 ± 4,200 | 31,400 ± 4,600 | <0.001 |
| Peak oxygen consumption (mL/kg/min) | 15.9 ± 3.7 | 23.5 ± 5.5 | 0.003 |
| Peak O2 pulse (mL/beats) | 7.8 ± 2.4 | 10.0 ± 2.5 | 0.08 |
| Peak work (watts) | 85.4 ± 30.1 | 131.8 ± 37.2 | 0.01 |
| HR recovery (beats/min) | 19.9 ± 14.6 | 18.3 ± 7.6 | 0.81 |
Data are expressed as mean ± SD.
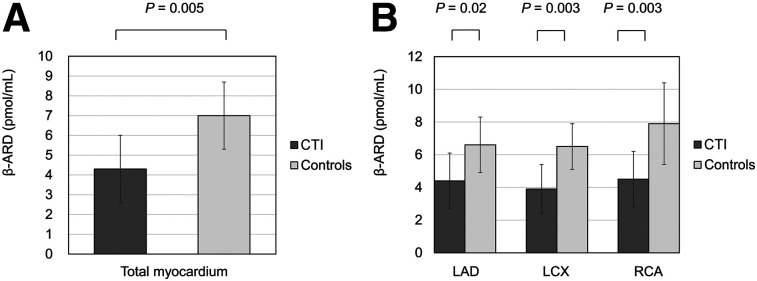
β-ARD significantly correlated with peak HR (r = 0.54, P = 0.02). However, other parameters did not correlate with β-ARD (Supplemental Table 3). Figure 1 shows the β-ARD in the total myocardium and in each region. β-ARD in the total myocardium was significantly lower in patients with CTI than in controls (4.3 ± 1.7 vs. 7.0 ± 1.7 pmol/mL, P = 0.005). β-ARD in the regions of the left anterior descending artery (4.4 ± 1.7 vs. 6.6 ± 1.8 pmol/mL, P = 0.02), left circumflex coronary artery (3.9 ± 1.5 vs. 6.5 ± 1.4 pmol/mL, P = 0.003), and right coronary artery (4.5 ± 1.7 vs. 7.9 ± 2.5 pmol/mL, P = 0.003) was also significantly lower in the CTI group than in controls.
FIGURE 1.
(A) Cardiac β-ARD of total myocardium. (B) β-ARD in left anterior descending artery (LAD), left circumflex coronary artery (LCX), and right coronary artery (RCA) regions.
DISCUSSION
This present study demonstrated cardiac β-adrenergic receptor downregulation in patients with CTI without HF; to our knowledge, this finding has not been reported previously. Our results indicate that decreased β-ARD is a common feature in CTI patients both with and without HF.
Postsynaptic Status
CTI in patients with HF has been suggested to be associated with downregulation of cardiac β-adrenergic receptors (2). On the other hand, Kawasaki et al. evaluated CTI using HR variability during CPX in patients without structural heart disease. They deduced that CTI in such patients was caused mainly by a pathophysiologic condition in which sympathetic activation was not well translated into an HR increase (7). The present study demonstrated downregulation of β-adrenergic receptors in patients with CTI without HF and showed a significant correlation between β-ARD and peak HR during CPX. Therefore, our results are consistent with the speculations of these previous studies. Notably, decreased β-ARD is a common feature in patients with CTI, whether with or without HF.
Presynaptic Status
Elevated sympathetic nervous system activity, evaluated with 123I-metaiodobenzylguanidine, has been reported in patients with indications for a pacemaker due to SND; these patients included those with CTI before pacemaker implantation, compared with healthy controls (8). The authors attributed the elevated norepinephrine to increased sympathetic nervous system activity due to abnormal adrenergic nerve function of the left ventricular myocardium in patients with SND. The increased sympathetic activity may also reflect the cardiac load caused by bradycardia in that study. In the current study, there was no significant increase in plasma B-type natriuretic peptide levels even in CTI patients; that is, the absence of bradycardia-induced cardiac load may have resulted in a lack of increase in plasma norepinephrine levels. On the other hand, Fukuoka et al. reported that cardiac sympathetic activity evaluated by 123I-metaiodobenzylguanidine did not differ between patients receiving atrial pacing and healthy individuals without cardiac pacing (9). Our findings were consistent with this report because there were no patients requiring ventricular pacing. Further investigation regarding presynaptic status is needed in patients with CTI without HF.
Mechanism Underlying CTI
HR during CPX is regulated by a reduction of vagal activity, an increase in sympathetic outflow, and the relative sensitivity of the sinoatrial node to catecholamines. In fact, the cardiac sympathetic nerves are particularly densely distributed around the sinoatrial node. The etiology of SND, which includes CTI, often involves age-dependent, progressive, degenerative fibrosis of the sinus nodal tissue and the surrounding atrial myocardium (10). Therefore, it is possible that both the cardiac sympathetic presynapses and the cardiac sympathetic postsynapses are impaired. The abovementioned study showed that patients with SND had disturbances of global and regional cardiac 123I-metaiodobenzylguanidine uptake before and after pacemaker implantation, indicating abnormal adrenergic nerve function (8). Contrary to sympathetic parameters, there was no difference in HR recovery as a parasympathetic parameter between the groups. Therefore, sympathetic dysfunction may be key to understanding the mechanisms underlying CTI in patients with CTI without HF. Moreover, the mechanism underlying CTI in such patients may involve both the presynaptic and postsynaptic states in the sympathetic nervous system.
Clinical Implications
To our knowledge, no effective treatment for CTI to improve prognosis has been established to date, and no study has been able to demonstrate improved prognosis by rate-adaptive pacing. This lack of evidence suggests that mechanically increasing HR would not be able to reverse β-adrenergic receptor downregulation. β-blockers are effective in chronic HF, for which downregulation of β-adrenergic receptor is also observed. However, if the β-adrenergic receptors themselves are already involved in degenerative fibrosis, the use of β-blockers is unlikely to be effective. The possibility of β-blocker use requires further study in patients with CTI without HF.
Despite the limited number of patients and the varying definitions of CTI, we believe that the results of this study, including the definition of CTI, are appropriate and allow for generalization.
CONCLUSION
Cardiac β-adrenergic receptor downregulation was observed in patients with CTI. Decreased β-ARD is a common feature in patients with CTI, irrespective of whether they have HF.
DISCLOSURE
This work was supported by a grant-in-aid for scientific research (15K09966) from the Japan Society for Promotion of Science. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Might β-adrenergic receptor downregulation be a mechanism underlying CTI without HF?
PERTINENT FINDINGS: In this case-control study, β-ARD of the total myocardium was statistically significantly lower in CTI patients without HF than in controls (4.3 ± 1.7 vs. 7.0 ± 1.7 pmol/mL).
IMPLICATIONS FOR PATIENT CARE: Clarifying the mechanisms underlying CTI will contribute to the development of therapeutic strategies for CTI.
REFERENCES
- 1. Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure: role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–323. [DOI] [PubMed] [Google Scholar]
- 3. Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. [DOI] [PubMed] [Google Scholar]
- 4. Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1–92. [PubMed] [Google Scholar]
- 5. Okin PM, Lauer MS, Kligfield P. Chronotropic response to exercise: improved performance of ST-segment depression criteria after adjustment for heart rate reserve. Circulation. 1996;94:3226–3231. [DOI] [PubMed] [Google Scholar]
- 6. Delforge J, Mesangeau D, Dolle F, et al. In vivo quantification and parametric images of the cardiac beta-adrenergic receptor density. J Nucl Med. 2002;43:215–226. [PubMed] [Google Scholar]
- 7. Kawasaki T, Kaimoto S, Sakatani T, et al. Chronotropic incompetence and autonomic dysfunction in patients without structural heart disease. Europace. 2010;12:561–566. [DOI] [PubMed] [Google Scholar]
- 8. Marketou ME, Simantirakis EN, Prassopoulos VK, et al. Assessment of myocardial adrenergic innervation in patients with sick sinus syndrome: effect of asynchronous ventricular activation from ventricular apical stimulation. Heart. 2002;88:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukuoka S, Nakagawa S, Fukunaga T, Yamada H. Effect of long-term atrial-demand ventricular pacing on cardiac sympathetic activity. Nucl Med Commun. 2000;21:291–297. [DOI] [PubMed] [Google Scholar]
- 10. Evans R, Shaw DB. Pathological studies in sinoatrial disorder (sick sinus syndrome). Br Heart J. 1977;39:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]