Abstract
The principle of pretargeted radioimmunoimaging and therapy has been investigated over the past 30 y in preclinical and clinical settings with the aim of reducing the radiation burden of healthy tissue for antibody-based nuclear medicine techniques. In the past few decades, 4 pretargeting methodologies have been proposed, and 2 of them—the bispecific antibody–hapten and the streptavidin–biotin platforms—have been evaluated in humans in phase 1 and 2 studies. With this review article, we aim to survey clinical pretargeting studies in order to understand the challenges that these platforms have faced in human studies and to provide an overview of how the clinical approval of the pretargeting system has proceeded in the past several decades. Additionally, we will discuss the successes of the pretargeting human studies and compare and highlight the pretargeting approaches and conditions that will advance clinical translation of the pretargeting platform in the future.
Keywords: molecular imaging, PET, radioimmunoimaging, radionuclide therapy, radiopharmaceuticals, PET imaging, pretargeting, radioimmunotherapy
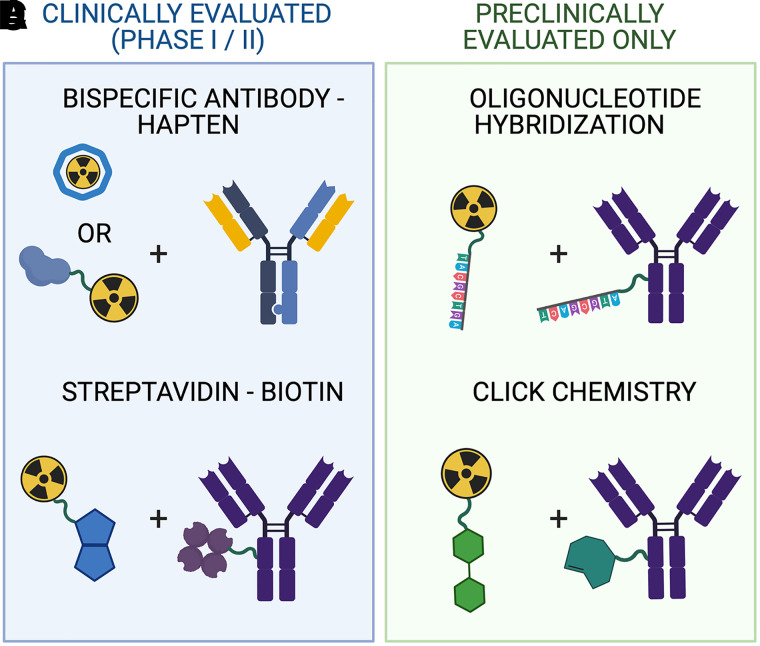
Pretargeted nuclear imaging and therapy is an alternative approach for conventional antibody-based nuclear medicine techniques (1). Pretargeting combines the specificity of a tumor-targeting antibody with the pharmacokinetic profile of a radiolabeled small molecule (radioligand) to reduce the overall radiation dose associated with directly radiolabeled antibody nuclear medicine agents. In 1985, Reardan et al. first introduced the concept of pretargeting in which a preadministered tumor-targeting antibody interacts in vivo with high specificity with a small molecule radioligand, commonly referred to as bioorthogonal reactivity (2). They reported a preclinical study of successful pretargeting using a bispecific antibody (bsAb) targeting a tumor antigen and an ethylenediaminetetraacetic acid chelate complex radioligand. Two years later, in 1987, Hnatowich et al. reported the use of another pretargeting approach in a preclinical model in which the pretargeting interaction between the tumor-bound antibody and the radioligand occurred via high-affinity avidin–biotin association (3). In total, 4 pretargeting mechanisms have been proposed and evaluated in vivo in preclinical studies (Fig. 1), and these have been reviewed by other groups previously (1,4).
FIGURE 1.
Pretargeting agents of the 4 main platforms that have been evaluated in clinical (A and C) or only in preclinical settings (B and D).
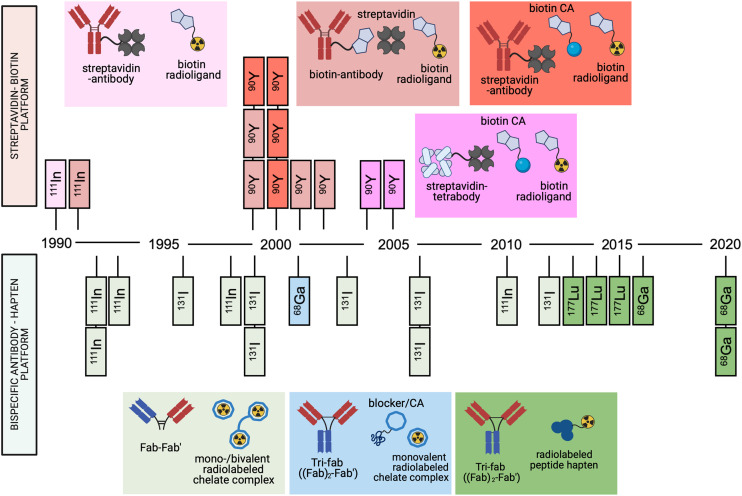
To date, the streptavidin–biotin and the bsAb–hapten pretargeting platforms are the only systems that have been evaluated in humans. Since the first reported human studies of the streptavidin–biotin and bsAb–hapten approaches in 1990 and 1993, respectively (5,6), more than 30 reports describing original pretargeting studies in humans have been published (Fig. 2). Both approaches have proven capable of lowering the radiation burden to healthy tissue compared with conventional antibody-based nuclear medicine. In this review, we discuss the challenges and successes of both of the approaches in the clinic and what possible directions upcoming pretargeting strategies need to take to result in a first clinical application of the approach.
FIGURE 2.
Timeline of clinical studies of bsAb and streptavidin–biotin pretargeting approaches. Each rectangle refers to a published report, with radionuclide note referring to primary radionuclide used in study. Pretargeting agents used in each report are color coded.
STREPTAVIDIN–BIOTIN PLATFROM IN THE CLINIC
The strong noncovalent interaction between streptavidin and biotin (K ∼ 1014 M−1) has made the molecule pair desirable for many applications in biomedicine, and the pair has been applied to pretargeting as well. The streptavidin–biotin approach has been evaluated in humans for pretargeted scintigraphy (5,7) and radioimmunotherapy (Table 1) (8–13). Most of the pretargeted streptavidin–biotin human studies have been performed using full-length tumor-targeting antibodies conjugated with either biotin or streptavidin along with a clearing agent (CA) that is given before the biotin radioligand administration.
TABLE 1.
Excerpts of Human Studies of Pretargeted Nuclear Medicine Using Streptavidin–Biotin System
| Antibody radioligand pair and pretargeting timeline | Radioligand dose | Target antigen and study population | Main findings | Reference |
|---|---|---|---|---|
|
Hour 0: Biotinylated antitenascin mAb BC4 Hour 36: (strept)avidin CA Hour 54–60: 90Y-DOTA-biotin |
2.22–2.96 GBq/m2 | Tenascin and high-grade glioma patients | Reduction in tumor in 25% patients, all patients developed immune response. | (8) |
|
Hour 0: SA-conjugated C2B8 mAb Hour 34: biotin CA Hour 52: 111In/90Y-DOTA-biotin |
3–5 mCi (111In), 30–50 mCi/m2 (90Y) | CD20 and non-Hodgkin lymphoma patients | Good tumor–to–whole-body ratios (38:1), mild hematologic toxicity, 28% of patients with CR. | (12) |
|
Hour 0: SA-conjugated NR-LU-10 mAb Hour 24–72: Biotin-galactose-HSA CA Hour 28–96: 111In/90Y-DOTA-biotin |
185 MBq (111In), 370 MBq/m2 (90Y) | Ep-CAM and adenocarcinoma patients (majority colorectal and lung) | Good tumor-to-marrow absorbed dose ratio (63:1), all tested patients developed immune response. | (16) |
|
Hour 0: Biotinylated antitenascin mAb BC4 Hour 24: avidin CA Hour 42: 90Y-DOTA-biotin (procedure repeated again 8–10 wk apart) |
0.555–1.110 GBq | Tenascin and recurrent high-grade glioma, anaplastic astrocytoma patients | 25% overall response to PRIT, no hematologic toxicity observed. | (14) |
|
Hour 0: Biotinylated antitenascin mAb BC4 Hour 24–36: avidin CA Hour 40–54: 90Y-DOTA-biotin |
2.2 GBq/m2 | Tenascin and high-grade glioma patients | Significantly higher OS in treated cohort than in control. | (15) |
|
Hour 0: SA-conjugated CC49-(scFv)4 Hour 48/72: biotin CA Hour 72/96: 111In/90Y-DOTA-biotin |
185 MBq (111In), 370 MBq/m2 (90Y) | TAG-72 and metastatic colorectal cancer | Tumor–to–normal-tissue dose ratio 54.5, immune response or toxicity not reported. | (11) |
CR = complete response; SA = streptavidin; OS = overall survival.
At the turn of the millennium, multiple human studies were performed evaluating pretargeted 90Y radioimmunotherapy in patients with non-Hodgkin lymphoma, glioma, and gastrointestinal carcinoma. In 1999, Paganelli et al. produced the first set of promising results showing reduction of tumor burden in 25% of their high-grade glioma patients after 1 cycle of pretargeted 90Y-biotin radioligand (8). Later, Paganelli et al. also reported an overall 25% response rate in recurrent grade II glioma and anaplastic astrocytoma patient cohorts (14). Around the same time, Grana et al. published a pretargeted 90Y radioimmunotherapy study in high-grade glioma patients resulting in a median survival of 33.5 mo compared with the 8 mo of the control cohort (15). These promising results established the feasibility of the approach in human therapy and led to greater interest in the pretargeting concept, accelerating research in the field.
Almost all the clinical trials that have been performed using a 3-step method have included the use of a streptavidin or avidin or a biotin-galactose–based CA (10,12,16). The use of biotin-galactose derivatives as CAs before radioligand injection has been shown to dramatically decrease the presence of accessible antibody conjugate in the blood pool and to efficiently decrease the required lag time between the antibody and radioligand administrations (10,12). Interestingly, despite the use of the 3-step approach and the evidence of its effect in reducing the antibody concentration in the blood pool, hematologic toxicity has appeared as a reoccurring difficulty in the streptavidin and biotin–based 90Y pretargeted radioimmunotherapy studies (8,9,15). To address the issue, Breitz et al. reported the effects of different pretargeting parameters by adjusting the interval time and the dosing of the streptavidin–antibody conjugate, CA, and 90Y-biotin (16). This optimization produced a pretargeting protocol that resulted in no hematologic toxicities in their patient population with various adenocarcinomas. However, the study did not report whether the optimized protocol resulted in a tumor uptake sufficient enough to show a clinical response.
In addition to the challenges with hematologic toxicity, the streptavidin–biotin platform has been marked by high incidence of immune response to the streptavidin- and avidin-based pretargeting agents. Development of antistreptavidin or antiavidin antibodies in patients has been observed in all of the clinical trials that reported an investigation of their immunogenicity studies (5,7–10,12–14,16). Despite several clinical evaluations of the platform’s safety and efficacy, the immunogenicity of the pretargeting agents has not been addressed. Preclinical investigation of the platform is still ongoing, but its clinical evaluation has ceased with the last reported clinical trial in 2005.
bsAb–HAPTEN PLATFORM IN THE CLINIC
In addition to the streptavidin–biotin methodology, the bsAb–hapten platform has been widely studied in the clinical setting (Table 2). Two different strategies for bsAb–hapten pretargeting approaches have been studied clinically. Starting in 1993, with the first clinical trial of bsAb–hapten pretargeting approach, most clinical trials have used fragmented bispecific antibodies (Fab-Fab' along with a radiolabeled mono- or bivalent chelate complex serving as radioligand). This contrasts with the use of a CA as with the streptavidin–biotin system. The use of fragmented antibodies leads to a more rapid blood-pool clearance of the antibody construct because of the smaller size. Additionally, upon elimination of the Fc region, the antibody fragment negates the interaction with the neonatal Fc receptor, further reducing the circulating half-life of the constructs. The overall effect of this approach is reduced intervals between administration of the targeting construct and radioligand, reduced potential for hematologic toxicity, and presumably an improvement in tumor-to-tissue uptake ratios.
TABLE 2.
Excerpts of Human Studies of Pretargeted Nuclear Medicine Using bsAb–Hapten System
| Antibody radioligand pair and pretargeting timeline | Radioligand dose | Target antigen and study population | Main findings | Reference |
|---|---|---|---|---|
|
Hour 0: anti-CEA × anti-DTPA indium bsAb Hour 96–120: 111In-bivalent DTPA hapten |
100–200 MBq | CEA and medullary thyroid carcinoma | Immunogenic response in 61% of the patients. 80% true-positive tumor visualization. | (23) |
|
Hour 0: hMN-14 × m734 bsAb Hour 120/168: 131I-bivalent hapten |
2.6–5.5 GBq | CEA and varied patient population with CEA-positive tumors | Tumor dose of 131I-bsAb and pretargeted 131I-hapten 2.0 Gy/GBq and 3.9 Gy/GBq, respectively. Tumor–to–whole-body ratio higher with 131I-hapten. | (28) |
|
Hour 0: TF2 bsAb Hour 24–30: 68Ga-IMP288 (premedicated with antihistamine and corticosteroid) |
150 MBq | CEA and HER2-negative metastatic breast cancer | Immuno-PET showed higher total lesion sensitivity (94.7%) than 18F-FDG PET (89.6%). Immunogenic response in 16% of the patients. | ((21); NCT01730612) |
|
Hour 0: TF2 bsAb Hour 30: 68Ga-IMP288 (premedicated with antihistamine and corticosteroid) |
150 MBq | CEA and metastatic colorectal cancer | Immuno-PET showed higher sensitivity (88%) and specificity (100%) than 18F-FDG PET (76% and 67%, respectively), no immunogenic response. | ((20); NCT02587247) |
|
Hour 0: TF2 bsAb Hour 24/120: 111In/177Lu-IMP288 |
185 MBq (111In) 2.5–7.4 GBq (177Lu) |
CEA and advanced colorectal malignancy | 10% patients experienced grade III–IV hematologic toxicity, no therapeutic effect detected. | ((17,18); NCT00860860) |
HER2 = human epidermal growth factor receptor 2.
In 2013, Schoffelen et al. reported the use of a bispecific trivalent antibody construct (Tri-Fab) in tandem with a histamine-succinyl-glycine (HSG) peptide–based hapten radioligand. Since then, all the reported bsAb–hapten clinical trials have used the Tri-Fab-HSG–hapten strategy. When the 2 approaches are compared, the Tri-Fab-HSG–hapten strategy has shown more promising results, providing excellent specificity and sensitivity for pretargeted PET imaging for patients with varying cancer profiles. Additionally, one of the major advantages of using peptide-based haptens, compared with the chelate complex haptens, is the ability to design a library of peptide haptens accompanied with different radionuclides with lowered risk of changing the haptens’ binding to the antibody.
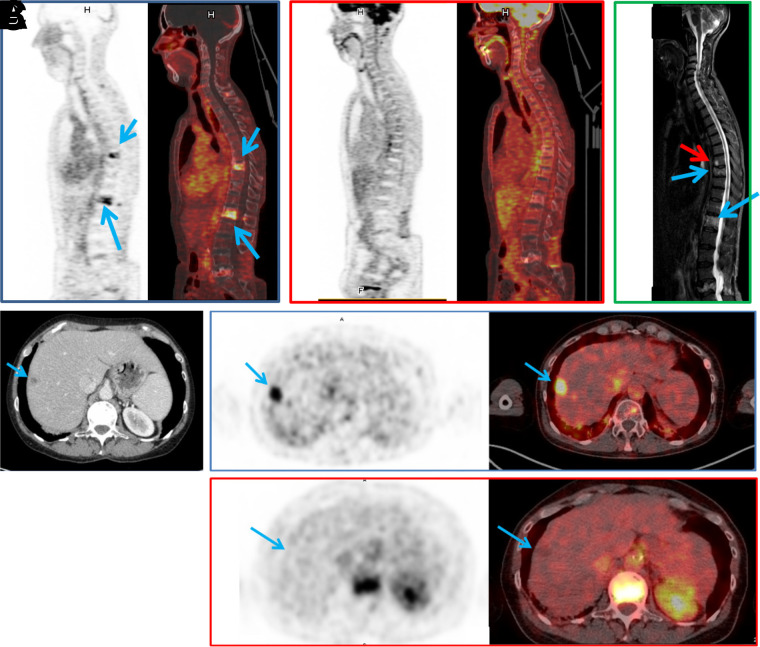
To date, the evaluation of the Tri-Fab-HSG–hapten approach in humans has included the use of only 1 antibody construct, a carcinoembryonic antigen (CEA) targeting humanized Tri-Fab bsAb called TF2 (NCT00860860) (17,18). This trivalent bsAb TF2 construct has been tested along with an HSG hapten called IMP288 for pretargeted PET imaging (68Ga-IMP288) ((19–21); NCT01730638, NCT01730 612) and radioimmunotherapy (111In/177Lu-IMP288) ((17,18,22); NCT00860860, NCT01221675) in patients with colorectal cancer, medullary thyroid carcinoma, epidermal growth factor receptor 2–negative breast cancer and metastatic lung cancer. 90Y- and 111In-radiolabled derivatives of IMP288 are being tested in a clinical setting as well ((20); NCT02587247, NCT02300922). The most recent work with the TF2-68Ga-IMP288 pretargeting pair has resulted in higher sensitivity and specificity in detecting tumor lesions in metastatic colorectal cancer patients compared with 18F-FDG PET ((20); NCT02587247). The CEA-targeted pretargeting pair was also shown to be highly capable of detecting lesions in patients with human epidermal growth factor receptor 2–negative metastatic breast cancer ((21); NCT01730612). In that setting, the pretargeted immuno-PET showed higher overall sensitivity (94.7%) relative to 18F-FDG PET (89.6%) (Fig. 3). The number of true-positive lesions detected in lymph nodes, bone, and liver was higher using immuno-PET than 18F-FDG PET. Those exciting results have clearly exhibited the potential for pretargeted PET imaging in cancer.
FIGURE 3.
(A–C) In patient 1, pretargeted immuno-PET with TF2 and 68Ga-IMP288 peptide images show 2 vertebral metastases (L1 and T9, arrows) (A), 18F-FDG PET discloses no vertebral abnormalities (B), and vertebral MRI confirms both lesions (blue arrows) and discloses another lesion (red arrow) at T8 (C). (D–F) In patient 2, CT shows suspected liver lesion (D), and pretargeted immuno-PET with TF2 and 68Ga-IMP288 peptide reveals high uptake by liver lesion (arrow) (E), which was not seen by 18F-FDG PET (F). (Reprinted from (21).)
As mentioned earlier, immunogenic response to the pretargeting agents has been a limitation of the streptavidin–biotin system. Immunogenicity of the bsAb constructs has been observed in the bsAb–hapten platform as well. Barbet et al. reported that a high percentage of their patients (61%) developed human antimouse antibodies (HAMA) when a fully murine anti-CEA × anti-diethylenetriaminepentaacetic acid (DTPA) bsAb was administrated as part of the pretargeting protocol (23). More recent trials have used mouse–human Fab-Fab' bsAbs, which has decreased the prevalence of HAMA development in patients ((24); NCT00467506). Yet, development of human antihuman antibodies (HAHA) against mouse–human pretargeting bsAb agents has been shown to occur as well ((24–26); NCT00467506). bsAb fragments tend to suffer from aggregation issues, which can induce an immunogenic response (27). However, Barbet et al. observed formation of high-molecular-weight aggregates and noticed a decrease in the HAMA induction after improving the production and purification process of the antibody fragment, indicating this problem can be overcome by appropriate formulation (23). Furthermore, premedication with an antihistamine and corticosteroid has been shown to reduce the prevalence of immunogenic response in patients. Using this approach, Touchefeu et al. reported that no patient (ntotal = 11) developed HAHA in their TF2-68Ga-IMP288 pretargeted PET study ((20); NCT02587247). Rousseau et al. shared similar results, observing immunogenic response in only 16% of patients (ntotal = 23) with the antihistamine and corticosteroid premedication ((21); NCT 01730612).
Many bsAb–hapten clinical trials have investigated the use of different pretargeting schedules and protocols to define the conditions that result in the highest tumor-to-background ratios and lowest radiation-induced toxicity ((17,19,22,25,28,29); NCT00860860, NCT01730638, NCT01221675). This is done by optimizing the amount of injected doses of each of the pretargeting components, the stoichiometric relation between these agents, and the interval time separating the administration of the doses. The interval time applied in the clinical pretargeting studies has varied between 1 and 7 d, and increasing the interval time has resulted in lower toxicity and better image quality to a certain extent. Schoffelen et al. showed that patients who received the 177Lu-IMP288 hapten radioligand 1 d after TF2 antibody injection experienced significantly higher red marrow doses than patients who received the radioligand 5 d after antibody injection ((17); NCT00860860). Bodet-Milin et al. reported that increasing the interval time from 24 to 30 h decreased the mediastinum blood-pool values, whereas a 42-h delay time resulted in lower tumor SUVmax (T-SUVmax) and T-SUVmax–to–mediastinum blood-pool ratios than the 30-h lag time ((19); NCT01730638).
From the point of view of adjusting both the interval time and the dosing, Kraeber-Bodéré et al. observed that a 5-d interval time resulted in a better tumor uptake of the hapten than the 7-d interval time. However, the tumor uptake increased and the tumor localization was visible even with the 7-d interval time when the bsAb dose was increased from 10 to 50 mg/m2 (28). Certainly, one of the challenges for all of the pretargeting platforms is that each combination of target antigen, antibody construct, and radioligand requires its own pretargeting protocol, which will be a challenge when pretargeting is applied to a variety of different cancers for diagnostic and therapeutic purposes.
Surprisingly, direct comparison of pretargeted to the directly labeled approach using the same targeting molecule within the same patient population has not been performed extensively. Kraeber-Bodéré et al. have compared the dosimetry of a CEA-targeting 131I-labeled bsAb with the pretargeted 131I-hapten. The dosimetry of the 131I-bsAb was determined first by scintigraphy over the several-day interval time, followed by an injection of the 131I-hapten and study of its dosimetry (28). With 2 different pretargeting conditions (75 mg/m2; 5-d interval or 100 mg/m2; 7-d interval), the tumor–to–whole-body ratios were significantly higher for the pretargeted hapten compared with the directly labeled antibody. Additionally, the calculated tumor radiation doses were higher with the pretargeted 131I-hapten than with the directly labeled 131I-bsAb (3.9 and 2.0 Gy/GBq, respectively). The study showed that their pretargeting approach was superior to the directly labeled approach, with a tumor–to–whole-body ratio of 55:1 for pretargeting (75 mg/m2; 5-d interval) and 16:1 for the conventional approach using just the 131I-bsAb. Perhaps more importantly, the study was a good example of how to optimize a pretargeting platform to achieve success in patients.
Over the past 30 y, the bsAb–hapten platform has been studied consistently, and upcoming clinical trials using the TF2-IMP288 pretargeting strategy are planned (NCT02300922, NCT01730638). The recent work with the platform holds promise in solidifying the use of the pretargeting approach as an alternative to the use of directly radiolabeled antibodies.
DISCUSSION
Clinical investigations of pretargeted nuclear imaging and therapy have shown the utility of the pretargeting approach in overcoming the high overall radiation doses of conventional radioimmunoimaging and therapy. Both of the discussed pretargeting platforms are successful at lowering the overall radiation dose, but they both have hurdles to overcome if their full potential is to be realized. In the case of the streptavidin–biotin approach, the main challenge has been the immunogenicity of the streptavidin and avidin pretargeting constructs. During its 15-y period of separate clinical studies, the high prevalence of immunogenic response to the pretargeting agents was not addressed. Also, the addition of a third molecule (e.g., CA) to the pretargeting protocol makes it a more complicated approach, because of the need to optimize the CA dosing. These concerns compared with pretargeting platforms such as the bsAb–hapten system, which have been shown to work efficiently without a CA, put the streptavidin–biotin approach at a major disadvantage.
One major drawback of the bsAb–hapten approach is that it lacks modularity. The development of the HSG-haptens has been an improvement in this regard, but each bsAb agent targeting a different antigen of interest needs to be designed and engineered even if a clinical antibody already exists. The development of novel working bsAb agents is a time-consuming and costly process. Additionally, the bsAb constructs have faced a lot of challenges in their clinical translation, and currently only 2 bispecific antibodies are approved for clinical use (27). Because of the increasing need for antibody-based imaging and therapeutic nuclear agents, ideally the pretargeting agents need to be developed and manufactured efficiently and affordably in order to access a wide variety of different tumor antigens.
In addition to the 2 platforms discussed in this review, other promising pretargeting methodologies are moving toward clinical evaluation as well. In the last decade the inverse electron-demand Diels–Alder (IEDDA) click chemistry pretargeting approach has been shown to work well in preclinical models, delivering the radioligand to the target site with great specificity. As a result, the platform’s first clinical trials, which will notably not use a CA, are reported to start in 2021 (30). Compared with the bsAb–hapten approach, the click chemistry pretargeting components—a transcyclooctene-conjugated antibody and a tetrazene-based radioligand—are highly modular, but the stability of the IEDDA pretargeting agents may prove a challenge for clinical translation (31,32). With the first clinical trials poised to begin within the year, the magnitude of this challenge will be revealed soon.
One of the main criticisms of all pretargeting approaches is the requisite use of noninternalizing or slowly internalizing antibodies, which limits the number of antibodies that can be used. Although the use of slowly internalizing antibodies such as CA19.9-targeting 5B1 and rapidly internalizing epidermal growth factor receptor–targeting cetuximab have been possible in a preclinical setting (33–35), it has yet to be reported in clinical studies. However, clinical translation will soon be attempted with the IEDDA-based approach and the slowly internalizing 5B1 antibody, which will help to determine more concretely what is possible in patients. It should also be noted that the process of antigen–antibody internalization is not always absolute. In preclinical studies, internalizing TF12 bsAb was shown to remain accessible for hapten binding because of the only partial internalization of the antibody construct (36). If those types of antibodies are successful in a clinical setting, it would increase the number of antibodies and molecular targets that can become part of the pretargeting tool kit, expanding the effectiveness of the approach.
In addition to imaging, pretargeting has immense potential to enhance radioimmunotherapy. Conventional radioimmunotherapy has shown good results in clinical response in patients with nonsolid tumors. However, solid tumors possess higher radio resistance and, relative to nonsolid tumors, 5- to 10-fold radiation doses are required to achieve a response (37). Because pretargeting produces faster delivery of the radiation source to the target site, larger doses could theoretically be administered with pretargeted radioimmunotherapy without inducing hematologic toxicities. It is exciting that a large portion of the clinical studies of pretargeting platforms have been for pretargeted radioimmunotherapy. However, phase 2 clinical trials have only shown modest efficacy for both platforms. In 2 different studies of bsAb–hapten pretargeting with a 131I-radiolabeled bivalent hapten radioligand in patients with CEA-positive cancer, Kraeber-Bodéré et al. reported no occurrence of complete or partial response (25,38). In another bsAb–hapten radioimmunotherapy study in patients with metastatic medullary thyroid carcinoma, a disease control rate of 76.2% (n = 32) was observed (24). Most of the patients enrolled in these pretargeted radioimmunotherapy studies were late-stage cancer patients with high tumor burden and had already unsuccessfully undergone other forms of therapy. Also, in these studies only a single dose of therapeutic radioligand was administrated as a standalone therapy. As clinical use of this approach expands, it may be useful to explore how pretargeted radioimmunotherapy would perform when joined with other therapies or when administered as multiple doses.
On average, more than 10 new cancer therapeutic antibodies enter late-stage clinical trials every year (39). As the role of antibodies in cancer therapeutics has increased, the potential for using antibody-based imaging agents in profiling patients’ tumor antigen landscape to predict therapeutic response is consequential and significant. For the past 30 y, pretargeting has been proposed as an alternative approach to conventional antibody-based nuclear imaging and therapy. The approval rate of directly radiolabeled antibodies for clinical use has been low, with only 2 Food and Drug Administration–approved radioimmunoconjugates, 131I-tositumomab and 90Y-ibritumomab, being approved in the early 2000s for non-Hodgkin lymphoma (37). According to a survey performed by Schaefer et al. in the United States, one of the bigger concerns for oncologists and hematologists in the use of 131I-tositumomab is the possible bone marrow damage that could preclude patients from further therapy (40). As our understanding of how to effectively implement pretargeted radioimmunotherapy expands, the preclinical data strongly suggest that these types of toxicities can be avoided, alleviating some of the concerns of physicians who want to use these strategies in the clinic.
Pretargeting is an approach that has shown significant promise in solving the challenge of relatively high radiation burden of the nontumorous tissue that is associated in the use of radioimmunoconjugates such as 131I-tositumomab and 90Y-ibritumomab. Yet, the clinical data on the use of pretargeting have not been straightforward. The challenges with toxicity, immunogenicity, and modularity have not been fully addressed, but progress is gaining momentum and the outlook for pretargeted imaging and therapy remains promising.
DISCLOSURE
This work was financially supported by Finnish Academy of Science and Letters (Vilho, Yrjö and Kalle Väisälä fund) and NIH (1R21EB027982-01A1). No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Bailly C, Bodet-Milin C, Rousseau C, Faivre-Chauvet A, Kraeber-Bodéré F, Barbet J. Pretargeting for imaging and therapy in oncological nuclear medicine. EJNMMI Radiopharm Chem. 2017;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reardan DT, Meares CF, Goodwin DA, et al. Antibodies against metal chelates. Nature. 1985;316:265–268. [DOI] [PubMed] [Google Scholar]
- 3. Hnatowich DJ, Virzi F, Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987;28:1294–1302. [PubMed] [Google Scholar]
- 4. Altai M, Membreno R, Cook B, Tolmachev V, Zeglis BM. Pretargeted imaging and therapy. J Nucl Med. 2017;58:1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalofonos HP, Rusckowski M, Siebecker DA, et al. Imaging of tumor in patients with indium-111-labeled biotin and streptavidin-conjugated antibodies: preliminary communication. J Nucl Med. 1990;31:1791–1796. [PubMed] [Google Scholar]
- 6. Le Doussal JM, Chetanneau A, Gruaz-Guyon A, et al. Bispecific monoclonal antibody-mediated targeting of an indium-111-labeled DTPA dimer to primary colorectal tumors: pharmacokinetics, biodistribution, scintigraphy and immune response. J Nucl Med. 1993;34:1662–1671. [PubMed] [Google Scholar]
- 7. Paganelli G, Magnani P, Zito F, et al. Three-step monoclonal antibody tumor targeting in carcinoembryonic antigen- positive patients. Cancer Res. 1991;51:5960–5966. [PubMed] [Google Scholar]
- 8. Paganelli G, Grana C, Chinol M, et al. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur J Nucl Med. 1999;26:348–357. [DOI] [PubMed] [Google Scholar]
- 9. Cremonesi M, Ferrari M, Chinol M, et al. Three-step radioimmunotherapy with yttrium-90 biotin: dosimetry and pharmacokinetics in cancer patients. Eur J Nucl Med. 1999;26:110–120. [DOI] [PubMed] [Google Scholar]
- 10. Knox SJ, Goris ML, Tempero M, et al. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin Cancer Res. 2000;6:406–414. [PubMed] [Google Scholar]
- 11. Shen S, Forero A, LoBuglio AF, et al. Patient-specific dosimetry of pretargeted radioimmunotherapy using CC49 fusion protein in patients with gastrointestinal malignancies. J Nucl Med. 2005;46:642–651. [PubMed] [Google Scholar]
- 12. Weiden PL, Breitz HB. Pretargeted radioimmunotherapy (PRIT) for treatment of non-Hodgkin’s lymphoma (NHL). Crit Rev Oncol Hematol. 2001;40:37–51. [DOI] [PubMed] [Google Scholar]
- 13. Forero A, Weiden PL, Vose JM, et al. Phase 1 trial of a novel anti-CD20 fusion protein in pretargeted radioimmunotherapy for B-cell non-Hodgkin lymphoma. Blood. 2004;104:227–236. [DOI] [PubMed] [Google Scholar]
- 14. Paganelli G, Bartolomei M, Ferrari M, et al. Pre-targeted locoregional radioimmunotherapy with 90Y-biotin in glioma patients: phase I study and preliminary therapeutic results. Cancer Biother Radiopharm. 2001;16:227–235. [DOI] [PubMed] [Google Scholar]
- 15. Grana C, Chinol M, Robertson C, et al. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: a pilot study. Br J Cancer. 2002;86:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breitz HB, Weiden PL, Beaumier PL, et al. Clinical optimization of pretargeted radioimmunotherapy with antibody-streptavidin conjugate and 90Y-DOTA-biotin. J Nucl Med. 2000;41:131–140. [PubMed] [Google Scholar]
- 17. Schoffelen R, Woliner-van der Weg W, Visser EP, et al. Predictive patient-specific dosimetry and individualized dosing of pretargeted radioimmunotherapy in patients with advanced colorectal cancer. Eur J Nucl Med Mol Imaging. 2014;41:1593–1602. [DOI] [PubMed] [Google Scholar]
- 18. Schoffelen R, Boerman OC, Golderberg DM, et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: first clinical results. Br J Cancer. 2013;109:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodet-Milin C, Faivre-Chauvet A, Carlier T, et al. Immuno-PET using anticarcinoembryonic antigen bispecific antibody and 68Ga-labeled peptide in metastatic medullary thyroid carcinoma: clinical optimization of the pretargeting parameters in a first-in-human trial. J Nucl Med. 2016;57:1505–1511. [DOI] [PubMed] [Google Scholar]
- 20. Touchefeu Y, Bailly C, Frampas E, et al. Promising clinical performance of pretargeted immuno-PET with anti-CEA bispecific antibody and gallium-68-labelled IMP-288 peptide for imaging colorectal cancer metastases: a pilot study. Eur J Nucl Med Mol Imaging. 2021;48:874–882. [DOI] [PubMed] [Google Scholar]
- 21. Rousseau C, Goldenberg DM, Colombie M, et al. Initial clinical results of a novel immuno-PET theranostic probe in human epidermal growth factor receptor 2-negative breast cancer. J Nucl Med. 2020;61:1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bodet-Milin C, Ferrer L, Rauscher A, et al. Pharmacokinetics and dosimetry studies for optimization of pretargeted radioimmunotherapy in CEA-expressing advanced lung cancer patients. Front Med (Lausanne). 2015;2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barbet J, Peltier P, Bardet S, et al. Radioimmunodetection of medullary thyroid carcinoma using indium-111 bivalent hapten and anti-CEA x anti-DTPA-indium bispecific antibody. J Nucl Med. 1998;39:1172–1178. [PubMed] [Google Scholar]
- 24. Salaun PY, Campion L, Bournaud C, et al. Phase II trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: biomarker response and survival improvement. J Nucl Med. 2012;53:1185–1192. [DOI] [PubMed] [Google Scholar]
- 25. Kraeber-Bodéré F, Rousseau C, Bodet-Milin C, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J Nucl Med. 2006;47:247–255. [PubMed] [Google Scholar]
- 26. Chatal JF, Campion L, Kraeber-Bodéré F, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24:1705–1711. [DOI] [PubMed] [Google Scholar]
- 27. Labrijn AF, Janmaat ML, Reichert JM, et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18:585–608. [DOI] [PubMed] [Google Scholar]
- 28. Kraeber-Bodéré F, Faivre-Chauvet A, Ferrer L, et al. Pharmacokinetics and dosimetry studies for optimization of anti-carcinoembryonic antigen x anti-hapten bispecific antibody-mediated pretargeting of Iodine-131-labeled hapten in a phase I radioimmunotherapy trial. Clin Cancer Res. 2003;9:3973S–3981S. [PubMed] [Google Scholar]
- 29. Aarts F, Boerman OC, Sharkey RM, et al. Pretargeted radioimmunoscintigraphy in patients with primary colorectal cancer using a bispecific anticarcinoembryonic antigen CEA X anti-di-diethylenetriaminepentaacetic acid F(ab’)2 antibody. Cancer. 2010;116:1111–1117. [DOI] [PubMed] [Google Scholar]
- 30. Peplow M. Click chemistry targets antibody-drug conjugates for the clinic. Nat Biotechnol. 2019;37:835–837. [DOI] [PubMed] [Google Scholar]
- 31. Rossin R, van den Bosch SM, Ten Hoeve W, et al. Highly reactive trans-cyclooctene tags with improved stability for Diels-Alder chemistry in living systems. Bioconjug Chem. 2013;24:1210–1217. [DOI] [PubMed] [Google Scholar]
- 32. Rossin R, van Duijnhoven SM, Lappchen T, van den Bosch SM, Robillard MS. Trans-cyclooctene tag with improved properties for tumor pretargeting with the Diels-Alder reaction. Mol Pharm. 2014;11:3090–3096. [DOI] [PubMed] [Google Scholar]
- 33. Houghton JL, Zeglis BM, Abdel-Atti D, Sawada R, Scholz WW, Lewis JS. Pretargeted immuno-PET of pancreatic cancer: overcoming circulating antigen and internalized antibody to reduce radiation doses. J Nucl Med. 2016;57:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keinänen O, Fung K, Pourat J, et al. Pretargeting of internalizing trastuzumab and cetuximab with a (18)F-tetrazine tracer in xenograft models. EJNMMI Res. 2017;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Houghton JL, Membreno R, Abdel-Atti D, et al. Establishment of the in vivo efficacy of pretargeted radioimmunotherapy utilizing inverse electron demand Diels-Alder click chemistry. Mol Cancer Ther. 2017;16:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharkey RM, van Rij CM, Karacay H, et al. A new tri-Fab bispecific antibody for pretargeting Trop-2–expressing epithelial cancers. J Nucl Med. 2012;53:1625–1632. [DOI] [PubMed] [Google Scholar]
- 37. Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumors. Nat Rev Cancer. 2015;15:347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kraeber-Bodéré F, Bardet S, Hoefnagel CA, et al. Radioimmunotherapy in medullary thyroid cancer using bispecific antibody and iodine 131-labeled bivalent hapten: preliminary results of a phase I/II clinical trial. Clin Cancer Res. 1999;5:3190s–3198s. [PubMed] [Google Scholar]
- 39. Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. MAbs. 2020;12:1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schaefer NG, Ma J, Huang P, Buchanan J, Wahl RL. Radioimmunotherapy in non-Hodgkin lymphoma: opinions of U.S. medical oncologists and hematologists. J Nucl Med. 2010;51:987–994. [DOI] [PubMed] [Google Scholar]