Visual Abstract
Keywords: fluorocholine, hyperparathyroidism, adenoma, PET, 99mTc-sestamibi
Abstract
Hyperparathyroidism is an endocrine disorder caused by one or more hyperfunctioning parathyroid glands. Current imaging consisting of ultrasound and 99mTc-sestamibi is imprecise, making localization difficult. 18F-fluorocholine (18F-FCH) PET has recently shown promise in presurgical localization of parathyroid adenomas. The primary aim of this study was to summarize the sensitivities and specificities of studies using 18F-FCH PET to localize hyperparathyroidism. A secondary aim was to summarize a subset of studies in which 99mTc-sestamibi scans were also used and to compare the performance of the 2 modalities. Methods: We searched the MEDLINE and EMBASE databases following the PRISMA (Preferred Reporting Items for Systematic Review and Metaanalysis) statement. Quality was assessed using the QUADAS-2 tool (Quality Assessment of Diagnostic Accuracy Studies). Twenty studies were included for quantitative assessment in our metaanalysis. A random-effects model and a hierarchic summary receiver-operating-characteristic model was used to summarize the sensitivity of 18F-FCH PET in detecting abnormal parathyroid adenomas. We used the same methodology to assess sensitivity of 99mTc-sestamibi, as a comparison to 18F-FCH PET. Results: 18F-FCH PET had a high sensitivity, 0.97 (range, 0.96–0.98), for the detection of abnormal parathyroid adenomas. In the subpopulation for which both 18F-FCH and 99mTc-sestamibi were reported, 18F-FCH also had a higher sensitivity, 0.96 (0.94–0.98), than the 0.54 (0.29–0.79) reported for 99mTc-sestamibi (P < 0.001). Conclusion: 18F-FCH PET demonstrates high localization accuracy in patients with hyperparathyroidism. This metaanalysis supports the use of 18F-FCH over 99mTc-sestamibi in patients with hyperparathyroidism.
Hyperparathyroidism is a common endocrine disorder in which one or more of the parathyroid glands become autonomously hyperfunctional, causing excessive secretion of parathyroid hormone into the bloodstream (1,2). It is a common endocrine disorder, with an estimated incidence of between 0.4 and 82 cases per 100,000 in the general population (3–6). The etiology is usually a benign overgrowth of parathyroid tissue in at least 1 of the 4 parathyroid glands. This occurs in a single gland in approximately 80% of cases and less frequently (15%–20% of cases) in multiple glands (7). Hyperparathyroidism is diagnosed biochemically and is associated with hypercalcemia and elevated parathyroid hormone (7); in turn, hypercalcemia, if left untreated, can cause significant morbidities ranging from skeletal complications to renal impairment, as well as complications such as nephrocalcinosis, polyuria, and polydipsia.
Surgical removal of the hyperfunctioning gland (i.e., a parathyroidectomy) remains the only curative treatment for hyperparathyroidism (8). Preoperative localization of the hyperfunctioning gland is necessary for a minimally invasive parathyroidectomy, which is associated with a reduced risk of complications and disability after surgery as compared with conventional bilateral cervical exploration (9). Preoperative localization is complex, and imaging recommendations vary considerably. Cervical ultrasonography and 99mTc-sestamibi SPECT are the most commonly used methods. However, their accuracy varies considerably depending on the location of the affected glands, the size of the adenoma, and the skill of individual sonographers (10,11).
Given the inconsistencies of currently approved imaging modalities, new approaches are actively being evaluated. Several studies support the utility of 18F-fluorocholine (18F-FCH) PET, and results from the literature are encouraging (12,13). For nearly 20 years, 18F-FCH has been used to detect metastatic prostate cancer. Choline is a precursor for the synthesis of phospholipids in the cell membrane and choline kinase, which results in the elevated phosphocholine that is overexpressed in prostate cancer (14). However, data on the utility of 18F-FCH in localizing hyperparathyroidism remain relatively sparse, and comparison of 18F-FCH PET to traditional tools is limited to single-center studies.
The primary aim of this study was to summarize studies that have used 18F-FCH PET to localize hyperparathyroidism and to assess their sensitivity and specificity after pathologic confirmation. A second aim was to analyze a subset of studies in which a 99mTc-sestamibi scan was also used and to compare the sensitivity and specificity with those of 18F-FCH PET imaging.
MATERIALS AND METHODS
Correct identification of hyperparathyroidism was defined on a per-patient level. The protocol for this metaanalysis was registered with PROSPERO (the International Prospective Register of Systematic Reviews).
Search Strategy
Two of us conducted independent literature reviews for article inclusion in the study. This review included electronic databases, as well as reference lists of relevant articles. The search was applied to the PubMed/MEDLINE and EMBASE databases and was last updated on August 25, 2020. We used a combination of the following terms: choline, fluorocholine, F-choline, FCH; PET, positron emission tomography; and parathyroid, hyperparathyroidism.
Eligibility Criteria
Two reviewers independently assessed article eligibility for inclusion in this study. Disagreements were resolved by consensus. Articles that met the following inclusion criteria were considered for the metaanalysis: studies evaluating the diagnostic accuracy of 18F-FCH PET in patients with hyperparathyroidism, and studies that used pathologic confirmation of hyperparathyroidism as the reference standard. No year or location restrictions were imposed on the studies. Articles were excluded if they risked overlap with other studies (including systematic reviews and other metaanalyses); were not available in English; were on unpublished studies; were on case reports; had fewer than 10 cases; did not use pathology as the reference standard for diagnosing hyperparathyroidism; or did not have data on the diagnosis of hyperparathyroidism at the per-patient level. We also subsequently performed the analysis by removing articles for which cases of secondary and tertiary hyperparathyroidism could not be separated from primary hyperparathyroidism.
Data Collection
The characteristics of the eligible studies are summarized in Table 1. Data were extracted, when available, from each eligible article on the following variables: National Clinical Trial number, prospective versus retrospective, consent performed, number of patients imaged with 18F-FCH, imaging modality (PET/CT or PET/MRI), number of patients with a pathologic correlate, number of imaging readers, whether the readers knew the results of patients’ pathology or clinical data, injected dose and range, uptake time, and details on any adverse event reporting.
TABLE 1.
| First author | Year | Prospective or retrospective? | NCT number | Consent obtained | Patients with 18F-FCH imaging | Patients with parathyroidectomy | Masked readers | Readers | Pathology correlation | PET/CT or PET/MRI? | Injected dose range (MBq) | Injected dose average (MBq) | Uptake time (min) | Primary HPT only? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alharbi | 2018 | Retrospective | No | Yes | 66 | 52 | No | 2 | Yes | Both | NR | 150 | 2 & 50 | Yes |
| Amadou | 2019 | Retrospective | No | No | 41 | 23 | No | NR | Yes | PET/CT | NR | 231 | 60 | Yes |
| Bossert | 2019 | Prospective | No | Yes | 34 | 17 | Unclear | 2 | Yes | PET/CT | NR | 3–3.5/kg | 9 & 60 | Yes |
| Broos | 2019 | Prospective | No | Yes | 271 | 139 | Yes | 3 | Yes | PET/CT | NR | 150 | 5 & 60 | Yes |
| Christakis | 2019 | Prospective | No | Yes | 12 | 12 | Yes | 1 | Yes | PET/CT | NR | 300 | 60 & 90 | Yes |
| Fischli | 2017 | Retrospective | No | Yes | 39 | 23 | No | 1 | Yes | PET/CT | IQR 180–149 | 160 | 45 | Yes |
| Grimaldi | 2018 | Prospective | No | No | 27 | 21 | Unclear | NR | Yes | PET/CT | 77–230 | 100 | 30 | Yes |
| Hocevar | 2017 | Retrospective | No | No | 151 | 151 | No | NR | Yes | PET/CT | NR | 100 | 5 & 60 | Yes |
| Huber | 2018 | Retrospective | No | Yes | 26 | 26 | Unclear | NR | Yes | Both | NR | 151 | 45 | No |
| Khafif | 2019 | Prospective | No | Yes | 19 | 19 | No | 2 | Yes | PET/MRI | NR | 93.75 | 16 | Yes |
| Kluijfhout | 2017 | Prospective | No | Yes | 10 | 10 | Yes | 2 | Yes | PET/MRI | 188 ± 26 | 188 | 0* | Yes |
| Kluijfhout | 2016 | Retrospective | No | Yes | 33 | 33 | Unclear | NR | Yes | PET/CT | NR | 2/kg | 30 | No |
| Lezaic | 2014 | Prospective | No | Yes | 24 | 24 | Unclear | 2 | Yes | PET/CT | NR | 100 | 5 & 60 | Yes |
| López-Mora | 2020 | Prospective | No | Yes | 33 | 33 | Unclear | 3 | Yes | PET/CT: digital vs. analog | NR | 0.1/kg | Unclear | Yes |
| Michaud | 2014 | Prospective | No | Yes | 12 | 12 | No | 1 | Yes | PET/CT | NR | 3/kg | 0† | No |
| Piccardo | 2019 | Prospective | No | Yes | 44 | 31 | Unclear | 2 | Yes | PET/CT | NR | 100 | 10 | Yes |
| Quak | 2018 | Prospective | NCT02432599 | Yes | 25 | 24 | Yes | NR | Yes | PET/CT | NR | 1.5/kg | 60 | Yes |
| Thanseer | 2017 | Prospective | No | Yes | 54 | 54 | Unclear | NR | Yes | PET/CT | 150–185 | 150–185 | 10–15 & 60 | Yes |
| Uslu-Beşli | 2020 | Retrospective | No | Yes | 105 | 81 | No | 2 | Yes | PET/CT | 325.1 ± 86.7 | 325.1 | 15 & 45 | No |
| Zajíčková | 2018 | Retrospective | No | Yes | 13 | 13 | Unclear | 2 | Yes | PET/CT | NR | 180 | 30 | Yes |
Dynamic imaging for 40 min.
Dynamic imaging for 10 min followed by static acquisition.
NCT = National Clinical Trial; HPT = hyperparathyroidism; NR = not reported; IQR = interquartile range.
Quantitative data points were then extracted from eligible studies. These included number of true-positive, false-positive, true-negative, and false-negative diagnoses of hyperparathyroidism based on 18F-FCH imaging as compared with a pathologic correlate on a per-patient basis. If available, the same results were collected for studies in which patients were also imaged with 99mTc-sestamibi. One reviewer extracted the data points from eligible studies, and the second reviewer reviewed the extracted data for quality assurance. For each study included in the analysis, bias was assessed qualitatively by 2 reviewers using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool (15).
Metaanalytic Methods
For this metaanalysis, we used a random-effects model and a hierarchic summary receiver-operating-characteristic model using Stata, version 12.0 (StataCorp). Sensitivity and specificity are summarized for 18F-FCH imaging accuracy in detecting hyperparathyroidism on a per-patient level using the pathologic correlate as the reference standard. We also assessed sensitivity and specificity for a subset of studies that additionally imaged patients with 99mTc-sestamibi. To include all the studies in the metaanalysis, a small number was added to the zero cells for this subset of studies. All point estimates of sensitivity and specificity from the metaanalysis are reported as the effect size and 95% CIs.
RESULTS
Eligible Studies
An electronic search of the PubMed and EMBASE libraries returned a total of 776 articles (Fig. 1). Twenty studies were deemed eligible for the metaanalysis and are summarized in Table 1. The number of patients assessed ranged from 10 to 151 in our selected studies. All 20 papers were used to evaluate the sensitivity and specificity of 18F-FCH PET in detecting hyperparathyroidism, and 10 studies included data on the results of 99mTc-sestamibi to use for comparison. The risk of bias and the applicability of each study to our current research were assessed using the QUADAS-2 tool (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). In several cases, the risk of bias of the index test and the flow and timing of the imaging protocol could not be determined from the information provided in the text. Bias concerned the retrospective nature of many studies included in this analysis, as well as the uncertain time between imaging and parathyroidectomy in several cases. For the purposes of this analysis, a time of less than 4 wk between imaging and surgery was considered to have a low risk of bias.
FIGURE 1.
PRISMA (Preferred Reporting Items for Systematic Review and Metaanalysis) flow diagram depicting process for selecting papers included in this metaanalysis.
Performance of 18F-FCH PET in Detecting Hyperparathyroidism
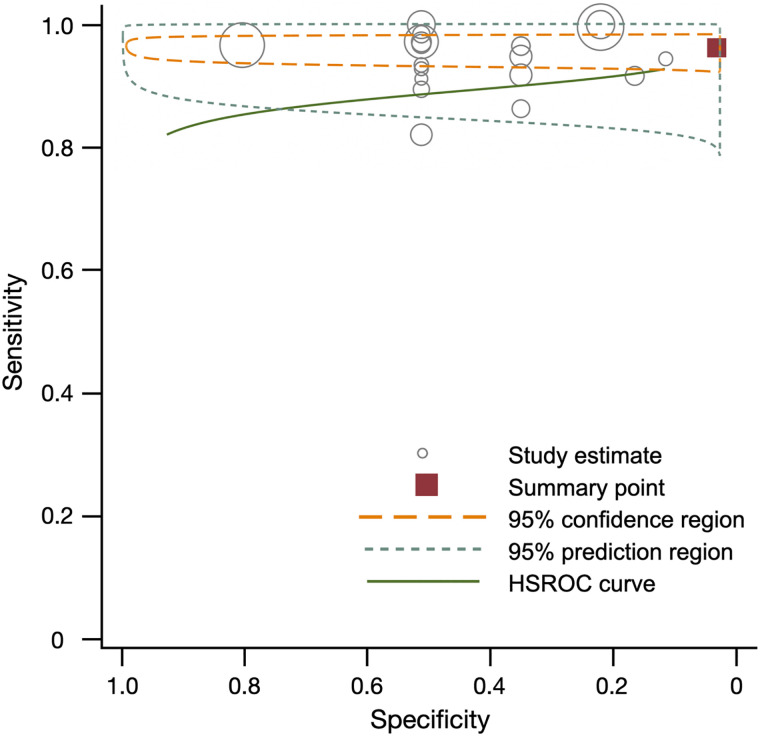
All 20 studies used 18F-FCH imaging (either by PET/CT, n = 18, or by PET/MRI, n = 4) to identify hyperparathyroidism (Table 2). The included studies were both retrospective (n = 8) and prospective (n = 12), consent was obtained for research participation in most cases (n = 17), and 1 study was registered with ClinicalTrials.gov before conducting research procedures. The average injected 18F-FCH dose ranged from 0.1 MBq/kg to a 325.1-MBq flat dose. The uptake time ranged from 0 min for dynamic imaging to 90 min for static imaging. The most common injected dose was 100 MBq, and the most common uptake time was 60 min. Across the 20 studies, including a total of 796 patients, the results of the random-effects metaanalysis of the sensitivity and specificity was 0.97 (range, 0.96–0.98) and 0.23 (range, 0.11–0.35), respectively (Fig. 2). The positive predictive value of 18F-FCH PET, compared with pathology, was 0.94 (range, 0.92–0.96). In studies that included only patients with primary hyperparathyroidism (n = 16), sensitivity and specificity were 0.94 (range, 0.92–0.97) and 0.14 (range, 0–0.36), respectively (Supplemental Fig. 1).
TABLE 2.
Overview of Studies Comparing Performance of 18F-FCH PET with Pathology in 20 Studies Reporting Total of 796 Patients
| First author | Year | Patients | TP | FP | TN | FN |
|---|---|---|---|---|---|---|
| Alharbi | 2018 | 52 | 52 | 0 | 0 | 0 |
| Amadou | 2019 | 23 | 21 | 1 | 0 | 1 |
| Bossert | 2019 | 17 | 15 | 0 | 0 | 2 |
| Broos | 2019 | 139 | 131 | 0 | 2 | 6 |
| Christakis | 2019 | 12 | 7 | 5 | 0 | 0 |
| Fischli | 2017 | 23 | 21 | 1 | NA | 1 |
| Grimaldi | 2018 | 21 | 17 | 1 | NA | 3 |
| Hocevar | 2017 | 151 | 144 | 4 | 1 | 2 |
| Huber | 2018 | 26 | 25 | 0 | 0 | 1 |
| Khafif | 2019 | 19 | 19 | 0 | 0 | 0 |
| Kluijfhout | 2017 | 10 | 9 | 0 | NA | 1 |
| Kluijfhout | 2016 | 33 | 30 | 1 | NA | 2 |
| Lezaic | 2014 | 24 | 23 | 0 | NA | 1 |
| López-Mora | 2020 | 33 | 29 | 1 | 0 | 3 |
| Michaud | 2014 | 12 | 11 | 0 | NA | 1 |
| Piccardo | 2019 | 31 | 25 | 0 | 0 | 6 |
| Quak | 2018 | 24 | 19 | 3 | NA | 2 |
| Thanseer | 2017 | 54 | 52 | 2 | NA | 0 |
| Uslu-Beşli | 2020 | 79 | 76 | NA | NA | 3 |
| Zajíčková | 2018 | 13 | 12 | 0 | 0 | 1 |
| Total | 796 | 738 | 19 | 3 | 33 |
TP = true positive; FP = false positive; TN = true negative; FN = false negative; NA = not applicable.
FIGURE 2.
Summary of sensitivity, specificity, and hierarchic summary receiver-operating-characteristic (HSROC) plot of sensitivity and specificity for 18F-FCH vs. pathology overall. Effect sizes for sensitivity and specificity were 0.97 (95% CI, 0.96–0.98) and 0.23 (95% CI, 0.11–0.35), respectively. Size of circles represents size of individual studies.
Comparison of 18F-FCH PET and 99mTc-Sestamibi in Detecting Hyperparathyroidism
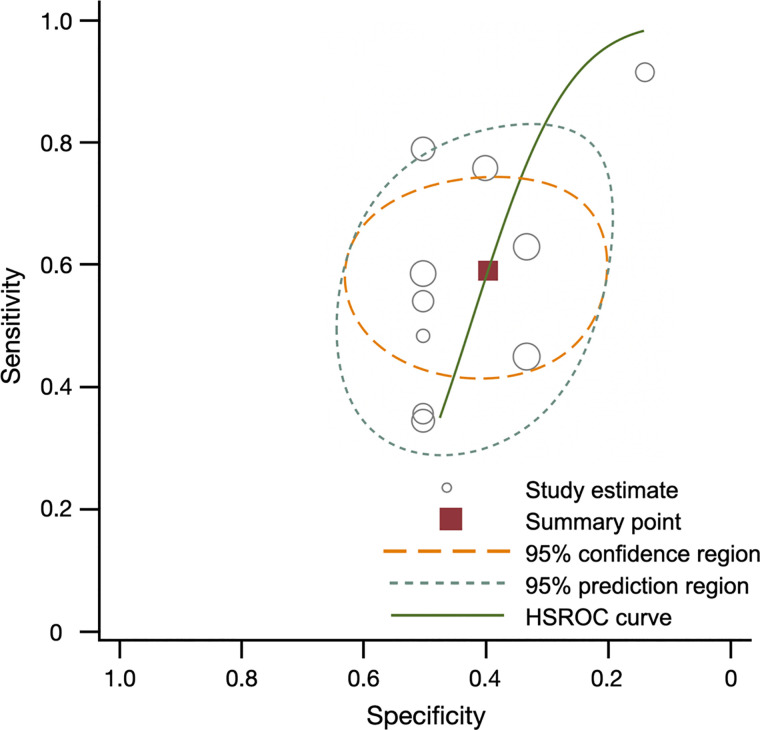
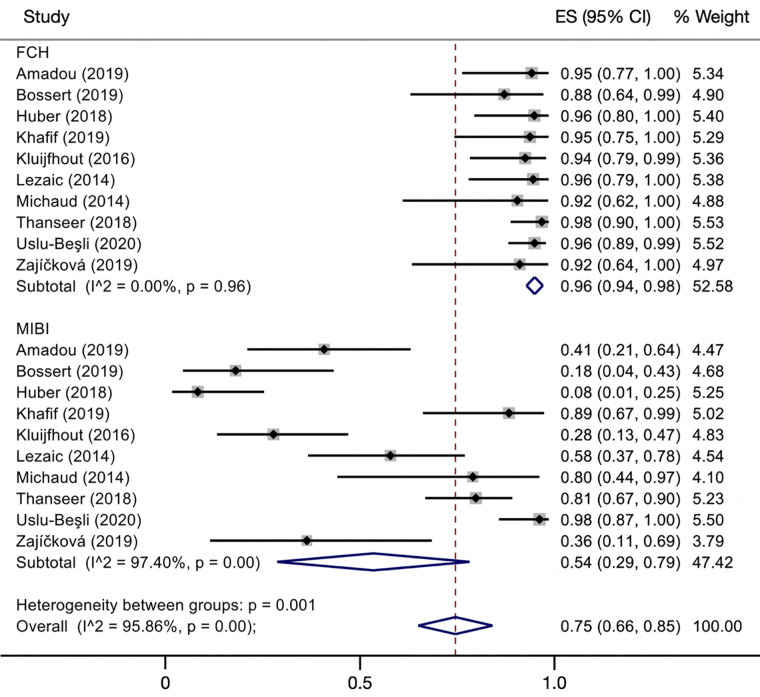
As a secondary analysis, we compared the performance of 18F-FCH PET and 99mTc-sestamibi in detecting cases of hyperparathyroidism before surgery. Ten studies, which included 301 patients, had these data available and were used in the comparison (Table 3; Supplemental Fig. 2). 18F-FCH PET had a superior sensitivity of 0.96 (range, 0.94–0.98), compared with 0.54 (range, 0.29–0.79) for 99mTc-sestamibi (P < 0.001) (Figs. 3 and 4). In studies limited to patients with primary hyperparathyroidism, 18F-FCH PET had a superior sensitivity of 0.97 (range, 0.94–1.00), compared with 0.55 (range, 0.32–0.78) for 99mTc-sestamibi.
TABLE 3.
Overview of Studies Comparing Performance of 99mTc-Sestamibi with Pathology
| 99mTc-sestamibi compared with pathology | ||||||
|---|---|---|---|---|---|---|
| First author | Year | Patients with pathology | TP | FP | TN | FN |
| Amadou | 2019 | 23 | 9 | 1 | 0 | 13 |
| Bossert | 2019 | 17 | 3 | 0 | 0 | 14 |
| Huber | 2018 | 26 | 2 | 0 | 0 | 24 |
| Khafif | 2019 | 19 | 17 | 0 | 0 | 2 |
| Kluijfhout | 2017 | 33 | 8 | 0 | 0 | 21 |
| Lezaic | 2014 | 24 | 14 | 0 | 0 | 10 |
| Michaud | 2014 | 12 | 8 | 2 | 0 | 2 |
| Thanseer | 2017 | 54 | 42 | 1 | 1 | 10 |
| Uslu-Beşli | 2020 | 80 | 39 | 1 | NA | NA |
| Zajíčková | 2018 | 13 | 4 | 2 | 0 | 7 |
| Total | 301 | 146 | 7 | 1 | 103 | |
TP = true positive; FP = false positive; TN = true negative; FN = false negative; NA = not applicable.
FIGURE 3.
Summary of sensitivity, specificity, and hierarchic summary receiver-operating-characteristic (HSROC) plot of sensitivity and specificity for 99mTc-sestamibi vs. pathology overall. Effect sizes for sensitivity and specificity were 0.54 (0.29–0.79) and 0.43 (0.30–0.57), respectively. Size of circles represents size of individual studies.
FIGURE 4.
Comparison of diagnostic sensitivities of 18F-FCH and 99mTc-sestamibi. Overall effect sizes (ES) were 0.96 (95% CI, 0.94–0.98) for 18F-FCH PET and 0.54 (95% CI, 0.29–0.79) for 99mTc-sestamibi. Size of squares represents size of individual studies. Reference numbers are in Supplemental Table 2.
DISCUSSION
Several individual cohort studies have reported 18F-FCH PET to be superior to traditional imaging modalities such as ultrasonography and 99mTc-sestamibi in detecting hyperparathyroidism in patients before parathyroidectomy. Individual studies are difficult to interpret because of their small sample sizes and variability between studies. In this metaanalysis, we pool the results of studies using 18F-FCH PET to localize abnormal parathyroid adenomas and using pathology as a reference standard. To acknowledge individual study bias, we assessed each paper using the QUADAS-2 tool and used a random-effects model to account for between-study variability in our quantitative analysis. Overall, the results of this metaanalysis lend further evidence to support the use of 18F-FCH PET as a superior imaging technique over 99mTc-sestamibi in the localization of hyperparathyroidism before parathyroidectomy.
To avoid loss of power and incorporate more studies into our analysis, we included studies using both PET/CT and PET/MRI. Diagnostic differences between these modalities for this indication have not been studied in previous literature, but we acknowledge that inclusion of PET/MRI may further bias this analysis. Our study did not consider results on a per-lesion basis, considering only whether imaging localized an overactive parathyroid gland on a per-patient basis. This approach may overestimate the accuracy of 18F-FCH PET as a presurgical tool in avoiding invasive open parathyroidectomies.
As with any metaanalysis, our approach is limited by the underlying data in the articles included. As reported, there was a wide range in acquisition parameters used. Most concerning was that masking of readers to the results of parathyroidectomies before image interpretation was not done or was unclear in most cases, and most studies were retrospective. This likely biased individual study results and may have skewed results in favor of 18F-FCH PET. Furthermore, several studies included patients with a history of thyroid or parathyroid surgery; it is unclear what effect this may have had on the accuracy of either 18F-FCH PET or 99mTc-sestamibi in detecting the affected parathyroid glands and may limit the applicability of these results to patients being imaged at baseline.
One other issue is the heterogeneity of the technique used for 99mTc-sestamibi imaging in our analysis, as each approach has varying sensitivities that can lead to inconsistencies across the articles used in the comparison analysis. Of the 10 studies included for comparison with 99mTc-sestamibi, 6 (including 41% of the analyzed patients) used dual-phase, dual-tracer 99mTc-sestamibi imaging with SPECT/CT. Three articles used SPECT, 2 of which used 99mTc-sestamibi alone, and 1 article did not describe the 99mTc-sestamibi imaging.
Despite these weaknesses, we believe this study is important in a setting that has seen little change in practice over many years. Furthermore, there are features of this study that we feel distinguish it from prior metaanalyses on this topic. We have taken care to define strict study eligibility criteria, including a minimum cohort size to limit patient selection bias, the requirement of a histopathologic correlate for all cases in the analysis, and a focus on 18F-FCH PET only, excluding studies that incorporate other choline tracers (13). Perhaps most notably, our study included 2 important subanalyses: a comparison of 18F-FCH PET to the standard-of-care 99mTc-sestamibi scan, making a strong clinical case for the adoption of this more novel technique; and a further study limited to cases with primary hyperparathyroidism. To our knowledge, it is also the largest study of this kind (12,16).
Beyond 99mTc-sestamibi, there are other imaging techniques being evaluated for the localization of abnormal parathyroids, such as 4-dimensional (4D) CT and 11C-choline PET. Both have also demonstrated utility in preoperative localization of parathyroid glands in patients with hyperparathyroidism. Literature on the use of 4D CT, with or without ultrasonography, has reported high but varying sensitivities in localizing adenomas (17,18). There are insufficient data at this time to compare 18F-FCH with 4D CT to perform a metaanalysis. However, there are several theoretic advantages of 18F-FCH over 4D CT, including obviation of intravenous iodinated contrast, as well as the lower total doses of radiation (19). 11C-choline PET, a similar radiotracer to 18F-FCH, received Food and Drug Administration approval in 2012 for use in prostate cancer (20) and has recently been used in preoperative localization for hyperparathyroidism. Because 11C-choline has a half-life of approximately 20 min, compared with 120 min for 18F (21), PET acquisition must occur very shortly after injection. The longer half-life of 18F-FCH allows for more flexible image acquisition and makes for more practical and favorable clinical use (22).
CONCLUSION
In patients with hyperparathyroidism, 18F-FCH PET demonstrates a high sensitivity (0.97) for parathyroid adenomas in patients with hyperparathyroidism. 18F-FCH PET also outperformed 99mTc-sestamibi, with a sensitivity of 0.96 for the former compared with 0.54 for the latter. This metaanalysis supports the use of 18F-FCH over 99mTc-sestamibi in patients with hyperparathyroidism.
DISCLOSURE
Thomas Hope receives grant support from the National Institutes of Health (R01CA212148). Isabel Allen is supported by the National Institute on Aging (R43AG066230). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does 18F-FCH PET aid in the localization of parathyroid adenomas in patients with hyperparathyroidism?
PERTINENT FINDINGS: In this metaanalysis, 18F-FCH PET had a high sensitivity for parathyroid adenomas and increased the sensitivity from 0.54 for 99mTc-sestamibi imaging to 0.96 for 18F-FCH PET.
IMPLICATIONS FOR PATIENT CARE: 18F-FCH PET is useful for localizing parathyroid adenomas and should be used when available.
REFERENCES
- 1. Khan AA, Hanley DA, Rizzoli R, Bollerslev J, Young JEM. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management—a Canadian and international consensus. Osteoporos Int. 2017;28:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El-Hajj Fuleihan G, Silverberg S. Patient education: primary hyperparathyroidism (beyond the basics). UpToDate website. https://www.uptodate.com/contents/primary-hyperparathyroidism-beyond-the-basics. Updated November 5, 2019. Accessed April 22, 2021.
- 3. Yeh MW, Ituarte PHG, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wermers RA, Khosla S, Atkinson EJ, et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21:171–177. [DOI] [PubMed] [Google Scholar]
- 5. Press DM, Siperstein AE, Berber E, et al. The prevalence of undiagnosed and unrecognized primary hyperparathyroidism: a population-based analysis from the electronic medical record. Surgery. 2013;154:1232–1237. [DOI] [PubMed] [Google Scholar]
- 6. Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinol. 2018;14:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilezikian JP, Bandeira L, Khan A, Cusano NE. Hyperparathyroidism. Lancet. 2018;391:168–178 [DOI] [PubMed] [Google Scholar]
- 8. Imperiale A, Taieb D, Hindie E. 18F-fluorocholine PET/CT as a second line nuclear imaging technique before surgery for primary hyperparathyroidism. Eur J Nucl Med Mol Imaging. 2018;45:654–657. [DOI] [PubMed] [Google Scholar]
- 9. Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253:585–591. [DOI] [PubMed] [Google Scholar]
- 10. Ruda JM, Hollenbeak CS, Stack BCJ. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359–372. [DOI] [PubMed] [Google Scholar]
- 11. Kobylecka M, Plazinska MT, Chudzinski W, et al. Comparison of scintigraphy and ultrasound imaging in patients with primary, secondary and tertiary hyperparathyroidism: own experience. J Ultrason. 2017;17:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim S-JJ, Lee S-WW, Jeong SY, et al. Diagnostic performance of F-18 fluorocholine PET/CT for parathyroid localization in hyperparathyroidism: a systematic review and meta-analysis. Horm Cancer. 2018;9:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Treglia G, Piccardo A, Imperiale A, et al. Diagnostic performance of choline PET for detection of hyperfunctioning parathyroid glands in hyperparathyroidism: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2019;46:751–765. [DOI] [PubMed] [Google Scholar]
- 14. Vali R, Loidl W, Pirich C, Langesteger W, Beheshti M. Imaging of prostate cancer with PET/CT using 18F-fluorocholine. Am J Nucl Med Mol Imaging. 2015;5:96–108. [PMC free article] [PubMed] [Google Scholar]
- 15. Whiting PF, Rutjes AWS, Westwood ME, et al.; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;154:253–260. [DOI] [PubMed] [Google Scholar]
- 16. Pardal-Refoyo JL, Tamayo-Alonso P, Ferreira-Cendon S, Martin-Gomez E. Pathological uptake with 18-fluorocholine versus 99mTc-MIBI in the location of the parathyroid glands in hyperparathyroidism: systematic review and meta-analysis. medRxiv website. https://www.medrxiv.org/content/10.1101/2020.07.25.20161927v1. Published July 27, 2020. Accessed April 22, 2021.
- 17. Yeh R, Tay Y-KD, Tabacco G, et al. Diagnostic performance of 4D CT and 99mTc-sestamibi SPECT/CT in localizing parathyroid adenomas in primary hyperparathyroidism. Radiology. 2019;291:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bann DV, Zacharia T, Goldenberg D, Goyal N. Parathyroid localization using 4D-computed tomography. Ear Nose Throat J. 2015;94:E55–E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, Carling T. Parathyroid four-dimensional computed tomography: evaluation of radiation dose exposure during preoperative localization of parathyroid tumors in primary hyperparathyroidism. World J Surg. 2012;36:1335–1339. [DOI] [PubMed] [Google Scholar]
- 20. FDA approves 11C-choline for PET in prostate cancer. J Nucl Med. 2012;53(12):11N–11N. [PubMed] [Google Scholar]
- 21. Grassi I, Nanni C, Allegri V, et al. The clinical use of PET with 11C-acetate. Am J Nucl Med Mol Imaging. 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- 22. Massaro A, Ferretti A, Secchiero C, et al. Optimising 18F-choline PET/CT acquisition protocol in prostate cancer patients. N Am J Med Sci. 2012;4:416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alharbi AA, Alshehri FM, Albatly AA, et al. [18F]fluorocholine uptake of parathyroid adenoma is correlated with parathyroid hormone level. Mol Imaging Biol. 2018;20:857–867. [DOI] [PubMed] [Google Scholar]
- 24. Amadou C, Bera G, Ezziane M, et al. 18F-fluorocholine PET/CT and parathyroid 4D computed tomography for primary hyperparathyroidism: the challenge of reoperative patients. World J Surg. 2019;43:1232–1242. [DOI] [PubMed] [Google Scholar]
- 25. Kluijfhout WP, Pasternak JD, Gosnell JE, et al. 18F fluorocholine PET/MR imaging in patients with primary hyperparathyroidism and inconclusive conventional imaging: a prospective pilot study. Radiology. 2017;284:460–467. [DOI] [PubMed] [Google Scholar]
- 26. Lezaic L, Rep S, Sever MJ, Kocjan T, Hocevar M, Fettich J. 18F-fluorocholine PET/CT for localization of hyperfunctioning parathyroid tissue in primary hyperparathyroidism: a pilot study. Eur J Nucl Med Mol Imaging. 2014;41:2083–2089. [DOI] [PubMed] [Google Scholar]
- 27. Michaud L, Burgess A, Huchet V, et al. Is 18F-fluorocholine-positron emission tomography/computerized tomography a new imaging tool for detecting hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism? J Clin Endocrinol Metab. 2014;99:4531–4536. [DOI] [PubMed] [Google Scholar]
- 28. Piccardo A, Trimboli P, Rutigliani M, et al. Additional value of integrated 18F-choline PET/4D contrast-enhanced CT in the localization of hyperfunctioning parathyroid glands and correlation with molecular profile. Eur J Nucl Med Mol Imaging. 2019;46:766–775. [DOI] [PubMed] [Google Scholar]
- 29. Thanseer N, Bhadada SK, Sood A, et al. Comparative effectiveness of ultrasonography, 99mTc-sestamibi, and 18F-fluorocholine PET/CT in detecting parathyroid adenomas in patients with primary hyperparathyroidism. Clin Nucl Med. 2017;42:e491–e497. [DOI] [PubMed] [Google Scholar]
- 30. Zajíčková K, Zogala D, Kubinyi J, et al. Parathyroid imaging by 18F-fluorocholine PET/CT in patients with primary hyperparathyroidism and inconclusive conventional methods: clinico-pathological correlations. Physiol Res. 2018;67(suppl):S551–S557. [DOI] [PubMed] [Google Scholar]
- 31. Quak E, Blanchard D, Houdu B, et al. F18-choline PET/CT guided surgery in primary hyperparathyroidism when ultrasound and MIBI SPECT/CT are negative or inconclusive: the APACH1 study. Eur J Nucl Med Mol Imaging. 2018;45:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khafif A, Masalha M, Landsberg R, et al. The role of F18-fluorocholine positron emission tomography/magnetic resonance imaging in localizing parathyroid adenomas. Eur Arch Otorhinolaryngol. 2019;276:1509–1516. [DOI] [PubMed] [Google Scholar]
- 33. López-Mora DA, Sizova M, Estorch M, et al. Superior performance of 18F-fluorocholine digital PET/CT in the detection of parathyroid adenomas. Eur J Nucl Med Mol Imaging. 2020;47:572–578. [DOI] [PubMed] [Google Scholar]
- 34. Romano R, Manzo N, Montefusco I, Romano A, Santini A. Liquid carbon dioxide use in the extraction of extra virgin olive oil from olive paste. J Food Res. 2014;3:119–128. [Google Scholar]
- 35. Beheshti M, Hehenwarter L, Paymani Z, et al. 18F-fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with 99mTc-MIBI or 99mTc-tetrofosmin SPECT/CT: a prospective dual-centre study in 100 patients. Eur J Nucl Med Mol Imaging. 2018;45:1762–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huber GF, Hüllner M, Schmid C, et al. Benefit of 18F-fluorocholine PET imaging in parathyroid surgery. Eur Radiol. 2018;28:2700–2707. [DOI] [PubMed] [Google Scholar]
- 37. Uslu-Beşli L, Sonmezoglu K, Teksoz S, et al. Performance of F-18 fluorocholine PET/CT for detection of hyperfunctioning parathyroid tissue in patients with elevated parathyroid hormone levels and negative or discrepant results in conventional imaging. Korean J Radiol. 2020;21:236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bossert I, Chytiris S, Hodolic M, et al. PETC/CT with 18F-choline localizes hyperfunctioning parathyroid adenomas equally well in normocalcemic hyperparathyroidism as in overt hyperparathyroidism. J Endocrinol Invest. 2019;42:419–426. [DOI] [PubMed] [Google Scholar]
- 39. Broos WAMM, Wondergem M, Knol RJJJ, et al. Parathyroid imaging with 18F-fluorocholine PET/CT as a first-line imaging modality in primary hyperparathyroidism: a retrospective cohort study. EJNMMI Res. 2019;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Christakis I, Khan S, Sadler GP, Gleeson FV, Bradley KM, Mihai R. 18Fluorocholine PET/CT scanning with arterial phase-enhanced CT is useful for persistent/recurrent primary hyperparathyroidism: first UK case series results. Ann R Coll Surg Engl. 2019;101:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischli S, Suter-Widmer I, Nguyen BT, et al. The significance of 18F-fluorocholine-PET/CT as localizing imaging technique in patients with primary hyperparathyroidism and negative conventional imaging. Front Endocrinol (Lausanne). 2018;8:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grimaldi S, Young J, Kamenicky P, et al. Challenging pre-surgical localization of hyperfunctioning parathyroid glands in primary hyperparathyroidism: the added value of 18F-fluorocholine PET/CT. Eur J Nucl Med Mol Imaging. 2018;45:1772–1780. [DOI] [PubMed] [Google Scholar]
- 43. Hocevar M, Lezaic L, Rep S, et al. Focused parathyroidectomy without intraoperative parathormone testing is safe after pre-operative localization with 18F-fluorocholine PET/CT. Eur J Surg Oncol. 2017;43:133–137. [DOI] [PubMed] [Google Scholar]
- 44. Kluijfhout WP, Vorselaars WMCM, van den Berk SAM, et al. Fluorine-18 fluorocholine PET-CT localizes hyperparathyroidism in patients with inconclusive conventional imaging: a multicenter study from the Netherlands. Nucl Med Commun. 2016;37:1246–1252. [DOI] [PubMed] [Google Scholar]