Visual Abstract
Keywords: personalized dosimetry, MIBG, PRRT, DOTATOC
Abstract
Peptide receptor radionuclide therapy (PRRT) is an effective treatment for metastatic neuroendocrine tumors. Delivering a sufficient tumor radiation dose remains challenging because of critical-organ dose limitations. Adding 131I-metaiodobenzylguanidine (131I-MIBG) to PRRT may be advantageous in this regard. Methods: A phase 1 clinical trial was initiated for patients with nonoperable progressive neuroendocrine tumors using a combination of 90Y-DOTATOC plus 131I-MIBG. Treatment cohorts were defined by radiation dose limits to the kidneys and the bone marrow. Subject-specific dosimetry was used to determine the administered activity levels. Results: The first cohort treated subjects to a dose limit of 1,900 cGy to the kidneys and 150 cGy to the marrow. No dose-limiting toxicities were observed. Tumor dosimetry estimates demonstrated an expected dose increase of 34%–83% using combination therapy as opposed to 90Y-DOTATOC PRRT alone. Conclusion: These findings demonstrate the feasibility of using organ dose for a phase 1 escalation design and suggest the safety of using 90Y-DOTATOC and 131I-MIBG.
Peptide receptor radionuclide therapy (PRRT), either as 177Lu-DOTATATE (Lutathera; Advanced Accelerator Applications) or as 90Y-DOTATOC, is well established as an effective form of treatment for patients with metastatic neuroendocrine tumors (1–3). Delivering a tumor radiation dose sufficient to result in a high percentage of overall response rates remains challenging because of limits imposed on administered activity levels by radiation-induced normal-organ toxicity (4). For 90Y-DOTATOC, the critical organ that limits the amount of deliverable administered activity is typically the kidney (5,6). Targeted radionuclide therapy with 131I-metaiodobenzylguanidine (131I-MIBG) has also demonstrated promise in some patients with advanced-stage neuroendocrine tumors (7,8). 131I-MIBG targets tumor sites in over 50% of patients with midgut neuroendocrine tumors through a mechanism distinctly different from that of PRRT agents (9). The amount of administered activity that can safely be delivered is limited primarily by radiation to the bone marrow as opposed to the kidneys (10). We have previously demonstrated that this difference enables the combination of large fractions of each agent (relative to amounts that can be delivered safely alone or individually) into a single treatment regimen that results in higher total tumor radiation doses without exceeding dose limits for either the marrow or the kidneys (11). Moreover, known differences in tumor distribution of 131I-MIBG and radiolabeled octreopeptides may prove to be advantages for combined therapy.
Traditionally, cancer trials on targeted radionuclide therapy have relied on a “one size fits all” approach to treating patients in terms of prescribed levels of administered activity. This approach to radionuclide-based therapy is considered by many to be less desirable than using personalized patient-specific dosimetry to guide treatment (12,13). We initiated a phase 1 clinical trial in which the escalation design was based on increasing the radiation dose limits to critical organs between cohorts as opposed to using cohorts defined by specific escalated levels of administered activity. Within this trial framework, we applied the technique previously described for addition of 131I-MIBG to PRRT using patient-specific dosimetry (14). We report here the results from this trial before a redesign wherein 90Y-DOTATOC is being replaced by 177Lu-DOTATATE and low-specific-activity 131I-MIBG is being replaced by high-specific-activity 131I-MIBG.
MATERIALS AND METHODS
The study was approved by the University of Iowa Biomedical Institutional Review Board (IRB-01), and all subjects provided written independent consent. Patients with nonoperable (metastatic or local), progressive neuroendocrine tumors of midgut origin with 68Ga-DOTATATE–positive tumors on PET were invited to participate. Combined imaging with 111In-pentetreotide (as a biodistribution surrogate for 90Y-DOTATOC) and 131I-MIBG was performed on each subject for dosimetric analysis and detailed tumor-targeting assessment. To be eligible to proceed to treatment, subjects had to demonstrate at least one of the following based on the results from the combined imaging/biodistribution studies: either one or more 131I-MIBG–positive and 90Y-DOTATOC–negative tumors, or one or more tumor sites where the expected tumor radiation dose is higher by at least 25% with a combination of 90Y-DOTATOC plus 131I-MIBG than with 90Y-DOTATOC alone.
Imaging and Dosimetry
Imaging and blood sampling were performed at 1, 4, 24, and 48 h after combined administration of 222 MBq of 111In-pentetreotide plus 74 MBq of 131I-MIBG. Planar and SPECT/CT images were acquired as multiisotope studies with a 20% window on the 364-keV photopeak of 131I and the 247-keV photopeak of 111In. High-energy collimation was used for all simultaneous imaging studies. Scatter correction was performed. Appropriate 1.85-MBq standards of 131I and 111In were placed within the field. Organ and tumor mass were measured from the CT scan. Dose was determined for the kidneys and bone marrow and for up to 2 soft-tissue tumor sites per organ system. Marrow dosimetry was based on the blood-to-marrow β-contribution and on the organ- or tumor-to-marrow γ-contribution. OLINDA, version 1.1, was used.
Therapy
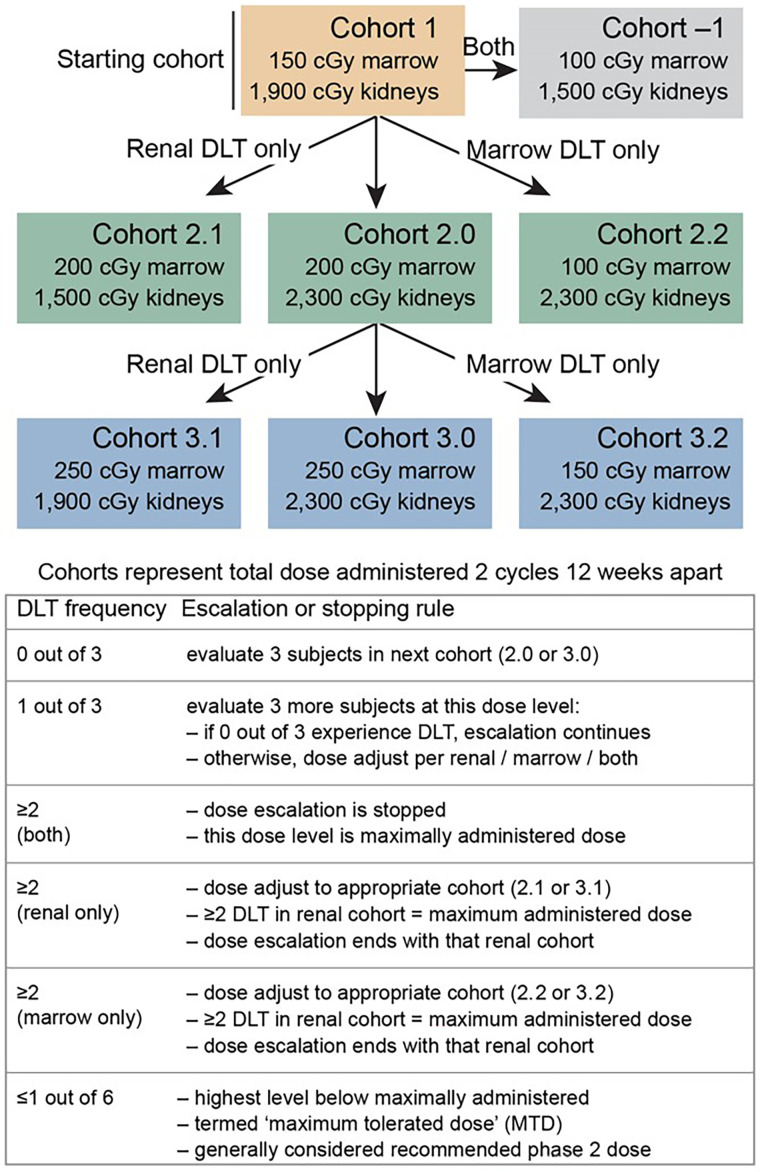
Cohort 1 subjects were treated with a combination of 131I-MIBG and 90Y-DOTATOC. The administered activity was an amount calculated to deliver a total expected cumulative renal radiation dose of 1,900 cGy and a bone marrow dose of 150 cGy (delivered over 2 equal treatment cycles separated by 10–12 wk). The concept and methods to accomplish these administered activity calculations have been described previously (11,15). The trial escalation paradigm is depicted in Figure 1.
FIGURE 1.
Trial design. DLT = dose-limiting toxicities.
Each cycle consisted of 90Y-DOTATOC delivered on an outpatient basis (day 1) followed by in-patient 131I-MIBG infusion (day 2). A compounded amino acid solution containing 25 g of lysine and 25 g of arginine was administered with the 90Y-DOTATOC infusion.
Blood counts, serum creatinine, and urinary protein were assessed regularly beginning at baseline and continuing through 6 mo after cycle 2 to evaluate for dose-limiting toxicity. Dose-limiting toxicities were based on the Common Terminology Criteria for Adverse Events, version 4.03.
RESULTS
Six patients consented to the trial; of these, one did not meet the second-phase eligibility criteria, a second had insurance deny clinical trial participation, and a third withdrew for personal reasons. There were 2 men and 1 woman in the cohort presented here, aged 50–68 y. The tumors were located in the liver or abdominal lymph nodes and, in one case, the anterior abdominal wall. The primary tumor (small bowel in all cases) had been excised from each patient. None of the subjects had bone metastases.
In each of the 3 treated subjects, it was determined that over 11,100 MBq (300 mCi) (total) of 131I-MIBG could safely be added to dosimetrically determined levels of 90Y-DOTATOC (Table 1). The pretherapy tumor dosimetry results revealed that the expected tumor-dose increases could be achieved through addition of 131I-MIBG to 90Y-DOTATOC, compared with what would have been the case for 90Y-DOTATOC given in maximum amounts alone. The calculated tumor-dose increases through the addition of 131I-MIBG ranged from 34% to 83% in 5 of the 6 target tumors evaluated. An example of one of these tumors is depicted in Figure 2. The calculated expected tumor-dose increase in the sixth tumor was an outlier, at 362%.
TABLE 1.
Calculated Administered Activity Levels to Achieve Dose Limit of 1,900 cGy to Kidneys Plus 150 cGy to Bone Marrow
| Maximum total activity 90Y-DOTATOC plus 131I-MIBG (GBq) | |||
|---|---|---|---|
| Subject no. | Maximum total activity 90Y-DOTATOC only (GBq) | 90Y-DOTATOC | 131I-MIBG |
| 1 | 10.8 | 8.7 | 11.4 |
| 2 | 7.8 | 5.6 | 18.3 |
| 3 | 5.0 | 2.8 | 18.7 |
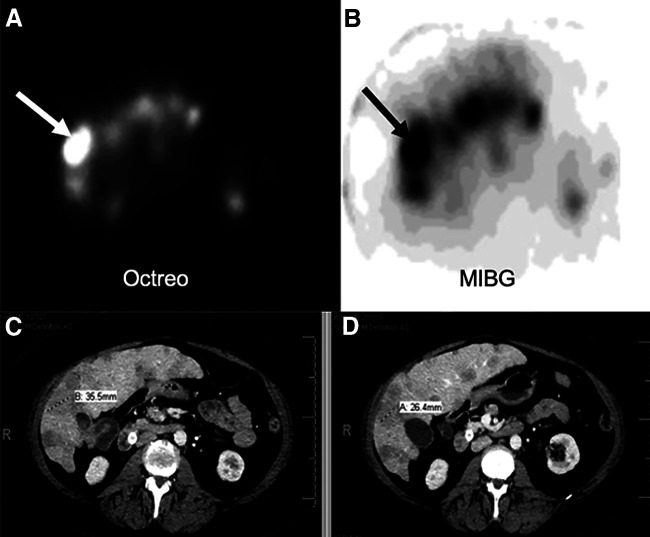
FIGURE 2.
Subject 1. (A) 111In-pentetreotide axial SPECT image through mid liver demonstrating multiple octreopeptide-positive metastases with focal intense uptake in target lesion (arrow). (B) 131I-MIBG SPECT axial slice at same level demonstrating intense uptake in same lesion (arrow). (C) Corresponding baseline venous phase CT scan depicting multiple liver metastases consistent with SPECT findings. Target lesion is 35.5 mm in maximum diameter. (D) Follow-up CT 6-mo after cycle 2 showing measurement of target lesion (maximum diameter, 26.4 mm).
No dose-limiting toxicities were observed during the 6-mo dose-limiting-toxicity window. One subject did register a temporary grade 3 thrombocytopenia after the second cycle, and another developed grade 2 kidney toxicity after therapy completion (creatinine level, 1.6 mg/dL), which remained stable at 1 y after treatment. Toxicity data are provided in Table 2. By RECIST, version 1.1, all 3 subjects showed stable disease 6 mo after cycle 2.
TABLE 2.
Posttreatment Renal and Bone Marrow Toxicity Assessment
| Cycle 1 | Cycle 2 | |||||
|---|---|---|---|---|---|---|
| Parameter | Baseline | 1 mo | 2 mo | 1 mo | 2 mo | 6 mo |
| Creatinine (mg/dL) | ||||||
| Subject 1 | 1.20 | 1.1 | 1.10 | 1.30 | 1.10 | 1.60 |
| Subject 2 | 1.10 | 0.86 | 0.94 | 0.98 | 1.13 | 1.00 |
| Subject 3 | 1.10 | 1.14 | 0.95 | 1.00 | 1.07 | 1.10 |
| Platelet (k/mm3) | ||||||
| Subject 1 | 396 | 151 | 215 | 191 | 216 | 165 |
| Subject 2 | 187 | 82 | 128 | 86 | 130 | 189 |
| Subject 3 | 253 | 107 | 150 | 111 | 47 | 173 |
| Absolute neutrophil count (cells/mm3) | ||||||
| Subject 1 | 5,050 | 6,510 | 4,310 | 5,630 | 4,560 | 4,100 |
| Subject 2 | 6,510 | 4,500 | 3,800 | 4,100 | 4,800 | 5,180 |
| Subject 3 | 3,230 | 3,393 | 1,575 | 3,281 | 1,332 | 3,520 |
DISCUSSION
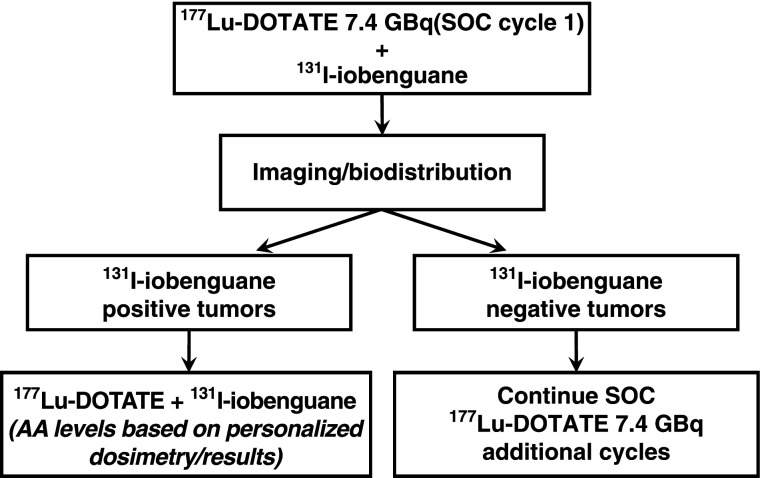
The opening of the trial was delayed to allow time for review and approval by the Centers for Medicare and Medicaid Services for compliance with billing for clinical trials; as the first study of its kind, the trial created a new billing pathway for radionuclide-based planning dosimetry. Enrollment was later hampered by the Food and Drug Administration (FDA) approval of 177Lu-DOTATATE, which meant potential participants had to choose between an FDA-approved commercial therapy or an experimental phase 1 clinical trial. The trial reported here was designed 6 years ago at a time when the only available cationic amino acid solution in the United States was highly emetogenic. Consequently, we did not wish to subject patients to an additional infusion of amino acids for the dosimetric evaluation phase of our trial. Thus, to partially adjust for this consideration, we applied a fixed 20% reduction to the 111In-pentetreotide–generated residence time for use in estimating the expected 90Y-DOTATOC kidney dose for each subject (16). Because the effect of the lysine/arginine solution on renal octreopeptide uptake may vary substantially from one individual to another, we have revised the protocol to account for this effect going forward. Subject biodistribution data can be obtained in future cohorts after 177Lu-DOTATATE treatment (eliminating the need for the pretreatment 111In-pentetreotide surrogate). Moreover, if biodistribution images are obtained after a therapeutic administration, the amino acid effect on renal uptake and radiation dose becomes patient-specific. Finally, high-specific-activity 131I-MIBG (Azedra; Progenics Pharmaceuticals, Inc.) is now an approved agent. High-specific-activity 131I-MIBG may be expected to deliver higher tumor dose levels through improved initial tumor uptake yet with marrow and renal dosimetry similar to that of low-specific-activity 131I-MIBG (17). The revised trial design is depicted in Figure 3.
FIGURE 3.
Modified trial design. AA = administered activity; DOTATE = DOTATATE; SOC = standard of care.
CONCLUSION
These results support the concept that adding 131I-MIBG to PRRT on the basis of individual patient dosimetry can be performed safely and with the possibility of increasing the delivered tumor dose beyond that achievable with 90Y-DOTATOC PRRT alone.
DISCLOSURE
Funding for this trial and support for the investigators was provided by the University of Iowa Department of Radiology, the Holden Comprehensive Cancer Center (3P30CA086862), and the Neuroendocrine SPORE (P50CA174521). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What are the maximum tolerated critical-organ dose limits for therapy with 131I-MIBG added to PRRT (90Y-DOTATOC)?
PERTINENT FINDINGS: Personalized combination of 131I-MIBG added to 90Y-DOTATOC, calculated to deliver 1,900 cGy to the kidneys and 150 cGy to the bone marrow, demonstrated no clinically significant toxicities. Tumors demonstrated an expected dose increase of 34%–83% (with one outlier of 362%) using combination therapy. 177Lu-DOTATATE (Lutathera) will replace 90Y-DOTATOC, and high-specific-activity 131I-MIBG (Azedra) will replace low-specific-activity 131I-MIBG in the next cohort.
IMPLICATIONS FOR PATIENT CARE: Once maximum tolerated organ dose limits for this treatment paradigm are established, a phase 2 trial may safely be initiated.
Acknowledgments
We are deeply grateful for the important contributions made by the following individuals: Kristin Gamari-Varner, Jeff Murguia, Dan Peterson, Mary Schall, Veronica Howsare, and Phil Danzer, in the Department of Radiology, University of Iowa Hospital and Clinics, as well as Teresa Ruggle in the University of Iowa Design Center. In addition, we sincerely thank the clinical trial participants, their families, and the caregivers for making this trial possible.
REFERENCES
- 1. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hicks RJ, Kwekkeboom DJ, Krenning E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms: peptide receptor radionuclide therapy with radiolabelled somatostatin analogues. Neuroendocrinology. 2017;105:295–309. [DOI] [PubMed] [Google Scholar]
- 3. Hope TA, Bergsland EK, Bozkurt MF, et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med. 2018;59:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. [DOI] [PubMed] [Google Scholar]
- 5. Sandström M, Garske-Roman U, Granberg D, et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54:33–41. [DOI] [PubMed] [Google Scholar]
- 6. Barone R, Borson-Chazot F, Valkema R, et al. Patient specific dosimetry in predicting renal toxicity with 90Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose–effect relationship. J Nucl Med. 2005;46(suppl):99S-106S. [PubMed] [Google Scholar]
- 7. Kane A, Thorpe MP, Morse MA, et al. Predictors of survival in 211 patients with stage IV pulmonary and gastroenteropancreatic MIBG-positive neuroendocrine tumors treated with 131I-MIBG. J Nucl Med. 2018;59:1708–1713. [DOI] [PubMed] [Google Scholar]
- 8. Ezziddin S, Sabet A, Logvinski T, et al. Long-term outcome and toxicity after dose-intensified treatment with 131I-MIBG for advanced metastatic carcinoid tumors. J Nucl Med. 2013;54:2032–2038. [DOI] [PubMed] [Google Scholar]
- 9. Bomanji JB. Treatment of neuroendocrine tumours in adults with 131I-MIBG therapy. Clin Oncol (R Coll Radiol) 2003;15:193–198. [DOI] [PubMed] [Google Scholar]
- 10. Pryma DA, Chin BB, Noto RB, et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med. 2019;60:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madsen MT, Bushnell D, Juweid M, et al. Potential increased tumor-dose delivery with combined 131I-MIBG and 90Y-DOTATOC treatment in neuroendocrine tumors: a theoretic model. J Nucl Med. 2006;47:660–667. [PubMed] [Google Scholar]
- 12. Del Prete M, Buteau FA, Arsenault F, et al. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging. 2019;46:728–742. [DOI] [PubMed] [Google Scholar]
- 13. Menda Y, Madsen MT, O’Dorisio TM, et al. 90Y-DOTATOC dosimetry-based personalized peptide receptor radionuclide therapy. J Nucl Med. 2018;59:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bushnell DL, Madsen MT, O’cdorisio T, et al. Feasibility and advantage of adding 131I-MIBG to 90Y-DOTATOC for treatment of patients with advanced stage neuroendocrine tumors. EJNMMI Res. 2014;4:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Besse IM, Madsen M, Bushnell D, Juweid M. Modeling combined radiopharmaceutical therapy: a linear optimization framework. Technol Cancer Res Treat. 2009;8:51–60. [DOI] [PubMed] [Google Scholar]
- 16. Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging. 2003;30:9–15. [DOI] [PubMed] [Google Scholar]
- 17. Barrett JA, Joyal JL, Hillier SM, et al. Comparison of high-specific-activity ultratrace 123/131I-MIBG and carrier-added 123/131I-MIBG on efficacy, pharmacokinetics, and tissue distribution. Cancer Biother Radiopharm. 2010;25:299–308. [DOI] [PubMed] [Google Scholar]