Introduction
Living-donor kidney transplantation (LDKT) is the best therapeutic option for patients with kidney failure. Extensive literature has documented the survival advantage of kidney transplantation (KT) over dialysis, the cost savings to health systems, and improved well-being of the patient. In addition, donors report several benefits that include increased self-esteem and decreased caregiver burden for those who care for the patient. However, patients and donors experience multiple barriers to accessing LDKT. These include challenges, inefficiencies, and disparities at multiple levels of the systems that are involved with delivering LDKT.1-4
Despite recognition of these issues for over a decade and implementation of well-intentioned and heavily resourced interventions, only a small proportion of patients with kidney failure receive LDKT each year.5,6 Average LDKT rates in Canada have not increased significantly over the past decade and are lower than many other high-income countries.1,7-10 The LDKT performance is also highly variable across provinces of Canada.5,11-13 For example, the proportion of LDKT to all kidney transplants performed is 50% to 60% annually in British Columbia (BC), which is significantly higher than Ontario (ON) and Quebec (QC) where the percentages are 30% to 40% and <15%, respectively. 5 The cause of this interprovincial disparity has not been systematically studied; however, our recent work has identified aspects of health system governance and organization that facilitate the delivery of LDKT to patients in BC. 14
There are also persistent disparities in access to LDKT. Groups marginalized by race and ethnicity experience significant inequities when accessing LDKT in Canada.15,16 Indigenous patients have particularly low access to LDKT. They are 52% less likely to receive LDKT compared with white Canadian patients; Indigenous children are 64% less likely.17,18 Overall, strategies to reduce socioeconomic, racial/ethnic, and other observed disparities remain elusive. Scholars from the United States have recently described the need to address structural and systemic factors to address racial disparities in transplantation. 3 We follow Purnell et al in considering that effective interventions to enhance access to LDKT must recognize and address structural, institutional, and interpersonal levels of influence. We suggest that a fundamental shift in how LDKT is approached as a health care service delivered to patients is needed.
In this article, we examine how current efforts to increase LDKT have often focused on individual levels of a health system in silos, and missed important organizational and environmental levels of practice. We go on to describe a full-system approach to LDKT and the possibilities that this might hold for addressing barriers to LDKT, increasing LDKT, and improving access to LDKT.
Current Approach to Health Systems in the Field of LDKT
Health systems comprise of all the organizations, institutions, and resources that are devoted to producing health actions with the primary purpose of improving health. 19 We outline the current understanding and organization of LDKT delivery in Canada in Figure 1. A patient with advanced kidney disease is followed by a multidisciplinary team of health professionals at dialysis centers and nephrology clinics. This is where discussions pertaining to LDKT and transplantation are often initiated. However, sometimes these teams see the patient right at the cusp of dialysis initiation. In these cases, discussions about LDKT may be challenging to conduct as health professionals have to manage complex and acute patient needs and discussions about LDKT may not be a priority. Also, patients have to transition to dialysis rapidly and deal with the social, psychological, and medical demands of being on dialysis and might be too overwhelmed to learn about LDKT. Following this stage of the patient’s trajectory, those interested and deemed eligible are referred to a transplant center; any interested donors are encouraged to contact the transplant center directly. Transplant centers will then evaluate the patient, readdress the benefits of pursuing LDKT, and provide resources and other educational tools. A separate team will concurrently evaluate an interested donor. Regional programs often assist both the donor and the patient. Organizations, such as Canadian Blood Services, may get involved should there be a need for paired kidney exchange (Figure 1).
Figure 1.
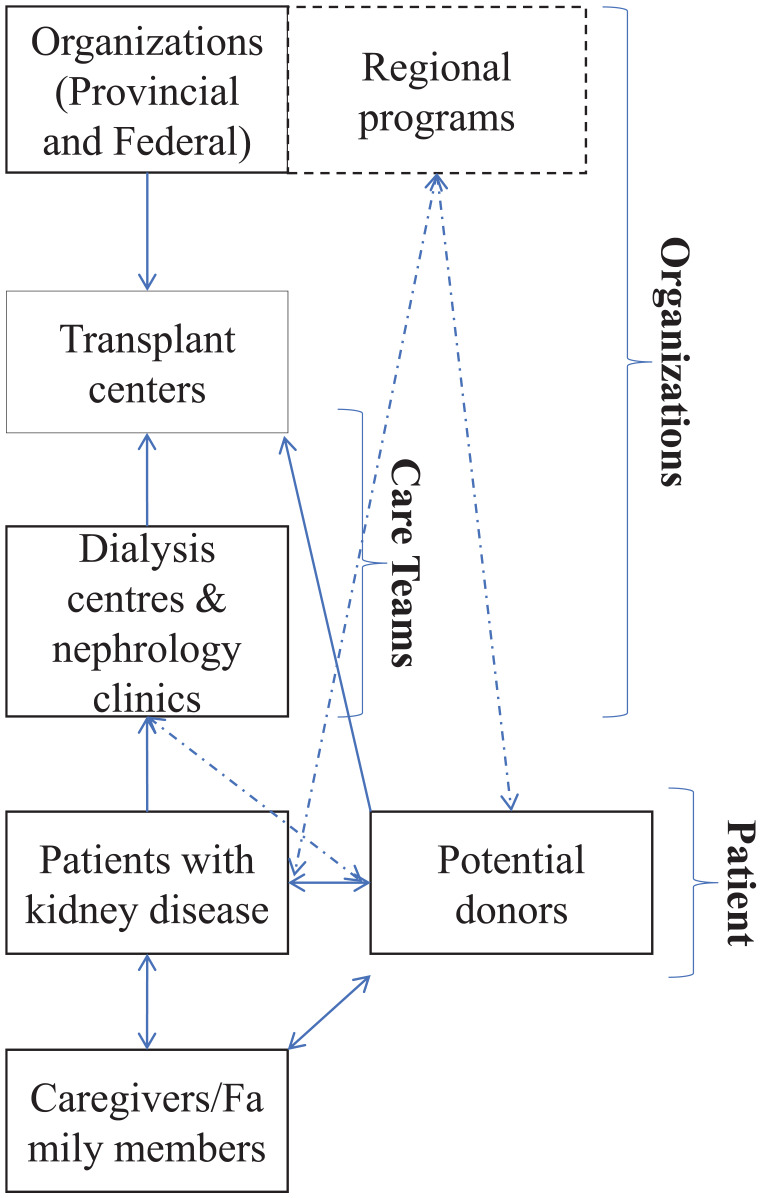
An outline of the current understanding and organization of living-donor kidney transplantation delivery.
Limitations of the Current Approach
Much of the present effort to increase LDKT tends to focus on studying individual levels, often examining them in silos. We now describe the limitations of current practices and approaches to increasing LDKT (Table 1).
Table 1.
Limitations of Current Practices and Approaches to Increasing LDKT.
| Overall approach • Focusing on each level of a health system in silos • Failure to recognize the complexity and interdependencies of each level • Implementing interventions that target one level Focusing primarily on patient-level barriers • Expecting an overwhelmed and sick patient to find donors • Short-term efficacy of interventions and high risk of selection bias • Amplifying, perhaps causing, disparities in access Limited understanding of barriers faced by care teams • Variable issues faced by different treating teams • Unmet needs and addressing concerns of health professionals • Inconsistent and inexplicit recommendations can cause disparities Poor grasp of organizational-level barriers • Poor infrastructure, limited resources, lack of strong leadership • Patient-identified issues and long donor evaluation time • Resources for meeting the needs of a diverse patient population Anecdotal work on an environment to support LDKT • Political and economic issues • Lack of a centralized source of information • Variable local/state and research mandates • Systemic hardships for donors, in particular, financial and access to long-term care |
Note. LDKT = Living-donor kidney transplantation.
Expecting an Overwhelmed Patient to Recruit Donors
Much of the current focus is placed on addressing patient-level barriers to LDKT, such as a patient’s discomfort to approach potential donors and lack of knowledge about LDKT. Interventions to address these via home-based interventions, teaching tools, and a social worker intervention have been developed.20-23 Some efforts have involved identifying a donor advocate or “champion.” 23 Although patients are encouraged to play a more active role in their care decisions and treatment options, many patients have been unable to adapt to this updated role effectively; many lack access to the information, tools, and other resources needed. 24 Thus, placing the onus of finding a donor on an already overwhelmed and sick patient who is dealing with dialysis and other complex treatment regimens may be of little yield.25,26
Short-Term Efficacy of Interventions and High Risk of Selection Bias
In 2017, a scoping review found that only 2 of 7 included studies reported statistically significant increases in the number of living donors and living-donor evaluations following individually targeted educational interventions. 27 A systematic review and meta-analysis reported that the overall effectiveness of patient-level interventions implemented to increase patient knowledge and comfort is modest. 28 The quality across these included studies was mixed and there was a high risk of selection bias. Several studies restricted patient participation by geography, language consideration, ability to use a computer, and physician preference.
Limited Understanding of Barriers Faced by Care Teams
Very limited work has been done on care teams, with most focusing on the inputs of nephrologists alone.29-31 Barriers experienced by frontline staff, such as dialysis nurses, and caregivers of the patients are largely unaddressed. The multidisciplinary professional teams involved in the care of patients both at the transplant center and at the referring dialysis centers/nephrology clinics can struggle with complex choices. They aim to achieve optimal recipient outcomes against the competing priority of justifying donor sacrifice. 29 Those involved with the donor assessment may struggle with the complex balance between safeguarding the donors’ welfare and respecting their autonomy. 31 When we specifically sought to identify barriers experienced by health professionals when discussing LDKT with their patients, several other themes emerged. 30 These included the lack of communication between transplant and dialysis teams, absence of referral guidelines, poor role perception and lack of multidisciplinary involvement, and lack of information and training.
Amplifying Disparities
Attempting to increase LDKT by only focusing on patient-level barriers may have created an inequitable 2-tier system, favoring those patients who have the socioeconomic means to learn the process and find donors.25,32 It was quantitatively assessed that a socioeconomically advantaged quartile of patients in Australia was 34% more likely to receive LDKT compared with the most disadvantaged quartile. 33 Not addressing the barriers experienced by care teams also contributes to disparities. The crucial role of health professionals, especially nurses, in a patient’s decision to pursue LDKT is well recognized. It is also known that their personal biases, lack of knowledge, and discomfort can lead to inconsistent and inexplicit recommendations and that this may intensify inequity to LDKT.29,30,34,35 A 2016 study showed that socioeconomically disadvantaged and ethnic minority patients were deemed by health professionals as less likely to have a suitable donor because of a higher incidence of obesity, cardiovascular disease, and diabetes. 29 On the contrary, patients of higher socioeconomic status were expected to be more likely to receive LDKT because they were “a good advocate for themselves.” 29 In our work, we noted that health professionals’ own accounts of encounters with patients reflected a propensity to pinpoint patients’ attitudes and characteristics as the main barrier to discussions about LDKT. 30
Poor Grasp of Organizational-Level Barriers
There is also a scarcity of literature on the barriers that organizations face while organizing, promoting, and delivering LDKT. Patients have identified organizational issues such as lengthy donor evaluation process, navigating a fragmented donor evaluation system, financial impact of donation, and lack of a centralized source of information. 1 Median duration of a donor evaluation in some centers in Canada and Australia ranges from 6.5 to 16.7 months. 36 While patients require free exchange of information and communication with their care teams, these teams are bound to organizations that provide the supporting infrastructure and resources. 24 Therefore, organizational barriers can affect access to LDKT. These include managerial and administrative and infrastructure issues, as well as structural and economic problems. For example, for-profit facilities had a lower chance of having patients receive LDKT compared with nonprofit facilities. 37 The factors contributing to this are largely speculative. In addition, barriers that are frequently considered as patient-level, with respect to cultural background, belief systems and language considerations often originate due to limited organizational resources directed toward a diverse patient population.
An Environment to Support LDKT
Finally, evidence about the role of the broader political and economic environments in facilitating LDKT has largely been anecdotal. 38 For example, a longitudinal study of 44 nations suggests that policies for presumed consent for deceased organ donation may negatively influence LDKT rates. 39 However, causality could not be established within the scope of this observational research. There are undoubtedly important environmental factors that significantly impact LDKT delivery. This includes the influences of provincial/state and national governmental bodies, and nongovernmental organizations. Significant gaps exist in legislation and policy frameworks to guide provincial transplant programs.13,7 Thus, in 2017 Prime Minister Justin Trudeau identified “facilitating collaboration on an organ and tissues donation and transplantation system that gives Canadians timely and effective access to care” as a priority to his then Minister of Health. 40 However, there is a lack of a centralized source of information and the organization of LDKT varies across different provinces. It is subject to mandates, structures, resources, and expenditure allocation that are localized to a particular province’s leadership. There is substantial variability in the support for living donors and they are known to incur out-of-pocket costs and those related to lost productivity.38,41 Although recognized, these barriers are not adequately addressed and attempts to attain financial neutrality for donors have been met with challenging ethical and legal considerations. This suggests the importance of attending to environmental factors that influence the execution of LDKT within the health system that delivers LDKT.
Advancing a Paradigm Shift to Approaching Health Systems in the Field of LDKT
Barriers and disparities in access to LDKT have multiple causes and some are well recognized.2-4 Elimination or reduction of these requires a better understanding of how a health system operates and calls for multilevel interventions to occur simultaneously or in close succession. 42 Leading health services researchers have argued that failure to understand the complexity of health systems is the reason why strategies and interventions based on isolated concepts or goals, such as access to a particular service, so often fall short of desired objectives.43,44 We believe that focusing on one level at a time leads to disjointed efforts that, despite being resource-intensive, have not increased LDKT and have amplified, perhaps caused, disparities in access to LDKT. Thus, we propose a comprehensive health systems approach to better understand the delivery of LDKT and how this can inform stronger and sustainable multilevel interventions.
The Concept of Complex Adaptive System (CAS)
The CAS thinking is an approach that has been used extensively and successfully to inform change and development in a range of sectors, such as education, engineering, and management. 44 It proposes that a system like health care is a dynamic network of agents acting in parallel, constantly reacting to what the other agents are doing, which in turn influences behavior and the network as a whole. 45 Health services researchers recommend CAS thinking to understand the constructs and dynamics of a health system.43,44 LDKT, as any other field of health care delivery, can be classified as a CAS because the various elements within it, such as organizations, transplant teams, referring teams, donors, and patients, are interconnected agents that work in nonlinear and evolving ways. 43
Understanding LDKT as a CAS
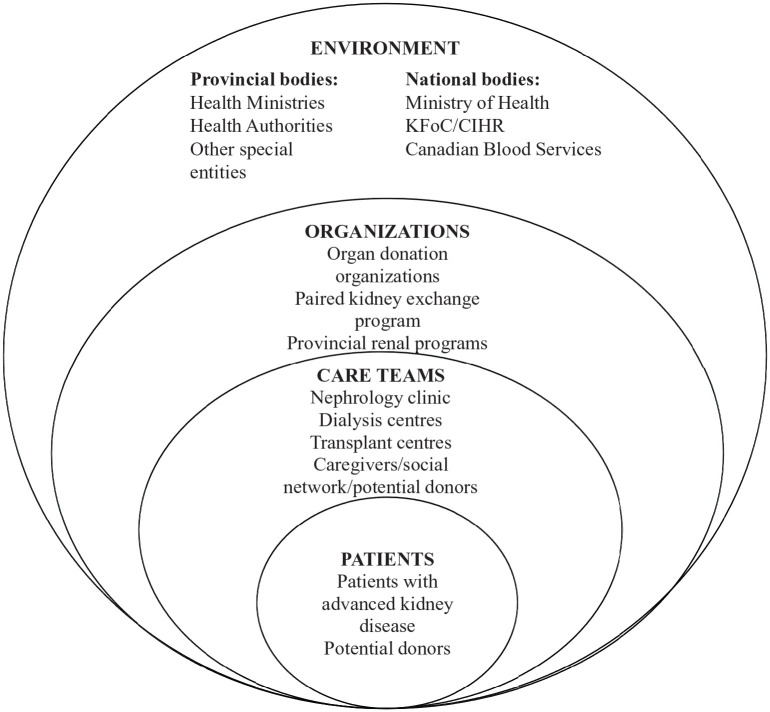
Health systems understanding then entails analyzing and unpacking a CAS to identify its parts and relationships. 43 A health system is divided into 4 nested and interconnected levels with the patient at its core. 24 These are (1) the individual patient; (2) the care teams, which include the multidisciplinary care providers and social support of the patient; (3) the organizations that provide infrastructure and resources; and (4) the political and economic environment. These levels provide the rough divisions of labor and interdependencies among major elements of the system, and most importantly, “the levers for change.” 24 We propose a similar approach to LDKT delivery described within a CAS framework (Figure 2). Such an approach can be used to understand how connected and multilevel interactions produce barriers and facilitators to LDKT. Many have argued for such approaches when designing and applying interventions and assessments in health care. 46 Indeed, recent work has utilized such forms of systems learning to address racial disparities in health care and transplantation. 3
Figure 2.
Advancing a paradigm shift to approaching health systems that governs living-donor kidney transplantation as a Complex Adaptive System that is made up of interconnected and nested levels with the patient at the core.
Source. Adapted from the 4-level model proposed by the National Academy of Engineering (US) and Institute of Medicine (US) Committee on Engineering and the Health Care System.
Note. CIHR = Canadian Institutes of Health Research; KFoC = Kidney Foundation of Canada.
Moving From One Level to Multileveled Interventions
In a high-performing health system, every level within it recognizes its dependence and influence on all other levels, and the imperative of addressing interventions targeting each level to optimize the performance of the system as a whole.14,24 Equally, multilevel interventions can help address determinants of health, and better address barriers that lead to disparities in access to care, such as those related to cultural background, belief systems, and language considerations.42,46 This is because the theoretical constructs of CAS emphasize understanding interdependent system dynamics. Thus, a CAS approach can be used to address systemic issues that persist in the field of LDKT, as it can inform multipronged and sensitive interventions that engage multiple stakeholders at all levels of a health system.14,29,30 As well as improving upon interventions that address recognized problems, such as inadequate resources for patient education, we consider that engaging with the complexity of a health system will be essential to targeting organizational and environmental barriers to LDKT as outlined in Figure 2.
Evidence of Efficacy in LDKT Literature
In several fields of health care, such as cancer care and diabetes management, it is widely recognized that effective interventions must influence multiple levels of a health system.47,48 There is some evidence that multilevel interventions to increase LDKT are more effective as well. For example, the Live Donor Champion Program developed at Johns Hopkins University offers a 2-level approach. 23 Herein, a 5-to-6-week educational program targeted the patient (n = 15) and a member of their care team identified by the patient as their champion. Following this intervention, 25 potential donors contacted the center, 4 participants received LDKT, and 3 additional participants had donors in evaluation, compared with zero among matched controls (P < .001). Perhaps the best example of CAS being used to inform multilevel intervention is the work from Gordon and colleagues, who developed a culturally competent and linguistically congruent program. 49 They intervened on multiple levels that went beyond the patient-provider interaction and identified this approach to be more holistic and effective in implementing their program. The program was associated with a 74% increase in the target population receiving LDKTs. Among their recommendations is the need to adjust institutional infrastructure to accommodate interventions, recognizing that effective interventions require understanding what factors challenge care delivery processes.
Anticipated Challenges to This Approach
Some issues within this paradigm shift are anticipated. First, research methodologies for studies pertaining to health systems learning are underdeveloped and proposed interventions are often contextual engaging few levels.42,46,50 Recently, we have employed a qualitative methodology with a CAS framework to conduct a case study of a high-performing provincial health system in Canada. 14 Several systemic factors were recognized as barriers and facilitators to LDKT. Although well recognized across many health care domains, qualitative methodologies remain underutilized in transplantation research. Second, the impact of multilevel interventions on individual patients may be gradual and one might not see the immediate effects. 42 Also, a CAS is not static but rather will evolve and adapt over time; models of care and interventions will need to be adjusted accordingly. 43 This requires long-term commitment and support that may prove challenging to secure. A scoping review of multilevel interventions in diabetes prevention and treatment in Canada identified buy-in from various key parties and organizations as a key challenge. 51
Finally, a multilevel approach puts a big impetus on the fourth level of the health system, that is, the environment under which the other 3 levels operate, such as regulatory, financial, and payment regimes. Implementing policy-level changes for interventions might result in greater health impacts, 42 which may require strong advocacy and lobbying from the transplant community. Federal agencies, such as the Canadian Institutes of Health Research and the Kidney Foundation of Canada, are some of the primary sources of funding for research. Support from them and other federal and state organizations will influence the trajectories of health care and research and support these multileveled interventions. 24 To create long-lasting and comprehensive improvements, buy-in from political leaders and federal and provincial representatives is needed. 42
Conclusion
We offer our personal viewpoint that current approaches to increasing LDKT are deficient, as they often put the impetus of finding a donor on the patient, contribute to disparities in access to LDKT, and do not adequately address barriers faced by care teams and organizations. Thus, we suggest that there is a need to engage with health systems as a CAS made up of dynamic, nested, and interconnected levels, with the patient at its core. This model may be used to better inform multilevel interventions and guide how interventions could be introduced and sustained. Overall, we recommend this paradigm shift to understanding how health systems deliver LDKT to increase rates and improve access to this gold-standard treatment.
Footnotes
List of Abbreviations: CAS, complex adaptive system; LDKT, living-donor kidney transplantation.
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Availability of Data and Materials: Not applicable.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.S. has received an education grant from Amgen Canada.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Gift of Life Institute Clinical Faculty Development Research Grant from the American Society of Transplantation. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, writing, review, or approval of the manuscript.
ORCID iD: Shaifali Sandal  https://orcid.org/0000-0003-1941-0598
https://orcid.org/0000-0003-1941-0598
References
- 1. Getchell LE, McKenzie SQ, Sontrop JM, Hayward JS, McCallum MK, Garg AX. Increasing the rate of living donor kidney transplantation in Ontario: donor- and recipient-identified barriers and solutions. Can J Kidney Health Dis. 2017;4:2054358117698666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Purnell TS, Hall YN, Boulware LE. Understanding and overcoming barriers to living kidney donation among racial and ethnic minorities in the United States. Adv Chronic Kidney Dis. 2012;19(4):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Purnell TS, Simpson DC, Callender CO, Boulware LE. Dismantling structural racism as a root cause of racial disparities in COVID-19 and transplantation. Am J Transplant. 2021;21(7):2327–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garg AX. Helping more patients receive a living donor kidney transplant. Clinical Journal of the American Society of Nephrology. 2018;13(12):1918–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Organ replacement in Canada: CORR annual statistics, 2020. https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020. Published 2020. Accessed February 11, 2021.
- 6. Hart A, Lentine KL, Smith JM, et al. OPTN/SRTR 2019 Annual Data Report: kidney. Am J Transplant. 2021;21(suppl 2):21–137. [DOI] [PubMed] [Google Scholar]
- 7. Canadian Blood S. Organ donation and transplantation in Canada, System Progress Report 2006–2015. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/15707/sam-shemie-part-1.pdf. Published 2016. Accessed February 4, 2022.
- 8. Anderson B, Khalil K, Evison F, Nath J, Sharif A. Hypoalbuminaemia at time of surgery is associated with an increased risk for overall graft loss after kidney transplantation. Nephrology (Carlton). 2019;24(8):841–848. [DOI] [PubMed] [Google Scholar]
- 9. Kim SJ, Fenton SSA, Kappel J, et al. Organ donation and transplantation in Canada: insights from the Canadian organ replacement register. Can J Kidney Health Dis. 2014;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norris S. Organ Donation and Transplantation in Canada. Canada: Legal and Social Affairs Division, Parliamentary Information and Research Service; 2018. https://publications.gc.ca/collections/collection_2018/bdp-lop/bp/YM32-2-2018-13-eng.pdf. Accessed October 31, 2021. [Google Scholar]
- 11. Arshad A, Anderson B, Sharif A. Comparison of organ donation and transplantation rates between opt-out and opt-in systems. Kidney Int. 2019;95(6):1453–1460. [DOI] [PubMed] [Google Scholar]
- 12. Organ donation and transplantation in Canada, System Progress Report 2006–2015. https://blood.ca/sites/default/files/ODT_Report.pdf. Published 2016. Accessed February 4, 2022.
- 13. Norris S. Organ Donation and Transplantation in Canada. Publication No. 2018-13-E. Ottawa, Canada: Library of Parliament. https://lop.parl.ca/sites/PublicWebsite/default/en_CA/ResearchPublications/202028E?. Published 2018. Accessed February 4, 2022. [Google Scholar]
- 14. Horton A, Nugus P, Fortin M, Landsberg D, Cantarovich M, Sandal S. Health system barriers and facilitators to living donor kidney transplantation: a qualitative case study of British Columbia. CMAJ Open. 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mucsi I, Bansal A, Famure O, et al. Ethnic background is a potential barrier to living donor kidney transplantation in Canada: a single-center retrospective cohort study. Transplantation. 2017;101(4):e142–e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Dassouki N, Wong D, Toews DM, et al. Barriers to accessing kidney transplantation among populations marginalized by race and ethnicity in Canada: a scoping review part 2-East Asian, South Asian, and African, Caribbean, and Black Canadians. Can J Kidney Health Dis. 2021;8:2054358121996834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeates KE, Schaubel DE, Cass A, Sequist TD, Ayanian JZ. Access to renal transplantation for minority patients with ESRD in Canada. Am J Kidney Dis. 2004;44(6):1083–1089. [DOI] [PubMed] [Google Scholar]
- 18. Samuel SM, Foster BJ, Tonelli MA, et al. Dialysis and transplantation among Aboriginal children with kidney failure. CMAJ. 2011;183(10):E665–E672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. The World Health Report 2000: health systems: improving performance. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 20. Massey EK, Gregoor PJ, Nette RW, et al. Early home-based group education to support informed decision-making among patients with end-stage renal disease: a multi-centre randomized controlled trial. Nephrol Dial Transplant. 2016;31(5):823–830. [DOI] [PubMed] [Google Scholar]
- 21. Gordon EJ, Feinglass J, Carney P, et al. A culturally targeted website for Hispanics/Latinos about living kidney donation and transplantation: a randomized controlled trial of increased knowledge. Transplantation. 2016;100(5):1149–1160. [DOI] [PubMed] [Google Scholar]
- 22. Rodrigue JR, Paek MJ, Egbuna O, et al. Making house calls increases living donor inquiries and evaluations for blacks on the kidney transplant waiting list. Transplantation. 2014;98(9):979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garonzik-Wang JM, Berger JC, Ros RL, et al. Live donor champion: finding live kidney donors by separating the advocate from the patient. Transplantation. 2012;93(11):1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Academy of E, Institute of Medicine Committee on E, the Health Care S. The national academies collection: reports funded by national institutes of health. In: Reid PP, Compton WD, Grossman JH, Fanjiang G. eds. Building a Better Delivery System: A New Engineering/health Care Partnership. Washington, DC: National Academies Press; 2005: 19-24. [Google Scholar]
- 25. Waterman AD, Stanley SL, Covelli T, Hazel E, Hong BA, Brennan DC. Living donation decision making: recipients’ concerns and educational needs. Prog Transplant. 2006;16(1):17–23. [DOI] [PubMed] [Google Scholar]
- 26. Kranenburg LW, Richards M, Zuidema WC, et al. Avoiding the issue: patients’ (non)communication with potential living kidney donors. Patient Educ Counsel. 2009;74(1):39–44. [DOI] [PubMed] [Google Scholar]
- 27. Barnieh L, Collister D, Manns B, et al. A scoping review for strategies to increase living kidney donation. Clin J Am Soc Nephrol. 2017;12(9):1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sandal S, Dendukuri N, Wang S, Guadagno E, Ekmekjian T, Alam A. Efficacy of educational interventions in improving measures of living-donor kidney transplantation activity: a systematic review and meta-analysis. Transplantation. 2019;103(12):2566–2575. [DOI] [PubMed] [Google Scholar]
- 29. Hanson CS, Chadban SJ, Chapman JR, Craig JC, Wong G, Tong A. Nephrologists’ perspectives on recipient eligibility and access to living kidney donor transplantation. Transplantation. 2016;100(4):943–953. [DOI] [PubMed] [Google Scholar]
- 30. Sandal S, Charlebois K, Fiore JF, Jr, et al. Health professional-identified barriers to living donor kidney transplantation: a qualitative study. Can J Kidney Health Dis. 2019;6:2054358119828389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tong A, Chapman JR, Wong G, Craig JC. Living kidney donor assessment: challenges, uncertainties and controversies among transplant nephrologists and surgeons. Am J Transplant. 2013;13(11):2912–2923. [DOI] [PubMed] [Google Scholar]
- 32. Giles S. An antidote to the emerging two tier organ donation policy in Canada: the public cadaveric organ donation program. J Med Ethics. 2005;31(4):188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grace BS, Clayton PA, Cass A, McDonald SP. Transplantation rates for living—but not deceased—donor kidneys vary with socioeconomic status in Australia. Kidney Int. 2013;83(1):138–145. [DOI] [PubMed] [Google Scholar]
- 34. Anderson K, Devitt J, Cunningham J, Preece C, Jardine M, Cass A. If you can’t comply with dialysis, how do you expect me to trust you with transplantation? Australian nephrologists’ views on indigenous Australians “non-compliance” and their suitability for kidney transplantation. Int J Equity Health. 2012;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandal S, Chen T, Cantarovich M. Evaluation of transplant candidates with a history of nonadherence: an opinion piece. Can J Kidney Health Dis. 2021;8:2054358121990137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Habbous S, Arnold J, Begen MA, et al. Duration of living kidney transplant donor evaluations: findings from 2 multicenter cohort studies. Am J Kidney Dis. 2018;72(4):483–498. [DOI] [PubMed] [Google Scholar]
- 37. Gander JC, Zhang X, Ross K, et al. Association between dialysis facility ownership and access to kidney transplantation. JAMA. 2019;322(10):957–973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Tushla L, Rudow DL, Milton J, Rodrigue JR, Schold JD, Hays R. Living-donor kidney transplantation: reducing financial barriers to live kidney donation—recommendations from a consensus conference. Clin J Am Soc Nephrol. 2015;10(9):1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horvat LD, Cuerden MS, Kim SJ, Koval JJ, Young A, Garg AX. Informing the debate: rates of kidney transplantation in nations with presumed consent. Ann Intern Med. 2010;153(10):641–649. [DOI] [PubMed] [Google Scholar]
- 40. Trudeau J. Minister of Health Mandate Letter. https://pm.gc.ca/en/mandate-letters/2017/10/04/archived-minister-health-mandate-letter. Published 2017. Accessed January 20, 2021.
- 41. Barnieh L, Klarenbach S, Arnold J, et al. Nonreimbursed costs incurred by living kidney donors: a case study from Ontario, Canada. Transplantation. 2019;103(6):e164–e171. [DOI] [PubMed] [Google Scholar]
- 42. Paskett E, Thompson B, Ammerman AS, Ortega AN, Marsteller J, Richardson D. Multilevel interventions to address health disparities show promise in improving population health. Health Aff (Millwood). 2016;35(8):1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuziemsky C. Decision-making in healthcare as a complex adaptive system. Healthc Manage Forum. 2016;29(1):4–7. [DOI] [PubMed] [Google Scholar]
- 44. Complex adaptive systems. https://www.health.org.uk/publications/complex-adaptive-systems. Published 2010. Accessed July 20, 2021.
- 45. Holland JH. Studying complex adaptive systems. J Syst Sci Complex. 2006;19(1):1–8. [Google Scholar]
- 46. Agurs-Collins T, Persky S, Paskett ED, et al. Designing and assessing multilevel interventions to improve minority health and reduce health disparities. Am J Public Health. 2019;109(suppl 1):S86–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gittelsohn J, Rowan M. Preventing diabetes and obesity in American Indian communities: the potential of environmental interventions. Am J Clin Nutr. 2011;93(5):1179S-83S. [DOI] [PubMed] [Google Scholar]
- 49. Gordon EJ, Romo E, Amórtegui D, et al. Implementing culturally competent transplant care and implications for reducing health disparities: a prospective qualitative study. Health Expect. 2020;23(6):1450–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stange KC, Breslau ES, Dietrich AJ, Glasgow RE. State-of-the-art and future directions in multilevel interventions across the cancer control continuum. J Natl Cancer Inst Monogr. 2012;2012(44):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stotz SA, McNealy K, Begay RL, DeSanto K, Manson SM, Moore KR. Multi-level diabetes prevention and treatment interventions for native people in the USA and Canada: a scoping review. Curr Diab Rep. 2021;21(11):46. [DOI] [PMC free article] [PubMed] [Google Scholar]