Abstract
The heterogeneity in disease pathology, the unpredictability in disease prognosis, and the variability in response to therapy make mantle cell lymphoma (MCL) a focus of novel therapeutic development. MCL is characterized by dysregulated expression of cyclin D1 through a chromosome t(11;14) translocation. MCL international prognostic index (MIPI), ki-67 proliferation index, and TP53 mutation status are currently utilized for prognostication. With advances in pharmacokinetic analysis and drug discovery, treatment strategy has evolved from chemotherapy to combination of targeted, epigenetic, and immune therapies. In this review, we discuss investigational and newly approved treatment approaches. In a short time, the US Food and Drug Administration (FDA) has approved five agents for the treatment of MCL: lenalidomide, an immunomodulatory agent; bortezomib, a proteasome inhibitor; and ibrutinib, acalabrutinib, and zanubrutinib, all Bruton kinase inhibitors. Epigenetic agents (e.g. cladribine and vorinostat), mammalian target of rapamycin (mTOR) inhibitors (e.g. temsirolimus and everolimus), and monoclonal antibodies and/or antibody-drug conjugates (e.g. obinutuzumab, polatuzumab, and ublituximab) are promising therapeutic agents currently under clinical trial investigation. Most recently, chimeric antigen receptor (CAR)-T cell therapy and bispecific T-cell engager (BiTE) therapy even open a new venue for MCL treatment. However, due to its intricate pathology nature and high relapse incidence, there are still unmet needs in developing optimal therapeutic strategies for both frontline and relapsed/refractory settings. The ultimate goal is to develop innovative personalized combination therapy approaches for the purpose of delivering precision medicine to cure this disease.
Keywords: allogeneic hematopoietic stem cell transplant, BiTE therapy, CAR-T therapy, cyclin D1, epigenetics, immunotherapy, mantle cell lymphoma, targeted therapies
Introduction
Mantle cell lymphoma (MCL) is a rare subtype of lymphoid malignancies that accounts for 2.5–6% of all non-Hodgkin lymphomas (NHLs), with an annual incidence of 1 in 200,000 people.1–3 MCL is more common in men than women (3 to 1), and the median age at diagnosis is between 60 and 70 years of age. 4 To date, MCL remains a largely incurable disease with a median overall survival (OS) of 5 years.
MCL is characterized by a t(11;14)(q13; q32) translocation that places the cyclin D1 (CCND1) gene, located at chromosome 11q13, under the influence of the immunoglobulin heavy chain gene (IGH) enhancers at chromosome 14q32. Thus, MCL pathogenesis is driven by the uncontrolled growth of B cells. Typically undetectable in lymphoid and hematopoietic tissue, 5 CCND1 controls the cells’ transition from G1 to S phase of cell cycle. As the main pathological driver of disease, CCND1 has become a valuable marker for diagnosis and, more recently, treatment of MCL.6,7 Epigenetic deregulation of cyclin D1 has also been reported to accelerate cell cycle and tumorigenesis. 8 As such, MCL can be regarded as the paradigm of lymphoma with increased proliferation due to a dysregulated cell cycle.
Many factors are involved in the prognostication of MCL, including age, organ involvement, lactate dehydrogenase (LDH) level, leukocyte count, severity of anemia, TP53 gene mutation, Ki-67 proliferation index, and the MCL international prognostic index (MIPI) score.2,9–12 The MIPI stratifies patients into three risk categories: low, indeterminate, and high. This distinction has helped to provide quantitative prognostic values with regard to mean OS, with the low-risk group conferring a mean OS that has not yet been reached; over 60% of the patients have reached the 5-year survival mark. Meanwhile, the intermediate and high-risk groups reached a median OS of 51 and 29 months, respectively. 11 Efforts have been made to incorporate Ki-67, a MIPI-independent surrogate marker of cellular proliferation, into MCL prognosis stratification. In specific patient populations, including those receiving high-dose chemotherapy and autologous stem cell transplant (ASCT), Ki-67 has been found to be a better predictor of survival than MIPI. 13 In addition, the Ki-67 index has a better predictive value than MIPI in randomized trials of rituximab-containing regimens. 14
Identifying prognostic values has become more critical with the advent of personalized medicine and the increasing economic feasibility of next-generation sequencing (NGS). On a singular gene basis, specific genes such as Sox 11 were found to be a biomarker predicting response to treatment. 15 However, this approach can also be extended to the entire human genome where gene expression profiling of mRNA extracted from bulk tissue biopsies can be used to correlate with clinical outcomes. 16 TP53 mutations and CDKN2A deletion demonstrated a significant association with inferior clinical outcomes. Multivariate analysis further found that harboring TP53 mutations correlated significantly with other adverse risk features. Median progression-free survival (PFS) among patients harboring a TP53 mutation was 1 year, whereas those without a TP53 mutation achieved a median PFS of 12.7 years.16,17 Generally, patients with TP53 mutation do poorly with conventional therapy and consequently require alternative novel therapeutic approaches. More recently, the long-term follow-up data from patients enrolled in the Nordic Mantle Cell Lymphoma Trials, MCL2 and MCL3, demonstrated that patients with progression of disease before 24 months (POD24, n = 51, 34%) displayed a median OS of 6.6 months compared with 46 months for patients with later POD (n = 98, 66%; P < 0.001), which confirmed that POD24 is a novel biomarker for MCL death-risk stratification.18,19
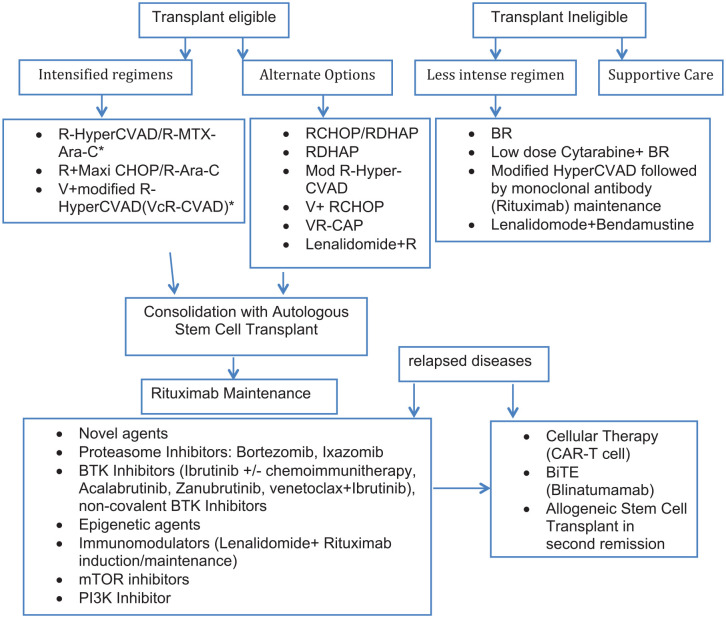
In this review, we discuss a multitude of novel therapeutic agents and strategies that have evolved in recent years, including bortezomib, Bruton’s tyrosine kinase (BTK) inhibitors, lenalidomide and other immunomodulatory agents, venetoclax, advances in ASCT, chimeric antigen receptor (CAR)-T cell, and bispecific T-cell engager (BiTE) therapies (Figure 1). We also discuss some promising novel molecules in evaluation.
Figure 1.
MCL treatment algorithm.
BiTE, bispecific T-cell engager; BR, bendamustine and rituximab; BTK, Bruton’s tyrosine kinase; CAR-T, chimeric antigen receptor-T cell; CHOP, cyclophosphamide, vincristine, doxorubicin, and prednisone; Mod, modified; mTOR, mammalian target of rapamycin; RDHAP, rituximab, dexamethasone, cytarabine, and cisplatin; R-HyperCVAD, rituximab in combination with hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone; V, bortezomib; VcR-CVAD, bortezomib with modified R-HyperCVAD; VR-CAP, bortezomib plus rituximab, cyclophosphamide, doxorubicin, and prednisone.
Efficacy of novel agents
With the advancement in developing innovative MCL therapies, the treatment paradigm is constantly evolving. From optimizing traditional methods by implementing new or repurposed drugs such as bortezomib or ibrutinib and other BTK inhibitors to the advent of novel approaches, including the advance of CAR-T cell therapy and BiTE therapy strategies, the field of MCL therapy has expanded into a wide variety of novel therapeutics in development. Alternatively, the traditional ASCT, with the purpose of prolonging remission, is also evolving. Autologous tumor cell vaccine study found that activation of CpG motif in tumor cells can enhance tumor responses and significantly impact the disease course. 20
Bortezomib
Bortezomib, a first-generation proteasome inhibitor, has seen limited efficacy in response rates in relapsed MCL patients as a monotherapy. 21 In combination settings with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP), bortezomib demonstrated an overall response rate (ORR) of 81–91%, complete response (CR) of 64%, and a median PFS of 23 months. 22 Vincristine was removed in that protocol due to neurotoxicity. A combination of bortezomib and R-CHP could accomplish a better median PFS than R-CHOP of 24.7 versus 14.4 months, respectively. 23 Initiating bortezomib as post-bortezomib-R-CHP treatment maintenance therapy could further enhance CR and PFS to 83% and 29.5 months, respectively. 24 However, patients usually do not tolerate bortezomib maintenance well due to neuro- and gastrointestinal toxicities.
Bortezomib has been shown to be safe in combination with intensive regimens such as R-HyperCVAD (rituximab in combination with hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone alternating with rituximab-methotrexate and cytarabine). 25 The addition of bortezomib to modified R-HyperCVAD or VcR-CVAD (bortezomib with modified R-HyperCVAD) made long-term remission possible, with ORR of 95% and CR of 68%. Post-induction comparison between 2 years of rituximab maintenance and ASCT showed similar OS and PFS. 26 Wisconsin Oncology Network analyzed long-term follow-up data after VcR-CVAD induction regimen and rituximab maintenance for 5 years, in which ORR and CR were 90% and 77%, respectively. The median PFS was 8.14 years and its OS was still not yet reached at the time of analysis. 27 A post-ASCT bortezomib and rituximab (BR) treatment study found a 2-year disease-free survival (DFS) and OS of 94.4% and 100%, respectively. 28 Currently, several clinical trials combining a second-generation oral proteasome inhibitor, Ixazomib, with other immunotherapy regimens and target therapy regimens in treating MCL are ongoing (https://clinicaltrials.gov; Table 1).
Table 1.
The ongoing clinical trials combining Ixazomib with other immunotherapy regimens and target therapy regimens in treating MCL.
| Trial phase | Setting | ClinicalTrials.gov identifier | Study titles |
|---|---|---|---|
| 1/2 | Relapsed/refractory MCL | NCT03323151 | A Study of Ixazomib and Ibrutinib in Relapsed/Refractory Mantle Cell Lymphoma |
| 2 | BTK inhibitor-resistant MCL | NCT04047797 | Ixazomib and Rituximab in Treating Patients With Relapsed or Refractory Mantle Cell Lymphoma |
| 1/2 | Post-autologous HSC transplant in MCL | NCT02632396 | Ixazomib & Rituximab after Stem Cell Transplant in Treating Patients with Mantle Cell Lymphoma in Remission |
| 2 | Indolent B-cell NHL | NCT02339922 | Ixazomib Citrate and Rituximab in Treating Patients with Indolent B-Cell Non-Hodgkin Lymphoma |
| 2 | Post-treatment maintenance therapy | NCT03616782 | Ixazomib Maintenance in Patients with Newly Diagnosed Mantle Cell Lymphoma (MCL) |
BTK, Bruton’s tyrosine kinase; MCL, mantle cell lymphoma; NHL, non-Hodgkin lymphoma.
In an 128 clinical centers participated open-label phase III study, the efficacy of frontline bortezomib plus rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP), and R-CHOP were compared among 487 transplant-ineligible MCL patients. The median OS for patients who received VR-CAP was significantly longer than for patients who received R-CHOP, 90.7 versus 55.7 months, with a manageable toxicity profile. 29
Ibrutinib and other BTK inhibitors
Ibrutinib is the US Food and Drug Administration (FDA)–approved original first-in-class BTK inhibitor preferred for relapsed/refractory (R/R) MCL in the standard care setting. The initial response rate and CR rates were 77% and 33%, respectively. 30 For R/R MCL, ibrutinib could only achieve a CR of 26.5% with a median PFS of 13 months. In contrast, among the patients with one prior line of chemotherapy, the median PFS increased to 33.6 months and median OS to 26.7 months. 31
Rule et al. 31 conducted a pooled analysis and reported a 3.5-year follow-up data of 370 patients with R/R MCL who received ibrutinib 560 mg once daily from 2011 to 2013. They found that patients who received second-line ibrutinib treatment and achieved CR would benefit most from ibrutinib without significant long-term toxicity. Similarly, this group also reported the results from the final analysis of an extended 38.7 months follow-up data from a randomized, open-label phase III RAY study that evaluated the efficacy and safety of ibrutinib compared with temsirolimus. 32 At this extended follow-up study, 33 patients remained in the ibrutinib group, and there were no crossover patients from the temsirolimus group to the ibrutinib group. Median PFS was significantly greater in the ibrutinib cohort than the temsirolimus cohort [15.6 versus 6.2 months; hazard ratio, HR = 0.45 (95% confidence interval, CI = 0.35–0.60); P < 0.0001]. Longer PFS was noted in ibrutinib cohort regardless of the number of prior lines of therapy.
Combination of ibrutinib with rituximab (IR) was also explored in relapsed MCL. Jain et al. 33 conducted the first single-center phase II clinical trial to analyze the efficacy and safety of IR combination as the first-line treatment for elderly MCL patients. With a median age of 71 years old, eastern cooperative oncology group (ECOG) performance score of 0–1 in 98% of the cohort, and a median number of IR cycles of 19, the best ORR was 95% (69% CR, 26% PR, 5% stable disease). Moreover, 65% achieved minimal residual disease (MRD) negative CR by flow cytometry. Predominant grade 3–4 toxicities were myalgia (17%), fatigue (14%), dyspnea (12%), neutropenia (9%), and new-onset atrial fibrillation (5%). Jain et al. 34 also reported the data from a 4-year follow-up of a single-arm, phase II IR trial among patients with R/R MCL. In this study, 58% of patients achieved CR in which 12 patients remained in the study at the time of analysis. High-risk features, such as blastoid morphology, high MIPI score, and high Ki-67, were noted to have inferior survival. This study concluded that IR combination could benefit R/R patients with a durable remission, especially those with low Ki-67.
Dual targets including BTK and B-cell lymphoma-2 (BCL-2) were recently explored in a phase II single-arm trial, in which 23 patients with R/R MCL and 1 with previously untreated MCL received ibrutinib 560 mg per day followed by addition of venetoclax in a stepwise fashion 4 weeks later with a maximum dose of 400 mg once daily. 35 In this study, 62% of patients achieved positron emission tomography (PET) confirmed CR, and 67% reached MRD via flow cytometry. Common adverse events (AEs) were diarrhea, fatigue, and nausea or vomiting in low-grade toxicity range.
Beyond Bruton’s tyrosine kinase inhibitor (BTKi) therapy, Eyre et al. 36 evaluated the efficacy of venetoclax monotherapy in patients with R/R MCL after receiving BTKi therapy. The study evaluated 20 patients whose median prior therapy lines were three, including high-dose cytarabine-based induction regimen (40%) and ASCT in first remission (30%). At first and subsequent relapse, all of them had received BTK inhibitor treatments (17 received ibrutinib). Before receiving venetoclax monotherapy, 95% had stage III/IV disease, 50% were classified as high-risk according to s-MIPI score system, and the median Ki-67 was 45%. In this study, venetoclax monotherapy ORR was 53%, with 18% achieving CR and 35% achieving PR. The median time to response was 48 days, median OS was 9.4 months, and PFS was 3.2 months.
A phase I trial combining palbociclib and ibrutinib in 27 patients with previously treated MCL demonstrated ibrutinib 560 mg daily plus palbociclib 100 mg on days 1–21 of a 28-day cycle could be safe. 37 With a median follow-up of 256 months, ORR and CR were noted in 67% and 37%, respectively. Grade 3–4 toxicities including neutropenia were noted in 41% of the cohort, thrombocytopenia in 30%, hypertension in 15%, febrile neutropenia in 15%, and pulmonary infection in 11%.
Various combination therapeutic strategies incorporating ibrutinib, rituximab, bendamustine, and R-CHOP have significantly improved treatment response rates in both the naïve and refractory patients.38–40 Rituximab with ibrutinib in a relapsed setting showed an ORR and CR of 88% and 44%, respectively. 41 In a phase I/Ib study, the combination of bendamustine and rituximab showed ORR of 94% and CR of 76%, with AEs encompassing predominantly cytopenia and rash (25%). 39 Finally, in the treatment naïve setting, the combination of ibrutinib with R-CHOP showed an ORR of 94% with only major grade 4 toxicity of neutropenia, although the study only included five patients. 40
In MANTLE-FIRST study, 261 patients with first R/R MCL after upfront high-dose cytarabine-containing regimens were recruited and analyzed.19,42 OS and PFS were analyzed from the time of salvage therapy. Second-line regimens consisted of rituximab and bendamustine (RB, 21%); rituximab, bendamustine, and cytarabine (R-BAC, 29%); ibrutinib (19%); and others (31%). R-BAC induced a higher rate of CR (63%) than other second-line regimens. Improved median PFS was noted in the ibrutinib and R-BAC cohorts (24 and 25 months, respectively). In patients who developed early disease progression, ibrutinib was associated with improved outcomes than comparator regimens, which makes it the only available non-chemoimmunotherapy drug in the relapsed setting.
Another multicenter open-label single-arm phase II trial, PHILEMON, reported efficacy and safety of ibrutinib in combination with lenalidomide and rituximab in 50 patients with R/R MCL enrolled between 2015 and 2016. 43 With a median follow-up of 17.8 months, 76% of patients had an ORR with CR and PR achieved in 56% and in 20%, respectively. The most prevalent grade 3–4 AEs were neutropenia (38%), infections (22%), and cutaneous toxicity (14%).
Another BTK inhibitor, acalabrutinib, is a highly selective and potent BTK inhibitor with limited off-target activity. A recent phase II study found an 81% ORR and 40% CR in the R/R setting. Besides its tolerance significantly better than ibrutinib, acalabrutinib also has a significantly better side effect profile with no increased grade 1–2 atrial fibrillation and bleeding events. 44 An extended 26-month follow-up of the multicenter phase II ACE-LY-004 study of acalabrutinib 100 mg twice daily showed an ORR and CR of 81% and 43%, respectively. 45 The estimated 24-month OS rate was 72.4%. Acalabrutinib was approved by the FDA for relapsed MCL second-line treatment in 2019.
More recently, another BTK inhibitor, zanubrutinib, has been approved by the FDA for the treatment of MCL patients who have received at least one prior treatment. 46 Zanubrutinib is a selective, irreversible, second-generation BTK inhibitor developed in China. A multicenter phase II study (NCT03206970) of zanubrutinib (160 mg twice daily by mouth) in 86 patients with previously treated MCL reported 84% ORR (59% CR); the median duration of response was 19.5 months. A similar ORR was reported in another zanubrutinib study that included 32 patients with previously treated MCL (NCT02343120). 47 The most common AEs (⩾20%) were cytopenia, rash, bruising, diarrhea, and cough. Headache, a side effect not commonly seen with other BTK inhibitors, was frequently reported and responded to caffeine.
ARQ-531 (nemtabrutinib), another novel BTK inhibitor in clinical trials, reversibly binds and inhibits the BTKs. This agent reportedly has activity in chronic lymphocytic leukemia (CLL) patients whose disease has progressed after ibrutinib treatment due to the BTK-C418 mutation, the most common mutation associated with resistance to BTK inhibitors. 48 However, the BTK-C418 mutation is not commonly seen in MCL patients. 48
LOXO-305 (pirtobrutinib), a highly selective, non-covalent BTK inhibitor, is currently under investigation in a BRUIN phase I/II multicenter trial to define safety and efficacy in multiple B-cell malignancies, including MCL. As of April 2020, 186 patients, including 38 patients with MCL, had been enrolled. 49 Among this cohort, 92% had previously received BTK inhibitor treatment. Of the 35 efficacy-evaluable MCL patients enrolled, ORR was 51% (9 patients with CR, 9 with PR, 7 with stable diease status [SD]). The study concluded that LOXO-305 poses a novel, promising therapeutic efficacy in heavily pretreated MCL patients with poor prognosis.
Orelabrutinib, which is a potent, irreversible, and highly selective BTK inhibitor, has been approved in China for the treatment of patients with MCL who have received at least one treatment in the past. 50 Clinical studies are underway in the United States in evaluating orelabrutinib efficacy, safety, and indication.
Lenalidomide and other immunomodulatory drugs
Lenalidomide is an antineoplastic agent with immunomodulatory capabilities. The ORR of R/R MCL to lenalidomide monotherapy was reported to be 28–57% and CR of 7.5–36%, with median PFS ranging from 4 to 5.7 months.51–53 However, in a study combining lenalidomide and bortezomib to treat relapsed MCL, there were issues related to toxicity-related dose reduction and inadequate treatment dosing. 54
In the relapsed setting, a combination of lenalidomide with rituximab showed some improvement in both median PFS and OS (11.1 and 24.3 months, respectively). 55 This combination therapy achieved an excellent response in induction, maintenance (ORR: 92%), and in the first-line setting (CR: 64%), with 50% of patients experiencing grade 3 or 4 neutropenia and 29% of patients with grade 3 or 4 rashes. Unfortunately, utilizing lenalidomide predisposes patients to treatment-related cancers, commonly non-invasive skin cancers, and more rarely, Merkel cell carcinoma and pancreatic cancer. 56
The use of lenalidomide and rituximab in a first-line setting is still under investigation. Ruan et al. 57 described a 5-year follow-up of phase II study of lenalidomide plus rituximab as initial treatment of 36 evaluable MCL patients. With a median follow-up period of 64 months, the OS rates were 89.5% and 77.4% at 3 and 5 years, respectively. Eight of 10 patients who had completed 3 or more years of treatment achieved MRD-negative CR. This study indicates that combination therapy of lenalidomide and rituximab as the first-line treatment could achieve a durable response with limited toxicity. At 2021 american society of hematology (ASH) meeting, Ribrag et al. 58 reported the results from the MCL R2 elderly clinical trial that Rituximab-Lenalidomide Maintenance Is Superior to Rituximab Maintenance after First Line Immunochemotherapy in Mantle Cell Lymphoma.
The efficacy of combination therapy using bendamustine, lenalidomide, and rituximab in a first-line setting was studied in elderly patients with a median age of 72. BR was used as the induction therapy with 52 weeks of lenalidomide maintenance. Post induction therapy, 64% of patients reached CR and 36% were MRD negative, with median PFS and OS of 42 and 53 months, respectively, with a median follow-up of 31 months. 59 The main shortfall of this study was a high incidence of serious infections, which indicates that this combination therapy may not fit well for the elderly population that this therapeutic strategy is intended for. Thus, while lenalidomide provides a promising avenue, further studies to evaluate the efficacy of combination therapies are required. Optimizing the treatment dosage and the duration will be essential before transitioning lenalidomide into a first-line treatment setting.
Other molecules structurally related to lenalidomide are also under investigation. Pomalidamide (CC-4047) has been FDA approved for the treatment of multiple myeloma and is being studied in other hematologic malignancies. Other similar immunomodulatory drugs/molecules (IMIDS), such as CC-220 (NCT03161483) and CC99282 (NCT04434196), showed additional immunomodulatory and immunostimulatory features in vitro and are in NHL clinical trials.
A single-center phase II study evaluating frontline sequential immunotherapy plus lenalidomide for MCL was recently explored. 60 The median follow-up duration was 2.8 years, and the 3-year PFS and OS were 64% and 85%, respectively. Among 47 patients evaluable for PFS enrolled in the trial, 45 completed maintenance, 43 achieved CR, and 1 was at stable disease status at the end of treatment with an ORR of 91%.
Venetoclax
Venetoclax is a BCL-2 inhibitor that was FDA approved for the treatment of CLL and acute myeloid leukemia (AML). In a phase I study, venetoclax showed activity against relapsed MCL with the single-agent activity of 75% and a median duration of response of 15.7 months. 61 The response to MCL patients who had failed ibrutinib therapy was lower, about 40–50%. The data regarding ibrutinib and venetoclax combination therapy were updated during the 2019 ASH annual meeting. In that study, the CR rates reached to 62% and the median PFS was 29 months. 62 MRD-negative remissions were also reported, and several patients discontinued treatment without evidence of progression. 35 Recently, a multicenter prospective phase I/II trial found that the combination of ibrutinib, obinutuzumab, and venetoclax had no dose-limiting toxicity. Meanwhile, this combination therapy reached the CR rates of 67% by cycle 6 in relapsed MCL patients and 86.6% in untreated patients with a 2-year PFS of 69.5%. 63 Further data on this combination are pending.
Data from a recent Nordic Lymphoma Group NLG-MCL7 (VALERIA) Phase I trial included 16 R/R MCL patients evaluable for efficacy who received venetoclax, lenalidomide, and rituximab. 64 In this study, ORR was noted to be 56%, with five patients achieving CR and four patients achieving PR. Four patients discontinued treatment after achieving molecular remission. Although the follow-up period was short, this study indicates that patients who achieved molecular remission could stop treatment under close monitoring.
mTOR and PI3K inhibitors
Mammalian target of rapamycin (mTOR) and phosphatidylinositol 3-kinase inhibitors such as rapamycin (sirolimus) and idelalisib have long been investigated. However, only in the most recent decade, these classes of molecules emerged in various clinical studies ranging from rheumatology to transplantation and to oncology largely due to its central role in metabolism modulation. mTOR inhibitors work by impairing lymphocyte activation and proliferation in response to antigenic and cytokine stimulation. 65 In cells, sirolimus binds to FK Binding Protein-12 (FKBP12) to generate an immunosuppressive complex. This complex then binds to and inhibits mTOR activation, suppressing cytokine-driven T-cell proliferation by inhibiting cell cycle progression from G1 to S phase. 66
Temsirolimus, a prodrug of sirolimus, has been evaluated in multiple settings of MCL treatment. In a phase III randomized controlled trial in R/R MCL patients, temsirolimus was tested at two dosages compared with the investigator’s choice. It was found that the cohort that received two dosages had better objective response rates (22%) than the investigator’s choice cohort (2%). The PFS in temsirolimus arm was also improved to 4.8 months, although OS in each arm was not statistically significant. 67 Temsirolimus monotherapy with alternative dose adjustments has been tested and demonstrated notable improvement in both ORR (38–41%) and median OS (12 months).68,69 Temsirolimus in combination with bendamustine and rituximab showed safe and effective in treating MCL in refractory settings. 70 Unfortunately, other mTOR inhibitors, such as everolimus, had limited success with an ORR of 8.6% (all partial responses). Similarly, median PFS and median OS were not improved (4.4 and 16.9 months, respectively). 71 The results of temsirolimus combined with cladribine and another active agent in a phase I/II MCL study were reported as well. 72
At 2020 ASH, parsaclisib, a next-generation oral phosphoinositide 3-kinas (PI3K) inhibitor, was reported to result in a high rate of rapid and durable responses in refractory NHL, including MCL (NCT03235544).73,74
Epigenetic agents
Since epigenetic deregulation of cyclin D1 is involved in molecular pathogenesis, epigenetic or cell cycle targeted therapies potentially offer precision medicine approaches. The molecular mechanisms involved remain to be elucidated, but some epigenetic agents have been investigated in MCL treatment. Epigenetics has become an important factor in MCL disease development. Vorinostat, a pan histone deacetylase (HDAC) inhibitor, has shown promising results with data suggesting that it possesses a less toxic profile and could be combined with rituximab to be clinically efficacious. 75 Vorinostat is FDA approved for the treatment of cutaneous T-cell lymphoma. DNA hypomethylating agents such as 5-azacytidine or decitabine have shown limited activity and are toxic in MCL and other B-cell lymphomas. 76 However, cladribine, but not fludarabine, is a cryptic hypomethylating agent that inhibits DNA, RNA, and histone methylation by inhibiting s-adenosyl methionine (SAM), the donor of methyl groups. 77 In MCL, cladribine has been shown to have activity as a single agent that is synergistic with rituximab.78,79 Cladribine was originally FDA approved for hairy cell leukemia treatment. In a phase II trial of 80 MCL patients treated with cladribine alone and in combination with rituximab, a durable CR was observed in about 50–60% of newly diagnosed patients. 78 The addition of vorinostat to cladribine and rituximab resulted in a 97% response rate in a phase II trial, with CR obtained in about 80% of patients. 80 Some of these responses are extremely durable, with patients on trial now than 10 years in remission, which suggests that this regimen may be curative. Another phase I study using bortezomib, cladribine, and rituximab (VCR) combination showed impressive result as well. 81 The ability of immuno- and epigenetic combination therapy to achieve long-term durable remissions is surprising, in contrast to conventional chemotherapy with maintenance or high-dose therapy/ASCT with maintenance. Further study is warranted.
In June 2020, the EZH2 histone methyl transferase (HMT) inhibitor, tazemetostat, was approved by FDA to treat EZH2 gene mutated and treatment-refractory follicular lymphoma. This molecule also has preclinical activity to MCL 82 and is incorporated into MCL clinical trial now (NCT03456726). Other EZH2 and HMT inhibitors are in preclinical and clinical development. EZH2 gene mutations are uncommon in MCL, but a deregulated expression of EZH2 and chromodomain-containing proteins, such as EED and SUZ12, has been reported in MCL. 83
Monoclonal antibodies and antibody-drug conjugates
Currently, there are no FDA-approved monoclonal antibodies or antibody-drug conjugates (ADCs) for MCL. Anti-CD20 antibodies rituximab and obinutuzimab are FDA approved for other types of NHL and are frequently used in MCL, usually in combination. Other monoclonal antibodies and ADC in development for MCL are polatuzumab vedotin 84 (CD79a; FDA approved for diffuse large B-cell lymphoma, DLBCL) and ublituximab, a glyco-engineered anti-CD20 antibody. 85
In addition, VLS-101 (zilovertamab), a ROR1 (receptor tyrosine kinase-like Orphan Receptor 1)-targeting ADC, showed efficacy in the first-in-human phase I study evaluating the efficacy and safety of the drug in 32 heavily pretreated MCL (15 patients) and DLBCL patients. 86 Objective tumor response was noted in seven patients with MCL (four PR and three CR).
As aforementioned, some monoclonal antibodies such as rituximab and obinutuzumab and small molecules such as venetoclax have been used for MCL maintenance therapy. Still, the exact duration and type of maintenance therapy remain to be optimized. Some MCL specialists continue maintenance therapy until progression, while others use a fixed 2-year, 3-year, or 5-year schedule.
Allogeneic stem cell transplant
Allogeneic stem cell transplant (allo-SCT) remains the only known and well-studied curative option for younger patients with limited comorbidities. Donor availability has largely been overcome using matched unrelated, cord blood, and haploidentical transplant approaches coupled with post-transplant cyclophosphamide to reduce graft versus host disease. However, this approach remains limited by preexisting comorbidities and complications such as graft versus host disease despite advances in allo-SCT. Allo-SCT employs graft-versus-leukemia effect to provide a durable disease clearance in patients with R/R MCL87,88 A recent study conducted by the European Bone Marrow Transplant Chronic Malignancies and Lymphoma Working Parties included 70 patients (48 with CLL and 22 with MCL) who were bridged to allo-SCT from ibrutinib. 89 At the time of transplant, 73% of patients were responsive to ibrutinib. In the MCL cohort, the 12-month non-relapse morality, PFS, and OS were 5%, 76%, and 86%, respectively. This study demonstrates that patients with ibrutinib-sensitive R/R MCL had an excellent disease control with allo-SCT. Ibrutinib may be a promising consolidation regimen for ibrutinib-sensitive MCL prior allo-SCT. In a recent retrospective MANTLE-FIRST study, 55 patients underwent allo-SCT for R/R MCL after treating with rituximab and high-dose cytarabine; the patients using BTK inhibitor as a bridge to allo-SCT achieved good disease control without an increase in toxicity. 90
CAR-T cell therapy and BiTE therapy
CAR-T cell therapy has revolutionized the field of immunotherapy. Three CD19 products are currently FDA approved for relapsed DLBCL (axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel)91,92 and one (tisagenlecleucel) approved for refractory B-cell acute lymphocytic leukemia (ALL). 93 A CD19 (KTE-C19; brexucabtagene autoleucel) CAR-T product recently received FDA approval for the treatment of R/R MCL. A 93% response rate with a 67% CR rate was reported. 94 However, concerns pertaining to the cost and accessibility of brexucabtagene autoleucel are emerging, similar to other approved CAR-T cell therapies.
BiTE therapy is a unique strategy that engages the cytotoxicity of T cells against cancer sites. BiTEs are fusion proteins consisting of an element that binds the CD3 receptors and the other to a tumor cell via a tumor-specific molecule. The mainstay of this class of drugs is blinatumomab, a bispecific CD19/CD3 antibody. In a phase I trial involving 24 MCL patients in the NHL cohort, blinatumomab demonstrated an ORR of 71%. 95 In a follow-up study of this cohort for long-term survival, median OS of 1560 days and median PFS of 204 days were noted. 96 More recently, mosunetuzumab, a bispecific CD20/CD3 T-cell engager, was developed and trialed in a phase I study presented in the 2018 ASH meeting that showed promising efficacy and safety with favorable response outcomes in patients who were thought to be refractory to anti-CD20 therapy. 97 However, limited data are available for mosunetuzumab as trials are currently underway. At 2021 ASH meeting, the early data from another bispecific CD20/CD3 T-cell engager, glofitamab, were presented. The data demonstrated that glofitamab step-up dosing (SUD) as monotherapy after glofitamab with obintuzumab pretreatment (Gpt) induced high response rates in patients with MCL (ORR: 81%, CR: 66.7%), most of whom had failed prior BTKi therapy. The cytokine release syndrome (CRS) rates were manageable and mostly low grade. Immune effector cell-associated neurotoxicity syndrome (ICANS)-like AEs were infrequent, low grade, and resolved within 1 day. No treatment discontinuations due to AEs were observed. 98
Young patients and TP53 mutation
While MCL is a disease that predominantly impacts elderly patients with the median age of diagnosis between 60 and 70 years of age, a significant proportion of MCL patients are young individuals. Younger patients are generally treated differently because of their age and performance status. Young patients can tolerate high-dose cytarabine-based regimens, which can be subsequently followed by ASCT. Studies from both French and North American groups found a considerable improvement in PFS when ASCT is followed by rituximab maintenance.99,100 More recently, a phase I/II trial demonstrated the efficacy of CpG-activated tumor cells in ASCT that amplified a CD8+ T-cell immune response, consequently resulting in a superior clinical outcome. 20 Historically, therapies have been directed at subgroups of young fit patients with limited comorbidities compared with older patients with significant comorbidities. For young fit SCT eligible patients who achieved a complete or partial response, high-dose chemotherapy with or without autologous stem cell rescue still remains a trending option. Autologous stem cell rescue is commonly used but is not curable and ineffective in high-risk patients with blastoid variant MCL. A randomized head-to-head trial will ultimately suggest the best approach.
A TP53 mutation has also been recently recognized as a very poor prognostic marker. One study found that patients harboring a specific TP53 mutation had inferior treatment outcomes. 101 One proposed solution to overcome this unfavorable prognostic feature is the implementation of allo-SCTs instead of the standard ASCT approach. 102 Agents active in TP53 mutated MCL are currently under investigation. In addition, agents specifically capable of refolding P53 molecules in hematologic malignancies are also under evaluation. 103
Conclusion
In summary, MCL is a group of heterogeneous B-cell lymphomas harboring unique pathological characteristics. 104 However, its heterogeneity in both disease presentation and response to therapy have made this entity a challenge for the oncology community. Aside from the aforementioned novel agents, many potentially promising new molecules and treatment approaches continuously emerge through the clinical trial pipeline (Table 2). With the advent of highly active novel agent combinations, new therapeutic modalities, and more targeted prognostic factors, there will be a need to personalize the therapies in the future. With the advent of whole-genome and transcriptome sequencing at both DNA and RNA levels, the scientific community is able to screen genetic profiling for prognostic factors. Furthermore, MRD has also been used as treatment outcome predictions, with findings that MRD positivity is highly predictive of shorter OS. 105 With such rapid advances in scientific discovery and innovative technology developments, the future is promising for curing MCL.
Table 2.
The ongoing clinical trials investigate promising new molecules and innovative therapeutic approaches.
| Name of molecule | Category | Trial phase | Target/mechanism | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| Tisagenlecleucel | CAR-T (CD19) | II | CD19 | NCT04234061 |
| CAR-T (CD19) | I, II | CD19 | NCT03676504 | |
| TC-110 | TCR fusion construction | I, II | CD19-CD3 | NCT04323657 |
| Epcoritamab | BiTE therapy | I, II | CD3 × CD20 | NCT03625037 |
| Obinutuzumab | Biologics | II | Anti-CD20 | NCT02736617 |
| Obinutuzumab | Biologics | II | Anti-CD20 | NCT03229382 |
| Ublituximab | Biologics | III, IV | Anti-CD20 | NCT02793583 |
| Palbociclib | CDK inhibitor | II | CDK4/6 | NCT03478514 |
| Parsaclisib | PI3K inhibitor | II | PI3K α/δ | NCT03235544 |
| LOXO-305 | BTK inhibitor | I, II | BTK | NCT03740529 |
| Orelabrutinib | BTK inhibitor | II | BTK | NCT05076097 |
| Chidamide | Histone deacetylation inhibitor | II | HDAC1/2/3/10 | NCT03629873 |
| ONC201 | Akt/ERK inhibitor | I, II | Akt/ERK | NCT02420795 |
| TQ-B3525 | PI3K inhibitor | II | PI3K α/δ | NCT04398953 |
| Parsaclisib | PI3K inhibitor | II | PI3K α/δ | NCT03235544 |
| BGB-10188 | PI3K inhibitor | I, II | PI3K α/δ | NCT04282018 |
| CLR 131 | Radioiodinated therapeutic | II | Cytotoxic radioisotope | NCT02952508 |
| SNS-062 | BTK/ITK inhibitor | I, II | BTK/ITK | NCT03037645 |
| CYT-0851 | RAD51 inhibitor | I, II | Inhibition of cytidine deaminases and DNA repair | NCT03997968 |
| Pembrolizumab | PD-1 antibody | II | Blocking PD-L1 and PD-L2 from interacting with PD-1 | NCT03153202 |
| NVG-111 | BiTE | II | Binding to ROR1/CD3 | NCT04763083 |
| TL-895 | ITK inhibitor | II | Blocking tyrosine kinase | NCT02825836 |
| IGM-2323 | An engineered bispecific IgM antibody | I | Block CD20 | NCT04082936 |
| BP1002 | L-Bcl-2 antisense oligonucleotide | I | Suppressing L-Bcl-2 | NCT04072458 |
BiTE, bispecific T-cell engager; BTK, Bruton’s tyrosine kinase; CAR-T, chimeric antigen receptor-T cell; ITK, irreversible tyrosine kinase; ROR1, receptor tyrosine kinase-like Orphan Receptor 1 ; TCR, T cell receptor; PD-1, program death-1.
Acknowledgments
The authors thank the patients and their family for contributing invaluable knowledge and experience for this study.
Footnotes
Author contributions: Jeffrey J. Pu: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Malvi Savani: Writing – original draft; Writing – review & editing.
Nick Huang: Data curation; Writing – original draft; Writing – review & editing.
Elliot M. Epner: Conceptualization; Writing – review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Aplastic Anemia & MDS International Foundation research grant to J.J.P. (146818), American Cancer Society grant to J.J.P. (124171-IRG-13-043-02), National Cancer Institute of the National Institutes of Health to J.J.P. (P30CA023074), and a University of Arizona Cancer Center research grant to J.J.P.
ORCID iD: Jeffrey J. Pu  https://orcid.org/0000-0001-7498-3159
https://orcid.org/0000-0001-7498-3159
Contributor Information
Jeffrey J. Pu, University of Arizona Cancer Center, 1515 N Campbell Avenue, Room #1968C, Tucson, AZ 85724, USA.
Malvi Savani, University of Arizona Cancer Center, Tucson, AZ, USA.
Nick Huang, State University of New York Upstate Medical University, Syracuse, NY, USA.
Elliot M. Epner, Penn State Hershey Cancer Institute, 100 University Drive, Hershey, PA, USA.
References
- 1. Sabattini E, Bacci F, Sagramoso C, et al. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 2010; 102: 83–87. [PubMed] [Google Scholar]
- 2. Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer 2008; 113: 791–798. [DOI] [PubMed] [Google Scholar]
- 3. Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol 2016; 34: 1256–1269. [DOI] [PubMed] [Google Scholar]
- 4. Fu S, Wang M, Lairson DR, et al. Trends and variations in mantle cell lymphoma incidence from 1995 to 2013: a comparative study between Texas and National SEER areas. Oncotarget 2017; 8: 112516–112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu H, Wang J, Epner EM. Cyclin D1 activation in B-cell malignancy: association with changes in histone acetylation, DNA methylation, and RNA polymerase II binding to both promoter and distal sequences. Blood 2004; 104: 2505–2513. [DOI] [PubMed] [Google Scholar]
- 6. Klapper W. Histopathology of mantle cell lymphoma. Semin Hematol 2011; 48: 148–154. [DOI] [PubMed] [Google Scholar]
- 7. Lee C, Huang X, Di Liberto M, et al. Targeting CDK4/6 in mantle cell lymphoma. Ann Lymphoma 2020; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang W, Kahn SM, Zhou P, et al. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene 1993; 8: 3447–3457. [PubMed] [Google Scholar]
- 9. Cortelazzo S, Ponzoni M, Ferreri AJ, et al. Mantle cell lymphoma. Crit Rev Oncol Hematol 2012; 82: 78–101. [DOI] [PubMed] [Google Scholar]
- 10. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the European mantle cell lymphoma network. J Clin Oncol 2016; 34: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 11. Weisenburger DD, Armitage JO. Mantle cell lymphoma – an entity comes of age. Blood 1996; 87: 4483–4494. [PubMed] [Google Scholar]
- 12. Mondello P, Steiner N, Willenbacher W, et al. 90Y-ibritumomab-tiuxetan consolidation therapy for advanced-stage mantle cell lymphoma after first-line autologous stem cell transplantation: is it time for a step forward? Clin Lymphoma Myeloma Leuk 2016; 16: 82–88. [DOI] [PubMed] [Google Scholar]
- 13. Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and Autologous Stem Cell Transplantation (ASCT). Blood 2010; 115: 1530–1533. [DOI] [PubMed] [Google Scholar]
- 14. Determann O, Hoster E, Ott G, et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood 2008; 111: 2385–2387. [DOI] [PubMed] [Google Scholar]
- 15. Balsas P, Palomero J, Eguileor A, et al. SOX11 promotes tumor protective microenvironment interactions through CXCR4 and FAK regulation in mantle cell lymphoma. Blood 2017; 130: 501–513. [DOI] [PubMed] [Google Scholar]
- 16. Rauert-Wunderlich H, Mottok A, Scott DW, et al. Validation of the MCL35 gene expression proliferation assay in randomized trials of the European Mantle Cell Lymphoma Network. Br J Haematol 2019; 184: 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood 2017; 130: 1903–1910. [DOI] [PubMed] [Google Scholar]
- 18. Eskelund CW, Dimopoulos K, Kolstad A, et al. Detailed long-term follow-up of patients who relapsed after the Nordic mantle cell lymphoma trials: MCL2 and MCL3. Hemasphere 2021; 5: e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Visco C, Tisi MC, Evangelista A, et al. Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br J Haematol 2019; 185: 940–944. [DOI] [PubMed] [Google Scholar]
- 20. Frank MJ, Khodadoust MS, Czerwinski DK, et al. Autologous tumor cell vaccine induces antitumor T cell immune responses in patients with mantle cell lymphoma: a phase I/II trial. J Exp Med 2020; 217: e20191712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher RI, Bernstein SH, Kahl BS, et al. Multi-center phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2006; 24: 4867–4874. [DOI] [PubMed] [Google Scholar]
- 22. Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol 2011; 29: 690–697. [DOI] [PubMed] [Google Scholar]
- 23. Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. New Engl J Med 2015; 372: 944–953. [DOI] [PubMed] [Google Scholar]
- 24. Till BG, Li H, Bernstein SH, et al. Phase II trial of R-CHOP plus bortezomib induction therapy followed by bortezomib maintenance for newly diagnosed mantle cell lymphoma: SWOG S0601. Br J Haematol 2016; 172: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romaguera JE, Fayad LE, McLaughlin P, et al. Phase I trial of bortezomib in combination with rituximab-HyperCVAD alternating with rituximab, methotrexate and cytarabine for untreated aggressive mantle cell lymphoma. Br J Haematol 2010; 151: 47–53. [DOI] [PubMed] [Google Scholar]
- 26. Chang JE, Li H, Smith MR, et al. Phase 2 study of VcR-CVAD with maintenance rituximab for untreated mantle cell lymphoma: an Eastern Cooperative Oncology Group study (E1405). Blood 2014; 123: 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang JE, Carmichael LL, Kim K, et al. VcR-CVAD induction chemotherapy followed by maintenance rituximab produces durable remissions in mantle cell lymphoma: a Wisconsin Oncology Network study. Clin Lymphoma Myeloma Leuk 2018; 18: e61–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen RW, Palmer JM, Tomassetti S, et al. Multi-center phase II trial of bortezomib and rituximab maintenance combination therapy in patients with mantle cell lymphoma after consolidative autologous stem cell transplantation. J Hematol Oncol 2018; 11: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robak T, Jin J, Pylypenko H, et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol 2018; 19: 1449–1458. [DOI] [PubMed] [Google Scholar]
- 30. Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013; 31: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rule S, Dreyling M, Goy A, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica 2019; 104: e211–e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rule S, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus: 3-year follow-up of patients with previously treated mantle cell lymphoma from the phase 3, international, randomized, open-label RAY study. Leukemia 2018; 32: 1799–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jain P, Romaguera J, Nomie K, et al. Combination of IBRUTINIB with RITUXIMAB (IR) is highly effective in previously untreated elderly (>65 years) patients (PTS) with mantle cell lymphoma (MCL) – phase II trial. Hematological Oncology 2019; 37(S2): 42–42. [Google Scholar]
- 34. Jain P, Romaguera J, Srour SA, et al. Four-year follow-up of a single arm, phase II clinical trial of ibrutinib with rituximab (IR) in patients with relapsed/refractory mantle cell lymphoma (MCL). Br J Haematol 2018; 182: 404–411. [DOI] [PubMed] [Google Scholar]
- 35. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. New Engl J Med 2018; 378: 1211–1223. [DOI] [PubMed] [Google Scholar]
- 36. Eyre TA, Walter HS, Iyengar S, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica 2019; 104: e68–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin P, Bartlett NL, Blum KA, et al. A phase 1 trial of ibrutinib plus palbociclib in previously treated mantle cell lymphoma. Blood 2019; 133: 1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol 2016; 17: 48–56. [DOI] [PubMed] [Google Scholar]
- 39. Maddocks K, Christian B, Jaglowski S, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood 2015; 125: 242–248. [DOI] [PubMed] [Google Scholar]
- 40. Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol 2014; 15: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 41. Rule S, Dreyling M, Goy A, et al. Median 3.5-year follow-up of ibrutinib treatment in patients with relapsed/refractory mantle cell lymphoma: a pooled analysis. Blood 2017; 130(Suppl. 1): 151–151. [Google Scholar]
- 42. Visco C, Di Rocco A, Evangelista A, et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: results from the MANTLE-FIRST study. Leukemia 2021; 35: 787–795. [DOI] [PubMed] [Google Scholar]
- 43. Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol 2018; 5: e109–e116. [DOI] [PubMed] [Google Scholar]
- 44. Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet 2018; 391: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang M, Rule S, Zinzani PL, et al. Durable response with single-agent acalabrutinib in patients with relapsed or refractory mantle cell lymphoma. Leukemia 2019; 33: 2762–2766. [DOI] [PubMed] [Google Scholar]
- 46. Song Y, Zhou K, Zou D, et al. Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton’s tyrosine kinase. Clin Cancer Res 2020; 26: 4216–4224. [DOI] [PubMed] [Google Scholar]
- 47. Tam CS, Wang M, Simpson D, et al. Updated safety and efficacy data in the phase I trial of patients with mantle cell lymphoma (MCL) treated with Bruton tyrosine kinase (BTK) inhibitor Zanubrutinib (BGB-3111). Hematol Oncol 2019; 37(S2): 245–247. [Google Scholar]
- 48. Woyach J, Stephens DM, Flinn IW, et al. Final results of phase 1, dose escalation study evaluating ARQ 531 in patients with relapsed or refractory B-cell lymphoid malignancies. Blood 2019; 134(Suppl._1): 4298–4298. [Google Scholar]
- 49. Wang M, Shah NN, Alencar AJ, et al. LOXO-305, a next generation, highly selective, non-covalent BTK inhibitor in previously treated mantle cell lymphoma, Waldenström’s macroglobulinemia, and other non-Hodgkin lymphomas: results from the phase 1/2 BRUIN study. Blood 2020; 136(Suppl. 1): 8–10.32614959 [Google Scholar]
- 50. Dhillon S. Orelabrutinib: first approval. Drugs 2021; 81: 503–507. [DOI] [PubMed] [Google Scholar]
- 51. Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol 2009; 145: 344–349. [DOI] [PubMed] [Google Scholar]
- 52. Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol 2011; 22: 1622–1627. [DOI] [PubMed] [Google Scholar]
- 53. Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013; 31: 3688–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morrison VA, Jung SH, Johnson J, et al. Therapy with bortezomib plus lenalidomide for relapsed/refractory mantle cell lymphoma: final results of a phase II trial (CALGB 50501). Leuk Lymphoma 2015; 56: 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012; 13: 716–723. [DOI] [PubMed] [Google Scholar]
- 56. Ruan J, Martin P, Shah B, et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. New Engl J Med 2015; 373: 1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood 2018; 132: 2016–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ribrag V, Safar V, Kluin-Nelemans H, et al. Rituximab-lenalidomide (R2) maintenance is superior to rituximab maintenance after first line immunochemotherapy in mantle cell lymphoma: results of the MCL R2 elderly clinical trial. Blood 2021; 138(Suppl. 1): 379. [Google Scholar]
- 59. Albertsson-Lindblad A, Kolstad A, Laurell A, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood 2016; 128: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 60. Epstein-Peterson ZD, Batlevi CL, Caron P, et al. Frontline sequential immunochemotherapy plus lenalidomide for mantle cell lymphoma incorporating MRD evaluation: phase II, investigator-initiated, single-center study. Blood 2020; 136(Suppl. 1): 11–12.32276273 [Google Scholar]
- 61. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 2017; 35: 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Handunnetti SM, Anderson MA, Burbury K, et al. Three year update of the phase II ABT-199 (venetoclax) and Ibrutinib in mantle cell lymphoma (AIM) study. Blood 2019; 134(Suppl._1): 756–756. [Google Scholar]
- 63. Le Gouill S, Morschhauser F, Chiron D, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase I/II trial. Blood 2021; 137: 877–887. [DOI] [PubMed] [Google Scholar]
- 64. Jerkeman M, Kolstad A, Niemann CU, et al. Venetoclax, lenalidomide and rituximab for patients with relapsed or refractory mantle cell lymphoma – data from the Nordic Lymphoma Group NLG-MCL7 (VALERIA) phase I trial: stopping treatment in molecular remission is feasible. Blood 2020; 136(Suppl. 1): 15. [Google Scholar]
- 65. Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991; 253: 905–909. [DOI] [PubMed] [Google Scholar]
- 66. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 2017; 168: 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 2009; 27: 3822–3829. [DOI] [PubMed] [Google Scholar]
- 68. Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 2005; 23: 5347–5356. [DOI] [PubMed] [Google Scholar]
- 69. Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer 2008; 113: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hess G, Keller U, Scholz CW, et al. Safety and efficacy of temsirolimus in combination with bendamustine and rituximab in relapsed mantle cell and follicular lymphoma. Leukemia 2015; 29: 1695–1701. [DOI] [PubMed] [Google Scholar]
- 71. Wang M, Popplewell LL, Collins RH, Jr, et al. Everolimus for patients with mantle cell lymphoma refractory to or intolerant of bortezomib: multicentre, single-arm, phase 2 study. Br J Haematol 2014; 165: 510–518. [DOI] [PubMed] [Google Scholar]
- 72. Inwards DJ, Fishkin PA, LaPlant BR, et al. Phase I trial of rituximab, cladribine, and temsirolimus (RCT) for initial therapy of mantle cell lymphoma. Ann Oncol 2014; 25: 2020–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mehta A, Trneny M, Walewski J, et al. Phase II study evaluating the efficacy and safety of parsaclisib in patients with relapsed or refractory mantle cell lymphoma not previously treated with a BTK inhibitor (CITADEL-205). Blood 2020; 136(Suppl. 1): 22–23. [Google Scholar]
- 74. Zinzani PLAM, Delwail V, Paneesha S, et al. Phase II study evaluating the efficacy and safety of parsaclisib in patients with relapsed or refractory mantle cell lymphoma previously treated with ibrutinib (CITADEL-205). Blood 2020; 136(Suppl. 1): 43–44. [Google Scholar]
- 75. Ogura M, Ando K, Suzuki T, et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol 2014; 165: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ribeiro ML, Reyes-Garau D, Armengol M, et al. Recent advances in the targeting of epigenetic regulators in B-cell non-Hodgkin lymphoma. Front Genet 2019; 10: 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hasanali ZS, Saroya BS, Stuart A, et al. Epigenetic therapy overcomes treatment resistance in T cell prolymphocytic leukemia. Sci Transl Med 2015; 7: 293ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Inwards DJ, Fishkin PA, Hillman DW, et al. Long-term results of the treatment of patients with mantle cell lymphoma with cladribine (2-CDA) alone (95-80-53) or 2-CDA and rituximab (N0189) in the North Central Cancer Treatment Group. Cancer 2008; 113: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Spurgeon SE, Pindyck T, Okada C, et al. Cladribine plus rituximab is an effective therapy for newly diagnosed mantle cell lymphoma. Leuk Lymphoma 2011; 52: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 80. Spurgeon SE, Sharma K, Claxton DF, et al. Phase 1-2 study of vorinostat (SAHA), cladribine and rituximab (SCR) in relapsed B-cell non-Hodgkin lymphoma and previously untreated mantle cell lymphoma. Br J Haematol 2019; 186: 845–854. [DOI] [PubMed] [Google Scholar]
- 81. Pu JJ, Ehmann WC, Liao J, et al. The results of a phase I study using velcade (bortezomib), cladribine, and rituximab (VCR) in treating mantle cell lymphoma. Blood 2016; 128: 1792. [Google Scholar]
- 82. Hood TL, Cosmopoulos K, Drew A, et al. Abstract 808: opportunity for therapeutic expansion in mantle cell lymphoma: tazemetostat combination synergy status in preclinical MCL models. Cancer Res 2018; 78(13 Suppl.): 808–808. [Google Scholar]
- 83. Demosthenous C, Gupta SK, Sun J, et al. Deregulation of polycomb repressive complex-2 in mantle cell lymphoma confers growth advantage by epigenetic suppression of cdkn2b. Front Oncol 2020; 10: 1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020; 38: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sawas A, Farber CM, Schreeder MT, et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br J Haematol 2017; 177: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang M, Barrientos JC, Furman RR, et al. VLS-101, a ROR1-targeting antibody-drug conjugate, demonstrates a predictable safety profile and clinical efficacy in patients with heavily pretreated mantle cell lymphoma and diffuse large B-cell lymphoma. Blood 2020; 136(Suppl. 1): 13–14. [Google Scholar]
- 87. Fenske TS, Zhang MJ, Carreras J, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. J Clin Oncol 2014; 32: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vaughn JE, Sorror ML, Storer BE, et al. Long-term sustained disease control in patients with mantle cell lymphoma with or without active disease after treatment with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Cancer 2015; 121: 3709–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dreger P, Michallet M, Bosman P, et al. Ibrutinib for bridging to allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia or mantle cell lymphoma: a study by the EBMT chronic malignancies and lymphoma working parties. Bone Marrow Transpl 2019; 54: 44–52. [DOI] [PubMed] [Google Scholar]
- 90. Arcari A, Morello L, Vallisa D, et al. Allogeneic stem cell transplantation in patients with mantle cell lymphoma: results from the MANTLE-FIRST study on behalf of Fondazione Italiana Linfomi. Leuk Lymphoma 2021; 62: 3474–3483. [DOI] [PubMed] [Google Scholar]
- 91. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New Engl J Med 2017; 377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. New Engl J Med 2019; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 93. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New Engl J Med 2018; 378: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2020; 382: 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Goebeler ME, Knop S, Viardot A, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol 2016; 34: 1104–1111. [DOI] [PubMed] [Google Scholar]
- 96. Dufner V, Goebeler M-E, Sayheli C, et al. Bispecific T-cell engager antibody construct blinatumomab shows durable response in a long-term follow-up analysis of 38 NHL patients treated in a phase I trial. Blood 2015; 126: 3974–3974. [Google Scholar]
- 97. Budde LE, Sehn LH, Assouline S, et al. Mosunetuzumab, a full-length bispecific CD20/CD3 antibody, displays clinical activity in relapsed/refractory B-cell non-Hodgkin lymphoma (NHL): interim safety and efficacy results from a phase 1 study. Blood 2018; 132(Suppl. 1): 399–399. [Google Scholar]
- 98. Phillips T, Dickinson M, Morschhauser F, et al. Glofitamab step-up dosing induces high response rates in patients (pts) with relapsed or refractory (R/R) mantle cell lymphoma (MCL), most of whom had failed prior Bruton’s tyrosine kinase inhibitor (BTKi) therapy. Blood 2021; 138(Suppl. 1): 130. [Google Scholar]
- 99. Gerson JN, Handorf E, Villa D, et al. Survival outcomes of younger patients with mantle cell lymphoma treated in the rituximab era. J Clin Oncol 2019; 37: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. New Engl J Med 2017; 377: 1250–1260. [DOI] [PubMed] [Google Scholar]
- 101. Lin RJ, Ho C, Hilden PD, et al. Allogeneic haematopoietic cell transplantation impacts on outcomes of mantle cell lymphoma with TP53 alterations. Br J Haematol 2019; 184: 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Malarikova D, Berkova A, Obr A, et al. Concurrent TP53 and CDKN2A gene aberrations in newly diagnosed mantle cell lymphoma correlate with chemoresistance and call for innovative upfront therapy. Cancers (Basel) 2020; 12: 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bykov VJ, Zhang Q, Zhang M, et al. Targeting of mutant p53 and the cellular redox balance by APR-246 as a strategy for efficient cancer therapy. Front Oncol 2016; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ladha A, Zhao J, Epner EM, et al. Mantle cell lymphoma and its management: where are we now? Exp Hematol Oncol 2019; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hoster E, Pott C. Minimal residual disease in mantle cell lymphoma: insights into biology and impact on treatment. Hematology Am Soc Hematol Educ Program 2016; 2016: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]