Summary
Background
Infantile spasms syndrome (IS) is a type of epilepsy affecting 1.6 to 4.5 per 10,000 children in the first year of life, often with severe lifelong neurodevelopmental consequences. Only two first-line pharmacological treatments currently exist for IS and many children are refractory to these therapies. In such cases, children are treated with the ketogenic diet (KD). While effective in reducing seizures, the diet can result in dyslipidemia over time.
Methods
Employing a neonatal Sprague-Dawley rat model of IS, we investigated how the KD affects hepatic steatosis and its modulation by a defined probiotic blend. A combination of multiple readouts, including malondialdehyde, fatty acid profiles, lipid metabolism-related enzyme mRNA expression, mitochondrial function, histone deacetylase activity, cytokines and chemokines were evaluated using liver homogenates.
Findings
The KD reduced seizures, but resulted in severe hepatic steatosis, characterized by a white liver, triglyceride accumulation, elevated malondialdehyde, polyunsaturated fatty acids and lower acyl-carnitines compared to animals fed a control diet. The KD-induced metabolic phenotype was prevented by the co-administration of a blend of Streptococcus thermophilus HA-110 and Lactococcus lactis subsp. lactis HA-136. This probiotic blend protected the liver by elevating pAMPK-mediated signaling and promoting lipid oxidation. The strains further upregulated the expression of caspase 1 and interleukin 18, which may contribute to their hepatoprotective effect in this model.
Interpretation
Our results suggest that early intervention with probiotics could be considered as an approach to reduce the risk of hepatic side effects of the KD in children who are on the diet for medically indicated reasons.
Funding
This study was funded by the Alberta Children's Hospital Research Institute and Mitacs Accelerate Program (IT16942).
Keywords: Epilepsy, Pediatric, Ketogenic diet, Steatosis, Probiotic, Nutrition
Research in context.
Evidence before this study
The ketogenic diet refers to a diet that is low in carbohydrate, modest in protein and high in fat. It aims to induce the production of ketone bodies that serve as an alternate energy source in the body for tissues that do not directly metabolize fatty acids. Clinically, the very-low-carbohydrate ketogenic diet is successfully used worldwide to treat epilepsy in those individuals unresponsive to drug treatment. It is highly effective in this regard, with more than half of children with pediatric epilepsy achieving a reduction in seizures of at least 50% following 6 months of treatment. While the ketogenic diet is useful in controlling seizures, poor diet quality and the high fat content of the diet can have negative implications for normal human growth and development including dyslipidemia and hepatic steatosis. As such, treatments to mitigate these potentially negative side effects are urgently needed.
Added value of this study
In this study, we examine a specific subtype of pediatric epilepsy called infantile spasms syndrome. We employ a validated model of infantile spasms syndrome in rodents to determine how the ketogenic diet affects hepatic steatosis and its modulation by a defined probiotic blend. Our rat model not only mimics infantile spasms, but also successfully recapitulates the neurodevelopmental deficits and neuropathology observed in children with the condition. Following seizure induction, we treated animals with the ketogenic diet with and without probiotics. Results showed that the ketogenic diet induced severe hepatic steatosis, characterized by a white liver, triglyceride accumulation, elevated malondialdehyde, polyunsaturated fatty acids and lower acyl-carnitines compared to animals fed a control diet. The ketogenic diet induced metabolic phenotype was prevented by the co-administration of a blend of Streptococcus thermophilus HA-110 and Lactococcus lactis subsp. lactis HA-136. This probiotic blend protected the liver by elevating pAMPK-mediated signaling and promoting lipid oxidation.
Implications of all available evidence
The data presented here help to understand the detrimental impacts of the ketogenic diet on the liver. Our results also suggest that early intervention with probiotics could be considered as an approach to reduce the risk of hepatic side effects in children with epilepsy and other metabolic disorders treated with ketogenic diet.
Alt-text: Unlabelled box
Introduction
Infantile spasms syndrome (IS), also known as West Syndrome, is a developmental epileptic encephalopathy syndrome characterized by epileptic spasms, hypsarrhythmia, and cognitive arrest or regression. Typically occurring in the first three years of life, spasms (seizures) often occur upon waking and in clusters, and some children can experience hundreds of seizures per day. Disease mortality is in the range of 30% of affected cases.1, 2, 3 In surviving cases, the condition is devastating to families and has important clinical implications because of frequent recurrences, risk for developing future epilepsy, developmental delay, autism and lifelong debilitation.4 First-line intervention for IS is pharmacological treatment. However, a significant number of IS cases (30–40%) are refractory to pharmacological treatment while rates of relapse are as high as 50% following initial positive responses.5, 6, 7 Furthermore, pharmacological treatment has significant side effects that are exaggerated in young children due to their early developmental stage and their rapid rates of growth.8 In these cases, clinicians turn to the ketogenic diet (KD), a high-fat, low-carbohydrate, normal protein diet to control seizures. Administered as a formula to infants, prospective studies show that the KD is equally as effective as adrenocorticotropic hormone (ACTH),9 a common IS medication. Likewise, a multicenter retrospective study examining the KD showed it to be effective in 55% of children with pediatric epilepsy, achieving a reduction in seizures of at least 50% following 6 months of treatment.10
While the KD is effective in controlling seizures, it has negative implications for normal human growth and development, especially in children. In particular, dyslipidemia has been reported, varying from 16% (17/104)11 to 30% (16/53)9, among IS patients after KD initiation. Dyslipidemia was further ranked first amongst the side effects following KD therapy at a ratio of 12% (20/171) in infants with drug-resistant epilepsy.12 Hepatosteatosis and cholelithiasis have also been reported in older children.13 With the increasing therapeutic application of the KD for a variety of neurodevelopmental and metabolic conditions,14,15 understanding how the diet alters liver metabolism is of great relevance for dissecting and ameliorating the potential side effects of the KD, especially during early childhood.
One potential approach to mitigate dyslipidemia and hepatosteatosis with the KD involves the administration of probiotics. Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit to the host.16 Administration of probiotics reduces lipid accumulation and hepatic steatosis in mice17, and enhances lipid metabolism by decreasing triglyceride accumulation and limiting oxidative damage in adult rats18 following high-fat diet administration. Whether probiotic inclusion affects the clinical (e.g. seizure reduction) and metabolic (e.g. dyslipidemia, hepatosteatosis) outcomes in IS is not known. Given this, the purpose of the present study was to examine the impact of a probiotic blend on liver metabolism in KD-fed epileptic neonatal rats, using a well-established infant model of symptomatic IS.19 This model mimics many neurodevelopmental features of IS and provides liver accessibility otherwise inaccessible in infants. We hypothesized that the probiotics may protect the neonatal liver from side effects related to lipid metabolism following KD treatment.
Methods
Animals and bacteria strains
The IS model was established using neonatal Sprague-Dawley rats as previously reported.19 All experimental procedures were approved by the Health Sciences Animal Care Committee of University of Calgary in accordance with the guidelines set forth by the Canadian Council on Animal Care. Male and female neonatal Sprague-Dawley rats were obtained from Sprague Dawley females (Charles River Laboratories, Wilmington, MA) bred in house. Briefly, at postnatal day (P) 4, neonatal rats were positioned in a stereotaxic apparatus for neonatal rat surgery (Benchmark Angle One, MyNeurolab.com, St. Louis MO). After hypothermia anesthesia, doxorubicin was injected into the right lateral ventricle at 5 µg/2.5µL, and lipopolysaccharide into the right parietal cortex at 3 µg/1.5µL. The coordinates used were as follows: doxorubicin, 2.68 mm anterior to lambda, 1.1 mm lateral to sagittal suture, 3.3 mm deep; lipopolysaccharide, 2.55 mm anterior to lambda, 1 mm lateral to sagittal suture, 1.7 mm deep. Animals were individually placed in beakers warmed in one water bath (45 °C) and filled with bedding material (31–33 °C). At P5 morning, animals were injected with 200 mg/kg p-chlorophenylalanine (PCPA) i.p. to induce infantile spasms. Neonatal rats were randomly allocated to different dietary treatments based on body weight and continuously fed either a control milk diet (CD, 1.7:1 ratio, fats : carbohydrate + protein) or the isocaloric ketogenic diet (KD, 4:1 ratio) from P4 to P8. Milk composition has been previously described20. The milk was delivered continuously through cheek cannulas connected to a time-controlled infusion pump via polyethylene 10 tube (KD Scientific Inc., MA, USA). To ensure comparable growth between groups, the infusion rate of milk was calculated daily based on the average body weight per group. Rats fed the same type of milk had equal content of intake, regardless of with or without probiotics. Loss of body weight < 20%, abdominal distension, and/or an infection at the incision site that prevents us from suturing were assumed a humane endpoint. Rats meeting the humane endpoint or dying due to the surgery were excluded from the study. Treatment and measurements were performed at the same time of experimental endpoints and in a random order to minimize potential confounders.
Two commercial probiotics, Streptococcus thermophilus HA-110 and Lactococcus lactis subsp. lactis HA-136, were provided by Lallemand Health Solutions (Montreal, QC, Canada). The blend was chosen based on the previous finding that the KD could effectively reduce spasms frequency20 as well as increase relative abundances of Streptococcus thermophilus and Lactococcus lactis species compared to the control diet.52 These strains are also documented psychobiotics, known to influence behaviour and neural activity.21,22 The purified powders were freshly rehydrated immediately before gavage in double-distilled water at 1010 colony-forming units per mL (S. thermophilus:L. lactis = 5:1, based on the their abundance in feces as observed in lab analysis). A total of 100 µL with 109 colony-forming units/dose was gavage-fed to each animal starting at the evening at P4 and each morning from P5 to P8. This limited experimental time frame was chosen due to ethical reasons including the highly invasive nature of the surgery and its severe developmental consequences in animals. It is also a period when animals experience a high rate of seizures and when the anti-seizure impacts of the KD mimic what is observed in children with seizure reductions in the range of 50%.20 The CD and KD groups were gavaged with an equivalent volume of double distilled water. The resulting experimental groups were as follows: control diet (CD), control diet + probiotics (CD + Pro), ketogenic diet (KD), and ketogenic diet +probiotics (KD + Pro). At P8, liver was collected, flash-frozen and stored at −80 °C for further analysis. All experiments were performed with the aim to keep animal pain, stress and numbers to a minimum as stated in our ethics approval.
Seizure quantification
Spasms (seizures) were characterized by rapid extension and flexion movements and were recorded in a blind manner using a video-system with Sirenia software (Pinnacle Technology, Lawrence, KS, USA) following published methods.19 Raw seizure data have been submitted for publication elsewhere and is shown here as a frame of reference for the novel data related to dyslipidemia and hepatosteatosis.52 As such, data is reported as a seizure score, calculated as the percentage of change relative to the CD group.
Fecal microbiome profiling
Fecal genomic DNA was extracted using the FastDNA® SPIN Kit for Feces (MP Biomedicals, Santa Ana, CA) and quantified using a high-sensitivity dsDNA Qubit Kit (Invitrogen, Carlsbad, CA, USA). The DNA library construction and sequencing were performed on an Illumina MiSeq platform with the MiSeq V3 600 cycle sequencing kit in the Core DNA facility at the University of Calgary. The sequence demultiplexing and removal of indices were performed using the bacterial metagenomics workflow in the MiSeq Reporter software (Illumina). Sequences were then processed with Mothur Version 1.35.1 following the online MiSeq standard operating procedure.23 Reads with exact barcode matching, two nucleotide mismatch in primer matching or containing ambiguous characters were removed. Very low abundant features were filtered using default options; minimum count 4 and low-count filter based on 20% prevalence in samples. To adjust for even sequencing depth, the numbers of sequences were rarefied to the same amount as the minimum number of sequence detected. Operational taxonomic units (OTUs) were clustered based on a 97% sequence similarity against Greengenes 13_5 database. The relative abundance per OTU was calculated for statistical analysis. Differences between CD versus CD + Pro or KD versus KD + Pro were analyzed with Mann-Whitney U test. The sequencing data is available under PRJNA795716 within NCBI Sequence Read Archive.
Biochemical assay
Measurements of hepatic triglyceride (Cat. 10010303) and malondialdehyde (Cat. 700870) were performed according to the manufacturer's instructions (Cayman Chemical Company, Ann Arbor, MI).
Metabolic fingerprinting with LC-QTOF-MS
Liver tissues (20 mg) were weighted and added into 200 μL cold methanol:water (2:1, LC/MS grade) for metabolite analysis. Purine was used as internal standard. Analyses of carnitines and fatty acids were conducted using an Agilent 6550 iFunnel HPLC Q-TOF MS system (Agilent, Santa Clara, CA, USA), following the previous report for positive24 and negative mode.25 Metabolite separation was performed on an ACQUITY UPLC HSS T3 column. Spectra were acquired over the mass range m/z 50–1000 at acquisition rate 3 spectra s-1. Reference ions for internal MS correction were introduced via the other sprayer and appear per spectra. Raw data were converted to mzXML format by ProteoWizard 3.0 package and uploaded to XCMS online (https://xcmsonline.scripps.edu/).26 Metabolites were identified against METLIN (https://metlin.scripps.edu) and Human Metabolome Database (http://www.hmdb.ca/). Data were normalized with internal standard, QC samples, and log-transformed for statistical analysis. A partial least squares discriminant analysis was performed using SIMCA 13.0 (Umetrics, Umea, Sweden). A radar plot was visualized using SigmaPlot version 14.0 software (Systat Software San Jose, CA).
RT-qPCR
RT-qPCR was performed as previously described.27,28 Briefly, Total mRNA and DNA were extracted from the liver tissues using the AllPrep DNA/RNA mini kit, in accordance with the manufacturer's protocol (Cat. 80204, Qiagen, Hilden, Germany). Total RNA concentration and purity were determined by using the Nanodrop 2000. Reverse transcription and quantitative PCR were performed as previously described.29 Ywhaz and CycA were used as reference genes. Primers targeting genes related to lipid metabolism and inflammasome were listed in Table S1.
Ratio of mtDNA/nDNA copy number
To assess whether the treatment was associated with increases in mitochondrial density, measurements of mtDNA/nDNA copy number ratios in the liver were performed via a quantitative PCR-based method as previously described.28
Western blot analyses
Depending on the target, 20 or 40 μg total protein was loaded onto Mini-PROTEAN® Precast Gels (Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad). Primary antibodies were as follows: Total OXPHOS cocktail (Cat. ab110413, RRID:AB_2629281, 1:1000, Abcam), AMPK (Cat. 2532, RRID:AB_330331, 1:1000, Cell Signaling), pAMPK (Cat. 2531S, RRID:AB_330330, 1:1000, Cell Signaling), Sirt3 (Cat. sc-49744, RRID:AB_2254693, 1:200, Santa Cruz), UCP2 (Cat. sc-6525, RRID:AB_2213585, 1:200, Santa Cruz), ACC (Cat. 3662, RRID:AB_2219400, 1:1000, Cell Signaling), pACC (Cat. 3661, RRID:AB_330337, 1:1000, Cell Signaling), and β-Actin (Cat. MAB1501, RRID:AB_2223041, 1:6000, Millipore). Bands were detected and quantified using a ChemiDOC MP gel imaging system (Bio-Rad). The expression levels of proteins were normalized to β-actin for tissue lysate.
Histone deacetylase (HDAC) activity
The nuclear extract was prepared from liver tissues following the following the manufacturer's instructions (Cat. 113474, Abcam, Cambridge, UK). About 100 µg of nuclear extract was used to measure HDAC activity with the HDAC Activity Colorimetric Assay Kit (Cat. K331-100, BioVision) following the manufacturer's instructions.
Cytokine and chemokine measurement
Liver tissues were homogenized in RIPA buffer and normalized to 2 mg protein/mL for Rat Cytokine and Chemokine Array 27 Plex by Eve Technologies (Calgary, AB) following manufacturer's instructions.
Statistical analysis
The normality of data distribution was assessed with Kolmogorov-Smirnov test. One-way ANOVA with Tukey's post hoc was used to determine differences between groups using GraphPad Prism (GraphPad Software, San Diego, CA). Data are presented as mean ± SEM. Results were considered significant when P < 0.05. A power calculation was performed using the G*Power 3.1.9.2. At least seven animals per group were needed to enable detection of an effect size at 0.7 for ANOVA (4 groups), with a power of 0.8, alpha value of 0.05 and a beta value of 0.2. The sample size was also decided considering the minimal use of newborn animals and the highly invasive nature of our intervention. To acquire unbiased data, the investigator who administered the treatment was the only person aware of the treatment group allocation. Another technician and research assistant evaluated the behavioral and biochemical analysis and were blinded to treatments.
Ethics statement
All procedures were performed under the guidelines of the Canadian Council on Animal Care with ethical approval by the Health Sciences Animal Care Committee at the University of Calgary (Approval ID. AC20-0013).
Role of funding source
Funders provided financial support for this study, and did not participate in study design, data collection, data analyses, interpretation, or writing of the manuscript.
Results
Effects of probiotics on seizure activity
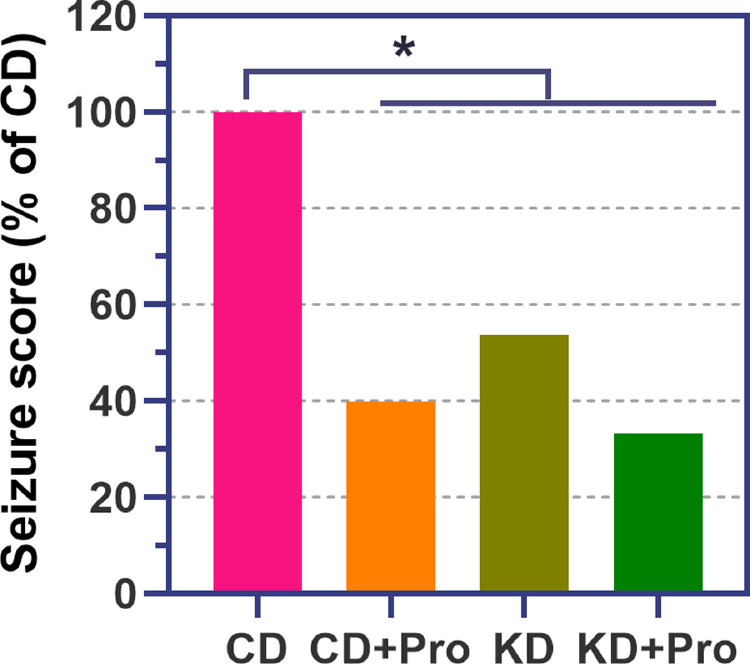
Seizure scores were significantly lower in KD compared to CD (P < 0.001, Figure 1). Analysis of CD+Pro and KD+Pro showed both treatments to lower seizures compared to CD (P < 0.001, Figure 1). No differences were observed between KD and KD+Pro, indicating that the probiotic administration did not interfere with the anti-seizure effects of the KD.
Figure 1.
Seizure scores in control diet (CD) and ketogenic diet (KD) animals administered with and without probiotics (Pro). *P < 0.05.
Confirmation of probiotic colonization
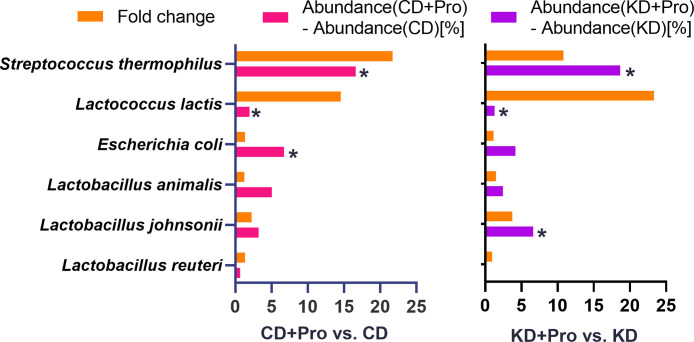
We employed 16S rRNA sequencing of the fecal microbiome following probiotic administration to confirm colonization. The relative abundances of S. thermophilus and L. lactis were significantly increased >10 fold in probiotics-treated animals compared to those without probiotics (Figure 2). In the present study, we focused on the impact of control and KD with and without probiotic manipulation. It is important to note that there are also minor differences in the gut microbiota between sham operated and lesion induced animals at P7. These differences are limited to greater Staphylococcus (P = 0.067) in lesioned animals, but no differences in diversity and other phyla (mainly Firmicutes and Proteobacteria) and genera (Fig. S1).52 As all animals were lesioned in the present study, these differences do not impact the present data about probiotic administration on hepatic steatosis. Detailed gut microbiota analysis of the administered probiotic have been reported elsewhere (Mu C, unpublished) and a sub-analysis is reported here for a frame of reference for the present data on hepatic steatosis.
Figure 2.
Probiotic administration promoted the colonization of Streptococcus thermophilus and Lactococcus lactis. N = 10-11 per group. Control diet (CD); ketogenic diet (KD); probiotics (PRO). *P < 0.05.
Probiotics ameliorated steatosis and reduced lipid accumulation in the liver
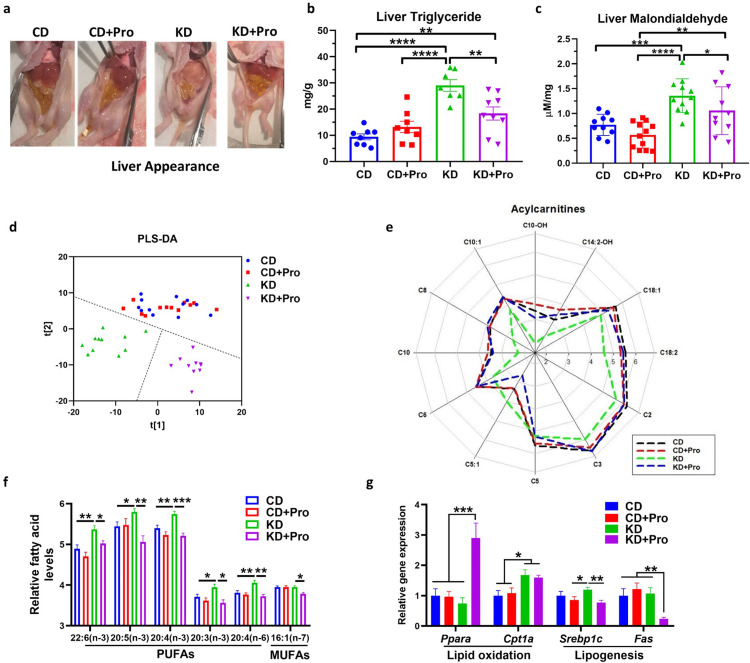
We show that KD formula administration induces hepatosteatosis in our model, as reflected by the shift in macroscopic liver appearance from red in the control diet (CD) to white in the KD in IS animals (Figure 3a). This was confirmed biochemically with a 2-fold increase in triglyceride concentration (P < 0.001, Figure 3b). The KD-induced alterations in hepatic lipid accumulation were reversed by the co-administration of a probiotic blend (Streptococcus thermophilus HA-110 and Lactococcus lactis ssp. lactis HA-136, Pro), which was confirmed by the lower triglyceride (P = 0.001) and malondialdehyde (P = 0.047) levels in the liver than KD + Pro animals (Figure 3b and c). Non-targeted metabolomics profiling of liver showed little distinction between CD and CD+Pro groups, but a large separation between KD and KD + Pro samples (Figure 3d), indicating the probiotic treatment had a greater impact on the liver metabolome of KD-fed animals. We found a group of metabolites including carnitines and fatty acids as major drivers of the metabolic shift. Relative to KD-fed animals, the KD+Pro restored the decreased abundance of long-(C18:2 [P < 0.001]), medium-(C10 [P < 0.001], C10:1 [P = 0.003], C8 [P < 0.001], C6 [P < 0.001]), and short-chain (C2 [P = 0.042], C3 [P = 0.004]) carnitines, to the level similar as CD (Figure 3e). Correspondingly, the concentrations of several polyunsaturated fatty acids and one monounsaturated fatty acid were significantly decreased by probiotics in KD-fed animals (e.g. P = 0.017 for 22:6(n-3), P = 0.007 for 20:3(n-3), P = 0.011 for 16:1(n-7); Figure 3f).
Figure 3.
Probiotics (Pro) ameliorate ketogenic diet induced steatosis. (a) Liver appearance after 4-day KD and probiotic treatment. (b) Triglyceride and (c) malondialdehyde concentrations in the liver. (d) PLS-DA plot from metabolomics profiling. (e) Carnitines and (f) unsaturated fatty acid concentrations in the liver. (g) mRNA expression of lipid metabolism genes. CD, control diet with cerebral lesion induction; KD, ketogenic diet with cerebral lesion induction. N = 7-13 per group for all tests. SEM represented, one-way ANOVA followed by Turkey's post-hoc test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Probiotics modulated the expression of key enzymes responsible for lipid metabolism
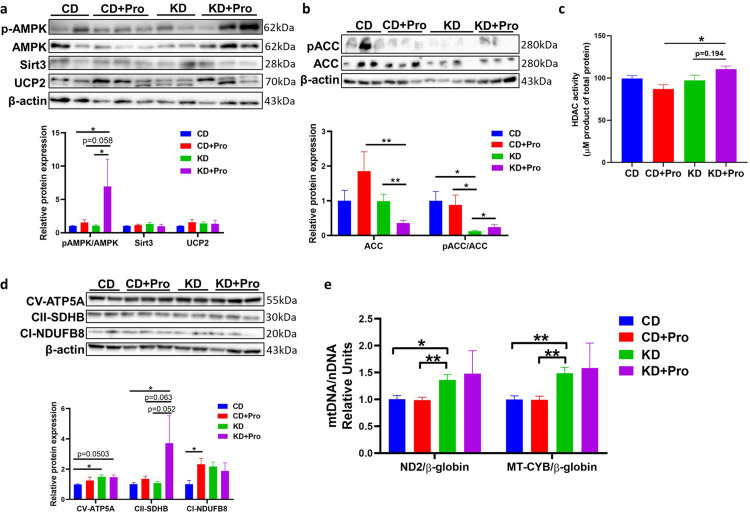
Although mRNA expression of Cpt1a was not different in KD-fed animals, probiotics upregulated the lipid oxidation marker, Pparα (P < 0.001), and downregulated the lipogenesis markers, Srebp1c (P = 0.004) and Fas (P = 0.003) (Figure 3g), indicating a shift in lipid metabolism towards utilization rather than synthesis. The lipid metabolism balance is tightly regulated by 5’-AMP kinase (AMPK) signaling that inhibits SREBP1c.30 Supporting the induction of lipid oxidation, the probiotics significantly enhanced the phosphorylation of AMPK on threonine-172 (P = 0.046) and its downstream target phosphorylation ACC (P = 0.041) in KD-fed animals (Figure 4a and b; see full blots in Supplemental Western blots). Activation of AMPK requires the stimulation of upstream deacetylase. KD+Pro group showed highest histone deacetylase (HDAC) activity, further supporting the activation of AMPK signaling (KD+Pro vs KD, P = 0.0194; Figure 4c). Taken together, our results indicate that the probiotic administration restores fatty acid oxidation through an AMPK-mediated mechanism.
Figure 4.
Probiotics regulate expression of proteins related to lipid oxidation and mitochondrial function in KD-fed pups. (a, b, c) Western blotting and the corresponding quantification of western blotting density below each gel. (d) Measurement of HDAC activity. (e) Quantification of mtDNA expression. CD, control diet with cerebral lesion induction; KD, ketogenic diet with cerebral lesion induction, probiotics (Pro). N = 7-13 per group for all tests. SEM represented, one-way ANOVA followed by Turkey's post-hoc test: #0.05<P < 0.1, *P < 0.05, **P < 0.01.
Probiotics affected the mitochondrial function
To investigate if the improved lipid oxidation was related to alterations in mitochondrial function, we measured the expression levels of mitochondrial respiratory chain complexes. Probiotic administration upregulated the protein expression of mitochondrial complex Ⅱ (P = 0.052) in KD-fed animals (Figure 4d). No difference was observed for mtDNA copy number between KD or KD+Pro groups (P = 0.718 for ND2/β-globin, P = 0.770 for MT-CYB/β-globin Figure 4e), indicating that the upregulation of mitochondrial complex Ⅱ was independent of mitochondrial abundance. These findings demonstrate that the probiotics enhance pAMPK-mediated signaling to activate lipid oxidation, which in turn ameliorates KD-induced steatosis.
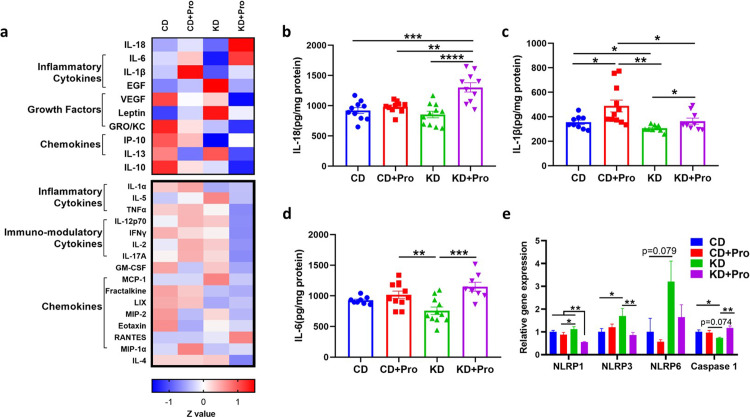
Probiotics affected the expression of cytokines and caspase 1
AMPK integrates a multitude of signals including inflammation and cytokine signaling to modulate lipid oxidation.31 Probiotics use several strategies to interact with host, one of which is immunological regulation. While discovering the upregulation of AMPK signaling, we were curious as to the possible trigger. As such, we were interested in exploring how probiotics alter cytokine expression, which may bridge the observed impacts of probiotics with pAMPK-mediated signaling. A cytokine panel-based screening showed that the KD+Pro induced a differential cytokine profile relative to KD (Figure 5a), specifically driven by upregulation of IL-18 (P < 0.001), IL-1β (P = 0.045) and IL-6 (P < 0.001) (Figure 5b–d). The gene expression of Caspase 1, a key protein catalyzing IL-18 and IL-1β maturation, was also upregulated (P = 0.002) by KD+Pro (Figure 5e).
Figure 5.
Probiotics alter cytokines in the liver. (a) Heatmap of cytokines and chemokines. Upper panel represents cytokines affected by KD or probiotic. (b, c, and d) Concentrations of IL-18, IL-1β, and IL-6. (e) mRNA expressions of inflammasome genes. CD, control diet with cerebral lesion induction; KD, ketogenic diet with cerebral lesion induction, probiotics (Pro). N = 8-11 per group. SEM represented, one-way ANOVA followed by Turkey's post-hoc test: #0.05<P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
While the KD is a well-recognized therapy to reduce seizures in drug resistant IS, this benefit can come at the expense of dyslipidemia with several studies reporting liver-related side effects in infants with IS.9,11 This is important as dyslipidemia may have lasting consequences in young children affecting metabolism, growth and development, as well as future metabolic disease risk.32 Herein we provide evidence that (i) probiotic administration can counteract some of the dyslipidemia caused by the KD in an IS model; (ii) a probiotic blend does not impact KD induced seizure mitigation and (iii) the mechanism by which probiotics are protective likely involves the upregulation of lipid oxidation involving the energy sensor AMPK.
Predictably, administration of the KD in our IS model promoted hepatic lipid accumulation, elevated malondialdehyde levels and reduced carnitines, all of which may contribute to the observed hepatosteatosis. Hepatosteatosis occurred following a 4-day KD induction, a relatively short time frame that likely reflects the immature nature of the infant liver and the inadequacy of their antioxidant defense system which is known to be insufficient to handle the lipid overload.33 Likewise, malondialdehyde levels were elevated with the KD. Malondialdehyde is a reactive metabolic product from lipid peroxidation and a well-established indicator for oxidative stress in various pathological conditions.34 Elevations are common with dietary insults with 12 week KD administration leading to lipid accumulation, steatosis and cellular injury in the liver of adult mice.35 Next, the KD resulted in reductions in liver carnitine levels in our model. Derived from amino acids, carnitine is an essential substance that transports long-chain fatty acids from the cytoplasm to the mitochondrial matrix and is key to regulating acetyl-CoA/CoA ratios and protecting against oxidative stress. Carnitine deficiency is well documented in non-alcoholic fatty liver disease (NAFLD) leading to reductions in fatty acid oxidation as well as triglyceride accumulation.36 In addition, carnitine is known to mediate inflammation with L-carnitine supplementation improving markers in children affected by chronic kidney disease.37 Given this, KD treatment could lead to carnitine deficiency in children with epilepsy, due to an enhanced demand for carnitines to promote the oxidation of fatty acids in mitochondria.38
Administered by gavage, colonization of mice with probiotics was confirmed through fecal 16S rRNA sequencing that demonstrated increases in both Streptococcus thermophilus HA-110 and Lactococcus lactis subsp. lactis HA-136 in treated animals. Results show probiotic administration was hepatoprotective, ameliorating KD induced lipid accumulation, hepatic oxidative stress and inflammation in our model of IS. Visually, probiotics resolved much of the fatty liver appearance from pink (fatty) to red (lean). Corresponding to the visual appearance of the livers, probiotics reduced triglycerides, malondialdehyde and restored carnitines in KD to levels observed in control diet animals. Although this is the first report employing probiotics to counteract KD induced dyslipidemia, the phenomenon has been previously observed in high fat induced obesity. Specifically, malondialdehyde reductions have been observed in diet-induced obese mice following administration of yogurt prepared from cultures of Streptococcus lactis and Lactobacillus bulgaricus.39 The decreased malondialdehyde after probiotic administration in the present model suggests a partially restored antioxidant defense in the liver. Likewise, the probiotics used here also altered the expression of lipid related enzymes involved in fatty acid oxidation and lipogenesis including Srebp1c, Fas and PPARα. Srebp1c and Fas, both involved in lipogenesis were reduced with KD + probiotic administration compared to KD alone. In contrast, KD + probiotic drastically increased PPARα expression. This is of interest as PPARα is a nuclear hormone receptor that regulates the transcription of genes stimulating fatty acid transport, mitochondrial ß-oxidation, ketogenesis, and triglyceride catabolism.40 This increase likely stimulated the observed elevations in mitochondrial complex II and V with the KD + probiotic group.41 While probiotics were effective in reducing KD induced lipidemia, it is important to note that the treatment had no impact on the anti-seizure effects of the diet. Given this, probiotics may be a viable option to counteract the negative impacts of the diet in young children.
Beyond metabolic improvements, we also noted probiotics influenced hepatic cytokines and chemokines. Higher concentrations of both hepatic IL-18 and caspase 1 were observed with KD + probiotic treatment. These signals are of interest as they are known to interact with the energy sensor AMPK. Specifically,IL-18 is known to activate AMPK signaling and promote lipid oxidation in the skeletal muscle of mice, counteracting high-fat diet-induced weight gain.42 Likewise, the induction of IL-18 from caspase 1 signaling has been found to be protective in the metabolic syndrome induced by a high-fat diet.43 The association between these two signaling molecules is that the maturation and secretion of IL-18 depends on the proteolytic cleavage of caspase 1 enzyme on pro-IL-18.44 AMPK upregulation after probiotics has also been previously reported in a mouse model of chronic-alcohol-induced hepatic steatosis45 and diet-induced NAFLD.46 Given these findings, the increase of IL-18 by probiotics in the present study provides a possible link to AMPK signaling. Although we observed these alterations, whether IL-18 directly contributes to the hepatoprotective effect of probiotics requires further investigation.
Another possible mediator of AMPK involves HDACs. Expression of HDAC2 could upregulate AMPK by suppressing miR-101b.47 However, upregulation of AMPK is also known to inhibit HDAC5 by promoting HDAC nuclear export via hyperphosphorylation.48 In the present study, we noted increases in total HDAC activity in our KD + probiotic group compared to CD + probiotic. It is not possible to make specific conclusions from this change as the interactions between HDAC and AMPK are complex. Due to tissue limitations, the HDAC assay used in our study detected all HDACs, including HDACs 1-11 and sirtuins. Different types of HDACs are involved in different functions. HDAC1, 2, and 3 are reported to be protective against NAFLD, while HDAC7, 8, and 11 promote NAFLD.49
In the present study, all animals were administered the same set of chemical drugs, which reduced the possibility that the chemicals used to induce IS in these rats affected signaling pathways studied in this investigation. As far as we are aware, there is no evidence showing intracerebral injection of doxorubicin and lipopolysaccharide affects the hepatic pathways reported here. Although there was one report that short-term inhibition of serotonin synthesis by intraperitoneal injection of 300 mg/kg PCPA could prevent hepatic lipid accumulation,50 the potential effects on the signaling pathways reported here were minimized in the current experimental setting as all animals were IS treated. Other limitations to be considered are the short experimental time frame of the experiment and the correlative nature of the signaling alterations found. Despite these limitations, our findings are clinically relevant and raise the possibility that KD induced side effects could be mitigated in children with epilepsy with probiotic administration.
In conclusion, the KD is often lifesaving in children with IS and other metabolic disorders,51 but it has the potential to negatively impact liver metabolism over time. The data presented here help to understand the detrimental impacts of the KD on the liver and highlight a potential approach to circumvent KD-induced steatosis through probiotic administration.
Contributors
C.M. and J.S. designed the research; C.M., N.N., T.A.T., J.M.R., M.H.S., and J.S. performed the experiments; N.N. and T.A.T. contributed new reagents; C.M. and J.S. analyzed data and wrote the paper; C.M. and J.S. directly accessed and verified the underlying data reported in the manuscript. All authors read and approved the final version of the manuscript.
Declaration of interests
C.M. received an Alberta Children's Hospital Research Institute Trainee Travel Award that provided payment for hotel and travel for meeting. T.A.T sits as a FSHN External Advisory Board Member (unpaid) of University of Florida and Advisory Council Member (unpaid) of V1 Studio-Concordia University. No conflicts of interest exist for any of the authors.
Acknowledgments
Acknowledgements
This work was supported by the funding from the Alberta Children's Hospital Research Institute and Mitacs Accelerate Program (IT16942). We thank the technical support from Matthew Rosin (Alberta Children's Hospital BioCORE) and Melinda Wang (Alberta Children's Hospital Research Institute), and Dr. Annie Tremblay (Rosell Institute for Microbiome and Probiotics, Lallemand Health Solutions) for manuscript language editing.
Data sharing statement
The sequencing data is available under PRJNA795716 within NCBI Sequence Read Archive. All supporting data are present in the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103838.
Appendix. Supplementary materials
References
- 1.Gaily E., Lommi M., Lapatto R., Lehesjoki A.E. Incidence and outcome of epilepsy syndromes with onset in the first year of life: a retrospective population-based study. Epilepsia. 2016;57(10):1594–1601. doi: 10.1111/epi.13514. [DOI] [PubMed] [Google Scholar]
- 2.Krijgh E., Catsman-Berrevoets C., Neuteboom R. Early seizure freedom is a prognostic factor for survival in patients with West syndrome. Neuropediatrics. 2018;49(04):279–282. doi: 10.1055/s-0038-1654708. [DOI] [PubMed] [Google Scholar]
- 3.Riikonen R. Infantile Spasms: outcome in clinical studies. Pediatr Neurol. 2020;108:54–64. doi: 10.1016/j.pediatrneurol.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Stafstrom CE. Febrile Seizures. Elsevier; 2002. The incidence and prevalence of febrile seizures; pp. 1–25. [Google Scholar]
- 5.Hrachovy R.A., Frost J.D., Kellaway P., Zion T.E. Double-blind study of ACTH vs prednisone therapy in infantile spasms. J Pediatr. 1983;103(4):641–645. doi: 10.1016/s0022-3476(83)80606-4. [DOI] [PubMed] [Google Scholar]
- 6.Karvelas G., Lortie A., Scantlebury M.H., Duy P.T., Cossette P., Carmant L. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18(3):197–201. doi: 10.1016/j.seizure.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Snead O.C., Benton J.W., Myers G.J. ACTH and prednisone in childhood seizure disorders. Neurology. 1983;33(8):966–970. doi: 10.1212/wnl.33.8.966. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y.H., Chen C., Chen S.H., Shen Y.C., Kuo Y.T. Effectiveness of corticosteroids versus adrenocorticotropic hormone for infantile spasms: a systematic review and meta-analysis. Ann Clin Transl Neurol. 2019;6(11):2270–2281. doi: 10.1002/acn3.50922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dressler A., Benninger F., Trimmel-Schwahofer P., et al. Efficacy and tolerability of the ketogenic diet versus high-dose adrenocorticotropic hormone for infantile spasms: a single-center parallel-cohort randomized controlled trial. Epilepsia. 2019;60(3):441–451. doi: 10.1111/epi.14679. [DOI] [PubMed] [Google Scholar]
- 10.Vining E.P., Freeman J.M., Ballaban-Gil K., et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55(11):1433–1437. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 11.Hong A.M., Turner Z., Hamdy R.F., Kossoff E.H. Infantile spasms treated with the ketogenic diet: prospective single-center experience in 104 consecutive infants. Epilepsia. 2010;51(8):1403–1407. doi: 10.1111/j.1528-1167.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- 12.Lyons L., Schoeler N.E., Langan D., Cross J.H. Use of ketogenic diet therapy in infants with epilepsy: a systematic review and meta-analysis. Epilepsia. 2020;61(6):1261–1281. doi: 10.1111/epi.16543. [DOI] [PubMed] [Google Scholar]
- 13.Arslan N., Guzel O., Kose E., et al. Is ketogenic diet treatment hepatotoxic for children with intractable epilepsy? Seizure. 2016;43:32–38. doi: 10.1016/j.seizure.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor D., Garg D., Sharma S. Emerging role of the ketogenic dietary therapies beyond epilepsy in child neurology. Ann Indian Acad Neurol. 2021;24(4):470–480. doi: 10.4103/aian.AIAN_20_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mu C., Corley M.J., Lee R.W.Y., et al. Metabolic framework for the improvement of autism spectrum disorders by a modified ketogenic diet: a pilot study. J Proteome Res. 2020;19(1):382–390. doi: 10.1021/acs.jproteome.9b00581. [DOI] [PubMed] [Google Scholar]
- 16.Gibson G.R., Hutkins R., Sanders M.E., et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Tang H., Zhang C., et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9(1):1–15. doi: 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna S., Walia S., Kondepudi K.K., Shukla G. Administration of indigenous probiotics modulate high-fat diet-induced metabolic syndrome in Sprague Dawley rats. Antonie Van Leeuwenhoek. 2020;113(9):1345–1359. doi: 10.1007/s10482-020-01445-y. [DOI] [PubMed] [Google Scholar]
- 19.Scantlebury M.H., Galanopoulou A.S., Chudomelova L., Raffo E., Betancourth D., Moshé S.L. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37(3):604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhary A., Mu C., Barrett K., et al. The link between brain acidosis, breathing, and seizures: a novel mechanism of action for the ketogenic diet in a model of infantile spasms. Brain Commun. 2021;3(4) doi: 10.1093/braincomms/fcab189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinan T.G., Stanton C., Cryan J.F. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar A., Lehto S.M., Harty S., Dinan T.G., Cryan J.F., Burnet P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schloss P.D., Westcott S.L., Ryabin T., et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayengbam S., Chleilat F., Reimer R.A. Dietary vitamin B6 deficiency impairs gut microbiota and host and microbial metabolites in rats. Biomedicines. 2020;8(11) doi: 10.3390/biomedicines8110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korver-Keularts I., Wang P., Waterval H., et al. Fast and accurate quantitative organic acid analysis with LC-QTOF/MS facilitates screening of patients for inborn errors of metabolism. J Inherit Metab Dis. 2018;41(3):415–424. doi: 10.1007/s10545-017-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tautenhahn R., Patti G.J., Rinehart D., Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem. 2012;84(11):5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newell C., Sabouny R., Hittel D.S., et al. Mesenchymal stem cells shift mitochondrial dynamics and enhance oxidative phosphorylation in recipient cells. Front Physiol. 2018;9:1572. doi: 10.3389/fphys.2018.01572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newell C., Shutt T.E., Ahn Y., et al. Tissue specific impacts of a ketogenic diet on mitochondrial dynamics in the BTBRT+ tf/j mouse. Front Physiol. 2016;7:654. doi: 10.3389/fphys.2016.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen J., Beveridge J.K., Wang M., Orr S.L., Noel M., Mychasiuk R. A pilot study investigating the role of gender in the intergenerational relationships between gene expression, chronic pain, and adverse childhood experiences in a clinical sample of youth with chronic pain. Epigenomes. 2021;5(2):9. doi: 10.3390/epigenomes5020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith B.K., Marcinko K., Desjardins E.M., Lally J.S., Ford R.J., Steinberg G.R. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metabol. 2016;311(4):E730–EE40. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg G.R. Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89(3):1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 32.Neves G.S., Lunardi M.S., Lin K., Rieger D.K., Ribeiro L.C., Moreira J.D. Ketogenic diet, seizure control, and cardiometabolic risk in adult patients with pharmacoresistant epilepsy: a review. Nutr Rev. 2021;79(8):931–944. doi: 10.1093/nutrit/nuaa112. [DOI] [PubMed] [Google Scholar]
- 33.Gulbayzar S., Arica V., Hatipoglu S., Kaya A., Arica S., Karatekin G. Malondialdehyde level in the cord blood of newborn infants. Iran J Pediatr. 2011;21(3):313–319. [PMC free article] [PubMed] [Google Scholar]
- 34.Ore A., Akinloye O.A. Oxidative stress and antioxidant biomarkers in clinical and experimental models of non-alcoholic fatty liver disease. Medicina. 2019;55(2):26. doi: 10.3390/medicina55020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garbow J.R., Doherty J.M., Schugar R.C., et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G956–G967. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N., Zhao H. Role of carnitine in non-alcoholic fatty liver disease and other related diseases: an update. Front Med. 2021;8 doi: 10.3389/fmed.2021.689042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamedi-Kalajahi F., Imani H., Mojtahedi S., Shabbidar S. Effect of L-carnitine supplementation on inflammatory markers and serum glucose in hemodialysis children: a randomized, placebo-controlled clinical trial. J Ren Nutr. 2021 doi: 10.1053/j.jrn.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Linek M. University of Cincinnati; 2019. The Relationship Between Serum Carnitine Levels and Ketones in Children with Epilepsy Following a Ketogenic Diet. [Google Scholar]
- 39.Lasker S., Rahman M.M., Parvez F., et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci Rep. 2019;9(1):20026. doi: 10.1038/s41598-019-56538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefebvre P., Chinetti G., Fruchart J.C., Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116(3):571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroon T., Harms M., Maurer S., et al. PPARγ and PPARα synergize to induce robust browning of white fat in vivo. Mol Metab. 2020;36 doi: 10.1016/j.molmet.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindegaard B., Matthews V.B., Brandt C., et al. Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes. 2013;62(9):3064–3074. doi: 10.2337/db12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy A.J., Kraakman M.J., Kammoun H.L., et al. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab. 2016;23(1):155–164. doi: 10.1016/j.cmet.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 44.Vande Walle L., Lamkanfi M. Inflammasomes: caspase-1-activating platforms with critical roles in host defense. Front Microbiol. 2011;2:3. doi: 10.3389/fmicb.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M., Wang C., Wang C., et al. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J Nutr Biochem. 2015;26(4):337–344. doi: 10.1016/j.jnutbio.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Z., Wang C., Zhang L., et al. Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl Microbiol Biotechnol. 2019;103(14):5843–5850. doi: 10.1007/s00253-019-09703-4. [DOI] [PubMed] [Google Scholar]
- 47.Liu D., Tang H., Li X.Y., et al. Targeting the HDAC2/HNF-4A/miR-101b/AMPK pathway rescues tauopathy and dendritic abnormalities in Alzheimer's disease. Mol Ther. 2017;25(3):752–764. doi: 10.1016/j.ymthe.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gongol B., Sari I., Bryant T., Rosete G., Marin T. AMPK: an epigenetic landscape modulator. Int J Mol Sci. 2018;19(10):3238. doi: 10.3390/ijms19103238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu S., Yu M., Tan Y., Liu D. Role of histone deacetylase on nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2021;15(4):353–361. doi: 10.1080/17474124.2021.1854089. [DOI] [PubMed] [Google Scholar]
- 50.Namkung J., Shong K.E., Kim H., Oh C.M., Park S., Kim H. Inhibition of serotonin synthesis induces negative hepatic lipid balance. Diabetes Metab J. 2018;42(3):233–243. doi: 10.4093/dmj.2017.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gavrilovici C., Rho J.M. Metabolic epilepsies amenable to ketogenic therapies: indications, contraindications, and underlying mechanisms. J Inherit Metab Dis. 2021;44(1):42–53. doi: 10.1002/jimd.12283. [DOI] [PubMed] [Google Scholar]
- 52.Mu Chunlong, Choudhary Anamika, Mayengbam Shyamchand, et al. Seizure modulation by the gut microbiota and tryptophan-kynurenine metabolism in an animal model of infantile spasms. EBioMedicine. 2022 doi: 10.1016/j.ebiom.2022.103833. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.