Abstract
This experiment was conducted to evaluate whether a commercial mycotoxins-binder, XL, could effectively attenuate the negative effects of Aflatoxin B1 (AFB1) on growth performance, immunological function, and intestinal health in birds. Two hundred forty 1-day-old Arbor Acres broiler chickens were randomly divided into 4 treatments using a 2 × 2 factorial randomized design with 2 levels of dietary mycotoxins binder (0 or 2g /kg) and 2 AFB1 supplemented levels (0 or 200 μg/kg) from 0 to 42 d. Results showed that AFB1 exposure impaired growth performance by decreasing BWG in 1–21 d and 1–42 d, decreasing FI in 1–21 d, increasing FCR in 1–21 d and 1–42 d (P < 0.05). Broilers fed AFB1- contaminated diet impaired the immune function, as evident by decreasing IgA contents, Newcastle disease antibody titers in serum, and sIgA contents of jejunal mucosa at 21 d (P < 0.05). On the other hand, AFB1 challenge significantly increased the gene expression of proinflammatory factors in spleen at 21 d and liver at 42 d, and significantly decreased claudin-1 expression at 42 d and occludin expression at 21 d, and increased claudin-2 at 21 d in jejunum of broiler chickens (P < 0.05) compared to the basal diet group. Dietary XL supplementation significantly decreased the gene expression of IL-6 in spleen at 21 d and IL-1β in liver at 42 d, cytochrome P450 3A4 (CYP3A4) expression in liver at 21 d of broilers (P < 0.05) compared with the nonsupplemented birds, regardless of AFB1 challenged or not. Inclusion of 2 g/kg XL increased serum ALB at 42 d, IgM and IgA at 42 d, Newcastle disease antibody titer level at 35 d (P < 0.05). Dietary XL addition enhanced intestinal barrier function by increasing the expression of claudin-1 at 21 d and Occludin at 42 d (P < 0.05) in jejunum. Conclusively, 2 g/kg mycotoxins-binder can relieve the toxic effect of AFB1 on broilers.
Key words: mycotoxins binder, aflatoxin B1, broiler chickens, immunological function, intestinal barrier function
INTRODUCTION
Aflatoxin (AF) is a secondary metabolite of Aspergillus species, which can contaminate food and agricultural products (Olarte et al. 2012; Jallow et al. 2021). As one form of AF, aflatoxin B1 (AFB1) is well known as the potent and dangerous teratogen, carcinogen classified in group 1 by IARC and immune-suppressor produced naturally by Aspergillus flavus (A. flavus) or Aspergillus parasiticus (A. parasiticus) for human and animals (Fouad et al., 2019; Jallow et al., 2021). Chicken exposed to ABF1 is reported to depress feed intake, growth performance, immune response, damage organs, disturb the balance of gut microbiota, and increase mortality (Williams et al., 2011; Chang et al., 2020; Rashidi et al., 2020). Consequently, income of poultry producer and human health suffered hazard risk from AFB1 (Khlangwiset et al., 2011). Organ damage induced by AF common occurs in liver (Rashidi et al., 2020), kidney (Śliżewska et al., 2019), reproductive organs (Doerr and Ottinger, 1980; Ortatatli et al., 2002), digestive tract (Feng et al., 2017; Poloni et al., 2020), pancreas (Ortatatli et al., 2002), immune organs (Peng et al., 2014; Rasouli-Hiq et al., 2017), and bones (Raju et al., 2005) in poultry species. And the degree of organ damage depends on the degree of contamination and the susceptibility of the bird species to AFB1. Major serum biochemistries changed significantly, such as reduction of total protein, TG, albumin, Ca, P (Rashidi et al., 2020), globulin (Chen et al., 2016), oxidoreductase activities and concentration (Ali Rajput et al., 2017) and immunoglobulin content (Chen et al., 2014), are supposed to be indicators of aflatoxicosis. Immunosuppression induced by AFB1 including higher skin response to mitogens (Bagherzadeh Kasmani et al., 2012), decrease of specific immunity response to Newcastle disease (ND), avian influenza (AI) viruses (Rashidi et al., 2020), sheep red blood cells (SRBC) (Bhatti et al., 2017), and impaired cell-mediated immunity (CMI) (Giambrone et al., 1985; Hoerr, 2010) in birds has been demonstrated. Intestinal health impairments from AFB1 exposure, including mechanical, immune (Jiang et al., 2015; Liu et al., 2018b), chemical, microbial barrier (Chang et al., 2020), and also have been documented.
The broiler industry has been preceded only by the pig industry which is the largest animal husbandry, and supply the second most highly consumed meat in China (Xin et al., 2016). In order to control and degrade mycotoxins, biological, physical, and chemical methods for detoxicating have been selected in broiler production (Solis-Cruz et al., 2019; Rashidi et al., 2020). Mycotoxin binders, XL, is a kind of additive package composed of bentonite with a high content of smectite binding agent, inactive yeast cell wall fractions from a Saccharomyces cerevisiae strain, activated β-1,3/1,6-glucans (glucose biopolysaccharides). Core modes of action of major compositions for XL to detoxicate are binding mycotoxins, protecting intestine, and modulating immune system (Morales-López et al., 2009; Shannon et al., 2017; Pascual et al., 2020). A published report showed positive effects of XL on counteracting the harmful effects of mycotoxins on performance in laying hens (Zhao et al., 2021). However, whether the commercial mycotoxins-binder could effectively attenuate the toxic effects of AFB1 when exposed to broiler chickens remains unknown. The objective of this study was to evaluate the efficacy of XL to reduce the toxicity of AFB1 to broilers.
MATERIALS AND METHODS
Animal Ethics Statement
The broiler care and use protocol was approved by the China Agricultural University Animal Care and Use Committee, Beijing, P. R. China.
Experimental Design and Animal Management
Two hundred and forty 1-day-old Arbor Acres (AA) male broiler chickens were purchased from Beijing Arbor Acres Poultry Breeding Company randomly assigned to 4 treatments with 6 replicates of 10 birds each. The experiment lasted 42 d which included 2 phases (1–21 d and 22–42 d). Feed and water were given ad libitum for the 6-wk exposure period. The composition of the basal diet and nutrient levels are showed in Table S1.
The feeding experiment was designed as follows:
Group A: Basal diet.
Group B: Basal diet with 2 g /kg XL (Trouw Nutrition, Amersfoort, The Netherlands).
Group C: Basal diet with 200 μg/kg AFB1 (Sigma-Aldrich, St. Louis, MO).
Group D: Basal diet with 2 g /kg XL +200 μg/kg AFB1.
Broiler chickens were housed in wire cages and maintained under 23L:1D for this experiment after receiving continuous light for the first 24 h. The room temperature was maintained at 32°C to 34°C during the first 5 d and then gradually decreased by 2°C/wk to reach a final room temperature of 22°C to 24°C.
Measurement of Growth Performance and Organ Index
Body weight (BW), feed intake (FI), and mortality were recorded on the 0, 21, and 42 d, and average body weight gain (BWG), average FI, and feed conversion ratio (FCR) were calculated during this trial. All performance parameters were corrected according to mortality.
On the 21 and 42 d., 6 birds in each treatment (1 bird from each cage) were humanely euthanized and tissue samples were collected. Detoxification organ and immune organs of the liver, spleen, bursa, and thymus were collected and weighed. Calculate organ index as organ index = organ weight (mg)/body weight (g).
Determination of Serum Protein and Immunoglobulin Levels by ELISA
At 21 and 42 d, 6 birds in each treatment (1 bird from each cage) were selected to collect blood. An approximately 10 mL blood sample was collected from the jugular vein into a non-heparinized tube, placed at room temperature for 30 min, centrifuged at 3,000 g for 10 min, and the serum was separated and stored in 1.5 mL eppendorf tubes at -20°C until further analysis. According to the Elisa kit instruction, the levels of serum total protein (TP), albumin (ALB), globulin (GLO), IgG, IgA, and IgM were determined. Serum GLO content was calculated as the difference between TP and ALB.
The birds were vaccinated intramuscularly with inactivated Newcastle Disease vaccine at 7 d and 21 d, respectively. Six birds in each treatment at 21 d and 35 d were selected to collect Blood samples (5.0 mL). All antibodies and reference sera used in the assay were purchased from IDEXX Laboratories Inc.
Intestinal Morphology and Jejunal mucous sIgA
At 21, 42 d, one bird per replicate was randomly selected and slaughtered and the whole gastrointestinal tract was immediately exposed. Then 10-cm intestinal segments of jejunum were sniped to scrape mucous membrane. All intestine samples were wrapped in Eppendorf tubes and stored at −80°C until further analysis. Another section of jejunum which was fixed in the 4% PFA was cut into smaller sections for preparation of histological slides. The slides were stained with hematoxylin and eosin and then captured images under a microscope (Leica, DM750). The villus height (VH), crypt depth (CD), VH to CD (VH/CD) ratio, and mucosal thickness were measured from stained samples by using the ImagePro Plus 6.0 software (Media Cybernetics, Bethesda, MD).
The VH was measured from the tip to the bottom of the villi, and CD as the distance between its mouth and its base. Eight well-oriented villi and crypts were randomly selected on each slide to determine VH and CD. The average value of the 6 structures per chicken was used. Mucosal thickness was measured as the distance between the mucosal epithelium and the muscular layer (Rubio et al., 2010).
The content of jejunal mucous sIgA was measured in accordance with instructions introduced by a chicken secretory immunoglobulin A (sIgA) ELISA Kit (Bethyl Laboratories, Inc.). The absorbance will be read at wavelength of 450 nm by a multiskan spectrum microplate spectrophotometer (Spectra Max i3x). The content of sIgA was presented as μg/mL mucous suspension.
Total RNA Extraction, Reverse Transcription and Quantitative Real-Time PCR
Total RNA was extracted from spleen, liver, and jejunum (50 mg) using Trizol reagent according to the manufacturer's instructions. The 260:280 nm absorbance ratio in an ultraviolet spectrophotometer (NanoDrop-2000, Thermo Fisher Scientific) was used to estimate the concentration and purity of the total RNA (OD260/OD280: 1.8–2.0). Then total RNA was stored at −80°C or synthesize cDNA stored at -20°C until next procedure. The primer sequences were presented in Table S2. The parameters of PCR reactions were 95°C for 5 min for one cycle, and then 95°C for 30 s for 40 cycles, 60°C for 30 s. Samples were run in triplicate. The qPCR reaction was 10-μL reaction volume and conducted with a 7500 Real-Time PCR system (Xue et al., 2021) . Gene expressions for IL-1β, IL-6, CYP3A4, claudin-1, claudin-2, claudin-3, and occludin were normalized against the level of housekeeping gene expression (β-actin).
Statistical Analysis
All analyses were performed by the GLM procedure SPSS 16.0 software (SPSS Inc., Chicago, IL) as a 2 × 2 factorial arrangement (2 levels of AFB1 challenge and 2 levels of XL treatment). The data were analyzed by two-way ANOVA analysis of variance with AFB1 and XL as the fixed factors. When interactive effects differed significantly, Duncan's multiple comparisons test was used to separate means. Differences were considered significant at P < 0.05, although probability values up to 0.05 ≤ P < 0.10 are shown in the text if data suggest a trend. The results are expressed as treatment means with their pooled SEM.
RESULTS
Growth Performance
Growth performance results (including body weight gain, feed intake, and feed conversion ratio) of broiler chickens at different growth periods are shown in Table 1. AFB1 exposure significant decreased BWG during 1–21 d (P < 0.001) and 1–42 d (P < 0.01), decreased FI during 1–21 d (P < 0.05), increased FCR during 1–21 d (P < 0.001) and 1–42 d (P < 0.05) compared to the control group. Compared to nonsupplemented birds, the addition of XL did not significantly affect BWG, FI, and FCR. No significant differences were found in mortality rate across all stage by AFB1 or XL addition (Table S3). Besides, there was no interaction effect (P > 0.05) in growth performance between AFB1 exposure and XL supplementation on growth parameters.
Table 1.
| Groups | BWG (g) |
FI (g) |
FCR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–21 d | 21–42 d | 1–42 d | 1–21 d | 21–42 d | 1–42 d | 1–21 d | 21–42 d | 1–42 d | ||
| Control | 603 | 1,827 | 2,430 | 933 | 3,120 | 4,054 | 1.55 | 1.71 | 1.67 | |
| XL | 628 | 1,809 | 2,437 | 967 | 3,148 | 4,115 | 1.54 | 1.74 | 1.69 | |
| AFB1 | 555 | 1,796 | 2,351 | 91 | 3,083 | 3,995 | 1.64 | 1.72 | 1.70 | |
| XL+AFB1 | 548 | 1,807 | 2,355 | 923 | 3,108 | 4,031 | 1.69 | 1.72 | 1.71 | |
| SEM4 | 8.2 | 9.4 | 15.3 | 7.6 | 17.0 | 23.1 | 0.015 | 0.007 | 0.006 | |
| The main effect | ||||||||||
| AFB1 | - | 628 | 1,818 | 2,433 | 950 | 3,134 | 4,084 | 1.55 | 1.72 | 1.68 |
| + | 548 | 1,801 | 2,353 | 917 | 3,096 | 4,013 | 1.67 | 1.72 | 1.71 | |
| XL | - | 579 | 1,811 | 2,390 | 923 | 3,102 | 4,024 | 1.60 | 1.73 | 1.68 |
| + | 588 | 1,808 | 2,396 | 945 | 3,128 | 4,073 | 1.61 | 1.71 | 1.70 | |
| P-value5 | ||||||||||
| AFB1 | <0.001 | 0.413 | 0.008 | 0.024 | 0.282 | 0.132 | <0.001 | 0.688 | 0.021 | |
| XL | 0.362 | 0.862 | 0.849 | 0.109 | 0.463 | 0.298 | 0.261 | 0.207 | 0.163 | |
| AFB1 XL | 0.106 | 0.461 | 0.969 | 0.419 | 0.965 | 0.785 | 0.105 | 0.312 | 0.728 | |
a-bMeans with different superscripts in the same column differ significantly (P < 0.05).
Each value represents the mean values of 6 pens of 10 animals each (n = 6).
Abbreviations: AFB1, aflatoxin B1; BWG, body weight gain; FI, feed intake; FCR, feed conversion ratio.
− represents without AFB1 or XL supplementation; + represents supplemented with AFB1 or XL.
SEM, standard error of the mean.
P-values represent the main effect of AFB1 exposure, the main effect of the dietary XL, and the interaction between AFB1 exposure and XL treatments.
Organ Index and Relative mRNA Expression of Inflammatory Cytokines and CYP3A4 in the Spleen and Liver
Relative organ weights including liver, spleen, bursa of Fabricius, and thymus from broiler were calculated in this study are shown in Table S4. Neither mycotoxins binder nor AFB1 addition affected the organ weight of broiler chickens (P > 0.05). Notably, bursary of Fabricius index and thymus index in 21 d was almost more than 2 times and 4 times as weight as the corresponding organ index in 42 d, respectively; while spleen index in 21 d was about half of that in 42d.
The effects of AFB1 contamination and mycotoxins binder addition on IL-6 expression in spleen of broiler chickens are shown in Table 2. AFB1-contaminated diet significantly increased IL-6 gene expression at 21d (P < 0.001) and tended to increase IL-6 at 42 d (P = 0.052). The addition of mycotoxins binder significantly decreased (P < 0.001) IL-6 expression at 21 d. There was a significant (P < 0.05) interaction effect on IL-6 expression at 21 d between AFB1 exposure and XL supplementation.
Table 2.
Effects of dietary AFB1 and XL on relative mRNA expression of IL-1β, IL-6, and CYP3A4 in the spleen and liver of broiler chickens.123
| Groups | Spleen |
Liver |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
IL-6 |
IL-1β |
IL-6 |
CYP3A4 |
||||||
| 21 d | 42 d | 21 d | 42 d | 21 d | 42 d | 21 d | 42 d | ||
| Control | 1.00b | 1.00 | 1.00 | 1.00b | 1.00 | 1.00 | 1.00 | 1.00 | |
| XL | 1.28b | 0.54 | 1.21 | 1.40b | 0.38 | 0.14 | 0.34 | 0.34 | |
| AFB1 | 8.43a | 1.24 | 1.09 | 3.52 | 0.67 | 2.30 | 0.67 | 1.01 | |
| XL+AFB1 | 0.80b | 2.38 | 0.86 | 1.24b | 0.46 | 0.69 | 0.27 | 0.37 | |
| SEM4 | 0.748 | 0.274 | 0.198 | 0.294 | 0.088 | 0.235 | 0.091 | 0.125 | |
| The main effect | |||||||||
| AFB1 | - | 1.14 | 0.77 | 1.10 | 1.20 | 0.69 | 0.53 | 0.69 | 0.65 |
| + | 4.62 | 2.38 | 1.47 | 2.38 | 0.56 | 1.50 | 0.56 | 0.69 | |
| XL | - | 4.71 | 1.12 | 1.55 | 2.34 | 0.83 | 1.71 | 0.83 | 1.00 |
| + | 1.04 | 1.46 | 1.03 | 1.31 | 0.42 | 0.41 | 0.42 | 0.34 | |
| P-value5 | |||||||||
| AFB1 | <0.001 | 0.052 | 0.323 | 0.020 | 0.417 | 0.014 | 0.417 | 0.864 | |
| XL | <0.001 | 0.50 | 0.176 | 0.058 | 0.014 | 0.002 | 0.014 | 0.007 | |
| AFB1 XL | <0.001 | 0.127 | 0.064 | 0.009 | 0.203 | 0.291 | 0.203 | 0.889 | |
Means with different superscripts in the same column differ significantly (P < 0.05).
Each value represents the mean values of 6 pens of 10 animals each (n = 6).
AFB1, aflatoxin B1; BWG, body weight gain; FI, feed intake; FCR, feed conversion ratio.
− represents without AFB1 or XL supplementation; + represents supplemented with AFB1 or XL.
SEM, standard error of the mean.
P-values represent the main effect of AFB1 exposure, the main effect of the dietary XL, and the interaction between AFB1 exposure and XL treatments.
As noted in Table 2, results revealed that AFB1-contaminated diet increased IL-1β and IL-6 expression in liver at 42 d (P < 0.05). Inclusion of XL to diet significantly decreased IL-6 at 42 d (P = 0.002), tended to decrease IL-1β at 42 d (P = 0.058), decreased CYP3A4 expression at 21 (P = 0.014) and 42 d (P = 0.007) in liver. Moreover, there were significant interactions to IL-1β expression at 42 d (P = 0.009) rather than IL-1β at 21 d or IL-6, CYP3A4 gene expression between AFB1 exposure and XL treatment.
Serum Proteins and Immunoglobulins
AFB1 exposure tended to decrease GLO at 21 d (P < 0.1) compared with the unchallenged birds. Chickens received XL diets showed higher content of ALB at 42 d (P < 0.05) compared to the nonsupplemented birds. There was significant interaction effect for ALB at 42 d (P = 0.027) and GLO at 21 d (P = 0.031) between AFB1 exposure and XL addition (Table 3).
Table 3.
| Items | TP (g/L) |
ALB (g/L) |
GLO (g/L) |
||||
|---|---|---|---|---|---|---|---|
| 21 d | 42 d | 21 d | 42 d | 21 d | 42 d | ||
| Control | 36.21 | 37.924 | 17.09 | 17.93ab | 20.25a | 18.54 | |
| XL | 33.97 | 37.14 | 17.91 | 17.60ab | 17.77ab | 21.63 | |
| AFB1 | 34.77 | 35.224 | 19.90 | 15.49b | 12.98b | 17.93 | |
| XL+AFB1 | 36.25 | 37.90 | 20.72 | 22.16a | 18.75a | 19.17 | |
| SEM4 | 0.573 | 0.578 | 1.018 | 0.849 | 1.010 | 0.773 | |
| The main effect | |||||||
| AFB1 | - | 35.09 | 37.53 | 17.50 | 18.82 | 19.01 | 20.09 |
| + | 35.51 | 36.56 | 20.31 | 17.77 | 15.60 | 18.55 | |
| XL | - | 36.49 | 36.57 | 18.49 | 16.71 | 16.61 | 18.24 |
| + | 35.11 | 37.52 | 19.32 | 19.88 | 18.21 | 20.40 | |
| P-value5 | |||||||
| AFB1 | 0.719 | 0.406 | 0.193 | 0.480 | 0.092 | 0.330 | |
| XL | 0.745 | 0.414 | 0.697 | 0.043 | 0.364 | 0.175 | |
| AFB1 XL | 0.122 | 0.145 | 0.999 | 0.027 | 0.031 | 0.554 | |
Means with different superscripts in the same column differ significantly (P < 0.05).
Each value represents the mean values of 6 pens of 10 animals each (n = 6).
Abbreviations: ALB, albumin; GLO, globulin; TP, total protein.
− represents without AFB1 or XL supplementation; + represents supplemented with AFB1 or XL.
SEM, standard error of the mean.
P-values represent the main effect of AFB1 exposure, the main effect of the dietary XL, and the interaction between AFB1 exposure and XL treatments.
In addition, AFB1 exposure significantly increased content of IgA (P = 0.015) as compared to the control. Compared with the nonsupplemented birds, supplementation of XL significantly increased serum IgM (P < 0.001) and IgA (P = 0.005) concentrations at 42 d, also showed an increased trend in IgA content at 21 d (P < 0.1), but showed no significant effects on IgG. No significant interaction effects were found on IgG, IgM, and IgA between AFB1 exposure and XL addition (Table 4).
Table 4.
| Items | IgG (g/L) |
IgM (g/L) |
IgA (g/L) |
||||
|---|---|---|---|---|---|---|---|
| 21 d | 42 d | 21 d | 42 d | 21 d | 42 d | ||
| Control | 3.96 | 4.24 | 1.65 | 1.57 | 2.25 | 2.16 | |
| XL | 3.92 | 4.26 | 1.64 | 1.63 | 2.28 | 2.21 | |
| AFB1 | 3.99 | 4.21 | 1.58 | 1.57 | 2.20 | 2.18 | |
| XL+AFB1 | 4.10 | 4.22 | 1.64 | 1.64 | 2.24 | 2.25 | |
| SEM4 | 0.035 | 0.016 | 0.014 | 0.010 | 0.010 | 0.010 | |
| The main effect | |||||||
| AFB1 | - | 3.94 | 4.25 | 1.64 | 1.59 | 2.26 | 2.19 |
| + | 4.04 | 4.21 | 1.61 | 1.61 | 2.22 | 2.21 | |
| XL | - | 3.97 | 4.23 | 1.61 | 1.57 | 2.22 | 2.18 |
| + | 4.00 | 4.24 | 1.64 | 1.63 | 2.26 | 2.22 | |
| P-value5 | |||||||
| AFB1 | 0.14 | 0.291 | 0.209 | 0.597 | 0.015 | 0.365 | |
| XL | 0.621 | 0.716 | 0.255 | 0.000 | 0.093 | 0.005 | |
| AFB1 XL | 0.278 | 0.972 | 0.169 | 0.537 | 0.681 | 0.170 | |
a-bMeans with different superscripts in the same column differ significantly (P < 0.05).
Each value represents the mean values of 6 pens of 10 animals each (n = 6).
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; IgA, immunoglobulin A.
− represents without AFB1 or XL supplementation; + represents supplemented with AFB1 or XL.
SEM, standard error of the mean.
P-values represent the main effect of AFB1 exposure, the main effect of the dietary XL, and the interaction between AFB1 exposure and XL treatments.
Antibody Response to Newcastle Disease
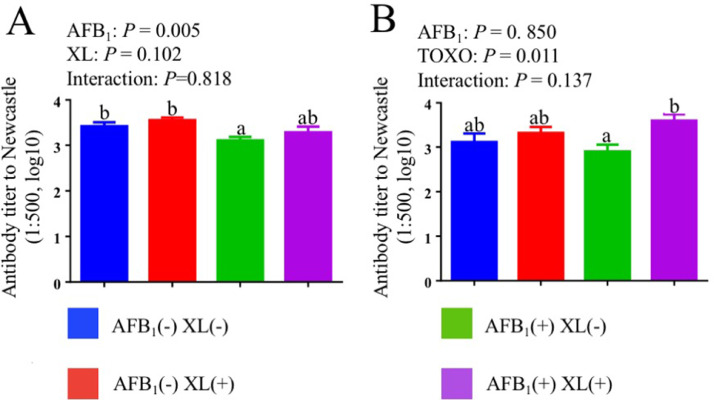
The effects of AFB1 challenge and mycotoxins binder addition on serum antibody response to Newcastle Disease of broiler chickens are shown in Figure 1. No significant cooperative effect was found between AFB1 exposure and XL addition in terms of serum antibody titer to Newcastle in broiler (P > 0.05). AFB1 exposure significant decreased (P = 0.005) serum antibody titer to Newcastle at 21 d when compared with the nonsupplemented birds. Compared to nonsupplemented groups, the addition of XL increased antibody titer to Newcastle at 35 d (P = 0.011).
Figure 1.
Effects of dietary AFB1 and mycotoxins binder on serum antibody response to Newcastle Disease of broiler chickens at day 21 (A) and day 35 (B).12 a-b Means with different superscripts in the same column differ significantly (P < 0.05) 1 Each value represents the mean values of 6 pens of 10 animals each (n = 6). 2P-values represent the main effect of AFB1 exposure, the main effect of the dietary XL, and the interaction between AFB1 exposure and XL treatments.
The Content of sIgA in Jejunal Mucous
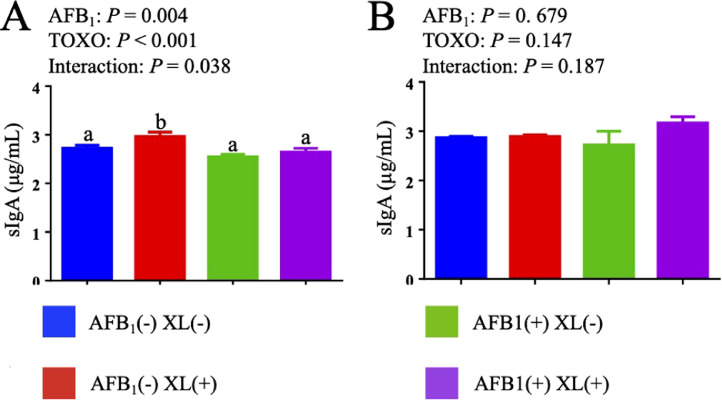
The effects of dietary AFB1 and mycotoxins binder addition on the content of sIgA in jejunal mucous of broiler chickens are shown in Figure 2. AFB1 challenge significant decreased sIgA contents of jejunal mucous at 21 d (P < 0.05). In addition, there were significant interactions to sIgA at 21 d (P < 0.05) between AFB1 exposure and XL supplementation.
Figure 2.
Effects of dietary AFB1 and mycotoxins binder on the content of jejuna mucous sIgA of broiler chickens at 21 d (A) and 42 d (B).123 a-b Means with different superscripts in the same column differ significantly (P < 0.05). 1 Each value represents the mean values of 6 pens of 10 animals each (n = 6). 2 sIgA = secretory immunoglobulin A. 3P-values represent the main effect of AFB1 exposure, the main effect of the dietary XL, and the interaction between AFB1 exposure and XL treatments.
mRNA Expression of Tight Junctions in Jejunum
No significant cooperative effect was observed between AFB1 exposure and XL supplementation in terms of jejunal mucosa morphology of broiler chickens (P > 0.05). Neither mycotoxins binder nor AFB1 poisoning affected villus height, crypt depth, and VH/CD in the jejunal mucosa of broiler chickens (P > 0.05; Table S5).
The effects of dietary AFB1 and XL addition on tight junction gene expression in jejunum of broiler chickens are shown in Table 5. AFB1 exposure significantly decreased Occludin expression (P = 0.007) at 21 d, and decreased claudin-1 expression at 42 d (P < 0.001), while increased claudin-2 expression at 21 d compared to contaminated groups. Compared with the non-supplemented group, the addition of XL significantly increased claudin-1 (P < 0.001) at 21 d, increased Occludin (P = 0.035) expression at 42 d, decreased claudin-2 (P = 0.003) gene expression at 42 d. There were significant interactions to expression of claudin-2, claudin-3, and Occludin at 42 d between AFB1 or XL.
Table 5.
Effects of dietary AFB1 and mycotoxins binder on relative mRNA expression of tight junctions in the jejunum of broiler exposure to AFB1.12
| Items |
Claudin-1 |
Claudin-2 |
Claudin-3 |
Occludin |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 21 d | 42 d | 21 d | 42 d | 21 d | 42 d | 21 d | 42 d | ||
| Control | 1.00 | 1.00 | 1.00 | 1.00ab | 1.00 | 1.00ab | 1.00 | 1.00a | |
| XL | 2.40 | 1.44 | 0.68 | 0.80bc | 1.43 | 0.67b | 1.10 | 1.00a | |
| AFB1 | 0.73 | 0.46 | 4.71 | 1.33 a | 1.02 | 0.53b | 0.58 | 0.49b | |
| XL+AFB1 | 2.32 | 0.56 | 3.56 | 0.44c | 1.38 | 1.28a | 0.72 | 1.14a | |
| SEM3 | 0.211 | 0.108 | 0.220 | 0.102 | 0.105 | 0.098 | 0.076 | 0.084 | |
| The main effect | |||||||||
| AFB1 | - | 1.70 | 1.22 | 0.84 | 0.90 | 1.21 | 0.83 | 1.05 | 1.00 |
| + | 1.53 | 0.51 | 4.13 | 0.89 | 1.20 | 0.90 | 0.65 | 0.82 | |
| XL | - | 0.87 | 0.73 | 2.85 | 1.17 | 1.01 | 0.76 | 0.79 | 0.75 |
| + | 2.36 | 1.00 | 2.12 | 0.62 | 1.40 | 0.97 | 0.91 | 1.07 | |
| P-value4 | |||||||||
| AFB1 | 0.574 | <0.001 | <0.001 | 0.930 | 0.941 | 0.675 | 0.007 | 0.211 | |
| XL | <0.001 | 0.091 | 0.203 | 0.003 | 0.071 | 0.220 | 0.372 | 0.035 | |
| AFB1 XL | 0.759 | 0.278 | 0.467 | 0.049 | 0.878 | 0.004 | 0.867 | 0.035 | |
Means with different superscripts in the same column differ significantly (P < 0.05).
Each value represents the mean values of 6 pens of 10 animals each (n = 6).
− represents without AFB1 or XL supplementation; + represents supplemented with AFB1 or XL.
SEM, standard error of the mean.
P-values represent the main effect of AFB1 exposure, the main effect of the dietary XL, and the interaction between AFB1 exposure and XL treatments.
DISCUSSION
Poultry are highly sensitive to aflatoxicosis (Arafa et al., 1981; Huff et al., 1986) induced by one of the most common carcinogenic pollutants, AFB1, in broiler feed. AFB1 poisoning induced liver damage, serum biochemical variables imbalanced, immunity inhibition, intestinal dysfunction, and inflammation increase the susceptibility of broilers to diseases (Yuan et al., 2016; Kraieski et al., 2017; Zhang et al., 2019; Rosim et al. 2020), suppress the broiler production (Rashidi et al., 2020) and increase the morbidity and mortality of broilers, resulting in the increase of poultry breeding cost (Bintvihok and Kositcharoenkul, 2006). In accord with previous studies (Denli et al., 2009; Liu et al., 2018a), our results also showed that 200 μg/kg AFB1 in diets significantly reduced the body weight gain and feed intake of Arbor Acres broiler chickens, increased feed conversion ratio after the first 21 days of feeding, but caused a nonsignificant mortality difference, declaring subclinical aflatoxicosis occurred to birds in early stage.
Mycotoxins binders have been reported to be the promising, effective, economical approach counteracting with contamination induced by AFB1. In the current study, addition of 2 g/kg commercial mycotoxins binder (XL) containing smectite binding agent, specific glucose biopolymers and activated β-1,3/1,6-glucans showed no significant effect on growth performance. These results were inconsistent with some previous study (Liu et al., 2018a), but in accordance with others (Morales-López et al., 2009; Liu et al., 2018c). The different effects of adsorbents on production performance may be largely attributable to inclusion concentration of binders and AFB1, varied quality, and poultry breeds (Zhao et al., 2010, 2021; Liu et al., 2018a).
The spleen, bursa of Fabricius, and thymus are important components of the immune system of poultry, and these organ indexes can indirectly evaluate the immune status of poultry. While the current results showed no significant effect of AFB1 on immune organ index (spleen and bursa of Fabricius), which was confirmed by previous study (Gómez-Espinosa et al., 2017). Gómez-Espinosa et al. (2017) reported that 6-day-old Turkey fed with diet exposed to AFB1 and AFB2 for 2 weeks, results show that no significant relative spleen and bursa of Fabricius weight changes were noted, but severe depletion of lymphoid cells in the bursa of Fabricius and spleen were observed from histopathological examination, indicating sensitivity of lymphoid organs to AFB1. This also means that 200 μg/kg AFB1 may have caused organ damage in broilers, but the damage was not severe enough to cause organ index change. Additionally, supplementation of 2g/kg mycotoxins binders also did not affect immune organ weight change in current study. It was documented that inclusion of 3g/kg yeast cell walls improved the relative weights of Fabricius and thymus (P < 0.01; Zhang et al., 2012). It is speculated the dose of mycotoxins binders used in this study was not enough to cause significant change of immune organ index.
The spleen is the peripheral immune organ of poultry and can produce a variety of antibodies. It is primarily responsible for immune response and plays an anti-inflammatory and protective role. Proinflammatory cytokine IL-6 can induce the immune response, alleviate the damage caused by toxins to the body and actively release other inflammatory mediators (Wei et al., 2012). In this study, AFB1 increased the mRNA expression level of the proinflammatory factor IL-6, further confirming the results of previous studies (Long et al., 2016). Although low dose AFB1 in this study could not change the spleen index, it may damage the spleen by increasing and induce spleen inflammation (Meissonnier et al., 2008). However, adding XL to the diet can inhibit the high expression of IL-6 induced by AFB1, suggesting that XL may relieve spleen injury by regulating the expression of pro-inflammatory factor genes.
Liver is the main organ for toxin metabolism in poultry, which can promote detoxification and protect the body from toxicity (Fouad and El-Senousey, 2014). AFB1 has strong hepatotoxicity in a dose-dependent manner (Yarru et al., 2009), which can cause liver lipid metabolism imbalance, promote lipid deposition in the liver and lead to liver enlargement (Siloto et al., 2013). In addition, it can inhibit the activity of antioxidant enzymes and secretion of anti-inflammatory cytokines induces lipid peroxidation and secretion of pro-inflammatory cytokines, and finally leads to liver cell damage and necrosis (Wang et al., 2019). The results showed that the mRNA expression levels of proinflammatory cytokines IL-1β and IL-6 in the liver of broilers were significantly increased compared with those in the control group, but the organ index was not significantly changed. The results were consistent with those in the spleen, suggesting that low dose AFB1 can cause inflammatory response in the liver of broilers, but its structure and function were less damaged. mycotoxins binder was found to relieve liver inflammation by downregulating IL-1β and IL-6 mRNA levels compared to control levels. In addition, mycotoxins binder can also regulate the mRNA expression level of liver CYP3A4, which is the isoenzyme of CYP450s, mainly exists in the liver of animals and plays a major role in activation and he metabolism of AFB1 (Crespi et al., 1991; Shen et al., 1996). It can participate in the metabolic activation of AFB1 to produce the ultimate carcinogenic intermediate, (AFB1-8,9-epoxide, AFBO) which interacts with macromolecules, particularly DNA (Swenson et al., 1977), thereby destroying the cell structure, causing cell damage and apoptosis (Fasullo et al., 2017). In addition, reactive oxygen species generated during metabolism can also cause oxidative damage to cells, further exacerbating the hepatotoxicity of AFB1 (Deng et al., 2018). The expression level of CYP3A4 in broilers' liver was decreased after inclusion of mycotoxins binder to the diet, indicating that mycotoxins binder could inhibit the activity of CYP3A4, and thus alleviate the toxic effect of AFB1 metabolites on broilers.
Liver is the main site of protein synthesis and metabolism in the body, and concentrations of serum total protein, albumin and globulin, in part, reflect hepatic injury and function (Abdel-Wahhab et al., 1999; Mathur et al., 2001). Compared with the control group, the serum globulin content of broilers in AFB1 group tended to drop which proved again that liver damage of broilers would be caused by AFB1 contamination level of 200 μg/kg in the diet. Inclusion of XL increased serum ALB, indicating its possible role as immunopotentiator and liver protector.
As indicators of humoral immune responses, serum immunoglobulin such as IgG, and IgM, IgA are determined frequently. Consistent with the reduced tendency observed in serum protein contents in birds exposed to AFB1 in this study, IgA reduction was also found, which confirmed previous reports (Chen et al., 2014). Not only in chicken, AFB1-contaminated diets were found a significant effect on titer serum concentrations of the IgG, and IgM, IgA in mice (Long et al., 2016), pigs (Marin et al., 2002), dairy cow (Xiong et al., 2018), and human (Turner et al., 2003). While dietary supplementation with XL increased serum ALB and IgM, IgA, which in agreement with previous studies, indicating components in XL can improve immune response in chicks (Liu et al., 2018a).
Epidemiological data showed that the outbreak of Newcastle disease in broiler chickens was highly correlated with AF contamination of feed (Yunus et al., 2009). In our study, diet contaminated with 200 μg/kg AFB1 significantly reduced serum Newcastle disease antibody titer of broilers which confirms previous reports (Bagherzadeh Kasmani et al., 2012), indicating that AFB1 reduced the immune response of broilers Newcastle disease vaccine. In addition, it was also observed a significant reduction of the content of sIgA in jejunal mucosa in birds exposed to AFB1, and the results of this study on humoral and mucosal immunity were consistent with previous studies (Jiang et al., 2015; Liu et al., 2017). Interestingly, the serum anti-Newcastle disease titer of broilers increased after the addition of mycotoxins binder in the diet, indicating that mycotoxins binder could relieve the suppression of AFB1 on the immune function of broilers.
The physical barrier in small intestinal mucosa, consisting of intestinal epithelial cells, intestinal mucus, commensal bacteria, sIgA, gut-associated lymphoid tissue, refers to the total structure and function of the intestine that can prevent hazardous substances such as harmful bacteria and toxins from passing through the intestinal mucosa and entering other tissues, organs and blood circulation in the body (Barbara, 2006; Branca et al., 2019). sIgA, secreted by IgA plasma cells in the intestinal mucosa, is one of the most abundant immunoglobulins on mucosal surfaces, plays a vital role in protecting the mucosal surfaces against pathogens and maintaining homeostasis with the commensal microbiota (Salerno-Goncalves et al., 2016; He et al., 2020, Rochereau et al., 2021). Tight junctions (TJs) largely determine the barrier function and the baseline level of inflammation in the body, which is correlated to intestinal permeability (Suzuki, 2013). TJ permeability is partly determined by the properties of claudins (Barrett, 2020). The claudin family is a key factor in determining the characteristics of paracellular transfer pathways, forming a tightly connected skeleton with proteins such as occludin, which can maintain the barrier function of intestinal mucosa (Grenier and Applegate, 2013). Claudin-1, a key constituent of the tight junction complex, maintains the integrity of the paracellular barrier and regulates water homeostasis. Claudin-1 has been shown to be dysregulated in inflammatory bowel disease (Kucharzik et al., 2001; Weber et al., 2008). Claudin-2, known as a channel-forming TJ protein permeable to small cations and water, is distinctively upregulated in most inflammatory and infectious diseases of the intestine indicator (Rosenthal et al., 2010; Zhang et al., 2013) . Claudin-3 strongly associated with intestinal barrier disruption, is a potential marker for intestinal barrier failure induced (Lu et al., 2013; Shim et al., 2015). Occludins, together with claudins, interacts with each other on their extracellular sides to promote junction assembly (Costantini et al., 2009). Therefore, the secretion of sIgA and the mRNA profiles of claudin-1, claudin-2, claudin-3, and occludin can directly reflect the intestinal mucosal barrier function to an extent.
Our results demonstrated that AFB1 exposure decreased sIgA and mRNA expression of claudin-1 and occludin, increase claudin-2 expression, which is consistent with previous findings (Liu et al., 2018b). Previous study reports that broilers' small intestinal mucosa could be damaged under long-term exposure and low-level of AFB1 contamination (Kana et al., 2010), and the intestinal mucosal barrier also be damaged, following by the loss of intestinal mucosal barrier function (Chen et al., 2016). Impaired intestinal mucosal barrier will lead to increased intestinal permeability and bacterial migration (Maresca et al., 2008). However, inclusion of XL to the diet could increase the mRNA expression level of claudin-1 and Occludin, decrease claudin-2 expression in broilers jejunum, indicating that XL can maintain the integrity of the tight junction of broilers' intestinal tract, and thus enhance the function of intestinal mucosal barrier in broilers to some extent. This result displays similar effect of dietary XL on laying hens (Zhao et al., 2021).
CONCLUSIONS
In summary, broiler chickens fed with diet contaminated with 200 μg/kg AFB1 presented poor growth performance, especially in early stage. These adverse effects of aflatoxicosis may closely associate with impaired liver, immune dysfunction, inflammation of immune organs and gut barrier induced by AFB1. Even though the supplementation of 2 g/kg mycotoxins binder did not significantly counteract the negative production outcome induced by AFB1, could alleviate the liver impairment and immune suppression, enhance the intestinal mucosal barrier function and improve intestinal health by improving serum protein and immunoglobulin levels, the titer of the antibody to Newcastle disease, down-regulating the expression of CYP3A4 in liver and pro-inflammatory factor in liver and spleen, up-regulating the expression of claudin-1 and occludin and decreasing claudin-2 gene expression and increase sIgA secretion in jejunum. However, further studies are needed to explore the protective roles of dietary mycotoxins binder supplementation on AFB1 challenges in broilers.
ACKNOWLEGMENTS
This study was supported by the National Natural Science Foundation of China (No.32072750) and the 2115 Talent Development Program of China Agricultural University.
Institutional review board statement: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Animal Care and Use Committee of China Agricultural University.
Author contributions: Yujiao Lai, Meng sun, Bingkun Zhang and Yuming Guo conceived and designed the experiments; Meng sun and Jiaqi Lei performed the experiments; Yujiao Lai, Meng sun, Jiaqi Lei and Yang he analyzed the data; Yujiao Lai and Mengsun wrote the paper, Bingkun Zhang, Yang He, Yanming Han, Yuanyuan Wu and Dongying Bai reviewed and edited. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Data availability statement: The datasets used and/or analyzed during the current study are publicly available.
DISCLOSURES
All authors declare that there are no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101683.
Appendix. Supplementary materials
REFERENCES
- Abdel-Wahhab M.A., Nada S.A., Amra H.A. Effect of aluminosilicates and bentonite on aflatoxin-induced developmental toxicity in rat. J. Appl. Toxicol. 1999;19:199–204. doi: 10.1002/(sici)1099-1263(199905/06)19:3<199::aid-jat558>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Ali Rajput S., Sun L., Zhang N., Mohamed Khalil M., Gao X., Ling Z., Zhu L., Khan F.A., Zhang J., Qi D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B₁. Toxins (Basel) 2017;9:371. doi: 10.3390/toxins9110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa A.S., Bloomer R.J., Wilson H.R., Simpson C.F., Harms R.H. Susceptibility of various poultry species to dietary aflatoxin. Br. Poult. Sci. 1981;22:431–436. doi: 10.1080/00071688108447906. [DOI] [PubMed] [Google Scholar]
- Bagherzadeh Kasmani F., Torshizi M.A.Karimi, Allameh A., Shariatmadari F. A novel aflatoxin-binding Bacillus probiotic: Performance, serum biochemistry, and immunological parameters in Japanese quail. Poult. Sci. 2012;91:1846–1853. doi: 10.3382/ps.2011-01830. [DOI] [PubMed] [Google Scholar]
- Barbara G. Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am. J. Gastroenterol. 2006;101:1295–1298. doi: 10.1111/j.1572-0241.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- Barrett K.E. Claudin-2 pore causes leak that breaches the dam in intestinal inflammation. J. Clin. Invest. 2020;130:5100–5101. doi: 10.1172/JCI140528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti S.A., Khan M.Z., Saleemi M.K., Saqib M., Khan A., Ul-Hassan Z. Protective role of bentonite against aflatoxin B(1)- and ochratoxin A-induced immunotoxicity in broilers. J. Immunotoxicol. 2017;14:66–76. doi: 10.1080/1547691X.2016.1264503. [DOI] [PubMed] [Google Scholar]
- Bintvihok A., Kositcharoenkul S. Effect of dietary calcium propionate on performance, hepatic enzyme activities and aflatoxin residues in broilers fed a diet containing low levels of aflatoxin B1. Toxicon. 2006;47:41–46. doi: 10.1016/j.toxicon.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Branca J.J.V., Gulisano M., Nicoletti C. Intestinal epithelial barrier functions in ageing. Ageing Res. Rev. 2019;54 doi: 10.1016/j.arr.2019.100938. [DOI] [PubMed] [Google Scholar]
- Chang J., Wang T., Wang P., Yin Q., Liu C., Zhu Q., Lu F., Gao T. Compound probiotics alleviating aflatoxin B(1) and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol. Environ. Saf. 2020;194 doi: 10.1016/j.ecoenv.2020.110420. [DOI] [PubMed] [Google Scholar]
- Chen K., Fang J., Peng X., Cui H., Chen J., Wang F., Chen Z., Zuo Z., Deng J., Lai W., Zhou Y. Effect of selenium supplementation on aflatoxin B₁-induced histopathological lesions and apoptosis in bursa of Fabricius in broilers. Food Chem. Toxicol. 2014;74:91–97. doi: 10.1016/j.fct.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Chen X., Naehrer K., Applegate T.J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016;95:1312–1325. doi: 10.3382/ps/pew022. [DOI] [PubMed] [Google Scholar]
- Costantini T.W., Deree J., Loomis W., Putnam J.G., Choi S., Baird A., Eliceiri B.P., Bansal V., Coimbra R. Phosphodiesterase inhibition attenuates alterations to the tight junction proteins occludin and ZO-1 in immunostimulated Caco-2 intestinal monolayers. Life Sci. 2009;84:18–22. doi: 10.1016/j.lfs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Crespi C.L., Penman B.W., Steimel D.T., Gelboin H.V., Gonzalez F.J. The development of a human cell line stably expressing human CYP3A4: role in the metabolic activation of aflatoxin B1 and comparison to CYP1A2 and CYP2A3. Carcinogenesis. 1991;12:355–359. doi: 10.1093/carcin/12.2.355. [DOI] [PubMed] [Google Scholar]
- Deng J., Zhao L., Zhang N.-Y., Karrow N.A., Krumm C.S., Qi D.-S., Sun L.-H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res/Rev Mutat. Res. 2018;778:79–89. doi: 10.1016/j.mrrev.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Denli M., Blandon J.C., Guynot M.E., Salado S., Perez J.F. Effects of dietary AflaDetox on performance, serum biochemistry, histopathological changes, and aflatoxin residues in broilers exposed to aflatoxin B(1) Poult. Sci. 2009;88:1444–1451. doi: 10.3382/ps.2008-00341. [DOI] [PubMed] [Google Scholar]
- Doerr J.A., Ottinger M.A. Delayed reproductive development resulting from aflatoxicosis in juvenile Japanese quail. Poult. Sci. 1980;59:1995–2001. doi: 10.3382/ps.0591995. [DOI] [PubMed] [Google Scholar]
- Fasullo M., Freedland J., St John N., Cera C., Egner P., Hartog M., Ding X. An in vitro system for measuring genotoxicity mediated by human CYP3A4 in Saccharomyces cerevisiae. Environ. Mol. Mutagen. 2017;58:217–227. doi: 10.1002/em.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G.D., He J., Ao X., Chen D.W. Effects of maize naturally contaminated with aflatoxin B1 on growth performance, intestinal morphology, and digestive physiology in ducks. Poult. Sci. 2017;96:1948–1955. doi: 10.3382/ps/pew420. [DOI] [PubMed] [Google Scholar]
- Fouad A.M., El-Senousey H.K. Nutritional factors affecting abdominal fat deposition in poultry: a review. Asian-Australas. J. Anim. Sci. 2014;27:1057–1068. doi: 10.5713/ajas.2013.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad A.M., Ruan D., El-Senousey H.K., Chen W., Jiang S., Zheng C. Harmful effects and control strategies of Aflatoxin B₁ produced by aspergillus flavus and aspergillus parasiticus strains on poultry: review. Toxins (Basel) 2019;11:176. doi: 10.3390/toxins11030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambrone J.J., Diener U.L., Davis N.D., Panangala V.S., Hoerr F.J. Effects of aflatoxin on young turkeys and broiler chickens. Poult. Sci. 1985;64:1678–1684. doi: 10.3382/ps.0641678. [DOI] [PubMed] [Google Scholar]
- Gómez-Espinosa D., Cervantes-Aguilar F.J., Del Río-García J.C., Villarreal-Barajas T., Vázquez-Durán A., Méndez-Albores A. Ameliorative effects of neutral electrolyzed water on growth performance, biochemical constituents, and histopathological changes in Turkey poults during Aflatoxicosis. Toxins (Basel) 2017;9:104. doi: 10.3390/toxins9030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier B., Applegate T.J. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins (Basel) 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lin Y., Lian S., Sun D., Guo D., Wang J., Wu R. Selenium deficiency in chickens induces intestinal mucosal injury by affecting the mucosa morphology, SIgA secretion, and GSH-Px activity. Biol. Trace Elem. Res. 2020;197:660–666. doi: 10.1007/s12011-019-02017-6. [DOI] [PubMed] [Google Scholar]
- Hoerr F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010;54:2–15. doi: 10.1637/8909-043009-Review.1. [DOI] [PubMed] [Google Scholar]
- Huff W.E., Kubena L.F., Harvey R.B., Corrier D.E., Mollenhauer H.H. Progression of aflatoxicosis in broiler chickens. Poult. Sci. 1986;65:1891–1899. doi: 10.3382/ps.0651891. [DOI] [PubMed] [Google Scholar]
- Jallow A., Xie H., Tang X., Qi Z., Li P. Worldwide aflatoxin contamination of agricultural products and foods: from occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021;20:2332–2381. doi: 10.1111/1541-4337.12734. [DOI] [PubMed] [Google Scholar]
- Jiang M., Fang J., Peng X., Cui H., Yu Z. Effect of aflatoxin B₁ on IgA⁺ cell number and immunoglobulin mRNA expression in the intestine of broilers. Immunopharmacol. Immunotoxicol. 2015;37:450–457. doi: 10.3109/08923973.2015.1081933. [DOI] [PubMed] [Google Scholar]
- Kana J.R., Teguia A., Tchoumboue J. Effect of dietary plant charcoal from Canarium schweinfurthii Engl. and maize cob on aflatoxin B1 toxicosis in broiler chickens. Adv. Anim. Biosci. 2010;1:462–463. [Google Scholar]
- Khlangwiset P., Shephard G.S., Wu F. Aflatoxins and growth impairment: a review. Crit. Rev. Toxicol. 2011;41:740–755. doi: 10.3109/10408444.2011.575766. [DOI] [PubMed] [Google Scholar]
- Kraieski A.L., Hayashi R.M., Sanches A., Almeida G.C., Santin E. Effect of aflatoxin experimental ingestion and Eimeira vaccine challenges on intestinal histopathology and immune cellular dynamic of broilers: applying an intestinal health index. Poult. Sci. 2017;96:1078–1087. doi: 10.3382/ps/pew397. [DOI] [PubMed] [Google Scholar]
- Kucharzik T., Walsh S.V., Chen J., Parkos C.A., Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001;159:2001–2009. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zuo Z., Zhu P., Zheng Z., Peng X., Fang J., Cui H., Zhou Y., Ouyang P., Geng Y., Deng J., Sun Y. Sodium selenite prevents suppression of mucosal humoral response by AFB(1) in broiler's cecal tonsil. Oncotarget. 2017;8:54215–54226. doi: 10.18632/oncotarget.17105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Ding K., Wang J., Deng Q., Gu K., Wang J. Effects of lactic acid bacteria and smectite after aflatoxin B(1) challenge on the growth performance, nutrient digestibility and blood parameters of broilers. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018;102:953–961. doi: 10.1111/jpn.12901. [DOI] [PubMed] [Google Scholar]
- Liu N., Wang J.Q., Jia S.C., Chen Y.K., Wang J.P. Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B1 and necrotic enteritis. Poult. Sci. 2018;97:477–484. doi: 10.3382/ps/pex342. [DOI] [PubMed] [Google Scholar]
- Liu N., Wang J.Q., Jia S.C., Chen Y.K., Wang J.P. Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B1 and necrotic enteritis. Poult. Sci. 2018;97:477–484. doi: 10.3382/ps/pex342. [DOI] [PubMed] [Google Scholar]
- Long M., Zhang Y., Li P., Yang S.H., Zhang W.K., Han J.X., Wang Y., He J.B. Intervention of grape seed proanthocyanidin extract on the subchronic immune injury in mice induced by aflatoxin B1. Int. J. Mol. Sci. 2016;17:516. doi: 10.3390/ijms17040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Ding L., Lu Q., Chen Y.H. Claudins in intestines: distribution and functional significance in health and diseases. Tissue Barriers. 2013;1:e24978. doi: 10.4161/tisb.24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca M., Yahi N., Younès-Sakr L., Boyron M., Caporiccio B., Fantini J. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: stimulation of interleukin-8 secretion, potentiation of interleukin-1beta effect and increase in the transepithelial passage of commensal bacteria. Toxicol. Appl. Pharmacol. 2008;228:84–92. doi: 10.1016/j.taap.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Marin D.E., Taranu I., Bunaciu R.P., Pascale F., Tudor D.S., Avram N., Sarca M., Cureu I., Criste R.D., Suta V., Oswald I.P. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J. Anim. Sci. 2002;80:1250–1257. doi: 10.2527/2002.8051250x. [DOI] [PubMed] [Google Scholar]
- Mathur S., Constable P.D., Eppley R.M., Waggoner A.L., Tumbleson M.E., Haschek W.M. Fumonisin B(1) is hepatotoxic and nephrotoxic in milk-fed calves. Toxicol. Sci. 2001;60:385–396. doi: 10.1093/toxsci/60.2.385. [DOI] [PubMed] [Google Scholar]
- Meissonnier G.M., Pinton P., Laffitte J., Cossalter A.M., Gong Y.Y., Wild C.P., Bertin G., Galtier P., Oswald I.P. Immunotoxicity of aflatoxin B1: impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008;231:142–149. doi: 10.1016/j.taap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Morales-López R., Auclair E., García F., Esteve-Garcia E., Brufau J. Use of yeast cell walls; β-1, 3/1, 6-glucans; and mannoproteins in broiler chicken diets. Poult. Sci. 2009;88:601–607. doi: 10.3382/ps.2008-00298. [DOI] [PubMed] [Google Scholar]
- Olarte R.A., Horn B.W., Dorner J.W., Monacell J.T., Singh R., Stone E.A., Carbone I. Effect of sexual recombination on population diversity in aflatoxin production by Aspergillus flavus and evidence for cryptic heterokaryosis. Mol. Ecol. 2012;21:1453–1476. doi: 10.1111/j.1365-294X.2011.05398.x. [DOI] [PubMed] [Google Scholar]
- Ortatatli M., Ciftci M.K., Tuzcu M., Kaya A. The effects of aflatoxin on the reproductive system of roosters. Res. Vet. Sci. 2002;72:29–36. doi: 10.1053/rvsc.2001.0516. [DOI] [PubMed] [Google Scholar]
- Pascual A., Pauletto M., Giantin M., Radaelli G., Ballarin C., Birolo M., Zomeño C., Dacasto M., Bortoletti M., Vascellari M., Xiccato G., Trocino A. Effect of dietary supplementation with yeast cell wall extracts on performance and gut response in broiler chickens. J. Anim. Sci. Biotechnol. 2020;11:40. doi: 10.1186/s40104-020-00448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Zhang K., Bai S., Ding X., Zeng Q., Yang J., Fang J., Chen K. Histological lesions, cell cycle arrest, apoptosis and T cell subsets changes of spleen in chicken fed aflatoxin-contaminated corn. Int. J. Environ. Res. Public Health. 2014;11:8567–8580. doi: 10.3390/ijerph110808567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni V., Magnoli A., Fochesato A., Cristofolini A., Caverzan M., Merkis C., Montenegro M., Cavaglieri L. A Saccharomyces cerevisiae RC016-based feed additive reduces liver toxicity, residual aflatoxin B1 levels and positively influences intestinal morphology in broiler chickens fed chronic aflatoxin B1-contaminated diets. Anim. Nutr. 2020;6:31–38. doi: 10.1016/j.aninu.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M.V., Rama Rao S.V., Radhika K., Panda A.K. Effect of amount and source of supplemental dietary vegetable oil on broiler chickens exposed to aflatoxicosis. Br. Poult. Sci. 2005;46:587–594. doi: 10.1080/00071660500255968. [DOI] [PubMed] [Google Scholar]
- Rashidi N., Khatibjoo A., Taherpour K., Akbari-Gharaei M., Shirzadi H. Effects of licorice extract, probiotic, toxin binder and poultry litter biochar on performance, immune function, blood indices and liver histopathology of broilers exposed to aflatoxin-B(1) Poult. Sci. 2020;99:5896–5906. doi: 10.1016/j.psj.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli-Hiq A.A., Bagherzadeh-Kasmani F., Mehri M., Karimi-Torshizi M.A. Nigella sativa (black cumin seed) as a biological detoxifier in diet contaminated with aflatoxin B(1) J. Anim. Physiol. Anim. Nutr. (Berl.) 2017;101:e77–e86. doi: 10.1111/jpn.12562. [DOI] [PubMed] [Google Scholar]
- Rochereau N., Roblin X., Michaud E., Gayet R., Chanut B., Jospin F., Corthésy B., Paul S. NOD2 deficiency increases retrograde transport of secretory IgA complexes in Crohn's disease. Nat. Commun. 2021;12:261. doi: 10.1038/s41467-020-20348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R., Milatz S., Krug S.M., Oelrich B., Schulzke J.D., Amasheh S., Günzel D., Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- Rosim R.E., Faria Filho D.E., Almeida T.W., Corassin C.H., Oliveira C.A.F. Determination of serum aflatoxin B(1)-lysine and biochemical parameters in broiler chicks fed an aflatoxin B(1)-contaminated diet. Drug. Chem. Toxicol. 2020;43:623–629. doi: 10.1080/01480545.2019.1578786. [DOI] [PubMed] [Google Scholar]
- Rubio L.A., Ruiz R., Peinado M.J., Echavarri A. Morphology and enzymatic activity of the small intestinal mucosa of Iberian pigs as compared with a lean pig strain. J. Anim. Sci. 2010;88:3590–3597. doi: 10.2527/jas.2010-3040. [DOI] [PubMed] [Google Scholar]
- Salerno-Goncalves R., Safavie F., Fasano A., Sztein M.B. Free and complexed-secretory immunoglobulin A triggers distinct intestinal epithelial cell responses. Clin. Experiment. Immunol. 2016;185:338–347. doi: 10.1111/cei.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon T.A., Ledoux D.R., Rottinghaus G.E., Shaw D.P., Daković A., Marković M. The efficacy of raw and concentrated bentonite clay in reducing the toxic effects of aflatoxin in broiler chicks. Poult. Sci. 2017;96:1651–1658. doi: 10.3382/ps/pew408. [DOI] [PubMed] [Google Scholar]
- Shen H.M., Shi C.Y., Shen Y., Ong C.N. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radic. Biol. Med. 1996;21:139–146. doi: 10.1016/0891-5849(96)00019-6. [DOI] [PubMed] [Google Scholar]
- Shim S., Lee J.G., Bae C.H., Lee S.B., Jang W.S., Lee S.J., Lee S.S., Park S. Claudin-3 expression in radiation-exposed rat models: a potential marker for radiation-induced intestinal barrier failure. Biochem. Biophys. Res. Commun. 2015;456:351–354. doi: 10.1016/j.bbrc.2014.11.084. [DOI] [PubMed] [Google Scholar]
- Siloto E.V., Oliveira E.F., Sartori J.R., Fascina V.B., Martins B.A., Ledoux D.R., Rottinghaus G.E., Sartori D.R. Lipid metabolism of commercial layers fed diets containing aflatoxin, fumonisin, and a binder. Poult. Sci. 2013;92:2077–2083. doi: 10.3382/ps.2012-02777. [DOI] [PubMed] [Google Scholar]
- Śliżewska K., Cukrowska B., Smulikowska S., Cielecka-Kuszyk J. The effect of probiotic supplementation on performance and the histopathological changes in liver and kidneys in broiler chickens fed diets with aflatoxin B₁. Toxins (Basel) 2019;11 doi: 10.3390/toxins11020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Cruz B., Hernandez-Patlan D., Petrone V.M., Pontin K.P., Latorre J.D., Beyssac E., Hernandez-Velasco X., Merino-Guzman R., Owens C., Hargis B.M., Lopez-Arellano R., Tellez-Isaias G. Evaluation of cellulosic polymers and curcumin to reduce aflatoxin B1 toxic effects on performance, biochemical, and immunological parameters of broiler chickens. Toxins (Basel) 2019;11:121. doi: 10.3390/toxins11020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson D.H., Lin J.K., Miller E.C., Miller J.A. Aflatoxin B1-2,3-oxide as a probable intermediate in the covalent binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal RNA in vivo. Cancer Res. 1977;37:172–181. [PubMed] [Google Scholar]
- Turner P.C., Moore S.E., Hall A.J., Prentice A.M., Wild C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003;111:217–220. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.H., Li W., Wang X.H., Han M.Y., Muhammad I., Zhang X.Y., Sun X.Q., Cui X.X. Water-soluble substances of wheat: a potential preventer of aflatoxin B1-induced liver damage in broilers. Poult. Sci. 2019;98:136–149. doi: 10.3382/ps/pey358. [DOI] [PubMed] [Google Scholar]
- Weber C.R., Nalle S.C., Tretiakova M., Rubin D.T., Turner J.R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Invest. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Hengmin C., Xi P., Jing F., Xiaodong L., Bangyuan W. Effect of vanadium on splenocyte apoptosis in broilers. Med. Chem. 2012;2:57–60. [Google Scholar]
- Williams J.G., Deschl U., Williams G.M. DNA damage in fetal liver cells of turkey and chicken eggs dosed with aflatoxin B1. Arch. Toxicol. 2011;85:1167–1172. doi: 10.1007/s00204-011-0653-x. [DOI] [PubMed] [Google Scholar]
- Xin X., Zhang Y., Wang J., Nuetah J.A. Effects of farm size on technical efficiency in china's broiler sector: a stochastic meta-frontier approach. Can. J. Agric. Econ./Rev Canad D'agroecon. 2016;64:493–516. [Google Scholar]
- Xiong J.L., Wang Y.M., Zhou H.L., Liu J.X. Effects of dietary adsorbent on milk aflatoxin M(1) content and the health of lactating dairy cows exposed to long-term aflatoxin B(1) challenge. J. Dairy Sci. 2018;101:8944–8953. doi: 10.3168/jds.2018-14645. [DOI] [PubMed] [Google Scholar]
- Xue C., Li Y., Lv H., Zhang L., Bi C., Dong N., Shan A., Wang J. Oleanolic acid targets the gut-liver axis to alleviate metabolic disorders and hepatic steatosis. J. Agric. Food Chem. 2021;69:7884–7897. doi: 10.1021/acs.jafc.1c02257. [DOI] [PubMed] [Google Scholar]
- Yarru L.P., Settivari R.S., Antoniou E., Ledoux D.R., Rottinghaus G.E. Toxicological and gene expression analysis of the impact of aflatoxin B1 on hepatic function of male broiler chicks. Poult. Sci. 2009;88:360–371. doi: 10.3382/ps.2008-00258. [DOI] [PubMed] [Google Scholar]
- Yuan S., Wu B., Yu Z., Fang J., Liang N., Zhou M., Huang C., Peng X. The mitochondrial and endoplasmic reticulum pathways involved in the apoptosis of bursa of Fabricius cells in broilers exposed to dietary aflatoxin B1. Oncotarget. 2016;7:65295–65306. doi: 10.18632/oncotarget.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus A.W., Nasir M.K., Aziz T., Böhm J. Prevalence of poultry diseases in district Chakwal and their interaction with Mycotoxicosis: effects of season and feed. J. Anim. Plant Ences. 2009;19:2009–2010. [Google Scholar]
- Zhang L.Y., Zhan D.L., Chen Y.Y., Wang W.H., He C.Y., Lin Y., Lin Y.C., Lin Z.N. Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of Kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: an in vitro, ex vivo and in vivo study. Arch. Toxicol. 2019;93:3305–3320. doi: 10.1007/s00204-019-02572-w. [DOI] [PubMed] [Google Scholar]
- Zhang S., Liao B., Li X., Li L., Ma L., Yan X. Effects of yeast cell walls on performance and immune responses of cyclosporine A-treated, immunosuppressed broiler chickens. Br. J. Nutr. 2012;107:858–866. doi: 10.1017/S000711451100362X. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-G., wu S., Xia Y., Sun J. Salmonella infection upregulates the leaky protein claudin-2 in intestinal epithelial cells. PLoS One. 2013;8:e58606. doi: 10.1371/journal.pone.0058606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Shirley R.B., Dibner J.D., Uraizee F., Officer M., Kitchell M., Vazquez-Anon M., Knight C.D. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult. Sci. 2010;89:2147–2156. doi: 10.3382/ps.2009-00608. [DOI] [PubMed] [Google Scholar]
- Zhao L., Feng Y., Wei J.T., Zhu M.X., Zhang L., Zhang J.C., Karrow N.A., Han Y.M., Wu Y.Y., Guo Y.M., Sun L.H. Mitigation effects of bentonite and yeast cell wall binders on AFB(1), DON, and OTA induced changes in laying hen performance, egg quality, and health. Toxins (Basel) 2021;13:156. doi: 10.3390/toxins13020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.