Abstract
Study Objectives:
Mandibular advancement devices (MADs) are a noninvasive treatment option for patients with obstructive sleep apnea (OSA) and act by increasing the upper airway volume. However, the exact therapeutic mechanism of action remains unclear. The aim of this study was to assess MAD mechanisms using functional imaging that combines imaging techniques and computational fluid dynamics and assess associations with treatment outcome.
Methods:
One hundred patients with OSA were prospectively included and treated with a custom-made MAD at a fixed 75% protrusion. A low-dose computed tomography scan was made with and without MADs for computational fluid dynamics analysis. Patients underwent a baseline and 3-month follow-up polysomnography to evaluate treatment efficacy. A reduction in apnea-hypopnea index ≥ 50% defined treatment response.
Results:
Overall, 71 patients completed both 3-month follow-up polysomnography and low-dose computed tomography scan with computational fluid dynamics analysis. MAD treatment significantly reduced the apnea-hypopnea index (16.5 [10.4–23.6] events/h to 9.1 [3.9–16.4] events/h; P < .001, median [quartile 1–quartile 3]) and significantly increased the total upper airway volume (8.6 [5.4–12.8] cm3 vs 10.7 [6.4–15.4] cm3; P = .003), especially the velopharyngeal volume (2.1 [0.5–4.1] cm3 vs 3.3 [1.8–6.0] cm3; P < .001). However, subanalyses in responders and nonresponders only showed a significant increase in the total upper airway volume in responders, not in nonresponders.
Conclusions:
MAD acts by increasing the total upper airway volume, predominantly due to an increase in the velopharyngeal volume. Responders showed a significant increase in the total upper airway volume with MAD treatment, while there was no significant increase in nonresponders. Findings add evidence to implement functional imaging using computational fluid dynamics in routine MAD outcome prediction.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Predicting Therapeutic Outcome of Mandibular Advancement Device Treatment in Obstructive Sleep Apnea; URL: https://clinicaltrials.gov/ct2/show/NCT01532050; Identifier: NCT01532050.
Citation:
Van Gaver H, Op de Beeck S, Dieltjens M, et al. Functional imaging improves patient selection for mandibular advancement device treatment outcome in sleep-disordered breathing: a prospective study. J Clin Sleep Med. 2022;18(3):739–750.
Keywords: OSA, CFD, MAD, obstructive sleep apnea, oral appliances
BRIEF SUMMARY
Current Knowledge/Study Rationale: Mandibular advancement devices (MADs) are increasingly being used to treat obstructive sleep apnea; however, treatment efficacy varies among patients and the exact treatment mechanism of action remains unclear. Various factors have been associated with treatment response, yet these associations only weakly predict MAD efficacy, emphasizing the need for alternative prediction methods, such as functional imaging, to select potential successful candidates for MAD treatment, preferably in an upfront setting.
Study Impact: This study demonstrates that MAD treatment acts by increasing the total upper airway volume, predominantly due to an increase in the velopharyngeal volume. However, this increase in the total upper airway volume was only significant in responders. Pending future validation, functional imaging might thus be useful for MAD patient selection.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder that is characterized by intermittent partial (hypopnea) or complete (apnea) collapse of the upper airway (UA) during at least 10 seconds, leading to hypoxemia and sleep fragmentation. OSA is highly prevalent and affects approximately 9% of middle-aged women and 17% of middle-aged men.1 Furthermore, undiagnosed or untreated OSA is an independent risk factor for cardiovascular disease and is associated with high rates of morbidity and mortality.2–4
Oral appliance therapy is a noninvasive treatment option for patients with OSA, with the mandibular advancement device (MAD) being the most frequently used class of oral appliance therapy.5,6 Oral appliances can be divided into 3 main categories, based on their mode of action.7 First, soft palate lifters aim to reduce vibrations from the soft palate by elevating both the soft palate and uvula. However, there is little evidence regarding their effectiveness.6,8 Second, tongue-retaining devices use suction pressure to hold the tongue in a forward position during sleep and thereby prevent the tongue from falling back into the pharyngeal airway.9,10 The third category is the oral appliance advancing the mandible and the attached tongue during the night, known as a mandibular advancement device (MAD). The MAD is the most common type of oral appliance therapy used for the treatment of OSA.11 An MAD is worn intraorally at night and maintains the lower jaw in a protruded position, resulting in an increase in the UA volume.12 However, the exact mechanism of action is not yet completely understood.
In a recent study, the efficacy of MAD therapy has been reported to be 64%, with approximately half of patients (37%) showing complete response (apnea-hypopnea index [AHI] < 5 events/h],13 resulting in still up to one-third of patients who will experience no or minimal therapeutic benefit.14,15 Improving the selection of potential successful candidates for MAD treatment is therefore desirable from both a therapeutic as well as a financial point of view.
Previous studies identified various factors that are associated with treatment response for MAD, such as younger age, female sex, supine-dependent OSA, lower body mass index (BMI), lower AHI, and low loop gain; however, these associations only partially predict MAD treatment efficacy.13,15–18
Functional imaging, by means of 3D models obtained from computed tomography (CT) scans coupled with computational fluid dynamics (CFD) simulations, gives information about the UA volume, UA resistance, and several cephalometric measurements.19 Several studies used functional imaging to investigate the pathophysiological aspects of OSA and the mechanism of action of MADs.20–25 The main findings of these studies are that MADs act by enlarging the UA volume and the minimal cross-sectional area.22,24 Furthermore, they suggest that an MAD causes a decrease in the UA resistance.22,23 Concerning the pathophysiological aspects of OSA, the smallest cross-sectional area and the UA resistance are correlated with OSA severity.25 However, little research has been done on the role of functional imaging findings in relation to MAD treatment response. This study aimed to prospectively investigate the role of functional imaging findings to assess the mechanism of action of MADs and to assess associations with MAD treatment outcome.
METHODS
Study population
The ethics committee at the Antwerp University Hospital and University of Antwerp approved this prospective clinical trial, and written informed consent was obtained from each participant. This study was registered on ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT01532050).
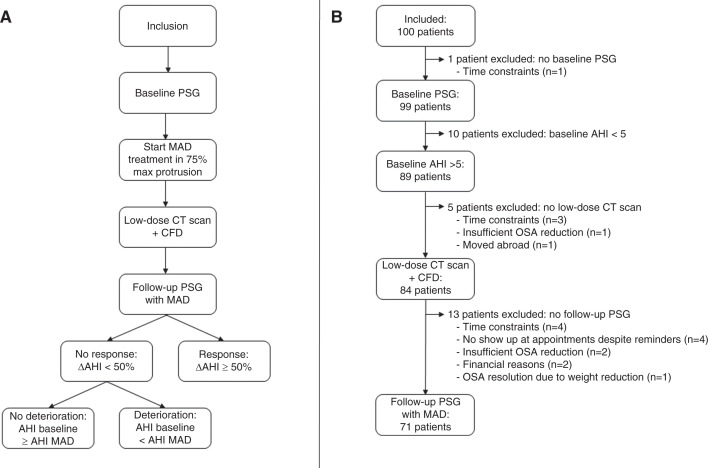
The study protocol was published in detail by Verbruggen et al26 (Figure 1A). Overall, 100 patients with an established OSA diagnosis were prospectively enrolled by a multidisciplinary team consisting of a dental sleep professional; an ear, nose, and throat (ENT) surgeon; and a sleep specialist in order to start MAD therapy. Eligibility criteria are shown in Table 1.
Figure 1. Study flowchart and patient flow.
(A) Flowchart. (B) Patient flow. AHI = apnea-hypopnea index, CFD = computational fluid dynamics, CT = computed tomography, MAD = mandibular advancement device, OSA = obstructive sleep apnea, PSG = polysomnography.
Table 1.
Eligibility criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
*RespiDent Butterfly MAD is manufactured by Orthodontic Clinics NV, Antwerp, Belgium, AHI = apnea-hypopnea index, BMI = body mass index, MAD = mandibular advancement device, OSA = obstructive sleep apnea, PSG = polysomnography.
Study protocol
At baseline, all patients underwent a standard full-night polysomnography (PSG) to verify the eligibility criteria (Table 1). If the patient fulfilled the eligibility criteria, custom-made titratable duobloc MAD treatment (RespiDent Butterfly MAD; Orthodontic Clinics NV, Antwerp, Belgium) was started.27 To allow standardization, the MAD was placed at 75% of each individual’s maximal protrusion.
One month after initiating the MAD treatment, a low-dose CT scan of the UA was made with and without the oral appliance in situ. These 2 CT scans were used to reconstruct 3-dimensional (3D) models of the UA with and without MAD. CFD simulations were performed on this 3D model. After 3 months, a full-night PSG with MAD was performed. Response was defined as ΔAHI ≥ 50%, deterioration as baseline AHI < AHI with MAD, and nondeterioration as baseline AHI ≥ AHI with MAD.
Functional imaging using CFD
All patients underwent a low-dose CT scan of the UA with and without MAD. The scanned area of the UA with and without MAD started at the nasopharynx down to the larynx. Scanning was performed while the patient was awake and in a supine position during 1 breath-hold at the end of a normal inspiration.
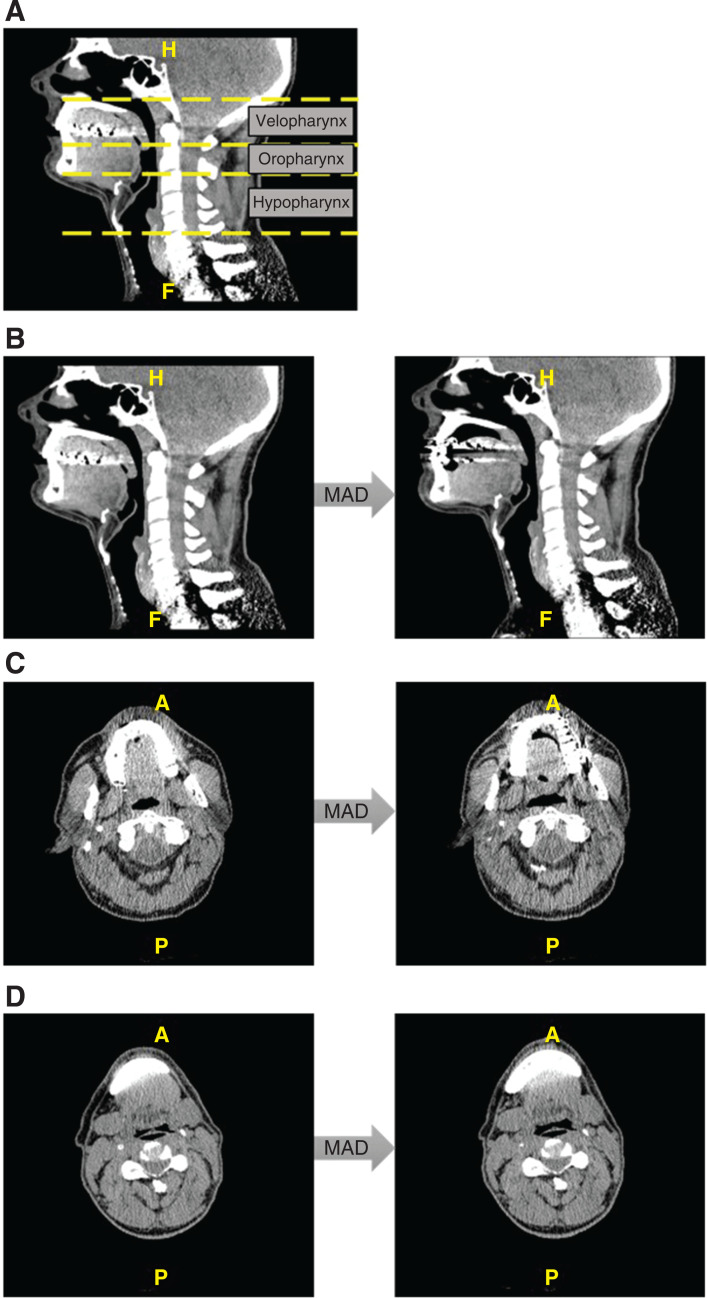
Based on these CT images, 3D computer-aided design models were reconstructed using Mimics software (Materialise, Leuven, Belgium).19,22 The anatomical parameters that were determined on this 3D model are as follows: UA volume (cm3), minimal cross-sectional area (cm2), UA length (cm), and distance from the spina mentalis to the hyoid (mm). The UA volume was expressed by the effective UA volume, only taking into account the UA volume through which the air flows. The total UA volume was measured, as well as the volume of the 3 subsegments: velopharynx (from the hard palate to the uvular tip), oropharynx (from the uvular tip to the epiglottic tip), and hypopharynx (from the epiglottic tip to the level of the vocal cords) (Figure 2A).
Figure 2. Low-dose CT scan images.
Subsegments of the UA on a midsagittal CT scan (A), UA changes due to MAD treatment on a midsagittal CT scan of a responder (B), velopharyngeal changes due to MAD treatment on an axial CT scan of a responder (C), and hypopharyngeal changes due to MAD treatment on an axial CT scan of a responder (D). A = anterior, CT = computed tomography, H = head, F = feet, MAD = mandibular advancement device, P = posterior, UA = upper airway.
Subsequently, CFD simulation was performed by FluidDa NV (Kontich, Belgium). Mimics software (Materialise, Leuven, Belgium) was used for the construction of 3D models based on Hounsfield units. The appropriate voxels had to be grouped in order to reconstruct 3D images based on Hounsfield units. Hence, masks were created that contain voxels with predefined Hounsfield units. Once the masks were created accurately with the correct Hounsfield unit bound (–1024, –400) for air and (226, 3071) for bone structure, the 3D models could be created using triangulation. After converting the UA volume into a 3D image, it was used to analyze the flow behavior inside the airway using CFD. To this end, a computational grid was created. The computational grid for each patient consisted of 600,000 to 800,000 tetrahedral cells depending on the complexity and volume of the UA models. The grids were made using TGrid 4.0.16 (Ansys, Lebanon, New Hampshire, USA). A grid dependency showed near-identical results for increasing grid sizes. The walls of the UA were assumed to be rigid, and the simulations were performed using the commercial code of Fluent v6.3 (Ansys, Lebanon, USA). The Reynolds Averaged Navier-Stokes equations were solved iteratively, and the flow in the models was assumed to be incompressible, steady, and isothermal. To obtain high orders of accuracy, the pressure-based solver was used with a node-based Green-Gauss gradient treatment. The pressure-velocity coupling was solved using the Semi-Implicit Method for Pressure-Linked Equations (SIMPLE) scheme. The flow regime was turbulent because of the narrowness of the UA region. The calculated Reynolds number was approximately 6000. Therefore, a 2-equation model was used to represent the flow turbulence. For this type of problem, the low Reynolds number (LRN) k-ω has been used, since this model can accurately predict pressure drops and velocity profiles. In addition, the LRN k-ω model can obtain an accurate laminar solution when the turbulent viscosity approaches zero.28 For the inlet, a velocity inlet condition, and for the outlet a pressure outlet boundary condition was used for all models. The patient-specific velocity profile was taken from the mass flow rates measured by the pneumotach in an in vivo study.19 More details on the model and simulation can be found in De Backer et al.19
Finally, the UA resistance (Pa/L) was extracted from the outcome of the CFD analysis. The UA resistance (R) was measured using the pressure drop over the UA (Δp) and the volume flow rate in the UA (Fua):
| (1) |
As resistance can become infinite, due to, for example, an occluded airway, the resistance-based radius (rres) was calculated for analysis purposes. This combines the Poiseuille equation, given as equation (2), with equation (1):
| (2) |
In this equation, is the pressure drop over the UA (Pa), μ is the dynamic viscosity (Pa.s), L is the length of the pipe (m), Fua is the volume flowrate in the UA (m3/s), rres is the resistance-based radius (m), and R is the UA resistance (Pa/L)
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (IBM Corp., Armonk, N.Y., USA). The Shapiro-Wilk test was conducted to test for normality. Descriptive statistics are presented as mean ± SD or median (quartile [Q] 1–Q3). Paired and unpaired t tests were used to compare normally distributed continuous variables, whereas Wilcoxon signed-rank tests and Mann-Whitney U tests were used for nonnormally distributed continuous variables. All P values are 2-sided and a P value < .05 was considered statistically significant.
RESULTS
Data characteristics and treatment outcome
One hundred patients with OSA (83% males; age: 47.6 ± 10.0 years; BMI 26.9 ± 3.3 kg/m2; AHI 21.0 ± 11.2 events/h sleep) were selected for this study and underwent a new baseline PSG. One patient did not complete the baseline PSG and 10 of the participants had an AHI of < 5 events/h; hence, OSA was confirmed in 89/100 patients. The low-dose CT scan was not performed in 5 of the patients with confirmed OSA and 13 patients did not undergo a follow-up PSG, resulting in a final dataset of 71 patients (Figure 1B). No significant differences in clinical characteristics at baseline were found between patients lost to follow-up (n = 18) and patients completing both low-dose CT scan with CFD analysis and 3-month follow-up PSG (n = 71). Thirty-five patients (49.3%) were diagnosed with mild (5 ≤ AHI < 15 events/h), 27 (38%) with moderate (15 ≤ AHI < 30 events/h), and 9 (12.7%) with severe (AHI ≥ 30 events/h) OSA. Comparing the clinical characteristics at baseline and 3-month follow-up showed significant improvements in AHI, apnea index, hypopnea index, AHI supine, AHI nonsupine, minimal O2 saturation, and oxygen desaturation index (Table 2). Furthermore, there was a statistical, but not clinical, significant difference in BMI before and after 3 months of treatment. In total, there were 33 (46%) responders and 38 (54%) nonresponders. Within the nonresponder group, 17 patients (45% [17/38], which is 24% of all included patients [17/71]) deteriorated under MAD treatment. Responders had an AHI of 3.9 (2.2–8.3) events/h with an MAD, whereas nonresponders had an AHI of 12.7 (9.1–23.3) events/h with an MAD (median [Q1–Q3], P < .001). No significant differences in clinical characteristics were found between responders and nonresponders at baseline (Table 3).
Table 2.
Clinical characteristics at baseline and 3-month follow-up (n = 71).
| Baseline PSG (n = 71) | Follow-up PSG (n = 71) | P | |
|---|---|---|---|
| AHIa (events/h) | 16.5 (10.4–23.6) | 9.1 (3.9–16.4) | < .0001 |
| VAS snoringa (0–10) | 6.0 (5.0–9.0) | 6.0 (3.8–9.0) | .027 |
| ESSa (0–24) | 7.0 (5.0–14.0) | 5.5 (3.0–10.0) | < .0001 |
| BMIb (kg/m2) | 27.8 ± 3.2 | 28.1 ± 3.3 | .019 |
| Sleep-onset latencya (min) | 13.6 (8.0–22.4) | 12.6 (7.5–19.2) | .372 |
| Sleep efficiency indexa (% TST/SPT) | 88.4 (81.1–92.6) | 88.4 (81.9–91.8) | .390 |
| AIa (events/h) | 1.4 (0.1–4.4) | 0.1 (0.0–1.5) | < .0001 |
| HIa (events/h) | 12.7 (8.4–19.2) | 8.2 (3.9–13.0) | < .0001 |
| ArIa (events/h) | 24.0 (15.7–34.0) | 22.4 (13.8–33.3) | .324 |
| ODIa (events/h) | 4.4 (2.2–11.3) | 2.0 (0.7–5.0) | < .0001 |
| Mean SaO2a (%) | 95.2 (94.1–96.1) | 95.3 (94.2–95.9) | .281 |
| Minimum SaO2a (%) | 86.7 (83.2–90.0) | 89.0 (85.5–91.0) | .003 |
| Time SaO2a < 90% (min) | 0.5 (0.0–3.6) | 0.2 (0.0–2.5) | .102 |
| AHI supinea (events/h) | 35.4 (18.1–53.5) | 13.3 (3.7–30.3) | < .0001 |
| AHI nonsupinea (events/h) | 8.9 (3.7–17.2) | 4.8 (2.8–10.8) | .009 |
aMedian (Q1–Q3) and Wilcoxon signed-rank test. bMean ± SD and paired t test. AHI = apnea-hypopnea index, AI = apnea index, ArI = arousal index, BMI = body mass index, ESS = Epworth Sleepiness Scale, HI = hypopnea index, ODI = oxygen desaturation index, PSG = polysomnography, Q = quartile, SaO2 = oxygen saturation, SD = standard deviation, SPT = sleep period time, TST = total sleep time, VAS = visual analog scale.
Table 3.
Clinical characteristics and airway parameters in responders and nonresponders at baseline.
| Responders (n = 33) | Nonresponders (n = 38) | P | |
|---|---|---|---|
| Clinical characteristics | |||
| AHIa (events/h) | 17.3 (10.6–25.3) | 14.7 (10.2–23.2) | .588 |
| VAS snoringa (0–10) | 7.0 (6.0–9.0) | 6.0 (5.0–9.0) | .190 |
| ESSa (0–24) | 7.0 (5.0–15.5) | 7.5 (4.0–11.75) | .429 |
| Ageb (y) | 48.2 ± 9.6 | 48.8 ± 10.1 | .779 |
| BMIb (kg/m2) | 28.1 ± 3.0 | 27.7 ± 3.5 | .599 |
| Sleep-onset latencya (min) | 11.1 (6.6–26.0) | 14.4 (10.0–21.4) | .271 |
| Sleep efficiency indexa (% TST/SPT) | 88.4 (81.5–93.0) | 87.5 (80.2–92.3) | .729 |
| AIa (events/h) | 0.7 (0.1–3.6) | 1.6 (0.1–5.1) | .455 |
| HIa (events/h) | 14.1 (9.6–21.8) | 11.2 (8.2–17.4) | .143 |
| ArIa (events/h) | 29.5 (18.7–33.6) | 21.2 (13.9–38.0) | .254 |
| ODIa (events/h) | 4.0 (2.1–10.4) | 5.3 (2.3–11.6) | .645 |
| Mean SaO2a (%) | 94.9 (94.0–95.9) | 95.3 (94.1–96.1) | .496 |
| Minimum SaO2a (%) | 87 (81.5–90.0) | 86.0 (84.0–90.0) | .894 |
| Time SaO2a < 90% (min) | 0.2 (0.0–8.0) | 0.8 (0.0–2.6) | .564 |
| AHI supinea (events/h) | 28.4 (16.7–43.3) | 39.7 (20.2–69.7) | .118 |
| AHI nonsupinea (events/h) | 9.9 (4.1–18.3) | 8.9 (3.7–16.1) | .687 |
| Airway parameters | |||
| Total UA volumea (cm3) | 8.3 (5.7–11.5) | 9.3 (5.4–15.7) | .305 |
| Delta UA volumea (%) | 22.0 (−3.6 to 56.8) | 9.0 (−17.8 to 32.9) | .195 |
| Velopharynx volumea (cm3) | 1.9 (0.4–3.6) | 2.1 (0.6–5.3) | .375 |
| Delta velopharynx volumea (%) | 44.8 (11.1–120.5) | 31.3 (−2.5 to 116) | .425 |
| Oropharynx volumea (cm3) | 3.4 (1.8–4.2) | 3.3 (1.9–4.7) | .653 |
| Delta oropharynx volumea (%) | 4.2 (−14.4 to 45.3) | 1.1 (−27.9 to 36.0) | .646 |
| Hypopharynx volumea (cm3) | 2.7 (1.6–5.0) | 3.2 (2.3–5.8) | .177 |
| Delta hypopharynx volumea (%) | 16.2 (−15.9 to 74.3) | –10.9 (−25.8 to 27.5) | .059 |
| UA resistancea,* (Pa/L) | 0.11 (0.06–0.15) | 0.09 (0.05–0.30) | .912 |
| UA resistance-based radiusa (mm) | 2.3 (0.0–2.7) | 2.0 (0.0–2.7) | .819 |
| Minimum cross-sectional areaa (cm2) | 0.25 (0.0–0.58) | 0.17 (0.0–0.60) | .756 |
| Airway lengthb (cm) | 6.9 ± 0.8 | 7.1 ± 0.8 | .418 |
| Distance spina mentalis–hyoida (mm) | 35.0 (31.1–40.2) | 36.4 (33.2–41.2) | .333 |
aMedian (Q1–Q3) and Mann-Whitney U test. bMean ± SD and unpaired t test. *Analysis on 44/71 patients. AHI = apnea-hypopnea index, AI = apnea index, ArI = arousal index, BMI = body mass index, ESS = Epworth Sleepiness Scale, HI = hypopnea index, ODI = oxygen desaturation index, Q = quartile, SaO2 = oxygen saturation, SD = standard deviation, SPT = sleep period time, TST = total sleep time, UA = upper airway, VAS = visual analog snoring scale.
Effect of MADs on the UA
Functional imaging results showed that MAD treatment significantly increased the total UA volume (8.6 [5.4–12.8] cm3 vs 10.7 [6.4–15.4] cm3; median [Q1–Q3], P = .003). This occurred predominantly due to an increase in the velopharyngeal volume (2.1 [0.5–4.1] cm3 vs 3.3 [1.8–6.0] cm3; median [Q1–Q3], P < .001). Detailed data are shown in Table 4. No significant difference between the UA resistance with and without MAD was found; however, it has to be mentioned that only patients with an incomplete collapse could be included in this part of the analysis (44/71 patients).
Table 4.
Airway parameters without and with an MAD.
| Without MAD (n = 71) | With MAD (n = 71) | P | |
|---|---|---|---|
| Total UA volumea (cm3) | 8.6 (5.4–12.8) | 10.7 (6.4–15.4) | .003 |
| Velopharynx volumea (cm3) | 2.1 (0.5–4.1) | 3.3 (1.8–6.0) | < .0001 |
| Oropharynx volumea (cm3)a | 3.3 (2.0–4.5) | 3.2 (1.7–4.7) | .492 |
| Hypopharynx volumea (cm3) | 3.0 (1.8–5.3) | 3.1 (1.7–5.5) | .547 |
| UA resistancea,* (Pa/L) | 0.11 (0.06–0.23) | 0.10 (0.05–0.21) | .595 |
| UA resistance based radiusa (mm) | 2.1 (0.0–2.7) | 2.4 (1.5–2.9) | .164 |
| Minimal cross-sectional areaa (cm2) | 0.23 (0.0–0.58) | 0.32 (0.05–0.66) | .140 |
| Airway lengthb (cm) | 7.0 ± 0.8 | 6.9 ± 0.8 | .250 |
| Distance spina mentalis–hyoida (mm) | 36.2 (32.0–40.6) | 36.1 (31.2–41.3) | .571 |
aMedian (Q1–Q3) and Wilcoxon signed-rank test. bMean ± SD and paired t test. *Analysis on 44/71 patients. MAD = mandibular advancement device, Q = quartile, SD = standard deviation, UA = upper airway.
Comparison of airway parameters in responders and nonresponders
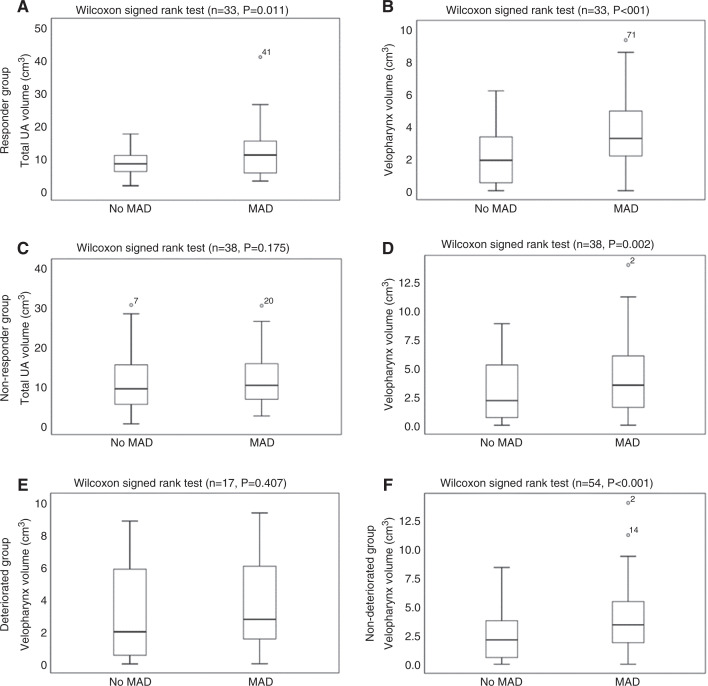
There were no significant differences at baseline between responders and nonresponders regarding UA volume, UA resistance, minimal cross-sectional area, and cephalometric parameters (Table 3). Analyses in both groups showed a significant increase in the total UA volume in responders (without MAD: 8.3 [5.7–11.5] cm3; with MAD: 11.0 [5.4–15.3] cm3; median [Q1–Q3], P = .011) (Figure 3A), but not in nonresponders (without MAD: 9.3 [5.4–15.7] cm3; with MAD: 10.2 [6.7–16.1] cm3; median [Q1–Q3], P = .175) (Figure 3C). The velopharyngeal volume was significantly increased in both responders (without MAD: 1.8 [0.4–3.6] cm3; with MAD: 3.2 [2.0–5.2] cm3; median [Q1–Q3], P < .001) (Figure 3B) and nonresponders (without MAD: 2.1 [0.6–5.3] cm3; with MAD: 3.5 [1.5–6.2] cm3; median [Q1–Q3], P = .002) (Figure 3D). Detailed results are shown in Table 5. The effect of the MAD on the UA of a responder is visualized in Figure 2B, Figure 2C, and Figure 2D. Other parameters did not show any significant differences between both groups.
Figure 3. Change in UA volume.
Total UA volume without and with MAD in responders (A), velopharynx volume without and with MAD in responders (B), total UA volume without and with MAD in nonresponders (C), velopharynx volume without and with MAD in nonresponders (D), velopharynx volume without and with MAD in deteriorated patients (E), and velopharynx volume without and with MAD in nondeteriorated patients (F). The outliers are plotted as individuals points. MAD = mandibular advancement device, UA = upper airway.
Table 5.
Airway parameters without and with an MAD according to treatment outcome (responders vs nonresponders).
| Responders (n = 33) | Nonresponders (n = 38) | |||||
|---|---|---|---|---|---|---|
| Without MAD | With MAD | P | Without MAD | With MAD | P | |
| Total UA volumea (cm3) | 8.3 (5.7–11.5) | 11.0 (5.4–15.3) | .011 | 9.3 (5.4–15.7) | 10.2 (6.7–16.1) | .175 |
| Velopharynx volumea (cm3) | 1.8 (0.4–3.6) | 3.2 (2.0–5.2) | < .001 | 2.1 (0.6–5.3) | 3.5 (1.5–6.2) | .002 |
| Oropharynx volumea (cm3) | 3.4 (1.8–4.2) | 3.5 (1.5–4.9) | .427 | 3.3 (1.9–4.7) | 3.2 (1.9–4.7) | .833 |
| Hypopharynx volumea (cm3) | 2.7 (1.6–5.0) | 3.2 (1.4–5.0) | .098 | 3.2 (2.3–5.8) | 3.0 (1.8–6.2) | .500 |
| UA resistancea,* (Pa/L) | 0.11 (0.06–0.15) | 0.09 (0.05–0.20) | .888 | 0.09 (0.05–0.30) | 0.12 (0.05–0.27) | .374 |
| UA resistance-based radiusa (mm) | 2.3 (0.0–2.7) | 2.4 (0.7–2.9) | .274 | 2.0 (0.0–2.7) | 2.4 (1.5–2.8) | .417 |
| Minimum cross-sectional areaa (cm2) | 0.25 (0.0–0.58) | 0.35 (0.04–0.62) | .362 | 0.17 (0.0–0.60) | 0.31 (0.05–0.70) | .301 |
| Airway lengthb (cm) | 6.9 ± 0.8 | 6.9 ± 0.9 | .955 | 7.1 ± 0.8 | 7.0 ± 0.8 | .165 |
| Distance spina mentalis–hyoida (mm) | 35.0 (31.1–40.2) | 36.1 (31.2–39.2) | .598 | 36.4 (33.2–41.2) | 36.5 (32.3–42.5) | .948 |
aMedian (Q1–Q3) and Wilcoxon signed-rank test. bMean ± SD and paired t test. *Analysis on 44/71 patients. MAD = mandibular advancement device, Q = quartile, SD = standard deviation, UA = upper airway.
Comparison of airway parameters in deteriorated and nondeteriorated patients
When comparing deteriorated (baseline AHI < AHI with MAD) with nondeteriorated patients, there was a significant increase in the velopharyngeal volume present in the nondeteriorated group (without MAD: 2.1 [0.6–3.8] cm3; with MAD: 3.4 [1.9–5.6] cm3; median [Q1–Q3], P < .001) (Figure 3F), but not in the deteriorated group (without MAD: 2.0 [0.4–6.0] cm3; with MAD: 2.8 [1.5–6.6] cm3; P = .407, median [Q1–Q3)]) (Figure 3E). Furthermore, there was a significant difference in the change in velopharyngeal volume between both groups (deterioration: 5.4% [–32.8% to 33.9%]; no deterioration: 45.2% [12.3%–119.1%]; median [Q1–Q3], P = .049]). Other parameters did not show any significant differences between both groups.
DISCUSSION
The key finding of this study is that MAD treatment acts by increasing the total UA volume, predominantly due to an increase in the velopharyngeal volume. Subanalyses in responders and nonresponders showed a significant increase in total UA volume with MADs in responders, while there was no significant increase in nonresponders. A second finding is that there was no significant increase in the velopharyngeal volume in deteriorating patients, while a significant increase was present in the nondeteriorating group.
MADs are currently the most frequently prescribed oral appliances to treat OSA. However, response to MAD treatment is patient-dependent, with a response rate of approximately 64%, with approximately half (37%) showing complete response (AHI < 5 events/h).13 In the past few years, several studies have been conducted to search for factors that are associated with MAD treatment outcome.26,29,30 These studies often had a retrospective study design and found anthropometric, polysomnographic, physiologic, and anatomic factors to be associated with treatment success. The current study suggests that imaging techniques might play an important role in preselection as well.
The results of our study suggest that MAD acts by increasing the total UA volume, predominantly due to an increase in the velopharyngeal volume. These results confirm the findings of 2 other functional imaging studies.12,22 Several other studies, which used videoendoscopy31–34 and cephalograms,35 confirmed that MAD mainly enlarges the velopharyngeal region. When evaluating the direction of the velopharyngeal widening, previous research12,32,33,36 found that the lateral dimensions, more than the anteroposterior dimensions, are increased. A dynamic imaging study37 suggests that this increase in the lateral airway dimensions is a consequence of a direct tissue connection between the lateral walls of the velopharynx and the lateral ramus of the mandible, probably the pterygomandibular raphe.
Subanalyses in responders and nonresponders showed a significant increase in the total UA volume in responders, but not in nonresponders. These associations between the increase in the total UA volume and responder/nonresponder status suggest that MAD efficacy is related to a multilevel enlargement in the UA caliber. This confirms the results of previous research.12 A weak correlation between the change in AHI and the change in UA volume was found in 2 previous studies with small patient populations.19,22 However, our study and several other studies12,36,38 did not find a direct linear correlation between these parameters. This is not completely surprising, as it seems unlikely that volume changes alone will be sufficient for predicting the clinical outcome of MAD treatment.38
The velopharyngeal volume was increased in both responders and nonresponders, indicating that it is possibly not only the effect on the velopharynx that is associated with treatment outcome but rather the sum of velopharyngeal and multilevel increase in UA volume. This may be explained by the fact that we saw a trend to an increase in the hypopharyngeal volume in responders, as well as a near-significant difference in the change in the hypopharyngeal volume between responders and nonresponders (Table 3), indicating that patients who show a significant increase in that part of the UA, ideally in combination with an enlargement at the level of the palate, are more likely to respond to MAD treatment. These findings are in line with the results of a previous study, showing a significant increase in the hypopharyngeal region in responders together with a significant enlargement of the velopharyngeal and total UA volume.12 This effect on the hypopharyngeal area was also reported using videoendoscopy.33
Furthermore, our results indicate that in particular, the deteriorated patients did not show a significant increase in the velopharyngeal volume, and there was a significant difference in the change in the velopharyngeal volume between patients who deteriorated and patients who did not. These results indicate that the absence of an increase in the velopharyngeal volume with mandibular advancement is associated with deterioration. These patients should be excluded from MAD therapy prospectively in order to gain time to efficacious treatment and to avoid time-consuming and expensive delays to OSA treatment in that specific patient.
In our study, the response rate was 46%. However, as previously mentioned, the efficacy of MAD therapy has been reported to be 64% in a recent study. This difference can be explained by the fact that in this study, there was a consistent but nonoptimal mandibular repositioning (MAD was fixed at 75% of the individual’s maximal protrusion to standardize the results). Furthermore, a sufficient number of nonresponders were required for this study to allow comparison between responders and nonresponders. For that reason, all patients were given MAD treatment regardless of whether their evaluation (physical examination and/or drug-induced sleep endoscopy [DISE]) suggested potential benefit.
Strengths of this study
First, the data were collected in a prospective way: Patients started MAD therapy and underwent a low-dose CT. The results of the CT evaluation were blinded for both the patient and the clinical multidisciplinary team throughout the study. Therefore, the finding on the low-dose CT scan had no effect on the offered MAD treatment. This increases the predictive value of this study in comparison to the previous studies that mostly relied on retrospective analysis.
Second, compared with similar studies, the analyses were done on a large patient dataset. In addition, functional imaging data were blinded for both patients and physicians throughout the study.
Third, the used imaging method is easily available in clinical practice. Functional imaging can be based on magnetic resonance imaging (MRI) and CT images. Both imaging techniques have advantages and disadvantages. The advantages of MRI are that it does not involve radiation and it is the most accurate method to visualize soft tissue structures. However, imaging time is several minutes, which can lead to a diminished image quality due to motion artifacts. CT scan, on the other hand, only takes a few seconds, making it more applicable for clinical practice. A disadvantage of this imaging technique is the radiation; however, the natural contrast between the air and the surrounding soft tissues makes it possible to use a lower amount of radiation—in other words, to perform a low-dose CT scan.39 No large-scale studies have been conducted yet to compare the results of CT and MRI images regarding 3D reconstruction and segmentation.40 In this study, low-dose CT scans were used since the aim of our study, and other current research, is to find a tool that can easily be used in clinical practice to predict MAD treatment outcome.
Limitations of this study
The CT scans were taken during wakefulness, so the observed effects of mandibular advancement may differ from the changes that occur during sleep. However, imaging during wakefulness allows for a quick investigation without the need for overnight studies. Therefore, it is of great interest to define UA changes caused by mandibular advancement, as well as for identifying predictors for MAD treatment outcome that can be implemented into clinical practice. Another limitation of this study is that we did not take into account the dynamic behavior of the UA, such as the influence of neuromuscular factors. Fluid-Structure Interaction (FSI) technology can be used to analyze the impact of surrounding soft tissue structures and air in the respiratory tract.22,40
Second, all patients were given MAD regardless of whether their evaluation (physical exam and/or DISE) suggested potential benefit. The data described in this paper are part of a prospective, single-center, cohort study called the “PRedicting therapeutic Outcome of Mandibular Advancement Device treatment in obstructive sleep apnea” (PROMAD) trial, aimed at identifying the predictive power of awake endoscopy including the Muller maneuver, DISE, and CT-scan–based CFD in treatment outcomes with MAD. Patients started MAD therapy and underwent all the different investigations with and without MAD. However, the results of the evaluations were blinded for both the patient and the clinical multidisciplinary team throughout the study, so that the results had no effect on the offered MAD treatment. This particular paper focused on the results of the low-dose CT scans, without taking into account the results of the other investigations. However, we do realize that a combination of the different selection methods such as DISE and anthropometric data will improve the patient selection. However, this was not the aim of this subanalysis.
Third, in the absence of a gold-standard protocol for MAD titration,41 the MAD was fixed at 75% of the individual’s maximal protrusion. Further titration in our study population could even have improved treatment response. However, the authors state that the applied approach was imperative for a more objective comparison between baseline and MAD treatment outcomes.
Furthermore, the assessment of MAD treatment outcome may have been affected by night-to-night variability in respiratory events. Several studies42–44 show that there is a remarkable intraindividual variability in AHI recordings. Ideally, multinight sleep studies are required to average out the variability. Therefore, the baseline PSG was repeated after inclusion in this study protocol to reconfirm the diagnoses of OSA. However, the follow-up sleep study was not repeated due to the fact that the study protocol was already quite intensive for the patients. To minimize the internight variability, patients were re-evaluated in the same sleep center with the same equipment in order to minimize the foreign sleep environment and optimize the habituation to the process of the in-laboratory PSG. Furthermore, interrater variability was eliminated since all PSGs were scored by 2 experienced scorers. Additionally, a remarkable number of patients seem to fluctuate with more than 10 respiratory events per hour from night to night when assessing the effect of interventions on OSA severity. Therefore, in our study, responders were defined as patients with a decrease in AHI of ≥ 50%, which is higher than the overall internight variability.
The patients referred for MAD therapy in the current trial are less obese and experience more severe OSA compared with the patients generally referred to MAD therapy, possibly affecting the generalizability of the results. Future prospective studies are needed to further validate our findings.
Another limitation of this study is that the UA resistance is not statistically different with and without MAD. This can be explained by the fact that 27 of the 71 included patients had a complete UA collapse during the CT scan, which causes the UA resistance to be infinite. Only patients with an incomplete collapse could be analyzed, which results in a selection bias for this part of the analysis.
We acknowledge the potentially increased type I error due to multiple hypothesis testing. However, given the exploratory nature of the study, no correction for multiple testing was applied, but results should be interpreted with care.
Finally, as it is the presence or absence of an increase in the UA volume during mandibular advancement that is found to be associated with treatment outcome, baseline parameters alone are insufficient to predict MAD treatment outcome. This indicates the need for an oral appliance or any other tool to simulate this effect during functional imaging. Custom-made MADs were manufactured in our study, which is quite expensive and time consuming. A previous study17 used a simulation bite during DISE to predict MAD treatment outcome. A simulation bite, for example, could provide a reliable and reproducible mandibular position, suggesting that this bite would also be useful for future functional imaging studies without prefabrication of the final custom-made MAD. Once the optimal position is determined with the simulation bite, it could further be used to construct the required protrusive position of the MAD for that particular patient.
CONCLUSIONS
In conclusion, the results of this prospective study suggest that based on functional imaging, MAD acts by increasing the total UA volume, predominantly due to an increase in the velopharyngeal volume. Subanalyses in responders and nonresponders showed that MAD treatment only caused a significant increase in the total UA volume in responders. This might indicate that the efficacy of the oral appliance is associated with an increase in the total UA volume. Additionally, we found that the absence of an increase in the velopharyngeal volume is associated with deterioration during MAD therapy. Therefore, functional imaging might be of added value for upfront MAD patient selection. Future prospective studies are needed to further explore the role of functional imaging in predicting MAD treatment outcome.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Antwerp University Hospital (UZA), University of Antwerp. This study was funded by a 3-year grant of the Flemish Government Agency for Innovation by Science and Technology (IWT-090864). M.D. holds a postdoctoral fellowship at Research Foundation Flanders (FWO)—12H4516N. J.V. reports grants from SomnoMed outside the submitted work and sits on the advisory board of ResMed Narval. M.J.B. reports grants from SomnoMed outside the submitted work and sits on the advisory boards of ResMed and SomnoMed. O.M.V. reports grants from Philips, grants from SomnoMed, personal fees from SomnoMed, other support from Inspire Medical Systems, personal fees from Inspire Medical Systems, other support from Zephyr, other support from NightBalance, and other support from Galvani, outside the submitted work. O.M.V. holds a Senior Clinical Investigator Fellowship from Research Foundation Flanders (FWO)—2016-2021. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the secretarial staff of the Special Dentistry Care, the Multidisciplinary Sleep Disorders Center, and the Ear-Nose-Throat, Head and Neck Surgery Department at Antwerp University Hospital.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CFD
computational fluid dynamics
- CT
computed tomography
- DISE
drug-induced sleep endoscopy
- MAD
mandibular advancement device
- OSA
obstructive sleep apnea
- PSG
polysomnography
- Q
quartile
- SaO2
oxygen saturation
- UA
upper airway
REFERENCES
- 1. Peppard PE , Young T , Barnet JH , Palta M , Hagen EW , Hla KM . Increased prevalence of sleep-disordered breathing in adults . Am J Epidemiol. 2013. ; 177 ( 9 ): 1006 – 1014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dempsey JA , Veasey SC , Morgan BJ , O’Donnell CP . Pathophysiology of sleep apnea . Physiol Rev. 2010. ; 90 ( 1 ): 47 – 112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vanderveken OM , Boudewyns A , Ni Q , et al . Cardiovascular implications in the treatment of obstructive sleep apnea . J Cardiovasc Transl Res. 2011. ; 4 ( 1 ): 53 – 60 . [DOI] [PubMed] [Google Scholar]
- 4. Bradley TD , Floras JS . Obstructive sleep apnoea and its cardiovascular consequences . Lancet. 2009. ; 373 ( 9657 ): 82 – 93 . [DOI] [PubMed] [Google Scholar]
- 5. Kushida CA , Morgenthaler TI , Littner MR , et al . Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005 . Sleep. 2006. ; 29 ( 2 ): 240 – 243 . [DOI] [PubMed] [Google Scholar]
- 6. Ramar K , Dort LC , Katz SG , et al . Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015 . J Clin Sleep Med. 2015. ; 11 ( 7 ): 773 – 827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dieltjens M , Vanderveken O . Oral appliances in obstructive sleep apnea . Healthcare (Basel). 2019. ; 7 ( 4 ): 141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marklund M , Franklin KA . Dental appliances in the treatment of snoring: a comparison between an activator, a soft-palate lifter, and a mouth-shield . Swed Dent J. 1996. ; 20 ( 5 ): 183 – 188 . [PubMed] [Google Scholar]
- 9. Chan AS , Cistulli PA . Oral appliance treatment of obstructive sleep apnea: an update . Curr Opin Pulm Med. 2009. ; 15 ( 6 ): 591 – 596 . [DOI] [PubMed] [Google Scholar]
- 10. Fleetham JA , de Almeida FR . Oral appliances. In: European Respiratory Monograph. Lausanne, Switzerland: European Respiratory Society; 2010:267–285.
- 11. Marklund M , Verbraecken J , Randerath W . Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy . Eur Respir J. 2012. ; 39 ( 5 ): 1241 – 1247 . [DOI] [PubMed] [Google Scholar]
- 12. Chan AS , Sutherland K , Schwab RJ , et al . The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea . Thorax. 2010. ; 65 ( 8 ): 726 – 732 . [DOI] [PubMed] [Google Scholar]
- 13. Sutherland K , Takaya H , Qian J , Petocz P , Ng AT , Cistulli PA . Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea . J Clin Sleep Med. 2015. ; 11 ( 8 ): 861 – 868 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanderveken OM , Devolder A , Marklund M , et al . Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea . Am J Respir Crit Care Med. 2008. ; 178 ( 2 ): 197 – 202 . [DOI] [PubMed] [Google Scholar]
- 15. Sutherland K , Vanderveken OM , Tsuda H , et al . Oral appliance treatment for obstructive sleep apnea: an update . J Clin Sleep Med. 2014. ; 10 ( 2 ): 215 – 227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards BA , Andara C , Landry S , et al . Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea . Am J Respir Crit Care Med. 2016. ; 194 ( 11 ): 1413 – 1422 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vroegop AV , Vanderveken OM , Dieltjens M , et al . Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome . J Sleep Res. 2013. ; 22 ( 3 ): 348 – 355 . [DOI] [PubMed] [Google Scholar]
- 18. Op de Beeck S , Dieltjens M , Azarbarzin A , et al . Mandibular advancement device treatment efficacy is associated with polysomnographic endotypes . Ann Am Thorac Soc. 2021. ; 18 ( 3 ): 511 – 518 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Backer JW , Vanderveken OM , Vos WG , et al . Functional imaging using computational fluid dynamics to predict treatment success of mandibular advancement devices in sleep-disordered breathing . J Biomech. 2007. ; 40 ( 16 ): 3708 – 3714 . [DOI] [PubMed] [Google Scholar]
- 20. Yucel A , Unlu M , Haktanir A , Acar M , Fidan F . Evaluation of the upper airway cross-sectional area changes in different degrees of severity of obstructive sleep apnea syndrome: cephalometric and dynamic CT study . AJNR Am J Neuroradiol. 2005. ; 26 ( 10 ): 2624 – 2629 . [PMC free article] [PubMed] [Google Scholar]
- 21. Stuck BA , Maurer JT . Airway evaluation in obstructive sleep apnea . Sleep Med Rev. 2008. ; 12 ( 6 ): 411 – 436 . [DOI] [PubMed] [Google Scholar]
- 22. Song B , Li Y , Sun J , et al . Computational fluid dynamics simulation of changes in the morphology and airflow dynamics of the upper airways in OSAHS patients after treatment with oral appliances . PLoS One. 2019. ; 14 ( 11 ): e0219642 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Holsbeke C , De Backer J , Vos W , et al . Anatomical and functional changes in the upper airways of sleep apnea patients due to mandibular repositioning: a large scale study . J Biomech. 2011. ; 44 ( 3 ): 442 – 449 . [DOI] [PubMed] [Google Scholar]
- 24. Suga H , Iwasaki T , Mishima K , Nakano H , Ueyama Y , Yamasaki Y . Evaluation of the effect of oral appliance treatment on upper-airway ventilation conditions in obstructive sleep apnea using computational fluid dynamics . Cranio. 2019. ; 39 ( 3 ): 209 – 217 . [DOI] [PubMed] [Google Scholar]
- 25. Vos W , De Backer J , Devolder A , et al . Correlation between severity of sleep apnea and upper airway morphology based on advanced anatomical and functional imaging . J Biomech. 2007. ; 40 ( 10 ): 2207 – 2213 . [DOI] [PubMed] [Google Scholar]
- 26. Verbruggen A , Vroegop A , Dieltjens M . Predicting therapeutic outcome of mandibular advancement device treatment in obstructive sleep apnoea (PROMAD): study design and baseline characteristics . J Dent Sleep Med . 2016. ; 3 ( 4 ): 119 – 138. [Google Scholar]
- 27. Dieltjens M , Vanderveken OM , Hamans E , et al . Treatment of obstructive sleep apnea using a custom-made titratable duobloc oral appliance: a prospective clinical study . Sleep Breath. 2013. ; 17 ( 2 ): 565 – 572 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilcox DC . Turbulence Modeling for CFD, 2nd ed . La Canada, CA: : DCW Industries Inc; .; 1998. . [Google Scholar]
- 29. Liu Y , Lowe AA , Fleetham JA , Park YC . Cephalometric and physiologic predictors of the efficacy of an adjustable oral appliance for treating obstructive sleep apnea . Am J Orthod Dentofacial Orthop. 2001. ; 120 ( 6 ): 639 – 647 . [DOI] [PubMed] [Google Scholar]
- 30. Marklund M , Stenlund H , Franklin KA . Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success . Chest. 2004. ; 125 ( 4 ): 1270 – 1278 . [DOI] [PubMed] [Google Scholar]
- 31. Isono S , Tanaka A , Sho Y , Konno A , Nishino T . Advancement of the mandible improves velopharyngeal airway patency . J Appl Physiol 1985. 1995. ; 79 ( 6 ): 2132 – 2138 . [DOI] [PubMed] [Google Scholar]
- 32. Sasao Y , Nohara K , Okuno K , Nakamura Y , Sakai T . Videoendoscopic diagnosis for predicting the response to oral appliance therapy in severe obstructive sleep apnea . Sleep Breath. 2014. ; 18 ( 4 ): 809 – 815 . [DOI] [PubMed] [Google Scholar]
- 33. Ryan CF , Love LL , Peat D , Fleetham JA , Lowe AA . Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx . Thorax. 1999. ; 54 ( 11 ): 972 – 977 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan AS , Lee RW , Srinivasan VK , Darendeliler MA , Grunstein RR , Cistulli PA . Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea . Eur Respir J. 2010. ; 35 ( 4 ): 836 – 842 . [DOI] [PubMed] [Google Scholar]
- 35. Tsuiki S , Hiyama S , Ono T , et al . Effects of a titratable oral appliance on supine airway size in awake non-apneic individuals . Sleep. 2001. ; 24 ( 5 ): 554 – 560 . [DOI] [PubMed] [Google Scholar]
- 36. Sutherland K , Deane SA , Chan AS , et al . Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea . Sleep. 2011. ; 34 ( 4 ): 469 – 477 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown EC , Cheng S , McKenzie DK , Butler JE , Gandevia SC , Bilston LE . Tongue and lateral upper airway movement with mandibular advancement . Sleep. 2013. ; 36 ( 3 ): 397 – 404 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao M , Barber T , Cistulli P , Sutherland K , Rosengarten G . Computational fluid dynamics for the assessment of upper airway response to oral appliance treatment in obstructive sleep apnea . J Biomech. 2013. ; 46 ( 1 ): 142 – 150 . [DOI] [PubMed] [Google Scholar]
- 39. Boiselle PM , Dippolito G , Copeland J , et al . Multiplanar and 3D imaging of the central airways: comparison of image quality and radiation dose of single-detector row CT and multi-detector row CT at differing tube currents in dogs . Radiology. 2003. ; 228 ( 1 ): 107 – 111 . [DOI] [PubMed] [Google Scholar]
- 40. De Backer JW , Vos WG , Verhulst SL , De Backer W . Novel imaging techniques using computer methods for the evaluation of the upper airway in patients with sleep-disordered breathing: a comprehensive review . Sleep Med Rev. 2008. ; 12 ( 6 ): 437 – 447 . [DOI] [PubMed] [Google Scholar]
- 41. Dieltjens M , Vanderveken OM , Heyning PH , Braem MJ . Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing . Sleep Med Rev. 2012. ; 16 ( 2 ): 177 – 185 . [DOI] [PubMed] [Google Scholar]
- 42. Roeder M , Bradicich M , Schwarz EI , et al . Night-to-night variability of respiratory events in obstructive sleep apnoea: a systematic review and meta-analysis . Thorax. 2020. ; 75 ( 12 ): 1095 – 1102 . [DOI] [PubMed] [Google Scholar]
- 43. Aarab G , Lobbezoo F , Hamburger HL , Naeije M . Variability in the apnea-hypopnea index and its consequences for diagnosis and therapy evaluation . Respiration. 2009. ; 77 ( 1 ): 32 – 37 . [DOI] [PubMed] [Google Scholar]
- 44. Ahmadi N , Shapiro GK , Chung SA , Shapiro CM . Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography . Sleep Breath. 2009. ; 13 ( 3 ): 221 – 226 . [DOI] [PubMed] [Google Scholar]
- 45. The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research . Sleep. 1999. ; 22 ( 5 ): 667 – 689 . [PubMed] [Google Scholar]