Abstract
Study Objectives:
Sleep is one of the most common factors related to health, yet a standard definition of sleep quality has not been identified. Polysomnography provides important information about objective sleep variables. However, the relationship between objective sleep variables and perception of sleep quality remains unclear. The purpose of this review was to (1) summarize the current methods of measuring objective sleep macrostructure and microstructure, including electroencephalography arousals, spectral frequency, cyclic alternating pattern, and self-report sleep quality, and (2) investigate the relationship between objective measures of sleep physiology and self-report sleep quality in healthy adults.
Methods:
A literature search was conducted using Medline, PubMed, and PsycInfo databases and cited reference searches. Eligible studies included a comparison between self-report sleep quality and polysomnography sleep measures in healthy adults.
Results:
Thirteen studies were identified. Measurement of self-report sleep quality varied widely across studies. Total sleep time and sleep efficiency were most consistently related to sleep quality, while other objective sleep variables, including electroencephalography spectral analysis, were not reliably predictive of self-report sleep quality in healthy adults. There is preliminary support that microstructural sleep analysis with cyclic alternating pattern may be related to self-reported sleep quality.
Conclusions:
Further research is needed to define and standardize self-report measures of sleep quality and investigate the microstructure of sleep. Objective measures of sleep and experiences of “quality” sleep are not as closely related as one may expect in healthy individuals, and understanding this relationship further is necessary to improve the clinical utility of sleep physiology.
Citation:
Cudney LE, Frey BN, McCabe RE, Green SM. Investigating the relationship between objective measures of sleep and self-report sleep quality in healthy adults: a review. J Clin Sleep Med. 2022;18(3):927–936.
Keywords: polysomnography, sleep physiology, self-report
INTRODUCTION
Humans spend approximately one-third of their lives sleeping. Sleep is an inherent physiological process controlled by homeostasis and circadian rhythms. It is a central part of maintaining overall mental and physical well-being, yet the exact functions of sleep remain poorly understood.1 The important role of sleep across several health domains (eg, mental health disorders, sleep disorders, quality of life, and functioning) has underscored the need for valid and reliable methods to measure sleep. The relationship between the self-perception of sleep quality and physiologically recorded sleep has been identified as an important clinical marker for identifying sleep disorders;2 however, this relationship is also not well-defined in healthy populations. A discrepancy between objective sleep estimations and perception of sleep quality has been highlighted in clinical populations, including insomnia disorder. Understanding the relationship between sleep perception and objective measures in healthy individuals allows for a basis when interpreting sleep disturbances in the context of medical illness or psychopathology and provides insight into potential interventions to improve the perceived experiences of sleep.
Sleep quality is often referred to as an important health factor in the literature, but it is not typically or consistently defined by individual studies. There are several scales commonly used to evaluate sleep quality, ranging from single-item questions that ask participants to rate how “good” or “bad” sleep was (eg, “how would you rate the quality of your sleep?”)3 to scales with multiple domains/subscales.4 Multiquestion scales capture broad information and comprise various subscales related to sleep over a specified timeframe, such as the Pittsburgh Sleep Quality Index.5 A drawback to this approach is that the factors expected to impact judgments of sleep quality and the weight of importance for each factor are predefined.4
Studies that attempt to understand which factors correspond with a good night’s sleep in healthy sleepers have shown that the top determinants of sleep quality ratings were total sleep time (TST), wake after sleep onset (WASO), the state of mind before bedtime (eg, experience of stress,6 feeling refreshed upon waking, and mood the day after sleep).4 Qualitative interviews have shown that self-reported feelings the next day (eg, waking up feeling restored, rested, or alert) were ranked as the most important variables that individuals use for the appraisal of the quality of their sleep.7 This suggests that judgments of sleep quality may reflect “nonsleep phenomena” that may not be captured in questions related to timing/quantity of sleep.2 Recording responses first thing in the morning with the use of a sleep diary minimizes the impact of these phenomena on sleep quality ratings.8,9
Sleep quality is not necessarily directly related to sleep quantity, as there are some individuals who have sleep complaints but show objective sleep parameters similar to those without sleep complaints.2 Krystal and Edinger2 presented several objective measures of sleep physiology and their potential utility for measuring aspects important for sleep quality, including traditional polysomnography (PSG) macrostructural sleep measures, as well as methods which further analyze the microstructures of PSG-measured sleep, such as electroencephalography (EEG) arousals and spectral activity, and cyclic alternating patterns (CAP). These methods are briefly described below.
Methods for measuring sleep objectively include PSG, which consists of EEG, electro-oculography, and electromyography. PSG can detect patterns of sleep architecture, including the cycling between rapid eye movement (REM) sleep and nonrapid eye movement (NREM) sleep, which occurs several times throughout the night in about 90- to 110-minute cycles.10 PSG measures include TST, sleep efficiency (SE), sleep onset latency (SOL), WASO, and number of awakenings, among other variables which are considered to comprise the macrostructure of sleep (see Table S1 in the supplemental material for a list of variables obtained from PSG).
The traditional PSG scoring method—the Rechtschaffen and Kales method—divides sleep into stages: REM and NREM subdivided into 4 stages from light sleep in stage 1 to slow-wave sleep in stages 3 and 4.11 These scoring methods persisted until the American Association of Sleep Medicine updated them in 2007.12 One of the major changes to sleep stage scoring was combining stage 3 and stage 4 to one stage labeled as N3, often called slow-wave sleep (SWS), as well as minor changes to the EEG placement.13 Sleep stage scoring continues to be controversial in the field of sleep medicine.13,14
Comparing data from studies scored according to different standards has natural drawbacks, although the new sleep stage scoring methods have improved interrater reliability of scoring.15 A notable example of this stems from a recent meta-analysis of PSG studies (that used American Academy of Sleep Medicine scoring) including over 5,000 healthy individuals across the lifespan and identified decreased TST and SE as well as increased WASO and SOL occurring with older age.16 These findings confirm age-related changes in sleep from an earlier meta-analysis17 and further support the comparability of sleep scoring methods. Therefore, it should be noted that demographic factors, such as sex- and age-related differences in physiological sleep, are important to consider when evaluating self-report sleep ratings. Declines in percentage of time in SWS and increased time in lighter stages of sleep (stage 1 and stage 2) have also been shown across the lifespan,18 and good sleep has been identified as an important aspect of quality of life in older adults.19
In addition to measuring the macrostructure of sleep, the EEG component of PSG allows for measurements of oscillations on a continuum of sleep–wake states.13 Although several bands of activity may be present at any given time, the type of oscillation that dominates any given period allows for understanding stages of sleep. EEG arousal events are scored manually based on American Sleep Disorder Association criteria and indicate sleep fragmentation, based on either total number of arousals or arousal indices for each individual sleep stage.20 Microstructural evaluation with EEG frequency spectral analysis allows for measurement of sleep more continuously than sleep stages by generating indices of activity signals in each frequency band. Spectral analysis consists of Fourier transformations of EEG signals to quantify the amplitudes of different frequencies.21 This separates the slower and faster EEG frequencies so that SWS/delta waves are discriminated from other frequencies that are present.21 In this regard, spectral analysis measures frequencies that are not quantified using sleep scoring methods, since sleep scoring methods report on the most dominant frequency and not the other frequencies that are present but of lesser amplitude. It has been hypothesized in early studies that individuals with insomnia who show no differences in macrostructural PSG measures compared to healthy controls may show different EEG spectral indices.2 This suggests that EEG spectral analysis may be another potential objective marker of sleep quality.
Another method has been developed to investigate and model microstates of sleep called probabilistic sleep modeling. This is another way of mathematically modeling sleep as a continuum and has resulted in a measure of sleep fragmentation, which may be important for sleep quality.22
Finally, CAP analysis is a method of examining the physiological activity in NREM sleep and identifying microstructures of sleep physiology.23 A CAP is a specific periodic activity that occurs in NREM and consists of phase A—transient events that last between 2 and 60 seconds and stand out from the background rhythm, phase B. There are 3 subtypes of phase A: A1 consists of high-amplitude slow waves (including delta bursts and K-complexes; see Table S2), A2 is a mix of slow and fast rhythms, and A3 is composed of primarily rapid low-voltage rhythms. The CAP rate is a ratio of the total amount of time in CAP to the total time in NREM sleep. Just as sleep macrostructure changes across the lifespan, CAP parameters also show age-related variability.24 A recent meta-analysis of CAP in normative samples showed that the CAP rate changes across development and is increased in elderly populations.25 An increased CAP rate has been associated with higher arousal instability in sleep26 and may be associated with sex differences in sleep.
In sum, there are a number of PSG-derived macrostructure and microstructure measures of sleep, although it is still unknown how these are related to sleep quality in the healthy population. The aim of this review is to investigate the relationship between macrostructure and microstructure physiology measures of sleep with ratings of sleep quality in healthy adults. This will provide a better understanding of how self-report measures of sleep quality correspond with objective sleep physiology measures.
METHODS
Review design
We conducted a review using the Participants, Intervention, Comparisons, Outcome27 study design approach to generate the research question.
Inclusion and exclusion criteria
Observational studies were included if the aim of the study was related to studying sleep in a nonclinical (healthy) adult population and reported sleep objectively measured with PSG and a self-report sleep quality measure.
Full inclusion and exclusion criteria are shown in Table S3. In summary, a literature search was conducted and included studies that met the following criteria:
Included nonclinical adult participants (aged 18 years or older) for the purpose of studying sleep in a healthy population (not merely as a healthy control group);
Included a measure of self-report sleep quality;
Included assessment of sleep characteristics measured with at least 1 full night of PSG or electrophysiology (1 or more of the following variables reported: TST, SOL, SE, WASO, sleep stage durations, REM latency, EEG spectral power frequency, EEG arousal number, EEG arousal index, CAP);
Included and reported a comparison between the self-report sleep quality and PSG-measured sleep characteristic;
Excluded participants with sleep, clinical, and neurological disorders;
Published in peer-reviewed journals in the English language.
Studies which employed actigraphy as the sole objective measure of sleep were not included in our search and are outside the scope of this article, as it does not provide electrophysiology or sleep stage data. Articles were excluded if they did not explicitly report a comparison between the objective sleep measures and the measure of self-report sleep quality.
Literature search
Databases utilized were PubMed, PsycInfo, Medline(R). We first searched “sleep quality,” since inclusion of a sleep quality measure was our main criterion and no synonyms were identified in the databases. Next, we added key terms for objective sleep measures including “sleep physiology,” “sleep architecture,” “sleep stages,” “polysomnography,” “electroencephalography,” and “cyclic alternating pattern.” We also wanted to limit studies to those examining sleep patterns in healthy adult samples so we included “adult,” “research subjects,” and “healthy volunteers.” Results were limited to human participants and English publications.
The final search strategy subject headings and search syntax were adapted for each of the databases separately. We also hand-searched for additional relevant cited articles within the search-generated articles. The screening process was conducted independently by the lead author (L.E.C.) based on the study inclusion criteria outlined. Duplicates were removed, and titles and abstracts were screened to eliminate nonrelevant studies. Full texts were screened and if there was uncertainty about the inclusion of a study, the other authors were consulted.
RESULTS
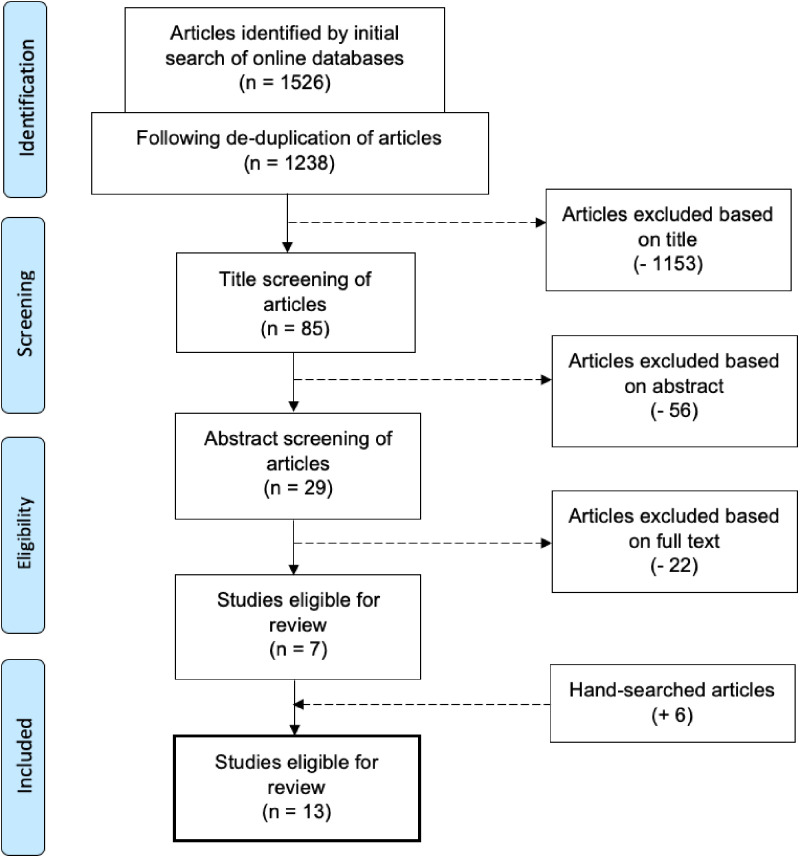
The search was conducted in August 2020 and resulted in 1,526 records [611 from PubMed, 319 from PsycInfo, and 596 from Medline(R)]. There were 1,238 records after the removal of duplicates. The first author (L.E.C.) screened 29 full-text records and included 13 studies in this review. Figure 1 shows the flow of the records through the screening process. All of the included articles reported using PSG for at least 1 full night.
Figure 1. Flowchart of the study selection process.
A summary of the study sample, sleep measures, and study findings related to the relationship between the objective sleep measure and self-report sleep quality measure are listed in Table 1. Ten of the studies used archival data that had been collected for other purposes. Ten of the 13 studies analyzed independent study samples, as 3 studies28–30 reported on data from the SIESTA project.31 The SIESTA sample consists of a sleep database of normative healthy participants who were screened for shift work, usual bedtime before midnight, and had no acute depressive or anxious symptoms.31
Table 1.
Summary of the sample, sleep measures, and findings for included studies.
| Study | Sample Description | Age Range, y (Mean ± SD) | Sample Size | Self-Reported Sleep Quality Measures | Objective Sleep Measures (Sleep Stage Scoring Method) | Findings |
|---|---|---|---|---|---|---|
| Akerstedt et al, 2016 (3) | Women randomly selected from representative community cohort sample in Sweden | 22–72 (divided at the median age of 51) 40.3 ± 0.7 58.0 ± 0.5 | 251 (0 males) | Single-item visual analog scale “How well did you sleep?” 0–100 | Single night in-home PSG (AASM 2007)
|
|
| Croy et al, 2017 (38) | Healthy young adults from 2 past studies on impact of noise on sleep | 19–30 (23.3 ± 2.9) | 47 (21 males) | 22-item verbal and numerical item questionnaire (4 items on overall sleep quality, 2 verbal on 5-point scale, 2 numerical on 11-point scale) | 2 nights in laboratory PSG (AASM 2007), including 1 control night, 1 night of exposure to noise
|
|
| Dos Santos Silva et al, 2015 (59) | Healthy older adults | 64–89 (73.7, no SD reported) | 40 (18 males) | Sleep habits questionnaire (part of the Comprehensive Geriatric Assessment, items marked as “yes” or “no”) | Single night laboratory PSG (R&K)
|
|
| Gabryelska et al, 2019 (37) | Healthy adult participants from archival database of controls | 19–73 (39.8 ± 12.9) | 206 (85 males) | Sleep Questionnaire A (sleep quality measure calculated as the mean of 7 subscales rated from 1–5) | 2 nights in laboratory PSG (R&K)
|
|
| Goelema et al, 2018 (30) | Healthy adults (subset from the SIESTA sample) | 51.5 ± 9.7 | 156 (71 males) | Subjective sleep and awakening scale (subscales: sleep quality, awakening quality, somatic complaints) | 2 nights in laboratory PSG (R&K)
|
|
| Hartmann et al, 2020 (33) | Community-dwelling older adults (males from Osteoporotic Fractures in Men study in males) | 65+ (Only median age reported: 76) | 2,811 (all males) | Males: 3 items rated on a 5-point Likert scale measuring “sleep depth,” “sleep restfulness,” and “sleep length” rated from a sleep diary | Single-night in-home PSG (R&K, modified to be similar to AASM 2007 by excluding stage 1, merging stages 3 and 4)
|
|
| Kaplan et al, 2017 (36) | Healthy midlife adults (subset from Sleep Heart Health Study) | 39+ (62.7 ± 11.3) | 3,173 (1,534 males) | 2 items rated on a 5-point Likert scale measuring “sleep depth” and “sleep restfulness” rated from a sleep diary (PSQI was measured and used as a predictor of the 2 scales) | Single-night in-home PSG (R&K)
|
|
| Kaplan et al, 2017 (32) | Community-dwelling older adults (males from Osteoporotic Fractures in Men study, females from Study of Osteoporotic Fractures study) | 65+ (males: 81.1 ± 4.6, females: 82.9 ± 3.3) | 1,483 (1,024 males) | 2-item rated on a 5-point Likert scale measuring “sleep depth” and “sleep restfulness” rated from a sleep diary (PSQI was measured and used as a predictor of the 2 scales) | Single-night in-home PSG (R&K)
|
|
| Keklund and Akerstedt, 1997 (35) | Healthy adults from 2 past studies (females were from a study on early morning work and males from a study on sleep in a truck berth) | 24–58 (37.0 ± 1.4) | 37 (14 males) | Karolinska Sleep Diary (factor analysis on 7 items to create a sleep quality index) | Single-night PSG (R&K): males in laboratory truck berth, females in-home PSG |
|
| Moser et al, 2010 (28) | Healthy adults (subset from the SIESTA sample) | 21–86 (53.3 ± 21.3) | 28 (13 males) | Self-rating scale for sleep and awakening quality (subscales: sleep quality, awakening quality, somatic complaints) | 2 nights in laboratory PSG (R&K)
|
|
| O’Donnell et al, 2009 (40) | Healthy older adults recruited from the community | 55–78 (males: 66.9 ± 6.7, females: 61.9 ± 5.1) | 24 (11 males) | Postsleep questionnaire (7 items on a 7-item Likert scale, 2 items related to sleep quality ratings) Rated morning after | 3 nights of laboratory PSG (R&K). 31 nights were included, but 27 nights included forced desynchrony
|
|
| Rosipal et al, 2013 (29) | Healthy adults (subset from the SIESTA sample) | 20–86 (51 ± 20) | 148 (67 males) | Self-rating scale for sleep and awakening quality (subscales: sleep quality, awakening quality, somatic complaints) | 2 nights in laboratory PSG (R&K)
|
|
| Westerlund et al, 2016 (39) | Healthy volunteers | 33–54 (44, no SD reported) | 33 (13 males) | Karolinska Sleep Questionnaire (4 items on sleep quality, 3 items on restoration) 6 months retrospective Rated 1 month prior to PSG | 2 nonconsecutive nights of in-home PSG (R&K)
|
|
AASM 2007 = American Academy of Sleep Medicine sleep stage scoring method, CAP = cyclic alternating pattern, EEG = electroencephalography, PSG = polysomnography, PSQI = Pittsburgh Sleep Quality Index, REM = rapid eye movement, R&K = Rechtschaffen and Kales sleep stage scoring manual, SD = standard deviation, SE = sleep efficiency, SWS = slow-wave sleep, SOL = sleep onset latency, TST = total sleep time, WASO = wake after sleep onset.
Two studies32,33 analyzed data from males from the Osteoporotic Fractures in Men study and females from the Study of Osteoporotic Fractures.34 Results are synthesized below by grouping the articles together based on the objective sleep parameters that were identified as significantly related to self-reported sleep quality. See Table S4 for a summary of the direction of association between each objective variable and the self-report sleep quality measure.
Macrostructural sleep
TST was the measure significantly associated with sleep quality most often among studies; however, the directions of these associations were inconsistent. Two studies found that TST was positively related to self-reported sleep quality,3,35,36 while 4 studies found TST was negatively correlated (eg, longer sleep duration was associated with decreased sleep quality28–30,37). Similarly, 2 studies found higher SE was associated with greater sleep quality;3,35 however, 2 other studies found the opposite relationship.29,30
Variables that signify difficulties with maintaining sleep during the night (WASO, percentage of time awake, and number of awakenings) were associated with poorer sleep quality in 3 studies.3,28,38 Goelema et al30 and Rosipal et al29 found the relationship with awakenings in the opposite direction (higher WASO was positively related with sleep quality), despite analyzing data from the same study of which Moser et al28 analyzed a smaller subset.
In terms of sleep stages, REM duration and N2 duration (see Table S2)30,37,39 were both primarily associated with sleep quality in a negative direction, other than 1 study with a small sample which showed a positive relationship with N2 duration.40 The only consistent association with sleep staging was that greater duration of SWS was significantly associated with higher self-reported sleep quality ratings in 3 studies.29,35,39
Findings from machine learning
Two studies of large datasets of over a thousand participants employed machine-learning techniques and showed that the most consistent predictors of self-reported sleep quality included SE, WASO, and age.32,36 SE and WASO were also noted to be highly collinear. Sleep staging variables were only weakly correlated with sleep quality.32 Regardless of the machine-learning model used, 11–17% of the variance in sleep quality ratings in older adults was explained by sleep, demographic, and clinical variables.32 Using the same techniques on a sample of midlife community-dwelling adults, only between 7 and 13% of the variance of self-reports of sleep quality was explained with PSG, EEG spectral power, and demographic variables.36 EEG spectral power frequency showed that microstructures of sleep did not contribute significantly to the machine-learning models.36 Finally, Gabryelska et al37 found a small effect size for lower N2 sigma 2 and REM delta 1 spectral power for predicting higher self-reported sleep quality.
CAP analyses and sleep quality
CAP analysis was performed in a small subset of the SIESTA study database (n = 28), finding CAP variables were not significantly associated with sleep quality ratings.28 More recently, CAP measures were automatized and calculated for a large sample of older male adults (n = 2,811).33 Decreased CAP rate was related to improved sleep quality measures. Those who reported higher sleep quality ratings also showed a decreased CAP A2 + A3 index.33
DISCUSSION
This review highlights the nuanced relationship between perceptions of sleep and physiological measures of sleep in healthy adults. Across the studies, TST and SE were the PSG-measured sleep variables that were most frequently significantly related to self-reported sleep quality. However, it is worth noting that TST and SE showed both positive3,35 and negative29,30 relationships with sleep quality. This indicates a meaningful relationship, since both long and short sleep durations can be associated with sleep difficulties. Objective measures of TST and SE may be useful in predicting self-reported sleep quality ratings but may also depend on the sample being studied. Although this review focused on a healthy population, the significant differences in sample selection and sleep quality measures across studies may influence interpretation of the direction of the results.
Other macrostructural sleep variables were not frequently related to sleep quality. Increased duration of SWS was associated with improved sleep quality, which is intuitive given SWS is regarded as the most “restorative” stage of sleep; however, this was only found in 3 studies with relatively small sample sizes.29,35,39 Greater N2 sleep stage duration was associated with poorer sleep quality ratings in 3 studies,30,37,39 which may be related to how more transient arousals (K-complexes/sleep spindles) occur during this stage.13
In terms of microstructural sleep variables, only a few studies have examined EEG spectral frequency analysis or CAP in relation to sleep quality. EEG spectral frequency analysis did not significantly predict self-reported sleep ratings in a large multisite study of older adults.36 A negative relationship between N2 sigma 2 (independent of N2 duration) and sleep quality with a small effect size was found.37 CAP rate was decreased significantly in older adult males with higher self-reported ratings of sleep depth, restfulness, and length.33 This recent study provides further evidence for the theory that CAP rate is a marker of instability and enhanced arousals within NREM sleep.26 CAP analysis has been automatized, indicating that it can now be more easily applied to previously collected PSG data and should continue to be investigated as a potentially more sensitive marker of self-reported sleep disruption.
Variations in self-reported sleep quality measures
This review reiterates the need for more standardized measurement of sleep quality. No two studies that examined different populations employed the same methods for measuring self-reported sleep quality. These ranged from single-item visual analog and Likert scales that are not validated measures to more commonly used validated measures that provide a single score composed of multiple items/subscales.29 Since many of the reviewed studies performed analyses on previously collected data, measures of sleep quality that may better correspond with objective sleep (such as the consensus sleep diary8) were not available at the time of collection, and many studies relied on single-item questions that may be less robust.36 This points to a major barrier in being able to properly quantify perceptions of sleep quality in the extant literature, which is a problem that has been identified for more than a decade.2 Identifying a core outcome set that is clinically meaningful for sleep studies including clinical trials would enhance the understanding of the key components of sleep quality.41
A recent panel of sleep experts systematically rated which sleep indicators were supported by appropriate evidence for indicating “good” sleep across the lifespan.42 Across all age groups, there was consensus among experts that good sleep quality generally consisted of sleep continuity measures and number of awakenings. Interestingly, panel-rated objective markers of good sleep quality based on objective measures of sleep are not equivalent to self-reported ratings of sleep quality rated by participants.42 Objective markers that correlate with “good” sleep have not been consistently identified, as seen in this review. Careful consideration about measurement of the individual differences that may be involved in one’s evaluation of sleep quality would further clarify the “nonsleep phenomenon” impacting perception of sleep experiences.2,43 This is illustrated in studies which show that other self-report measures of stress,44 depression, and anxiety45 were significantly better predictors of sleep quality than objective sleep measures.
Studies that measured objective sleep variables with actigraphy were not included in this review, because we were primarily interested in sleep architecture. However, several studies comparing actigraphy with self-reported sleep quality found discrepant results similar to those described here with PSG.46,47 The variance in sleep quality has been better explained by self-reported variables from a sleep diary, such as number of awakenings, SOL, and WASO, than by the actigraphy-based sleep measures.48 Sleep duration measures have also been shown to be significantly different between actigraphy, PSG, and sleep diary.49 It is possible that the discrepancy between sleep measures may reflect the methods for objective sleep do not adequately capture sleep quality. This general poor agreement between self-reported sleep quality and objective sleep methods (eg, PSG and actigraphy) indicates a need for more basic research on the brain activity throughout sleep–wake transitions50 in order to improve sleep measurement techniques.
Sleep across the adult lifespan
Five of the 13 studies reviewed were focused on understanding sleep in older adults. More disruption in sleep across the lifespan has been established based on large population studies51,52 and a recent meta-analysis.16 In general, shorter TST and longer SOL and WASO detected by PSG have been associated with older age, as well as more overall variability. The studies that examined large samples of adults across the lifespan found the same PSG-measured variables (SE and WASO) to be the most significant predictors of sleep quality in older age.32,36 These studies also showed that age was a significant predictor of sleep quality (direction of this relationship was not specified). Self-reported sleep quality ratings in older adults have also been shown to be more related to health-related quality of life than PSG measures of sleep.19 These studies suggest that the perception of sleep also shifts with age. In fact, Åkerstedt et al3 found that older women required an average of 85 minutes less TST than younger women to achieve the same rating of sleep quality. This may be explained by a shift in expectations about sleep, namely, a strategy of acceptance of age-related sleep architecture changes.3
Sex differences in sleep
Previous studies have shown that females reported poorer sleep quality than males, despite showing greater TST and shorter SOL and WASO.53 Kaplan et al32 found that females rated sleep quality higher than males directly after they woke up; however, females rated their overall sleep quality from the past month worse than males. This suggests a potential difference in the interpretation of sleep quality between the sexes, which is particularly relevant when considering the higher rates of depression, anxiety, and insomnia in females54 and may further explain discrepant findings due to studies not controlling for sex.
Hartmann et al33 found that females showed fewer A1 phases than males in SWS, despite exhibiting similar macrostructural properties of sleep. Although sex differences in sleep quality ratings were not explored in this study, the sex differences in microstructures as measured with CAP may help elucidate these differences even when objective macrostructural sleep does not. A recent meta-analysis found that CAP was indicated as a possible marker of hormonal and sex differences across age groups.25 Studies examining CAP indices in major depressive disorder have shown differences in CAP rate and cycle in depression, although sex differences were not explored.55,56 The role of CAP in the interaction between sex and psychopathology is promising yet requires further investigation. Future research on sex differences in microstructural sleep parameters and sleep quality are needed.
CONCLUSIONS
Objective markers and perceptions of sleep are not as closely related as one may expect, which is largely due to the idiosyncratic nature of sleep quality, which appears to extend well beyond the minutes spent in a sleep state or interrupted sleep. Since PSG is considered to be the “gold standard” measurement of sleep architecture, understanding the factors that contribute to the discrepancy with perception of sleep experiences is needed in order to improve techniques and clinical utility of sleep physiology. The findings of this review suggest that self-report remains the most consistent way of measuring sleep quality, since it is unclear which markers of objective sleep quality are the most consistent. This inconsistency has been found in “healthy” sleepers, as demonstrated in this review, and discrepancy between perceptions of sleep quality and objective sleep measures may not be a unique feature to sleep disorders. It is therefore not surprising that sleep quality in those with sleep complaints is also not consistently reflected in objective sleep measures (eg, “paradoxical insomnia”).21 This further explains why treating insomnia with cognitive behavioral therapy for insomnia—a gold-standard therapy that targets both sleep behaviors and thoughts—more clearly benefits perceptions of sleep rather than objective sleep measures.57 A better understanding of what healthy sleep looks like in the general population would provide invaluable insight into the assessment of sleep and treatment targets for the broad domain of sleep across many populations. This is necessary to obtain foundational knowledge which may be applied to clinical populations where sleep is disturbed.
Future directions based on this review include further research on defining, standardizing, and validating self-report sleep quality measures, including the definition of a core outcome set. Use of nonlaboratory methods of measuring objective sleep, such as actigraphy, may be useful in understanding the discrepancy between objective and self-reported sleep measures in more naturalistic settings.58 Finally, additional studies on the microstructure of sleep, such as CAP, are needed to clarify its utility as a potential “marker of instability” of sleep that is more correlated with self-reported ratings of sleep.
DISCLOSURE STATEMENT
All authors have read and approved the submitted manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: L.E.C. was involved in conception and design of the study, conducting the literature search and screening, interpreting the relevant literature, and drafting and revised the paper. B.N.F., R.E.M., and S.M.G. were involved in the conception and design of the study, as well as providing critical review of content and revisions of the final manuscript.
ABBREVIATIONS
- CAP
cyclic alternating pattern
- EEG
electroencephalography
- NREM
nonrapid eye movement
- PSG
polysomnography
- REM
rapid eye movement
- SE
sleep efficiency
- SOL
sleep onset latency
- SWS
slow-wave sleep
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1. Irwin MR . Why sleep is important for health: a psychoneuroimmunology perspective . Annu Rev Psychol. 2015. ; 66 ( 1 ): 143 – 172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krystal AD , Edinger JD . Measuring sleep quality . Sleep Med. 2008. ; 9 ( Suppl 1 ): S10 – S17 . [DOI] [PubMed] [Google Scholar]
- 3. Åkerstedt T , Schwarz J , Gruber G , Lindberg E , Theorell-Haglöw J . The relation between polysomnography and subjective sleep and its dependence on age - poor sleep may become good sleep . J Sleep Res. 2016. ; 25 ( 5 ): 565 – 570 . [DOI] [PubMed] [Google Scholar]
- 4. Ramlee F , Sanborn AN , Tang NKY . What sways people’s judgment of sleep quality? A quantitative choice-making study with good and poor sleepers . Sleep. 2017. ; 40 ( 7 ): zsx091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buysse DJ , Reynolds CF III , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 6. Goelema MS , de Bruijn R , Overeem S , Møst E , Haakma R , Markopoulos P . Conceptions of sleep experience: a layman perspective . BMC Res Notes. 2018. ; 11 ( 1 ): 494 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harvey AG , Stinson K , Whitaker KL , Moskovitz D , Virk H . The subjective meaning of sleep quality: a comparison of individuals with and without insomnia . Sleep. 2008. ; 31 ( 3 ): 383 – 393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carney CE , Buysse DJ , Ancoli-Israel S , et al . The consensus sleep diary: standardizing prospective sleep self-monitoring . Sleep. 2012. ; 35 ( 2 ): 287 – 302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monk TH , Buysse DJ , Rose LR , Hall JA , Kupfer DJ . The sleep of healthy people--a diary study . Chronobiol Int. 2000. ; 17 ( 1 ): 49 – 60 . [DOI] [PubMed] [Google Scholar]
- 10. Carskadon MA , Dement WC . Normal human sleep: an overview . In: Kryger M , Roth T , Dement WC , eds. Principles and Practice of Sleep Medicine. Philadelphia: : Elsevier; ; 2017. : 15 – 24 . [Google Scholar]
- 11. Rechtschaffen A , Kales A . A Manual of Standardized Terminology, Techniques and Scoring System of Sleep Stages of Human Subjects. Washington, DC: : US Government Printing Office; ; 1968. . [Google Scholar]
- 12. Iber C , Ancoli-Israel S , Chesson AL , Quan SF ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed . Westchester, IL: : American Academy of Sleep Medicine; ; 2007. . [Google Scholar]
- 13. Adamantidis AR , Gutierrez Herrera C , Gent TC . Oscillating circuitries in the sleeping brain . Nat Rev Neurosci. 2019. ; 20 ( 12 ): 746 – 762 . [DOI] [PubMed] [Google Scholar]
- 14. Schulz H . Rethinking sleep analysis . J Clin Sleep Med. 2008. ; 4 ( 2 ): 99 – 103 . [PMC free article] [PubMed] [Google Scholar]
- 15. Danker-Hopfe H , Anderer P , Zeitlhofer J , et al . Interrater reliability for sleep scoring according to the Rechtschaffen & Kales and the new AASM standard . J Sleep Res. 2009. ; 18 ( 1 ): 74 – 84 . [DOI] [PubMed] [Google Scholar]
- 16. Boulos MI , Jairam T , Kendzerska T , Im J , Mekhael A , Murray BJ . Normal polysomnography parameters in healthy adults: a systematic review and meta-analysis . Lancet Respir Med. 2019. ; 7 ( 6 ): 533 – 543 . [DOI] [PubMed] [Google Scholar]
- 17. Ohayon MM , Carskadon MA , Guilleminault C , Vitiello MV . Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan . Sleep. 2004. ; 27 ( 7 ): 1255 – 1273 . [DOI] [PubMed] [Google Scholar]
- 18. Kuo TB , Li JY , Kuo HK , Chern CM , Yang CC . Differential changes and interactions of autonomic functioning and sleep architecture before and after 50 years of age . Age (Dordr). 2016. ; 38 ( 1 ): 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Driscoll HC , Serody L , Patrick S , et al . Sleeping well, aging well: a descriptive and cross-sectional study of sleep in “successful agers” 75 and older . Am J Geriatr Psychiatry. 2008. ; 16 ( 1 ): 74 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boselli M , Parrino L , Smerieri A , Terzano MG . Effect of age on EEG arousals in normal sleep . Sleep. 1998. ; 21 ( 4 ): 351 – 357 . [PubMed] [Google Scholar]
- 21. Feige B , Baglioni C , Spiegelhalder K , Hirscher V , Nissen C , Riemann D . The microstructure of sleep in primary insomnia: an overview and extension . Int J Psychophysiol. 2013. ; 89 ( 2 ): 171 – 180 . [DOI] [PubMed] [Google Scholar]
- 22. Lewandowski A , Rosipal R , Dorffner G . Extracting more information from EEG recordings for a better description of sleep . Comput Methods Programs Biomed. 2012. ; 108 ( 3 ): 961 – 972 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferri R , Bruni O , Miano S , et al . The time structure of the cyclic alternating pattern during sleep . Sleep. 2006. ; 29 ( 5 ): 693 – 699 . [DOI] [PubMed] [Google Scholar]
- 24. Parrino L , Boselli M , Spaggiari MC , Smerieri A , Terzano MG . Cyclic alternating pattern (CAP) in normal sleep: polysomnographic parameters in different age groups . Electroencephalogr Clin Neurophysiol. 1998. ; 107 ( 6 ): 439 – 450 . [DOI] [PubMed] [Google Scholar]
- 25. Migueis DP , Lopes MC , Ignacio PSD , et al . A systematic review and meta-analysis of the cyclic alternating pattern across the lifespan . Sleep Med. 2021. ; 85 : 25 – 37 . [DOI] [PubMed] [Google Scholar]
- 26. Parrino L , Ferri R , Bruni O , Terzano MG . Cyclic alternating pattern (CAP): the marker of sleep instability . Sleep Med Rev. 2012. ; 16 ( 1 ): 27 – 45 . [DOI] [PubMed] [Google Scholar]
- 27. Liberati A , Altman DG , Tetzlaff J , et al . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration . PLoS Med. 2009. ; 6 ( 7 ): e1000100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moser D , Kloesch G , Fischmeister FP , Bauer H , Zeitlhofer J . Cyclic alternating pattern and sleep quality in healthy subjects--is there a first-night effect on different approaches of sleep quality? Biol Psychol. 2010. ; 83 ( 1 ): 20 – 26 . [DOI] [PubMed] [Google Scholar]
- 29. Rosipal R , Lewandowski A , Dorffner G . In search of objective components for sleep quality indexing in normal sleep . Biol Psychol. 2013. ; 94 ( 1 ): 210 – 220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goelema M , Leufkens T , Heekma R , Markopoulos P . Determinants of self-reported sleep quality in healthy sleepers and patients . Cogent Psychol. 2018. ; 5 : 1499197 . [Google Scholar]
- 31. Klösch G , Kemp B , Penzel T , et al . The SIESTA project polygraphic and clinical database . IEEE Eng Med Biol Mag. 2001. ; 20 ( 3 ): 51 – 57 . [DOI] [PubMed] [Google Scholar]
- 32. Kaplan KA , Hirshman J , Hernandez B , et al .; Osteoporotic Fractures in Men (MrOS), Study of Osteoporotic Fractures SOF Research Groups . When a gold standard isn’t so golden: lack of prediction of subjective sleep quality from sleep polysomnography . Biol Psychol. 2017. ; 123 : 37 – 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hartmann S , Bruni O , Ferri R , Redline S , Baumert M . Characterization of cyclic alternating pattern during sleep in older men and women using large population studies . Sleep. 2020. ; 43 ( 7 ): zsaa016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dean DA 2nd , Goldberger AL , Mueller R , et al . Scaling up scientific discovery in sleep medicine: the National Sleep Research Resource . Sleep. 2016. ; 39 ( 5 ): 1151 – 1164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keklund G , Akerstedt T . Objective components of individual differences in subjective sleep quality . J Sleep Res. 1997. ; 6 ( 4 ): 217 – 220 . [DOI] [PubMed] [Google Scholar]
- 36. Kaplan KA , Hardas PP , Redline S , Zeitzer JM ; Sleep Heart Health Study Research Group . Correlates of sleep quality in midlife and beyond: a machine learning analysis . Sleep Med. 2017. ; 34 : 162 – 167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gabryelska A , Feige B , Riemann D , et al . Can spectral power predict subjective sleep quality in healthy individuals? J Sleep Res. 2019. ; 28 ( 6 ): e12848 . [DOI] [PubMed] [Google Scholar]
- 38. Croy I , Smith MG , Gidlöf-Gunnarsson A , Persson-Waye K . Optimal questions for sleep in epidemiological studies: Comparisons of subjective and objective measures in laboratory and field studies . Behav Sleep Med. 2017. ; 15 ( 6 ): 466 – 482 . [DOI] [PubMed] [Google Scholar]
- 39. Westerlund A , Lagerros YT , Kecklund G , Axelsson J , Åkerstedt T . Relationships between questionnaire ratings of sleep quality and polysomnography in healthy adults . Behav Sleep Med. 2016. ; 14 ( 2 ): 185 – 199 . [DOI] [PubMed] [Google Scholar]
- 40. O’Donnell D , Silva EJ , Münch M , Ronda JM , Wang W , Duffy JF . Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints . J Sleep Res. 2009. ; 18 ( 2 ): 254 – 263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Williamson PR , Altman DG , Blazeby JM , et al . Developing core outcome sets for clinical trials: issues to consider . Trials. 2012. ; 13 ( 1 ): 132 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohayon M , Wickwire EM , Hirshkowitz M , et al . National Sleep Foundation’s sleep quality recommendations: first report . Sleep Health. 2017. ; 3 ( 1 ): 6 – 19 . [DOI] [PubMed] [Google Scholar]
- 43. Jackowska M , Dockray S , Hendrickx H , Steptoe A . Psychosocial factors and sleep efficiency: discrepancies between subjective and objective evaluations of sleep . Psychosom Med. 2011. ; 73 ( 9 ): 810 – 816 . [DOI] [PubMed] [Google Scholar]
- 44. Åkerstedt T , Orsini N , Petersen H , Axelsson J , Lekander M , Kecklund G . Predicting sleep quality from stress and prior sleep--a study of day-to-day covariation across six weeks . Sleep Med. 2012. ; 13 ( 6 ): 674 – 679 . [DOI] [PubMed] [Google Scholar]
- 45. Gould CE , Karna R , Jordan J , et al . Subjective but not objective sleep is associated with subsyndromal anxiety and depression in community-dwelling older adults . Am J Geriatr Psychiatry. 2018. ; 26 ( 7 ): 806 – 811 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akerstedt T , Axelsson J , Lekander M , Orsini N , Kecklund G . The daily variation in sleepiness and its relation to the preceding sleep episode--a prospective study across 42 days of normal living . J Sleep Res. 2013. ; 22 ( 3 ): 258 – 265 . [DOI] [PubMed] [Google Scholar]
- 47. Jackowska M , Ronaldson A , Brown J , Steptoe A . Biological and psychological correlates of self-reported and objective sleep measures . J Psychosom Res. 2016. ; 84 : 52 – 55 . [DOI] [PubMed] [Google Scholar]
- 48. Goelema MS , Regis M , Haakma R , van den Heuvel ER , Markopoulos P , Overeem S . Determinants of perceived sleep quality in normal sleepers . Behav Sleep Med. 2019. ; 17 ( 4 ): 388 – 397 . [DOI] [PubMed] [Google Scholar]
- 49. Matthews KA , Patel SR , Pantesco EJ , et al . Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample . Sleep Health. 2018. ; 4 ( 1 ): 96 – 103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stevner ABA , Vidaurre D , Cabral J , et al . Discovery of key whole-brain transitions and dynamics during human wakefulness and non-REM sleep . Nat Commun. 2019. ; 10 ( 1 ): 1035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mitterling T , Högl B , Schönwald SV , et al . Sleep and respiration in 100 healthy caucasian sleepers–a polysomnographic study according to American Academy of Sleep Medicine Standards . Sleep. 2015. ; 38 ( 6 ): 867 – 875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hertenstein E , Gabryelska A , Spiegelhalder K , et al . Reference data for polysomnography-measured and subjective sleep in healthy adults . J Clin Sleep Med. 2018. ; 14 ( 4 ): 523 – 532 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goel N , Kim H , Lao RP . Gender differences in polysomnographic sleep in young healthy sleepers . Chronobiol Int. 2005. ; 22 ( 5 ): 905 – 915 . [DOI] [PubMed] [Google Scholar]
- 54. Suh S , Cho N , Zhang J . Sex differences in insomnia: from epidemiology and etiology to intervention . Curr Psychiatry Rep. 2018. ; 20 ( 9 ): 69 . [DOI] [PubMed] [Google Scholar]
- 55. Farina B , Della Marca G , Grochocinski VJ , et al . Microstructure of sleep in depressed patients according to the cyclic alternating pattern . J Affect Disord. 2003. ; 77 ( 3 ): 227 – 235 . [DOI] [PubMed] [Google Scholar]
- 56. Lopes MC , Quera-Salva MA , Guilleminault C . Non-REM sleep instability in patients with major depressive disorder: subjective improvement and improvement of non-REM sleep instability with treatment (Agomelatine) . Sleep Med. 2007. ; 9 ( 1 ): 33 – 41 . [DOI] [PubMed] [Google Scholar]
- 57. Mitchell LJ , Bisdounis L , Ballesio A , Omlin X , Kyle SD . The impact of cognitive behavioural therapy for insomnia on objective sleep parameters: a meta-analysis and systematic review . Sleep Med Rev. 2019. ; 47 : 90 – 102 . [DOI] [PubMed] [Google Scholar]
- 58. Van de Water ATM , Holmes A , Hurley DA . Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography--a systematic review . J Sleep Res. 2011. ; 20 ( 1 Pt 2 ): 183 – 200 . [DOI] [PubMed] [Google Scholar]
- 59.Dos Santos Silva M, Bazzana CM, de Souza AL, Ramos LR, Tufik S, Lucchesi LM, Lopes GS. Relationship between perceived sleep and polysomnography in older adult patients. Sleep Sci. 2015;8(2):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]