Abstract
The ammonium ion is an indispensable nitrogen source for crops, especially paddy rice (Oryza sativa L. cv Nipponbare). Until now, it has been impossible to measure ammonium uptake and nitrogen movement in plants in real time. Using the new technologies of PETIS (positron emitting tracer imaging system) and PMPS (positron multi-probe system), we were able to visualize the real time translocation of nitrogen and water in rice plants. We used positron-emitting 13N-labeled ammonium (13NH4+) and 15O-water to monitor the movement. In plants cultured under normal conditions, 13NH4+ supplied to roots was taken up, and a 13N signal was detected at the discrimination center, the basal part of the shoots, within 2 minutes. This rapid translocation of 13N was almost completely inhibited by a glutamine synthetase inhibitor, methionine sulfoximine. In general, nitrogen deficiency enhanced 13N translocation to the discrimination center. In the dark, 13N translocation to the discrimination center was suppressed to 40% of control levels, whereas 15O-water flow from the root to the discrimination center stopped completely in the dark. In abscisic acid-treated rice, 13N translocation to the discrimination center was doubled, whereas translocation to leaves decreased to 40% of control levels. Pretreatment with NO3− for 36 hours increased 13N translocation from the roots to the discrimination center to 5 times of control levels. These results suggest that ammonium assimilation (from the roots to the discrimination center) depends passively on water flow, but actively on NH4+-transporter(s) or glutamine synthetase(s).
More than 70% of the world's rice (Oryza sativa) is produced in intensively cultivated, irrigated lowland fields in Asia. In flooded lowland rice fields, the bulk of the soil is hypoxic or anaerobic, and the major form of nitrogen available to plants is NH4+. This is in marked contrast to most (aerobic) agricultural soils in which NO3− is the predominant inorganic nitrogen species. NH4+ is the preferred nitrogen species taken up by rice; it is superior to NO3− in terms of fertilizer efficiency in paddy fields (Yoshida, 1981).
Radioisotopes and stable isotopes are often used to study the uptake and translocation of nutrients in plants. Since nitrogen is the main nutrient of plants, many plant physiologists have used 15N, which is a stable isotope, to elucidate the biochemical processes in rice root cells (Yoneyama and Kumazawa, 1974a; Arima and Kumazawa, 1975). They proved that the formation of the amide-nitrogen of Gln is the primary process in the fixation of ammonium absorbed from rice roots using (15NH4)2SO4 as the sole nitrogen source. Preparing samples for 15N analysis is a very tedious process, and it is impossible to detect the excess percentage of 15N in plants in real time. To overcome these shortcomings, 13N has been adopted in plant physiology research (Glass et al., 1985; Ingemarsson et al., 1987). 13N is a positron-emitting nuclide. When a positron decays, it emits two γ-rays in opposite directions. Several researchers have used 13NH4+ and 13NO3− in plant nutrition research (Presland and McNaughton, 1986; Wang et al., 1993a, 1993b; Kronzucker et al., 1995a, 1995b, 1995c) and detected the positrons using a liquid scintillation counter. However, this is not real time analysis. Hamamatsu Photonics of Japan and the TIARA (Takasaki Ion Accelerators for Advanced Application) group recently developed a dynamic image measurement system called “PETIS” (Positron Emitting Tracer Imaging System). This system detects the γ-rays produced by positron-emitting nuclides with a scintillation camera and enables study of the movement of elements in plants in real time (Kume et al., 1997; Hayashi et al., 1998; Uchida et al., 1998; Matsunami et al., 1999; Nakanishi et al., 1999; Sato et al., 1999; Mori et al., 2000). In this study, we produced 13NH4+ and 15O-water in a cyclotron and compared 13N translocation with 15O-water flow (mass flow) in rice supplied with 13NH4+ under different conditions in real time using PETIS and PMPS (positron multi-probe system).
RESULTS
Absorption and Translocation of 13NH4+ from Roots in Control Rice Plants
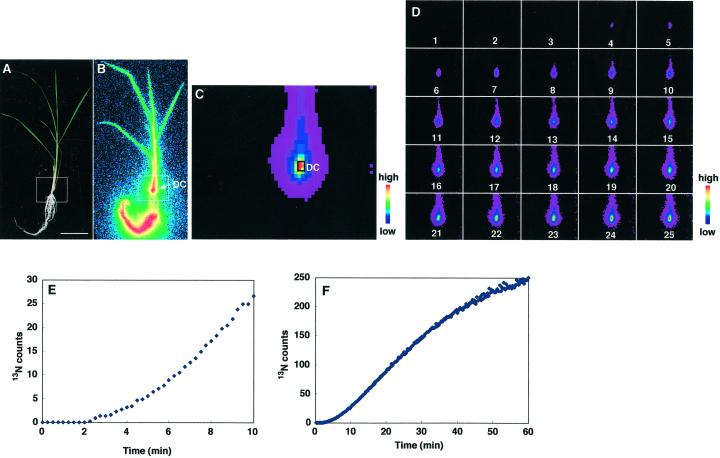
In control rice plants, 13NH4+ was absorbed from the roots and 13N was translocated to all parts of the plant within 60 min (Fig. 1B). BAS images showed that the discrimination center (DC), the basal part of the shoots, was strongly labeled (Fig. 1, B and C). The youngest leaf was more strongly labeled than older leaves (Fig. 1B). PETIS detectors were focused on the DC (Fig. 1A), and real time 13N translocation was monitored (Fig. 1, D–F). The image of the DC appeared 4 min after supplying 13NH4+ (Fig. 1D), and then images of the shoot appeared in the subsequent 3 to 4 min. The translocation curve made from the PETIS images revealed that the first 13N arrived at the DC 2 min after the beginning of absorption (Fig. 1E), and the amount increased with time throughout the experiment (Fig. 1F).
Figure 1.
13N translocation from roots to the DC of control rice supplied with 13NH4+. A, Gross image of a control rice plant. B, Image of 13N translocation using BAS-1500. Scale bar = 4 cm. C, Accumulative PETIS image after a 60-min analysis. D, The time course for the accumulation of radioactivity in the white squares in A and B was followed over time by PETIS. The images shown are for 1-min intervals and the data were scored every 15 s. E, Curve showing the accumulation of radioactivity in the DC after 10 (C) and 60 (F) min by PETIS.
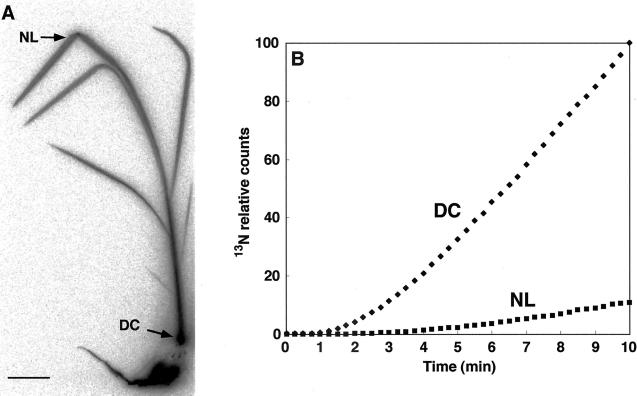
When the PMPS detectors were focused on the DC (Fig. 2A) and on the part of the newest leaf (NL in Fig. 2A) that was 34.3 cm above the DC of control plants, radioactivity was detected in the DC and NL 2 and 6 min, respectively, after 13NH4+ supply to the roots (Fig. 2B). Therefore, the velocity of transport of 13N to the newest leaf from the DC was 8.6 cm min−1 [=34.3 cm/(6 − 2 min)].
Figure 2.
13NH4+ translocation from roots to the DC of control rice. A, BAS image of 13N. The arrows at the NL (newest leaf) and DC indicate where the radioactivity was traced with PMPS. B, Time course for radioactivity accumulation in the NL and DC.
When 13NH4+ is absorbed from roots, the culture solution, and roots must be completely shielded with lead blocks to avoid direct irradiation of the PETIS camera or PMPS probes with positrons, otherwise, the high background radioactivity would affect the PETIS image or PMPS data. Therefore, it is impossible to observe the direct progression of 13NH4+ activity from outside to inside the roots using these methods.
13NH4+ Translocation from the Second Newest Leaf to the DC
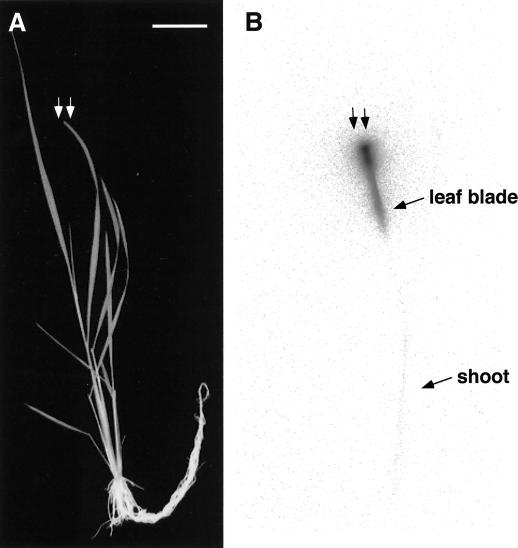
To study 13NH4+ translocation from the leaf, 13NH4+ was supplied to the cut end of the second newest leaf (SNL) of a control plant (Fig. 3, two arrows). Only a few 13N counts were detected at the DC (data not shown). The BAS image showed that a large amount of 13N remained halfway along the leaf blade, and a very small amount of 13N moved to the shoot (Fig. 3). 13NH4+ translocation from the cut leaf tip was also examined using 3-d nitrogen-deficient rice. Translocation was similar to that of controls (data not shown).
Figure 3.
Poor translocation of 13NH4+ from the cut part of the SNL in a control plant to other parts of the plant. A, Gross image of a plant. B, Image of 13N translocation using BAS-1500. 13NH4+ was absorbed from the SNL of a control plant (arrows) for 120 min. The rice plant was cultured in complete nutrient solution.
Effect of Met Sulfoximine (MSX)
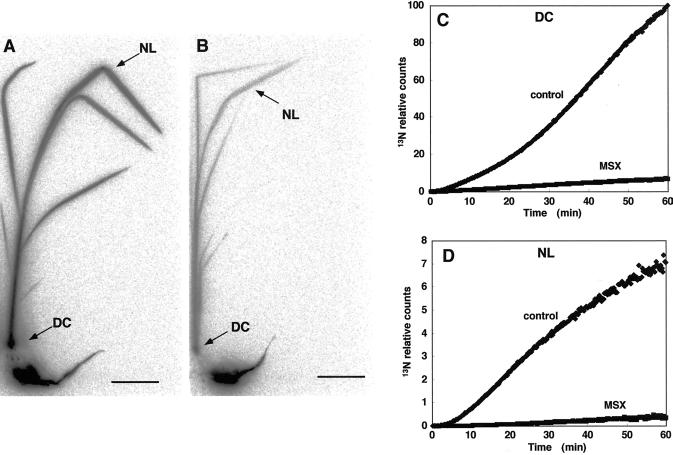
The amount of 13N in the DC in Met sulfoximine (MSX)-treated rice decreased drastically to 5% of control levels after 60 min of 13NH4+ supply (Fig. 4C). In the newest leaf, MSX also depressed 13N translocation to 5% of control levels after 60 min (Fig. 4D). The BAS images showed that the 13N signals of all the leaves and the DC in MSX-treated rice were much weaker than those of control rice (Fig. 4, A and B).
Figure 4.
Severe suppression of 13NH4+ translocation from rice roots to the DC with MSX treatment. Image of the translocation of 13N in control (A) and MSX-treated (B) rice plants using BAS-1500. Scale bar = 4 cm. Curves showing the accumulation of radioactivity in the DC (C) and newest leaf (D) using PMPS in two plants.
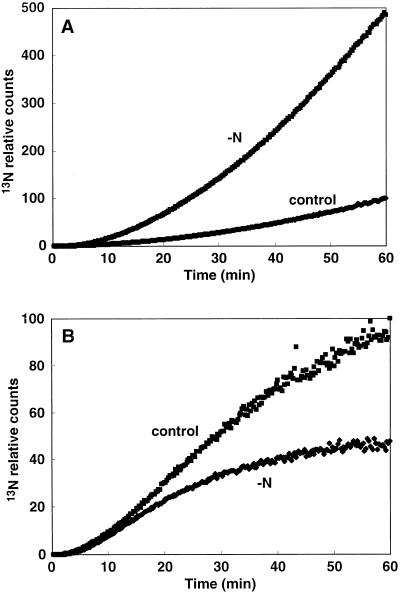
Effect of Nitrogen Deficiency
When 13NH4+ was supplied to the roots of nitrogen-deficient rice cultured under 500 μmol m−2 s−1, the amount of 13N in the DC increased to 5 times control levels after 60 min of 13NH4+ supply (Fig. 5A). However, it declined to 50% of control levels under pretreatment of stronger illumination (1,500 μmol m−2 s−1; Fig. 5B).
Figure 5.
Enhanced or depressed translocation of 13NH4+ from rice roots to the DC of 3-d nitrogen deficiency pretreated rice under natural light. Time course study of the translocation of 13NH4+ to the DC from roots of rice cultured under light intensities of 500 μmol m−2 s−1 (A) and 1,500 μmol m−2 s−1 (B), traced by PETIS.
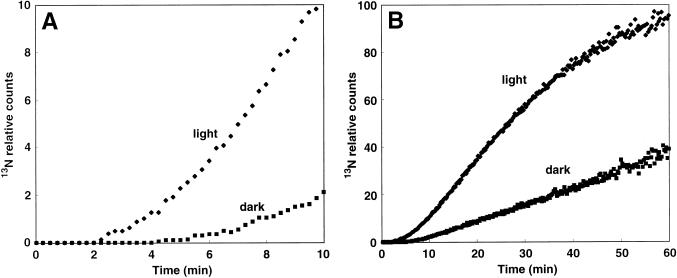
Effect of the Dark
In the dark, the initial 13N translocation from the roots to the DC occurred 5 min after 13NH4+ supply, 3 min later than in controls (Fig. 6A), and the radioactivity was reduced to 40% of control levels after 60 min of absorption (Fig. 6B).
Figure 6.
Suppression of the translocation of 13NH4+ from the roots to the DC of rice in the dark. Time course study of the translocation of 13N into the DC from the roots. Accumulation of radioactivity within 10 (A) and 60 (B) min.
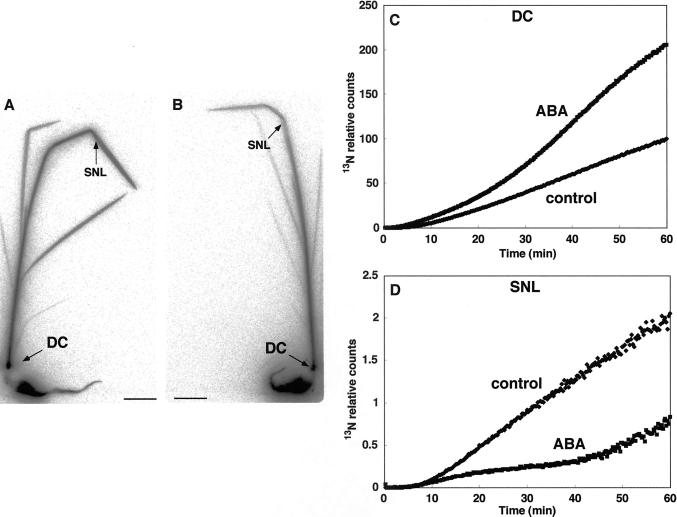
Abscisic Acid Treatment
The amount of 13N in the DC in abscisic acid (ABA)-treated rice was double that in control rice after 60 min of 13NH4+ supply (Fig. 7C), although there was no delay in the initial detection. However, 13N translocation to the SNL in ABA-treated rice was depressed to 40% of control levels after 60 min (Fig. 7D). BAS images showed that 13N signals in all the leaves of the ABA-treated rice were weaker than those of controls (Fig. 7, A and B).
Figure 7.
13NH4+ translocation from roots to the DC and to the SNL in rice with ABA treatment. Image of the translocation of 13N in control (A) and ABA-treated (B) rice plants using BAS-1500. The accumulation of radioactivity in the DC (C) and the SNL (D) over 60 min.
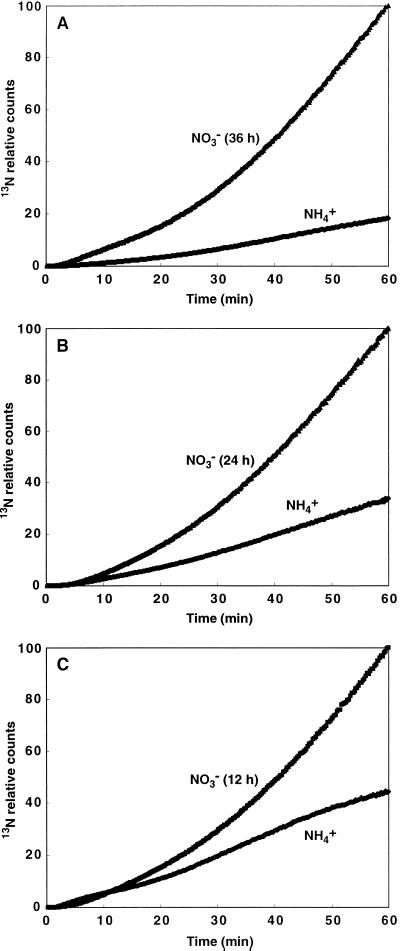
Pretreatment with NO3−
13N translocation to the DC in NO3− pretreated rice for 36, 24, and 12 h were 5, 3, and 2.3 times higher than in controls, respectively, after 60 min of 13NH4+ supply (Fig. 8).
Figure 8.
Enhancement of 13NH4+ translocation from roots to the DC of rice with NO3− pretreatment for 36, 24, and 12 h.
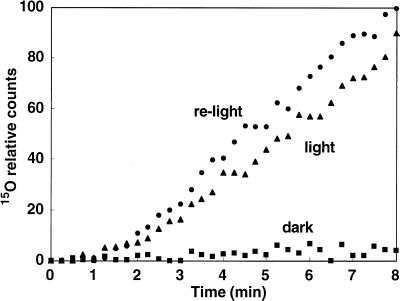
Effect of Light on 15O-Water Flow in Plants
In the dark (pretreatment for 30 min in dark conditions) 15O-water flow from the roots to the DC stopped completely (Fig. 9, dark and light). After 60 min of illumination following 60 min of darkness, the 15O-water flow rate from the roots to the DC recovered completely (Fig. 9, relight).
Figure 9.
Water flow from the roots to the DC. Conditions were changed sequentially from light → dark → light. In the dark, water flow was stopped completely.
DISCUSSION
Normal Translocation Image of 13N Supplied with 13NH4+ in Rice
In rice, 13NH4+ was absorbed by the roots and 13N was translocated to all parts of the plant within 60 min. BAS images showed that 13N-labeled younger leaves more strongly than older leaves, which indicates that younger leaves are stronger nitrogen sinks than older ones (Figs. 1B and 2A) (Yoneyama and Kumazawa, 1974b; Mori, 1998). In all cases, 13N accumulated in the DC. The absorption curve made from the PETIS analysis revealed that 13N reached the DC 2 min after 13NH4+ supply (Figs. 1E and 2B) and the tip of the newest leaf after 6 min (Fig. 2B). It took 4 min for 13N to move from the DC to the tip of the newest leaf.
The PETIS analysis showed that only negligible amounts of 13N supplied to leaves were detected in the DC (data not shown). This was also confirmed by the BAS image after 120 min of NH4+ supply, which showed that a large amount of 13N still remained halfway along the leaf blade, and that a very small amount of 13N was translocated to the shoot of the leaf (Fig. 3). In our study 13NH4+ was supplied to the SNL, which was a sink for nitrogen flow. Rice Gln synthetase, GS2, is thought to assimilate any NH4+ evolved from photorespiration in the leaf (Redinbaugh and Campbell, 1993). Hence, 13NH4+ supplied to the leaf might be immediately assimilated by leaf GS2 and metabolized to other nitrogen compounds through the GS/GOGAT cycle (Sechley et al., 1992). This nitrogen does not leave the cells to be translocated because this leaf is a sink. No NH4+ was found in phloem sap collected from the young rice leaf (sink) using the insect laser technique (Hayashi and Chino, 1985). On the other hand, rice cytosolic GS1 functions in the bundle sheath cells in the senescence leaf blade, but little GS1 activity was detected in the sink leaf (Kamachi et al., 1992). This explains the Gln transport from the old (senescence) leaf to the young (sink) leaf (Mae et al., 1983). Therefore, in young rice leaves either 13NH4+ assimilation to Gln by GS1 and incorporation of the 13N-labeled Gln into the phloem does not occur, or direct incorporation of 13NH4+ into the phloem is very weak (Fig. 3).
The DC, a Crucial Site for Material Transport in Graminaceous Plants
We recently reported the role of the DC in Fe and Met transport in barley using 59Fe(III)-epihydroxymugineic acid and [11C]Met, respectively (Mori, 1998; Nakanishi et al., 1999). When 59Fe or [11C]Met was supplied to roots or leaves, the DC was strongly labeled. 59Fe and [11C]Met subsequently were distributed to other parts of the plant. Therefore, this part of the plant seems to play a crucial role in the translocation of minerals and metabolites in graminaceous plants and has been named the “discrimination center.” In barley, [11C]Met was translocated from the SNL tip to the DC at a velocity of 2 cm min−1. In Fe-deficient barley, new chlorotic leaves were a strong Met sink, and leaf-to-leaf transfer through the DC occurred very rapidly (Nakanishi et al., 1999). 59Fe-epihydroxymugineic acid supplied to cut barley leaves was also translocated through the DC to other new chlorotic leaves and to the root tips within 45 min as detected by radioautography (Mori, 1998). Photosynthetic 11CO2 from an old leaf was translocated throughout the DC to the ears within 45 min at a velocity of 0.9 cm min−1 (Matsuhashi et al., 1998). Therefore, the translocation rate of the substrate depends on the substrate itself, the nutritional status of the plant, the plant's age, etc. Because the DC is also an important site controlling N translocation and partitioning as mentioned above, the structural characterization of the tissues involved using cytological methods should be considered in the future.
MSX Treatment
13N translocation from the roots to the DC and to the newest leaf decreased drastically in MSX-treated rice (Fig. 4, C and D). BAS images also showed that 13N translocation to the DC and to all leaves was suppressed by MSX treatment (Fig. 4, A and B). As we show in Figure 9, for 15O-water, the time required for radiation to travel from the roots to the DC under light conditions is approximately 1 min. Because there is no physical barrier in the xylem flow, the barriers to radial transport are the main limiting factor for the passage of each mineral element from outside the root cells to the DC. These factors include cell membranes transporters (or channels), plasmodesmata, Casparian strip, and so forth. In case of MSX treated rice, 13N-radioactivity appeared at the DC 10 min after supplying 13NH4+ under light conditions (MSX in Fig. 4C), whereas 13N-radioactivity appeared after 2 min in the control rice (control in Fig. 4C or Fig. 1E). Therefore, a delay up to 8 min occurs in the process of radial transport. This strongly suggests that the conversion of 13NH4+ to 13N-gluatamine by GS1 in the cytoplasm of root cells for xylem loading is the essential for process for 13NH4+ transport in rice.
Pretreatment with MSX is reported to completely inhibit Gln synthetase activity in rice roots (Kronzucker et al., 1998). Although data were not shown, it was also mentioned that the long distance translocation of 13NH4+ was markedly inhibited by MSX in rice (Kronzucker et al., 1998). In addition, the major nitrogen solute in the xylem of rice is Gln and not NH4+ (Fukumorita and Chino, 1982). Therefore, the 13N translocation that we observed in control rice reflects the amount of 13N-Gln synthesized by Gln synthetase (GS1) in roots after passage through an NH4+ transporter in the roots.
Nitrogen Deficiency Treatment
When 13NH4+ was supplied to the roots of 3-d nitrogen-deficient rice that had been cultured under 500 μmol m−2 s−1, the rate of 13N translocation to the DC was enhanced (Fig. 5A). It has been reported that nitrogen starvation increases 13NH4+ influx in the roots of rice (Wang et al., 1993b; Kronzucker et al., 1998), and that it induces one of the NH4+ transporter genes (AtAMT1) in the roots of Arabidopsis (Gazzarrini et al., 1999; Rawat et al., 1999) and tomato (LeAMT1:1) (von Wirén et al., 2000). Our results concur with these results. A rice NH4+ transporter gene is presumably induced by nitrogen deficiency; however, rice NH4+ transporter gene, OsAMT1–1, has not been characterized yet (von Wirén et al., 1997). In contrast, under natural light intensity (1,500 μmol m−2 s−1), nitrogen deficiency depressed 13N translocation to the DC (Fig. 5B). In this study, higher photosynthetic activity presumably caused severe nitrogen deficiency in the tops of plants very soon after the nitrogen deficiency treatment. This in turn resulted in the decreased translocation of an energy source (ATP) or carbon substrate (i.e. Suc, Glu, etc.) from the tops to the roots. These sequential processes might suppress 13N translocation to the DC. Long distance nitrogen translocation is reported to be influenced by the availability of carbon skeletons (Kronzucker et al., 1998).
Effect of Darkness
In the dark, 13N translocation to the DC decreased, but it was not completely stopped (Fig. 6, A and B). When translocation of 15O-water in rice was traced by the PETIS method, the flow of 15O-water from the roots to the DC was completely stopped by 30-min dark pretreatment (Fig. 9). Therefore, in rice, the dark may cause stomata closure, reducing the rate of flow of water in the xylem, resulting in low xylem loading. These sequential events might lead to the decrease in 13N translocation from the DC to the top and cause the delay in the initial detection of 13N from the roots at the DC. In tomato leaves, expression of the NH4+ transporters LeAMT1;2 and LeAMT1;3 showed reciprocal diurnal regulation with the highest transcription of LeAMT1;3 in the dark and the highest levels of LeAMT1;2 after the onset of illumination (von Wirén et al., 2000). In Arabidopsis, three NH4+ transporter genes (AtAMT1;1, AtAMT1;2, and AtAMT1;3) showed diurnal variation in expression. Of these, AtAMT1;3 transcript levels peaked with ammonium uptake at the end of the light period, suggesting that AtAMT1;3 links nitrogen assimilation and carbon provision in roots (Gazzarrini et al., 1999). Therefore, it is reasonable to assume that NH4+ absorption is not completely stopped in the dark, since some NH4+ transporter genes might be expressed diurnally, even if water flow in the xylem is stopped in the dark (Fig. 9). It is still unknown whether there is diurnally regulated expression of an NH4+-transporter in rice roots.
ABA Effect
ABA is a plant hormone that affects stomata closure. Therefore, we predicted that 13N movement would be reduced by lower water flow in the xylem, as occurs in the dark. In fact, ABA decreased 13N transport to the SNL (Fig. 7D); the BAS images also showed that 13N translocation to leaves was depressed in ABA-treated rice (Fig. 7, A and B). Unexpectedly, however, with ABA treatment the 13N accumulation observed at the DC was greater than in control rice (Fig. 7C). This was quite different from the results of the dark treatment, suggesting that ABA not only closes the stomata, but also stimulates ammonium assimilation. Treatment with ABA (1 or 10 mm) is reported to increase the activity of Gln synthetase in maize roots and shoots (Sengar and Srivastava, 1995). No direct or indirect effects of ABA on NH4+ transporters have been reported. Presumably, enhanced assimilation of 13NH4+ to Gln by ABA is the major reason for the enhanced NH4+ translocation to the DC from the roots.
NO3− Effect
NO3− pretreatment for 36, 24, and 12 h enhanced 13N translocation to the DC (Fig. 8). In radish (Ota and Yamamoto, 1989), Arabidopsis (Gazzarrini et al., 1999), and rice (Kronzucker et al., 1998, 1999) simultaneous application of nitrate and ammonium enhanced NH4+ assimilation and translocation to shoots. Our result also strongly suggests that nitrate regulates ammonium assimilation by rice roots, perhaps via enhanced expression of either NH4+-transporter or Gln synthetase, GS1, genes (Li et al., 1993; Cren and Hirel, 1999).
Summarizing results, NH4+ assimilation from the roots to the DC in rice depends passively on water flow, and actively on NH4+ transporter(s) or Gln synthetase(s) activity in the roots. Some of these genes may be regulated by NO3−, nitrogen deficiency, ABA, or diurnally. Cloning of these genes in rice is awaited.
For a time course study, using a scintillation counter to monitor 13N requires the preparation of many plants of the same age (Presland and McNaughton, 1986) as to stop the enzyme reactions plants must be killed at each sampling time. In contrast, PETIS enables visualization of the movement of labeled substances in a single intact plant body in real time, reproducibly. TIARA now produces nine positron-emitting nuclides for biological studies: 11C, 13N, 15O, 18F, 22Na, 48V, 52Mn, 52Fe, and 62Zn. Many transporter genes for heavy metal ions (Mori, 1999; Guerinot, 2000), potassium (Schachtman, 2000), sugars (Lemoine, 2000), phosphate (Raghothama, 2000), sulfate (Saito, 2000), and amino acids (Fischer et al., 1998; Ortiz-Lopez et al., 2000) recently have been isolated, and various transgenic plants harboring such genes will be developed in the future. Positron-emitting nuclide studies of such transgenic plants using the PETIS method will provide novel dynamic knowledge about the movement of nutrients and metabolites in plants in real time under various nutrient and environmental stresses. In other words, it will be easy to visualize where in a plant body some transporter gene is not functioning by using a transgenic plant that is defective in the transporter gene and vice versa.
MATERIALS AND METHODS
13NH4+ Synthesis
The radiotracer 13N (half-life = 9.96 min) was produced in the cyclotron (Sumitomo Cypris-HM, Japan) at Hamamatsu Photonics (Hamamatsu, Japan) by proton irradiation of water. This procedure produces mostly 13NO3− with high radiochemical purity (Kronzucker et al., 1995a). The irradiated solutions were supplied in sealed 20-mL glass vials. The procedures used to remove the radiocontaminants and convert 13NO3− to 13NH4+ using Devard's alloy have been described in detail elsewhere (Kronzucker et al., 1995a, 1995b, 1995c).
15O Water Synthesis
15O was produced by the 14N(d, n)15O reaction in a nitrogen gas target. The target gas contained 0.5% (v/v) oxygen as carrier and was kept in continuous flow at rates of 500 mL min−1 and a pressure of 3 kg cm−2. The gas in the target chamber was irradiated with 10 MeV deuteron beams at a current of 15 μA using the Sumitomo Cypris-HM cyclotron, and then transferred into an automated radio-synthesizing system supplied by Sumitomo Heavy Industries Ltd. The system purifies 15O2 using an Ascarite column to remove 15O-CO2 from the 15O-labeled gases. Then, 15O2 is converted into 15O-water in the form of vapor by the platinum-catalyzed reaction of 15O2 with hydrogen at 150°C. 15O-water is finally recovered from the vapor by passage of the vapor through distilled water. Almost 3 GBq of 15O-water could be produced from a 4-min irradiation.
Plant Materials and Growth Conditions
Rice (Oryza sativa L. cv Nipponbare) seeds were germinated at room temperature on paper towels soaked with distilled water. After germination, plantlets were transferred to a plastic net floating on tap water, pH 5.5, in a greenhouse under natural light. After 3 weeks, plants were transferred to nutrient solution consisting of 1 mm (NH4)2SO4, 0.3 mm NaH2PO4, 0.7 mm K2SO4, 2.0 mm CaCl2, 0.5 mm MgSO4, 10 μm H3BO3, 0.5 μm MnSO4, 0.2 μm CuSO4, 0.5 μm ZnSO4, 0.01 μm (NH4)6Mo7O24, and 0.1 mm Fe-EDTA. The nutrient solution was changed every week.
The 13NH4+ absorption experiments were carried out when the plants had one to three tillers and there were four to six leaves on the main shoots. (a) For nitrogen-deficiency treatment, plants were cultured under a light intensity of 500 μmol m−2 s−1 in a growth chamber under artificial light, or under 1,500 μmol m−2 s−1 in a greenhouse under natural light, and then transferred to culture solution without NH4+ for 3 d. In all cases, plants grown with complete nutrient solution under the same light conditions were used as controls. (b) For NO3− pretreatment, NH4+-fed plants were cultured for 36 h with NO3− as the sole nitrogen source in nutrient solution consisting of 2.0 mm Ca(NO3)2, 0.7 mm K2SO4, 0.1 mm KCl, 0.1 mm KH2PO4, 0.5 mm MgSO4, 0.1 mm Fe(III)-EDTA, 10 μm H3BO3, 0.5 μm MnSO4, 0.2 μm CuSO4, 0.5 μm ZnSO4, and 0.01 μm (NH4)6Mo7O24.
Absorption and Translocation of 13NH4+ in Plants
To study 13NH4+ absorption from roots, the roots of a single plant were placed in a polyethylene bag that contained 15 mL of culture solution without NH4+. To maintain geometry, the plant and bag were placed between two acrylic boards centered between the PETIS detectors. 13NH4+ (100–500 MBq, carrier-free in 1 mL) was added to the culture solution after synthesis with gentle aeration for immediate mixing. The light intensity was 500 μmol m−2 s−1 unless otherwise described. The PETIS detectors (the detection area was 50 × 60 mm) were focused on the DC at the basal part of the shoot (Nakanishi et al., 1999) or on the leaves. The γ-rays emitted from decaying positrons from 13N were counted over time using the coincident method with the paired detectors. The data were automatically corrected using 9.96 min as the half-life of 13N. After a 60-min trace analysis, the plant was removed from the polyethylene bag and the roots were gently washed for 1 min in 100 mL of 5× complete culture solution containing NH4+. Then the plant was placed inside the cassette of a BAS-imaging plate for 10 to 20 min. This is very sensitive to positrons and produced a clear radioautograph using the BAS-1500 Imaging System (Fuji Photo Film, Tokyo). In some cases, part of a leaf or the DC was placed between two PMPS probes to directly count the paired γ-rays from decaying positrons in the tissues using the coincidence method (Uchida et al., 1998). The spatial position of PMPS probe should be such that it escapes direct irradiation by 13NH4+ solution. For this reason the longest leaf (the newest leaf or the SNL) was selected as detection point of 13N translocation from roots.
To study 13NH4+ absorption from the leaf, we also used the SNL (not the newest leaf), because this leaf was the longest one. The leaf was cut at the tip in distilled water to avoid the intrusion of air into the exposed leaf tissues. The cut end of the leaf was dipped in 3 mL of culture solution (5× culture solution without NH4+) in the vial and 13NH4+ (1 GBq, carrier-free in 1 mL) was added. This vial was shielded with lead blocks to protect the probes of the PETIS camera from direct irradiation positrons from the 13NH4+ solution.
Dark (2-h pretreatment), nitrogen deficient (3-d pretreatment), and 1 mm MSX (30-min pretreatment), NO3− (36-, 24-, and 12-h pretreatment), and 0.1 mm ABA (30 min pretreatment) treatments were used to evaluate their effects on 13NH4+ assimilation/translocation.
15O Water Flow Experiments
15O-water (1 mL = 0.5 GBq) was supplied to 15 mL of 5× culture solution. The PETIS detectors were focused on the DC and the time course followed under illumination (500 μmol m−2 s−1) for 15 min. After 60 min in darkness, additional 15O-water (0.5 GBq) was supplied and the DC was monitored for 30 min in darkness. Then, after an additional 60 min of illumination, more 15O-water was supplied and the DC was monitored for 15 min with illumination (500 μmol m−2 s−1). The data were automatically corrected using 2.04 min as the half-life of 15O.
In all 13NH4+ and 15O-water experiments, each experiment was repeated at least three times to confirm the reproducibility of the results.
ACKNOWLEDGMENTS
The Research Center for Nuclear Science and Technology, The University of Tokyo, is duly acknowledged. We are grateful to Dr. N.K. Nishizawa for critical reading of the manuscript.
Footnotes
This work was supported by the Universities and Japan Atomic Energy Research Institute Joint Research Project.
LITERATURE CITED
- Arima Y, Kumazawa K. A kinetic study of amide and amino acid synthesis in rice seedling roots fed with 15N-labeled ammonium: physiological significance of glutamine on nitrogen absorption and assimilation in plants (part 2) J Soil Sci Manure Jpn. 1975;46:355–361. [Google Scholar]
- Cren M, Hirel B. Glutamine synthetase in higher plants: regulation of gene and protein expression from the organ to cell. Plant Cell Physiol. 1999;40:1187–1193. [Google Scholar]
- Fischer WN, Andre B, Rentsch D, Krolkiewics S, Tegeder M, Britkeuz K, Frommer WB. Amino acid transport in plants. Trends Plant Sci. 1998;3:188–195. [Google Scholar]
- Fukumorita T, Chino M. Sugar, amino acid, and inorganic contents in rice phloem sap. Plant Cell Physiol. 1982;23:273–283. [Google Scholar]
- Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, von Wirén N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell. 1999;11:937–947. doi: 10.1105/tpc.11.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass APM, Thompson RG, Bordeleau L. Regulation of NO3− influx in barley: studies using 13NO3−. Plant Physiol. 1985;77:379–381. doi: 10.1104/pp.77.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. The ZIP family of metal transporters. Biochim Biochem Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Chino M. Nitrate and other anions in the rice phloem sap. Plant Cell Physiol. 1985;26:325–330. doi: 10.1093/oxfordjournals.pcp.a029451. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Okada Y, Mano M, Kume T, Matsuhashi S, Ishioka N-S, Uchida H, Chino M. Detection and characterization of nitrate circulation through the sieve tubes and xylem vessels of rice plants. Plant Soil. 1998;196:233–237. [Google Scholar]
- Ingemarsson B, Oscarson P, aff Ugglas M, Larsson C-M. Nitrogen utilization in Lemna: II. Studies of nitrate uptake using 13NO3−. Plant Physiol. 1987;85:860–864. doi: 10.1104/pp.85.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi K, Yamaya T, Mae T, Ojima K. Vascular bundle-specific localization of cytosolic glutamine synthetase in rice leaves. Plant Physiol. 1992;99:1481–1486. doi: 10.1104/pp.99.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Schjoerring JK, Erner Y, Kirk GJD, Siddiqi MJ, Glass ADM. Dynamic interactions between root NH4+ influx and long-distance N translocation in rice: insights into feedback processes. Plant Cell Physiol. 1998;39:1287–1293. [Google Scholar]
- Kronzucker HJ, Siddiqi MJ, Glass ADM. Compartmentation and flux characteristics of nitrate in spruce. Planta. 1995a;196:674–682. [Google Scholar]
- Kronzucker HJ, Siddiqi MJ, Glass ADM. Nitrate induction in spruce: an approach using compartmental analysis. Planta. 1995b;196:683–690. [Google Scholar]
- Kronzucker HJ, Siddiqi MJ, Glass ADM. Compartmentation and flux characteristics of ammonium in spruce. Planta. 1995c;196:691–698. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM, Kirk GJD. Nitrate-ammonium synergism in rice: a subcellular flux analysis. Plant Physiol. 1999;119:1041–1045. doi: 10.1104/pp.119.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T, Matsuhashi S, Shimazu M, Ito H, Fujimura T, Adachi K, Uchida H, Shigeta N, Matsuoka H, Osa A. Uptake and transport of positron-emitting tracer (18F) in plants. Appl Radiot Isot. 1997;48:1035–1043. [Google Scholar]
- Lemoine R. Sucrose transporters in plants: update on function and structure. Biochem Biophys Acta. 2000;1465:246–262. doi: 10.1016/s0005-2736(00)00142-5. [DOI] [PubMed] [Google Scholar]
- Li MG, Villemur R, Hussey PJ, Silflow CD, Gantt JS, Snustad DP. Differential expression of six glutamine synthetase genes in Zea mays. Plant Mol Biol. 1993;23:401–407. doi: 10.1007/BF00029015. [DOI] [PubMed] [Google Scholar]
- Mae T, Makino A, Ohhira K. Changings in the amounts of ribulose bisphosphate carboxylase synthesized and degraded during the life span of rice leaves. Plant Cell Physiol. 1983;24:1079–1084. [Google Scholar]
- Matsuhashi S, Ito T, Kume T, Ishioka NS, Watanabe S, Osa A, Sekine T, Uchida H, Tsuji A. TIARA Annual Report 1997. Ibaraki, Japan: Japan Atomic Energy Research Institute; 1998. Application of positron emitting tracers for the study of living-plant functions: effect of environmental condition on the uptake of 11CO2 and translocation of photosynthetic products; pp. 44–46. [Google Scholar]
- Matsunami H, Arima Y, Watanabe K, Ishioka NS, Watanabe S, Osa A, Sekine T, Uchida H, Tsuji A, Matsuhashi S. 13N-nitrate uptake sites and rhizobium-infectible region in a single root of common bean and soybean. Soil Sci Plant Nutr. 1999;45:955–962. [Google Scholar]
- Mori S. Iron transport in graminaceous plants. In: Sigel A, Sigel H, editors. Iron Transport and Storage in Microorganisms, Plant and Animals. 35, Metal Ions in Biological Systems. New York: Marcel Dekker; 1998. pp. 215–237. [Google Scholar]
- Mori S. Iron acquisition by plants. Curr Opin Plant Biol. 1999;2:250–253. doi: 10.1016/S1369-5266(99)80043-0. [DOI] [PubMed] [Google Scholar]
- Mori S, Kiyomiya S, Nakanishi H, Ishioka NS, Watanabe S, Osa A, Matsuhashi S, Hashimoto S, Sekine T, Uchida H. Utilization of 15O-water flow in tomato and rice in the light and dark using a positron-emitting tracer imaging system (PETIS) Soil Sci Plant Nutr. 2000;46:975–979. [Google Scholar]
- Nakanishi H, Bughio N, Matsuhashi S, Ishioka N-S, Uchida H, Tsuji A, Osa A, Sekine T, Kume T, Mori S. Visualizing real time [11C]methionine translocation in Fe-sufficient and Fe-deficient barley using a positron emitting tracer imaging system (PETIS) J Exp Bot. 1999;50:637–643. [Google Scholar]
- Ortiz-Lopez A, Chang H, Bush DR. Amino acid transporters in plants. Biochem Biophys Acta. 2000;1465:275–280. doi: 10.1016/s0005-2736(00)00144-9. [DOI] [PubMed] [Google Scholar]
- Ota K, Yamamoto Y. Promotion of assimilation of ammonium ions by simultaneous application of nitrate and ammonium ions in radish plants. Plant Cell Physiol. 1989;30:365–371. [Google Scholar]
- Presland MR, McNaughton GS. Whole plant studies using radioactive 13-nitrogen. J Exp Bot. 1986;37:1619–1632. [Google Scholar]
- Raghothama KG. Phosphate transport and signalling. Curr Opin Plant Biol. 2000;3:182–187. [PubMed] [Google Scholar]
- Rawat SR, Silim SN, Kronzucker HJ, Siddiqi MJ, Glass ADM. AtAMT1 gene expression and NH4+ uptake in roots of Arabidopsis thaliana: evidence for regulation by root glutamine levels. Plant J. 1999;19:143–152. doi: 10.1046/j.1365-313x.1999.00505.x. [DOI] [PubMed] [Google Scholar]
- Redinbaugh MG, Campbell WH. Glutamine synthetase and ferredoxin-dependent glutamate synthase expression in the maize (Zea mays) root primary response to nitrate. Plant Physiol. 1993;101:1249–1255. doi: 10.1104/pp.101.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol. 2000;3:188–195. [PubMed] [Google Scholar]
- Sato T, Ohtake N, Ohyama T, Ishioka N-S, Watanabe S, Osa A, Sekine T, Uchida H, Tsuji A, Matsuhashi S. Analysis of nitrate absorption and transport in non-nodulated and nodulated soybean plants with 13NO3− and 15NO3−. Radioisotopes. 1999;48:450–458. [Google Scholar]
- Schachtman DP. Molecular insights into the structure and function of plant K+ transport mechanisms. Biochem Biophys Acta. 2000;1465:127–139. doi: 10.1016/s0005-2736(00)00134-6. [DOI] [PubMed] [Google Scholar]
- Sechley KA, Yamaya T, Oaks A. Compartmentation of nitrogen assimilation in higher plants. Int Rev Cytol. 1992;134:85–163. [Google Scholar]
- Sengar RS, Srivastava HS. Influence of abscisic acid on ammonium assimilation under water stress in roots and shoots of maize seedlings. Indian J Exp Biol. 1995;33:876–879. [Google Scholar]
- Uchida H, Omura T, Suzuki T, Tsuji A, Yamashita T, Fujimura T, Matsuhashi S. Positron imaging system for plant analysis. Radiat Ind. 1998;80:6–10. [Google Scholar]
- von Wirén N, Bergfeld A, Ninnemann O, Frommer WB. OsAMT1–1: a high affinity ammonia transporters from rice (Oryza sativa cv. Nipponbare) Plant Mol Biol. 1997;35:681. [Google Scholar]
- von Wirén N, Lauter FR, Ninnemann O, Gillissen B, Walch LP, Engels C, Jost W, Frommer WB. Differential regulation of three functional ammonium transporter genes by nitrogen in root hairs and by light in leaves of tomato. Plant J. 2000;21:167–175. doi: 10.1046/j.1365-313x.2000.00665.x. [DOI] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MJ, Ruth TJ, Glass ADM. Ammonium uptake by rice root: I. Flux and subcellular distribution of 13NH4+ Plant Physiol. 1993a;103:1249–1258. doi: 10.1104/pp.103.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MJ, Ruth TJ, Glass ADM. Ammonium uptake by rice root: II. Kinetics of 13NH4+ influx across the plasmalemma. Plant Physiol. 1993b;103:1259–1267. doi: 10.1104/pp.103.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama T, Kumazawa K. Differences in the distribution pattern of 15NO3-N and 15NH4-N absorbed by rice seedlings. J Soil Sci Manure. 1974a;43:329–332. [Google Scholar]
- Yoneyama T, Kumazawa K. A kinetic study of the assimilation of 15N-labeled ammonium in rice seedlings. Plant Cell Physiol. 1974b;15:655–661. [Google Scholar]
- Yoshida S. Fundamentals of Rice Crop Science. Manila, Philippines: International Rice Research Institute; 1981. [Google Scholar]