Take Home Message
Elderly men are more often diagnosed with higher-risk prostate cancer but are less likely to receive curative treatment options than younger men. Our analysis demonstrates that for men ≥80 yr of age with high-risk prostate cancer, definitive local therapy, including surgery or radiation therapy ± androgen deprivation therapy, is associated with a 50% reduction in overall mortality compared with observation or androgen deprivation therapy alone. We therefore recommend that elderly men, especially those with low comorbidity scores and aged 80–85 yr, should undergo discussion with their treatment provider regarding potentially definitive local therapy, as this may be associated with increased overall survival.
Keywords: Prostate cancer elderly, High-risk prostate cancer, Prostate cancer octogenarians, National Cancer Database
Abstract
Background
Elderly patients diagnosed with high-risk prostate cancer (PCa) present a therapeutic dilemma of balancing treatment of a potentially lethal malignancy with overtreatment of a cancer that may not threaten life expectancy.
Objective
To investigate treatment patterns and overall survival outcomes in this group of patients.
Design, setting, and participants
A retrospective cohort study was conducted. We queried the National Cancer Database for high-risk PCa in patients aged 80 yr or older diagnosed during 2004–2016.
Intervention
Eligible patients underwent no treatment following biopsy (ie, observation), androgen deprivation therapy (ADT) alone, radiation therapy (RT) alone, RT + ADT, or surgery.
Outcome measurements and statistical analysis
Kaplan-Meier, log rank, and multivariate Cox proportional hazard regression was performed to compare overall survival (OS).
Results and limitations
A total of 19 920 men were eligible for analysis, and the most common treatment approach was RT + ADT (7401 patients; 37.2%). Observation and ADT alone declined over time (59.3% in 2004 vs 47.5% in 2016). There was no observed difference in OS between observation and ADT alone (adjusted hazard ratio [HR] 1.04, 95% confidence interval [CI], 0.99–1.09; p = 0.105). Definitive local treatment was associated with improved OS compared with ADT alone (RT alone, HR 0.54, 95% CI, 0.50–0.59, p < 0.0001; ADT + RT, HR 0.48, 95% CI, 0.46–0.50, p < 0.0001; surgery, HR 0.50, 95% CI, 0.42–0.59, p < 0.0001).
Conclusions
This analysis demonstrates that the use of definitive local therapy, including surgery or RT ± ADT, is increasing and is associated with a 50% reduction in overall mortality compared with observation or ADT alone. While prospective validation is warranted, elderly men with high-risk disease eligible for definitive management should be counseled on the risks, including a possible compromise in OS, with deferring definitive management.
Patient summary
Elderly men are more often diagnosed with higher-risk prostate cancer but are less likely to receive curative treatment options than younger men. Our analysis demonstrates that for men ≥80 yr of age with high-risk prostate cancer, definitive local therapy, including surgery or radiation therapy and/or androgen deprivation therapy, is associated with a 50% reduction in overall mortality compared with observation or androgen deprivation therapy alone. We therefore recommend that life expectancy (ie, physiologic age) be taken into account, over chronologic age, and that elderly men with good life expectancy (eg, >5 yr; minimal comorbidity) should be offered definitive, life-prolonging therapy.
1. Introduction
Prostate adenocarcinoma (PCa) is the most common noncutaneous malignancy in men, accounting for over 20% of incidence cancer cases in American men in 2020 [1], [2]. Prostate-specific antigen (PSA) screening has allowed earlier detection in many of these cases, including those that may never have presented clinically within a patient’s lifetime [3], [4]. With PSA screening outside of standard recommendations [5], [6], [7], [8], [9] in elderly or comorbid men, there is a risk of overtreatment of cancers that may not threaten life expectancy but instead result in quality of life disturbances. Many elderly men who continue PSA screening may develop a clinically insignificant PCa; however, some with high-risk disease, defined by the National Comprehensive Cancer Network (NCCN) [9] as having clinical stage T3–4, PSA >20 ng/ml, and/or Gleason score 8–10, may ultimately develop life-threatening disease if untreated. In these men, aggressive, curative treatment may prevent prostate cancer dissemination and provide an overall survival (OS) benefit despite competing comorbidities. Nonetheless, advanced age has been associated with the receipt of suboptimal PCa management, and older men are less likely to receive curative treatment than their younger counterparts across risk groups [10], [11].
Based on NCCN guidelines, men with high-risk PCa with >5 yr life expectancy should be offered curative treatment, including radiation plus androgen deprivation therapy (ADT) or radical prostatectomy, while those with ≤5 yr expectancy may be better treated with observation or noncurative treatments, including ADT or observation [9]. Prior reports have suggested that conservative management, such as observation or ADT alone, is preferentially utilized in men aged 80 yr or older, and this approach may be used in over 80% of men over the age of 80 yr, regardless of calculated life expectancy [10]. Elderly patients, especially those >80 yr old, diagnosed with high-risk PCa often pose a therapeutic challenge given competing comorbidities and inability to estimate life expectancy accurately. Herein, we sought to evaluate utilization patterns and OS outcomes in men over 80 yr old with high-risk PCa undergoing current NCCN-endorsed treatment options.
2. Patients and methods
2.1. Data source and study population
The National Cancer Database (NCDB) Participant User File (PUF) for prostate cancer was evaluated to identify all patients aged 80 yr or older with a diagnosis of prostate cancer. The NCDB is a joint program of the American College of Surgeons and the American Cancer Society. Data from ∼70% of patients diagnosed at Commission on Cancer (CoC)-accredited cancer centers are captured and include patient, disease, and treatment characteristics. The PUF contains deidentified information and was therefore exempt from Institutional Review Board review.
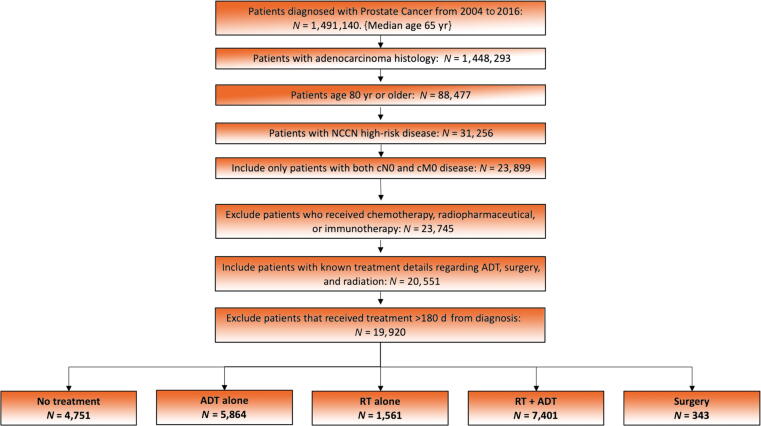
Men ≥80 yr old who were diagnosed with high-risk PCa, defined by NCCN stratification (clinical stage T3–4, PSA >20, and/or Gleason score 8–10) between 2004 and 2016 were evaluated. Survival data were available on patients diagnosed in 2004–2015. Only histologic diagnoses of PCa (ICD-0-3 8140) were included. Those with incomplete treatment data, node-positive disease, or metastatic disease were excluded. Patients with initiation of treatment, including start of ADT, >180 d after diagnosis were excluded to minimize the number of those patients who may have crossed over from observation to treatment. Patients missing the Charlson-Deyo comorbidity index (CCI) score information were excluded from the analysis. The CCI is an age-independent score to predict long-term survival, which incorporates multiple comorbidity conditions. A flow diagram of inclusion and exclusion criteria is depicted in Fig. 1.
Fig. 1.
Flow diagram of inclusion criteria. ADT = androgen deprivation therapy; NCCN = National Comprehensive Cancer Network; RT = radiation therapy.
Demographic and clinical data included the following: age, race, diagnosis year, clinical T stage, PSA, Gleason score, CCI, treatment center location, treatment facility type, primary patient insurance status, and treatment modality. The following treatment groups were included for analysis: observation only, ADT alone (ADT), radiation therapy (RT) alone, combined ADT and RT (ADT + RT), and surgery. The surgery cohort included only radical prostatectomy. Radiation cohorts included those who received treatment to the prostate only, prostate and pelvis, or pelvis (radiation treatment volume code 29/35/41) with total radiation dose ≥6000 cGy.
2.2. Statistical analysis
OS was determined from the date of diagnosis until patient death or last follow-up. The Kaplan-Meier survival analysis was used to estimate OS probabilities. Cox regression analyses were performed. In addition to these analyses, patient age at diagnosis, race, year of diagnosis, facility location and type, insurance status, CCI, clinical T stage, PSA, Gleason score, and treatment approach were used in the univariable analysis. Variables with p < 0.05 on univariable testing were entered into the multivariable analyses (MVAs) using the Cox proportional hazard model. Univariable analysis and MVA logistic regression modeling was used to identify the predictors of receiving local therapy (RT, ADT + RT, and surgery) and are reported as odds ratios (ORs). Statistical significance was considered with a value of p < 0.05. All measured levels of significance were two sided. SPSS Statistics version 27 (IBM, Armonk, NY, USA) was utilized for all statistical analyses.
3. Results
3.1. Demographic, patient, tumor, and treatment characteristics
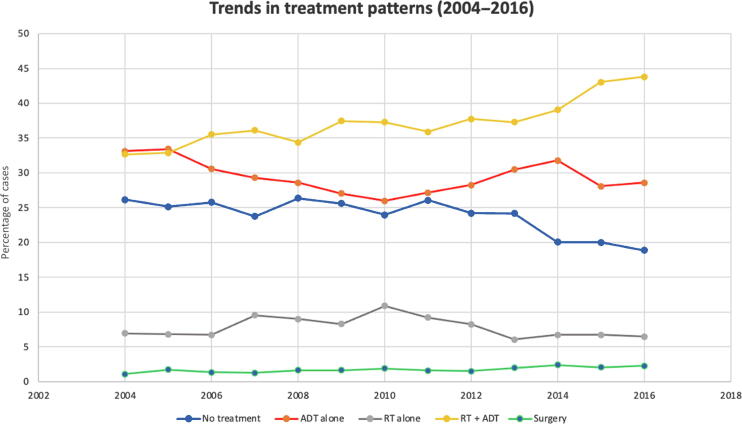
A total of 19 920 patients were identified with a median follow-up of 41.7 mo (range, 0–164.8 mo). A complete synopsis of patient demographic and characteristics is provided in Table 1. A summary of treatment approaches is found in Table 2. The median RT dose was 7740 cGy (interquartile range: 7560–7920 cGy). The most commonly utilized treatment modality was combined RT + ADT (7401 patients; 37.2%) followed by ADT alone (5864 patients; 29.4%), no treatment (4751 patients; 23.9%), RT alone (1561 patients; 7.8%), and surgery (343 patients, 1.7%; Table 1). A multivariable logistic regression analysis showed that increasing age and increasing CCI were associated with a decreased likelihood of receiving local therapy (OR for increasing age, 0.98 [95% confidence interval {CI}, 0.97–0.99], p = 0.003; OR for CCI >1, 0.87 [95% CI, 0.81–0.91], p < 0.0001). The year-to-year trend in each management is shown in Fig. 2. Among patients diagnosed in 2004, the earliest year analyzed, 40.7% received definitive treatment (RT, ADT + RT, or surgery) compared with 52.6% of patients diagnosed in 2016. In 2004, 59.3% of patients received either ADT alone or no treatment compared with 47.5% in 2016.
Table 1.
Demographics and clinical characteristics
| Patients, n (%) | |
|---|---|
| Age (yr) | |
| 80–84 | 13 433 (67.4) |
| ≥85 | 6487 (32.6) |
| Race | |
| White | 17 342 (87.1) |
| Black | 1727 (8.6) |
| Other | 652 (3.3) |
| Unknown | 199 (1.0) |
| Year of diagnosis | |
| 2004–2009 | 9574 (48.1) |
| 2010–2016 | 10 346 (51.9) |
| Charlson-Deyo comorbidity | |
| 0 | 15 839 (79.5) |
| 1 | 2885 (14.5) |
| ≥2 | 1196 (6.0) |
| Facility location | |
| Central | 6063 (30.4) |
| Northeast | 4442 (22.3) |
| South/southeast | 5936 (29.8) |
| West | 3479 (17.5) |
| Facility type | |
| Academic/research program | 5390 (27.1) |
| Community cancer program | 2478 (12.4) |
| Comprehensive community cancer program | 9612 (48.3) |
| Integrated network cancer program | 2440 (12.2) |
| Insurance status | |
| Medicaid | 277 (1.4) |
| Medicare | 17 137 (86.0) |
| Not insured | 116 (0.6) |
| Other government | 180 (0.9) |
| Private | 1915 (9.6) |
| Unknown | 295 (1.5) |
| Clinical T stage | |
| T1 | 8866 (44.5) |
| T2 | 6749 (33.9) |
| T3 | 1903 (9.6) |
| T4 | 514 (2.6) |
| TX | 1888 (9.5) |
| Gleason score | |
| 6 | 1154 (5.8) |
| 7 | 3277 (16.5) |
| 8–10 | 15 489 (77.8) |
| PSA | |
| <10 | 6163 (30.9) |
| 10–20 | 4639 (23.3) |
| >20 | 9118 (45.8) |
PSA = prostate-specific antigen.
Table 2.
Treatment characteristics
| Patients, n (%) |
|||||
|---|---|---|---|---|---|
| No treatment | ADT alone | RT alone | RT + ADT | Surgery | |
| Age (yr) | |||||
| 80–84 | 2658 (19.8) | 3120 (23.2) | 1224 (9.1) | 6138 (45.7) | 293 (2.2) |
| ≥85 | 2093 (32.3) | 2744 (42.3) | 337 (5.2) | 1263 (19.5) | 50 (0.8) |
| Race | |||||
| White | 4007 (23.1) | 5014 (28.9) | 1402 (8.1) | 6614 (38.1) | 305 (1.8) |
| Black | 563 (32.6) | 581 (33.6) | 105 (6.1) | 459 (26.6) | 19 (1.1) |
| Other | 132 (20.2) | 200 (30.7) | 43 (6.6) | 260 (39.9) | 17 (2.6) |
| Unknown | 49 (24.6) | 69 (34.7) | 11 (5.5) | 68 (34.2) | 2 (1.0) |
| Year of diagnosis | |||||
| 2004–2009 | 2435 (25.4) | 2908 (30.4) | 757 (7.9) | 3335 (34.8) | 139 (1.5) |
| 2010–2016 | 2316 (22.4) | 2956 (28.6) | 804 (7.8) | 4066 (39.3) | 204 (2.0) |
| Charlson-Deyo comorbidity | |||||
| 0 | 3505 (22.1) | 4351 (27.5) | 1343 (8.5) | 6378 (40.3) | 262 (1.7) |
| 1 | 846 (29.3) | 1007 (34.9) | 177 (6.1) | 789 (27.3) | 66 (2.3) |
| ≥2 | 400 (33.4) | 506 (42.3) | 41 (3.4) | 234 (19.6) | 15 (1.3) |
| Facility location | |||||
| Central | 1377 (22.7) | 2127 (35.1) | 415 (6.8) | 2049 (33.8) | 95 (1.6) |
| Northeast | 905 (20.4) | 1150 (25.9) | 342 (7.7) | 2001 (45.0) | 44 (1.0) |
| South/southeast | 1628 (27.4) | 1581 (26.6) | 556 (9.4) | 2075 (35.0) | 96 (1.6) |
| West | 841(24.2) | 1006 (28.9) | 248 (7.1) | 1276 (36.7) | 108 (3.1) |
| Facility type | |||||
| Academic/research program | 1160 (21.5) | 1644 (30.5) | 435 (8.1) | 2013 (37.3) | 138 (2.6) |
| Community cancer program | 705 (28.5) | 800 (32.3) | 202 (8.2) | 750 (30.3) | 21 (0.8) |
| Comprehensive community cancer program | 2248 (23.4) | 2729 (28.4) | 737 (7.7) | 3762 (39.1) | 136 (1.4) |
| Integrated network cancer program | 638 (26.1) | 691 (28.3) | 187 (7.7) | 876 (35.9) | 48 (2.0) |
| Insurance status | |||||
| Medicaid | 77 (27.8) | 100 (36.1) | 15 (5.4) | 83 (30.0) | 2 (0.7) |
| Medicare | 4090 (23.9) | 5047 (29.5) | 1332 (7.8) | 6365 (37.1) | 303 (1.8) |
| Not insured | 39 (33.6) | 43 (37.1) | 9 (7.8) | 23 (19.8) | 2 (1.7) |
| Other government | 30 (16.7) | 30 (16.7) | 19 (10.6) | 100 (55.6) | 1 (0.6) |
| Private | 440 (23.0) | 544 (28.4) | 165 (8.6) | 739 (38.6) | 27 (1.4) |
| Unknown | 75 (25.4) | 100 (33.9) | 21 (7.1) | 91 (30.8) | 8 (2.7) |
| Clinical T stage | |||||
| T1 | 2478 (27.9) | 2471 (27.9) | 797 (9.0) | 2993 (33.8) | 127 (1.4) |
| T2 | 1186 (17.6) | 1775 (26.3) | 565 (8.4) | 3105 (46.0) | 118 (1.7) |
| T3 | 216 (11.4) | 531 (27.9) | 129 (6.8) | 989 (52.0) | 38 (2.0) |
| T4 | 151 (29.4) | 260 (50.6) | 11 (2.1) | 91 (17.7) | 1 (0.2) |
| TX | 720 (38.1) | 827 (43.8) | 59 (3.1) | 223 (11.8) | 59 (3.1) |
| Gleason score | |||||
| 6 | 560 (48.5) | 283 (24.5) | 135 (11.7) | 152 (13.2) | 24 (2.1) |
| 7 | 976 (29.8) | 1046 (31.9) | 295 (9.0) | 914 (27.9) | 46 (1.4) |
| 8–10 | 3215 (20.8) | 4535 (29.3) | 1131 (7.3) | 6335 (40.9) | 273 (1.8) |
| PSA | |||||
| <10 | 1186 (19.2) | 1228 (19.9) | 662 (10.7) | 2919 (47.4) | 168 (2.7) |
| 10–20 | 827 (17.8) | 1238 (26.7) | 370 (8.0) | 2124 (45.8) | 80 (1.7) |
| >20 | 2738 (30.0) | 3398 (37.3) | 529 (5.8) | 2358 (25.9) | 95 (1.0) |
| Total patients | 4751 (23.9) | 5864 (29.4) | 1561 (7.8) | 7401 (37.2) | 343 (1.7) |
ADT = androgen deprivation therapy; PSA = prostate-specific antigen; RT = radiation therapy.
Fig. 2.
Year-to-year analysis of each treatment modality. ADT = androgen deprivation therapy; RT = radiation therapy.
3.2. Outcomes
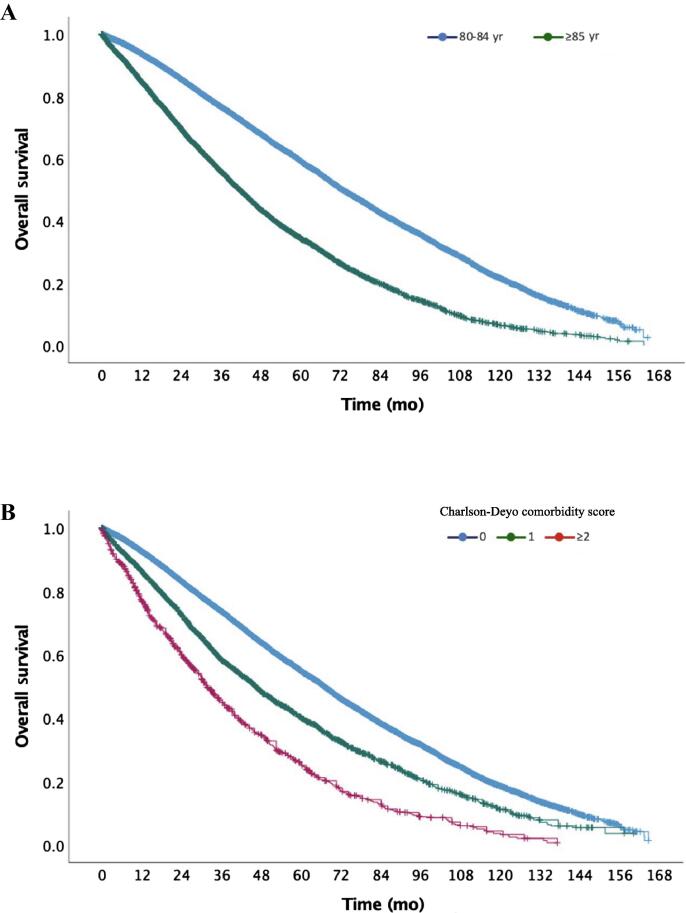
The median OS for the entire cohort was 61.6 mo (95% CI, 60.4–62.8 mo), and the estimated 5- and 10-yr OS were 51.2% and 16.8%, respectively. For men aged 80–84 yr, the median OS was 72.7 mo (95% CI, 71.2–74.2); for men aged ≥85 yr, the median OS was 41.6 mo (95% CI, 40.3–42.9; log-rank p < 0.0001; Fig. 3A). The median OS was 67.0 mo (95% CI, 65.7–68.2) for men with a CCI of 0, 46.3 mo (95% CI, 43.8–48.8) for those with a CCI of 1; and 31.5 mo (95% CI, 29.0–34.0; Fig. 3B; log-rank p < 0.0001 between all groups) for those with a CCI of ≥2. For patients diagnosed between 2004 and 2009, the median OS was 60.9 mo (95% CI, 59.4–62.5) versus 61.8 mo (95% CI, 60.0–63.6) for those diagnosed in 2010–2015 (log-rank p = 0.286).
Fig. 3.
(A) Kaplan-Meier overall survival curve for patients aged 80–84 yr old versus those aged ≥85 yr (log-rank p < 0.0001). (B) Kaplan-Meier overall survival curve for patients by Charlson-Deyo comorbidity scores (0, 1, and ≥2; log-rank p < 0.0001 between each group).
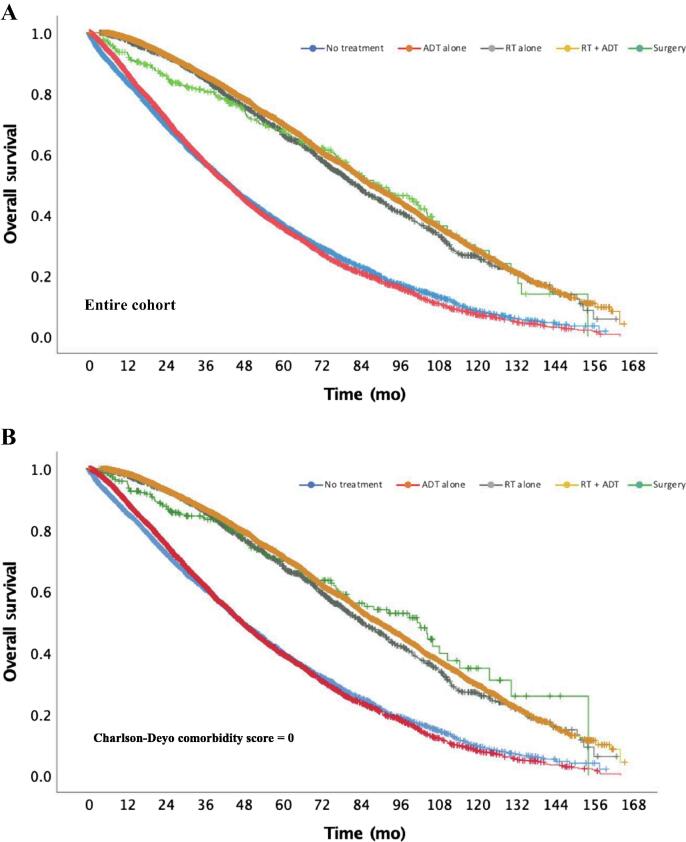
Kaplan-Meier analyses for patients per treatment modality are depicted in Fig. 4A. The median OS was 42.9 mo (95% CI, 41.2–44.6) for patients who underwent no treatment and 42.8 mo (95% CI, 41.3–44.2) for those with ADT alone (log-rank p = 0.47). The median OS periods for RT alone and RT plus ADT were 82.1 mo (95% CI, 77.6–86.6) and 86.5 mo (95% CI, 84.3–88.6), respectively (log-rank p = 0.045). Patients treated with surgery had median OS of 88.3 mo (95% CI, 74.7–101.9), which was not statistically different from that for patients treated with RT alone or ADT + RT (log-rank p = 0.93 and p = 0.38, respectively).
Fig. 4.
(A) Kaplan-Meier overall survival curve by treatment modality: no treatment versus androgen deprivation therapy (ADT) alone, p = 0.47; no treatment or ADT alone versus radiation therapy (RT) alone, RT + ADT, or surgery, p < 0.0001 for all; RT alone versus RT + ADT, p = 0.045; RT versus surgery, p = 0.93; and RT + ADT versus surgery, p = 0.38. (B) Kaplan-Meier overall survival curve by treatment modality for all patients with Charlson-Deyo comorbidity score of 0: no treatment versus ADT alone, p = 0.75; no treatment or ADT alone versus RT alone, RT + ADT, or surgery, p < 0.0001 for all; RT alone versus RT + ADT, p = 0.07; RT versus surgery, p = 0.27; and RT + ADT versus surgery, p = 0.73. ADT = androgen deprivation therapy; RT = radiation therapy.
In a subgroup analysis of patients with a CCI of 0, there was no difference in OS between RT, ADT + RT, and surgery (log-rank p = 0.274 for RT vs surgery, p = 0.726 for ADT + RT vs surgery, and p = 0.066 for RT vs ADT + RT). RT, ADT + RT, and surgery were all superior to no treatment or ADT alone (log-rank p < 0.0001 between each group) for patients with a CCI of 0 (Fig. 4B). Similar findings were observed in a subgroup of patients with a CCI of 1 (log-rank p < 0.01 between each local therapy and both observation and ADT alone).
An additional subgroup analysis was performed for patients aged 80–85 yr old. There was no difference in OS between RT, ADT + RT, and surgery (log-rank p = 0.102 for RT vs surgery, p = 0.807 for ADT + RT vs surgery, and p = 0.059 for RT vs ADT + RT). RT, ADT + RT, and surgery were all associated with improved OS compared with observation or ADT alone (log-rank p < 0.0001 between each group) for patients with age 80–85 yr.
3.3. Univariable analysis and MVA
Univariable and multivariable (MVA) Cox-regression survival analyses are shown in Table 3. On MVA, OS was influenced by age, CCI, facility type, insurance status, clinical T stage, Gleason score, PSA, and treatment modality. In MVA, treatment at an academic/research center was associated with increased OS compared with community cancer programs (hazard ratio [HR] 0.83, 95% CI, 0.78–0.88; p < 0.0001). Patients with Medicare insurance had worse OS (HR 1.13, 95% CI, 1.06–1.21; p < 0.0001) compared with private insurance. Patients with increasing CCI scores (CCI of 1, HR 1.33, 95% CI, 1.26–1.40, p < 0.0001; CCI of ≥2, HR 1.85; 95% CI, 1.72–1.99, p < 0.0001), and increasing or unknown T stage (cT3, HR 1.17, 95% CI, 1.09–1.26, p < 0.0001; cT4, HR 2.03; 95% CI, 1.82–2.27, p < 0.0001; cTx, HR 1.30; 95% CI, 1.23–1.38, p < 0.0001) had worse OS. Higher Gleason score (Gleason 7, HR 1.17, 95% CI, 1.07–1.278, p < 0.0001; Gleason 8–10, HR 1.64, 95% CI, 1.51–1.78, p < 0.0001) and increasing PSA (PSA 10–20, HR 1.13, 95% CI, 1.07–1.19, p < 0.0001; PSA >20, HR 1.35, 95% CI, 1.28–1.42, p < 0.0001) also correlated with decreased OS.
Table 3.
Univariable and multivariable analyses
| Variable | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (yr) | ||||
| 80–84 | Reference group | Reference group | ||
| ≥85 | 1.99 (1.91–2.06) | <0.0001 | 1.56 (1.50–1.63) | <0.0001 |
| Race | ||||
| White | Reference group | Reference group | ||
| Black | 1.11 (1.04–1.19) | 0.002 | 0.98 (0.92–1.05) | 0.607 |
| Other | 0.75 (0.66–0.85) | <0.0001 | 0.84 (0.74–1.02) | 0.078 |
| Unknown | 0.80 (0.65–0.99) | 0.039 | 0.81 (0.66–1.01) | 0.055 |
| Year of diagnosis | ||||
| 2004–2009 | Reference group | – | ||
| 2010–2015 | 0.98 (0.94–1.02) | 0.286 | – | |
| Charlson-Deyo comorbidity | ||||
| 0 | Reference group | Reference group | ||
| 1 | 1.49 (1.41–1.57) | <0.0001 | 1.33 (1.26–1.40) | <0.0001 |
| ≥2 | 2.36 (2.20–2.55) | <0.0001 | 1.85 (1.72–1.99) | <0.0001 |
| Facility location | ||||
| Northeast | Reference group | Reference group | ||
| Central | 1.21 (1.15–1.28) | <0.0001 | 1.03 (0.96–1.09) | 0.303 |
| South/southeast | 1.15 (1.09–1.21) | <0.0001 | 1.03 (0.98–1.09) | 0.241 |
| West | 1.02 (0.96–1.08) | 0.582 | 0.96 (0.90–1.01) | 0.089 |
| Facility type | ||||
| Community cancer program | Reference group | Reference group | ||
| Comprehensive community cancer program | 0.85 (0.80–0.90) | <0.0001 | 0.92 (0.87–0.98) | 0.009 |
| Academic/research program | 0.74 (0.69–0.79) | <0.0001 | 0.83 (0.78–0.88) | <0.0001 |
| Integrated network cancer program | 0.88 (0.81–0.95) | 0.001 | 0.97 (0.90–1.05) | 0.411 |
| Insurance status | ||||
| Private | Reference group | Reference group | ||
| Not insured | 1.12 (0.84–1.49) | 0.432 | 0.81 (0.61–1.08) | 0.151 |
| Medicaid | 0.91 (0.74–1.13) | 0.395 | 0.83 (0.67–1.02) | 0.077 |
| Medicare | 1.19 (1.11–1.27) | <0.0001 | 1.13 (1.06–1.21) | <0.0001 |
| Other government | 1.00 (0.79–1.28) | 0.975 | 1.23 (0.97–1.56) | 0.093 |
| Unknown | 1.11 (0.94–1.30) | 0.225 | 0.96 (0.82–1.13) | 0.615 |
| Clinical T stage | ||||
| T1 | Reference group | Reference group | ||
| T2 | 0.95 (0.91–1.00) | 0.050 | 1.04 (0.99–1.08) | 0.130 |
| T3 | 0.97 (0.91–1.04) | 0.426 | 1.17 (1.09–1.26) | <0.0001 |
| T4 | 2.69 (2.41–3.00) | <0.0001 | 2.03 (1.82–2.27) | <0.0001 |
| TX | 1.57 (1.48–1.67) | <0.0001 | 1.30 (1.23–1.38) | <0.0001 |
| Gleason score | ||||
| 6 | Reference group | Reference group | ||
| 7 | 1.09 (1.00–1.19) | 0.061 | 1.17 (1.07–1.28) | <0.0001 |
| 8–10 | 1.17 (1.08–1.26) | <0.0001 | 1.64 (1.51–1.78) | <0.0001 |
| PSA | ||||
| <10 | Reference group | Reference group | ||
| 10–20 | 1.17 (1.11–1.23) | <0.0001 | 1.13 (1.07–1.19) | <0.0001 |
| >20 | 1.45 (1.39–1.52) | <0.0001 | 1.35 (1.28–1.42) | <0.0001 |
| Treatment modality | ||||
| ADT alone | Reference group | Reference Group | ||
| No treatment | 0.98 (0.93–1.03) | 0.368 | 1.04 (0.99–1.09) | 0.105 |
| Radiation alone | 0.43 (0.39–0.46) | <0.0001 | 0.54 (0.50–0.59) | <0.0001 |
| Surgery | 0.42 (0.35–0.50) | <0.0001 | 0.50 (0.42–0.59) | <0.0001 |
| Radiation + ADT | 0.39 (0.37–0.41) | <0.0001 | 0.48 (0.46–0.50) | <0.0001 |
ADT = androgen deprivation therapy; CI = confidence interval; HR = hazard ratio; PSA = prostate-specific antigen.
There was no difference in OS between no treatment and ADT alone (HR for no treatment, 1.04, 95% CI, 0.99–1.09; p = 0.105). Definitive treatment was associated with improved survival compared with ADT alone (RT alone, HR 0.54, 95% CI, 0.50–0.59, p < 0.0001; ADT + RT, HR 0.48, 95% CI, 0.46–0.50, p < 0.0001; surgery, HR 0.50, 95% CI, 0.42–0.59, p < 0.0001). Similarly, all definitive treatment groups were associated with improved OS versus no treatment (p < 0.0001 between no treatment and RT, RT + ADT, surgery).
4. Discussion
With longer life expectancy and more widespread adoption of PSA screening over the past two decades, the number of elderly men diagnosed with PCa is increasing [10]. Appropriate management of genitourinary malignancies in this aging population is challenging [12]. Many of these men, especially those with small-volume low-grade prostate cancer, die of unrelated causes [13], [14], and aggressive treatment could result in quality of life disturbances and significant health care expenditures without any life-expectancy gain. However, older men are often diagnosed with higher-grade and higher-stage prostate cancer than younger men, Prostate Cancer due to less routine PSA screening with age [15], [16]. Furthermore, prior studies have shown that older men are at risk of undergoing noncurative treatment [10], [11], [17]. Many elderly men with PCa, especially those diagnosed with high-risk disease, are at risk for cancer progression and premature death. In fact, prostate cancer is the second leading cause of cancer death in men aged 80 yr or older, with 15 298 PCa deaths in the USA in 2017 [1].
In this observational analysis of men ≥80 yr old with high-risk prostate cancer, RT plus ADT was the single most common approach of the management groups; however, more men underwent either observation or ADT alone compared with some form of local treatment (ie, RT, RT + ADT, or surgery). Less than 2% of men underwent surgery. Yet, there was an ∼50% reduced risk of overall mortality with local therapy, including surgery, RT, or RT + ADT, compared with observation or ADT alone. Notably, there were no significant differences in long-term survival between surgery and RT ± ADT. The benefit of local therapy over observation and ADT alone persisted even after adjusting for comorbidity score, T stage, PSA, and Gleason score. Additionally, it was noted that men with increasing age and increasing CCI were less likely to receive local therapy (surgery ± ADT or surgery).
To some extent, there are limited randomized data to help guide treatment decisions in prostate cancer, especially in older men. A Swedish randomized trial of surgery (radical prostatectomy) versus observation in 695 men with just over 8 yr of follow-up found that surgery reduced overall mortality, in addition to earlier cancer-specific endpoints such as local and distant progression [18]. Subgroup analyses suggested that treatment preferentially benefited men <65 yr old; however, subgroup sample sizes were not sufficiently powered and the hazard ratio for death with treatment in older men was not reported. Furthermore, randomized data comparing RT with surgery are limited aside from the ProtecT trial in a cohort of younger men with low-risk and favorable-intermediate-risk PCa [19]. Interestingly, our study showed that the benefit of surgery was similarly advantageous to RT ± ADT, even after multivariable modeling. However, <2% of the cohort underwent surgery and <0.01% of patients were aged 85 yr or older.
Undoubtedly, aggressive local therapy with RT ± ADT and surgery are associated with more quality of life disturbances (eg, bowel, urinary, or sexual disturbances) than observation alone. Elderly men may be hesitant to pursue the associated toxicities of local therapy, especially if counseled that treatment may be unnecessary or the patient is likely to die of causes other than PCa [20]. However, studies have shown that prostate cancer mortality is independent of age at diagnosis but directly linked to risk group, ranging up to 35–40% in the high-risk group [21]. Elderly patients, especially those with good functional status and minimal medical comorbidities, need to be counseled on the potential risk of prostate cancer–specific mortality without aggressive, curative-intent local treatment. Furthermore, advanced techniques in treatment, such as robotic-assisted laparoscopic prostatectomy for surgery resulting in less perioperative morbidity and intensity modulation (intensity-modulated RT) or proton beam therapy for RT resulting in less bowel/urinary bother, have reduced treatment-associated morbidity substantially [22], [23], [24].
We found an increase in utilization of definitive management (surgery, RT ± ADT) in these men from 2004 to 2016. This trend is concordant with expert guidelines, which have placed more emphasis on life expectancy (ie, physiologic age) instead of chronologic age. The NCCN treatment guidelines, for example, now recommend aggressive, curative therapies for men with life expectancies of >5 yr regardless of age. Numerical age has historically been given emphasis in clinical trials and included in multiple prognostic scoring schemes, due to its impartiality and ubiquity. However, physiologic age, presence of comorbidities, and performance status, all of which can contribute to estimated life expectancy, may be equally or more important when determining which patients may benefit from definitive or more aggressive treatment. Based on the US Social Security Index actuarial life table, a 77-yr-old man of average health would still have near 10-yr life expectancy, while an 87-yr-old man would still have 5-yr life expectancy [25]. Age was associated with survival on the multivariable analysis; however, even after adjusting for age, aggressive local therapy was independently associated with an overall survival advantage over observation or ADT.
Interestingly, we did not observe any survival difference between men who underwent ADT alone and those who received no treatment. While ADT is effective at producing rapid biochemical responses, it is associated with substantial side effects and toxicity, including metabolic syndrome, cardiovascular events, and neurocognitive dysfunction, and has been associated with an increased risk of other-cause mortality [26], [27], [28], [29]. Some of these side effects can potentially be mitigated with utilization of intermittent ADT over continuous therapy; however, observational data have shown that older men with low-risk tumors treated with primary ADT may have worse survival than their counterparts who received no treatment in the 6 mo after receiving their diagnosis [30]. This finding was not observed in this analysis in men with higher-risk prostate cancer, likely due to worse outcomes with no treatment compared with a lower-risk group of patients. Nonetheless, the potential morbidity associated with ADT monotherapy in elderly men, especially those with competing medical risks, may result in minimal net survival gain due to an increase in noncancer mortality, and the short- and long-term toxicities of lifelong ADT need to be weighed against those associated with RT or surgery.
Our observational study utilizing a hospital-based registry has several limitations. Many of these limitations are inherent to the database, while others are specific to the analysis of a geriatric population. The NCDB PUF for prostate tumors includes only men who are diagnosed or treated at CoC-accredited treatment centers. Therefore, the results herein may not represent the overall cancer population in the USA; however, given that the NCDB includes ∼70% of all cancer diagnoses each calendar year, we suggest that this study is a significant representation of outcomes in men ≥80 yr old with high-risk prostate cancer in the USA that may not be included in the database. Outcome measures in the database are limited to only OS, and details regarding distant metastasis or cancer-specific survival are unavailable. However, in this elderly cohort with high-risk disease, these earlier endpoints may be less relevant than an overall survival endpoint. Notably, the database does not include variables for performance status, geriatric specific evaluation, or life expectancy. This limits the ability to predict a physiologic age or to account for which men may be candidates for definitive treatment compared with those whose performance status or other limitations may preclude such treatment. To account for such limitations, we utilized the Charlson-Deyo comorbidity score, which is an age-independent score, to predict long-term survival and found that, in patients with the lowest score (CCI = 0), the use of local definitive therapy improved OS significantly. Toxicity related to treatment and quality of life metrics is not captured in the database and therefore could not be analyzed; it is conceivable that the increased survival is at the cost of acute toxicity and worse quality of life. Duration and method (ie, continuous vs intermittent) of ADT are unavailable and could affect outcomes, especially in ADT-only group. Additionally, it is unknown whether a patient in the definitive ADT + RT group died while undergoing ADT and whether ADT was associated with significant toxicities and/or the cause of death. Lastly, given the observational and retrospective design using a database registry, this study will undoubtedly have selection biases and imbalances in some unmeasured variables. As cause of death is unavailable in our analysis, there is a risk of selection bias with unmeasured confounders associated with curative treatment over observation or ADT alone. However, we utilized multivariate modeling and included all available patient, treatment, and demographic variables associated with PCa outcomes, and the benefit in OS with local therapy remained.
5. Conclusions
Elderly men are more often diagnosed with higher-risk PCa but are less likely to undergo curative treatment options than younger men [10], [11]. Our analysis demonstrates that for men ≥80 yr of age with high-risk PCa, definitive local therapy, including surgery or RT ± ADT, is associated with a 50% reduction in overall mortality in comparison with observation or ADT alone. Utilization of curative treatment options for older men is increasing, and guidelines now focus more on life expectancy and performance status in lieu of age alone. We therefore recommend that elderly men, especially those with low comorbidity scores and aged 80–85 yr, should undergo discussion with their treatment provider regarding potential definitive local therapy, as this may be associated with increased overall survival. While these results warrant rigorous prospective validation, these findings are informative. Specifically, elderly men with high-risk disease eligible for definitive management should be counseled on the risks, including potential side effects related to local therapy but also a possible compromise in overall survival with deferring definitive management.
Author contributions: Benjamin W. Fischer-Valuck had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fischer-Valuck, Patel, Hershatter.
Acquisition of data: Rao.
Analysis and interpretation of data: Fischer-Valuck, Patel.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Liu, Fischer-Valuck, Patel.
Obtaining funding: Liu.
Administrative, technical, or material support: Fischer-Valuck.
Supervision: Fischer-Valuck, Patel.
Other: None.
Financial disclosures: Benjamin W. Fischer-Valuck certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was supported by the Breen Foundation and National Institutes of Health/National Cancer Institute and the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments: A portion of this work will be presented in February 2021 at the ASCO GU Symposium.
Associate Editor: Guillaume Ploussard
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Dy G.W., Gore J.L., Forouzanfar M.H., Naghavi M., Fitzmaurice C. Global burden of urologic cancers, 1990–2013. Eur Urol. 2017;71:437–446. doi: 10.1016/j.eururo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Etzioni R., Penson D.F., Legler J.M., et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 4.Lee D.J., Mallin K., Graves A.J., et al. Recent changes in prostate cancer screening practices and epidemiology. J Urol. 2017;198:1230–1240. doi: 10.1016/j.juro.2017.05.074. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Prostate cancer early detection (version 2.2020). 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf.
- 6.US Preventive Services Task Force Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901–1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 7.American Urological Association. Early detection of prostate cancer. 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline.
- 8.Carter H.B., Albertsen P.C., Barry M.J., et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Prostate cancer (version 2.2020). 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 10.Stangelberger A., Waldert M., Djavan B. Prostate cancer in elderly men. Rev Urol. 2008;10:111–119. [PMC free article] [PubMed] [Google Scholar]
- 11.Samet J., Hunt W.C., Key C., Humble C.G., Goodwin J.S. Choice of cancer therapy varies with age of patient. JAMA. 1986;255:3385–3390. [PubMed] [Google Scholar]
- 12.Fischer-Valuck B.W., Rao Y.J., Rudra S., et al. Treatment patterns and overall survival outcomes of octogenarians with muscle invasive cancer of the bladder: an analysis of the National Cancer Database. J Urol. 2018;199:416–423. doi: 10.1016/j.juro.2017.08.086. [DOI] [PubMed] [Google Scholar]
- 13.Newschaffer C.J., Otani K., McDonald M.K., Penberthy L.T. Causes of death in elderly prostate cancer patients and in a comparison nonprostate cancer cohort. J Natl Cancer Inst. 2000;92:613–621. doi: 10.1093/jnci/92.8.613. [DOI] [PubMed] [Google Scholar]
- 14.Wong Y.N., Mitra N., Hudes G., et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA. 2006;296:2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 15.Cooperberg M.R., Cowan J., Broering J.M., Carroll P.R. High-risk prostate cancer in the United States, 1990–2007. World J Urol. 2008;26:211–218. doi: 10.1007/s00345-008-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford E.D. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology. 2009;73(5 Suppl):S4–S10. doi: 10.1016/j.urology.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Konety B.R., Cowan J.E., Carroll P.R., Ca P.I. Patterns of primary and secondary therapy for prostate cancer in elderly men: analysis of data from CaPSURE. J Urol. 2008;179:1797–1803. doi: 10.1016/j.juro.2008.01.044. discussion 1803. [DOI] [PubMed] [Google Scholar]
- 18.Bill-Axelson A., Holmberg L., Ruutu M., et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 19.Hamdy F.C., Donovan J.L., Lane J.A., et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 20.Droz J.P., Albrand G., Gillessen S., et al. Management of prostate cancer in elderly patients: recommendations of a task force of the International Society of Geriatric Oncology. Eur Urol. 2017;72:521–531. doi: 10.1016/j.eururo.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Rider J.R., Sandin F., Andren O., Wiklund P., Hugosson J., Stattin P. Long-term outcomes among noncuratively treated men according to prostate cancer risk category in a nationwide, population-based study. Eur Urol. 2013;63:88–96. doi: 10.1016/j.eururo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Leow J.J., Chang S.L., Meyer C.P., et al. Robot-assisted versus open radical prostatectomy: a contemporary analysis of an all-payer discharge database. Eur Urol. 2016;70:837–845. doi: 10.1016/j.eururo.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 23.Sheets N.C., Goldin G.H., Meyer A.M., et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan H.Y., Jiang J., Hoffman K.E., et al. Comparative toxicities and cost of intensity-modulated radiotherapy, proton radiation, and stereotactic body radiotherapy among younger men with prostate cancer. J Clin Oncol. 2018;36:1823–1830. doi: 10.1200/JCO.2017.75.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Social Security Administration. Actuarial life table 2020. https://www.ssa.gov/OACT/STATS/table4c6.html.
- 26.O'Farrell S., Garmo H., Holmberg L., Adolfsson J., Stattin P., Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243–1251. doi: 10.1200/JCO.2014.59.1792. [DOI] [PubMed] [Google Scholar]
- 27.Saigal C.S., Gore J.L., Krupski T.L., et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 28.Keating N.L., O'Malley A.J., Freedland S.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motlagh R.S., Quhal F., Mori K., et al. The risk of new onset dementia and/or Alzheimer's disease among prostate cancer patients treated with androgen deprivation therapy: a systematic review and meta-analysis. J Urol. 2021;205:60–67. doi: 10.1097/JU.0000000000001341. [DOI] [PubMed] [Google Scholar]
- 30.Wong Y.N., Freedland S.J., Egleston B., Vapiwala N., Uzzo R., Armstrong K. The role of primary androgen deprivation therapy in localized prostate cancer. Eur Urol. 2009;56:609–616. doi: 10.1016/j.eururo.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]