Take Home Message
The efficacy of tools for selection of candidates for prostate biopsy after multiparametric magnetic resonance imaging (MRI) varies across Prostate Imaging-Reporting and Data System (PI-RADS) categories. The new Proclarix test performs better than prostate-specific antigen density and the European Randomized Study of Screening for Prostate Cancer MRI predictive model in the challenging PI-RADS 3 category. Proclarix guaranteed 100% detection of clinically significant prostate cancer (PCa), avoiding almost one-quarter of prostate biopsies and decreasing overdetection of insignificant PCa from 16.6% to 11.2%.
Keywords: Clinically significant prostate cancer, Multiparametric magnetic resonance imaging, Proclarix, Prostate-specific antigen density, European Randomized Study of Screening for Prostate Cancer predictive model
Abstract
Background
Prostate Imaging-Reporting and Data System (PI-RADS) category 3 is a challenging scenario for detection of clinically significant prostate cancer (csPCa) and some tools can improve the selection of appropriate candidates for prostate biopsy.
Objective
To assess the performance of the European Randomized Study of Screening for Prostate Cancer (ERSPC) magnetic resonance imaging (MRI) model, the new Proclarix test, and prostate-specific antigen density (PSAD) in selecting candidates for prostate biopsy among men in the PI-RADS 3 category.
Design, setting, and participants
We conducted a head-to-head prospective analysis of 567 men suspected of having PCa for whom guided and systematic biopsies were scheduled between January 2018 and March 2020 in a single academic institution. A PI-RADS v.2 category 3 lesion was identified in 169 men (29.8%).
Outcome measurement and statistical analysis
csPCa, insignificant PCa (iPCa), and unnecessary biopsy rates were analysed. csPCa was defined as grade group ≥2. Receiver operating characteristic (ROC) curves, decision curve analysis curves, and clinical utility curves were plotted.
Results and limitations
PCa was detected in 53/169 men (31.4%) with a PI-RADS 3 lesion, identified as csPCa in 25 (14.8%) and iPCa in 28 (16.6%). The area under the ROC curve for csPCa detection was 0.703 (95% confidence interval [CI] 0.621–0.768) for Proclarix, 0.657 (95% CI 0.547–0.766) for the ERSPC MRI model, and 0.612 (95% CI 0.497–0.727) for PSAD (p = 0.027). The threshold with the highest sensitivity was 10% for Proclarix, 1.5% for the ERSPC MRI model, and 0.07 ng/ml/cm3 for PSAD, which yielded sensitivity of 100%, 91%, and 84%, respectively. Some 21.3%, 26.2%, and 7.1% of biopsies would be avoided with Proclarix, PSAD, and the ERSPC MRI model, respectively. Proclarix showed a net benefit over PSAD and the ERSPC MRI model. Both Proclarix and PSAD reduced iPCa overdetection from 16.6% to 11.3%, while the ERSPC MRI model reduced iPCa overdetection to 15.4%.
Conclusions
Proclarix was more accurate in selecting appropriate candidates for prostate biopsy among men in the PI-RADS 3 category when compared to PSAD and the ERSPC MRI model. Proclarix detected 100% of csPCa cases and would reduce prostate biopsies by 21.3% and iPCa overdetection by 5.3%.
Patient summary
We compared three methods and found that the Proclarix test can optimise the detection of clinically significant prostate cancer in men with a score of 3 on the Prostate Imaging-Reporting and Data System for magnetic resonance imaging scans.
1. Introduction
Early detection of clinically significant prostate cancer (csPCa) decreases PCa-specific mortality [1]. Currently, suspicion of PCa is still based on detection of elevated serum levels of prostate-specific antigen (PSA) and/or an abnormal digital rectal examination (DRE) [2]. Suspected PCa has typically been confirmed via systematic biopsies of the prostate, but this approach results in a high rate of unnecessary biopsies and overdetection of insignificant PCa (iPCa) [3]. True improvement in the early detection of csPCa has come from multiparametric magnetic resonance imaging (mpMRI) and guided biopsies [2]. At present, the negative predictive value of mpMRI can reach 95%, so prostate biopsies can usually be avoided in men with a Prostate Imaging-Reporting and Data System (PI-RADS) score <3 [4], [5]. By contrast, most clinicians recommend prostate biopsy in men with PI-RADS >3 because the likelihood of PCa ranges from 62% to 92% [6]. PI-RADS category 3 is the most challenging scenario: 60–85% of prostate biopsies are unnecessary and up to 60% of PCa cases detected are insignificant [7], [8]. Therefore, tools such as PSA density (PSAD), modern markers, and predictive models are recommended for appropriate selection of candidates for prostate biopsy [2].
Proclarix is a new blood-based test that estimates the likelihood of csPCa by computing the risk according to measurement results for thrombospondin-1 (THBS1), cathepsin D (CTSD), total PSA, and percentage free PSA in serum, as well as patient age [9]. Recent studies have suggested that Proclarix can improve csPCa detection by reducing unnecessary biopsies in men with or without mpMRI [10]. However, data on the behaviour of Proclarix by PI-RADS category are lacking. Meanwhile, PSAD has become relevant as prostate volume can be accurately measured on MRI [11], [12], [13]. The externally validated European Randomized Study of Screening for Prostate Cancer (ERSPC) predictive model has recently incorporated the PI-RADS version 1 score and age in the “3+DRE” and “4+DRE” risk calculators [14]. However, no specific analyses of its behaviour regarding PI-RADS categories have been carried out.
In this study we compare the behaviour of PSAD, Proclarix, and the ERSPC MRI predictive model according to PI-RADS categories. Our main objective was to analyse the usefulness of these three tools for appropriate selection of candidates for prostate biopsy in the challenging setting of PI-RADS category 3.
2. Patients and methods
2.1. Design, setting, and participants
This was a prospective head-to-head study in which the likelihood of csPCa was assessed using the Proclarix test, PSAD, and the ERSPC MRI model. A cohort of 567 men with a suspicion of PCa because of PSA >3 ng/ml and/or abnormal DRE underwent prebiopsy 3-T mpMRI and had guided and systematic prostate biopsies scheduled between January 12, 2018 and March 15, 2020 at a single academic institution. A PI-RADS v.2 category 3 lesion was identified in 169 men (29.8%). Men with PCa on active surveillance and those with symptomatic benign prostatic hyperplasia treated with 5α-reductase inhibitors were previously excluded. Two- to three-core transrectal ultrasound-guided biopsies of suspected lesions and 12-core systematic biopsies were performed in all men using the transrectal approach. The study was approved by our institutional ethics committee (PR-AG129/2020).
2.2. Testing
Blood was obtained immediately before prostate biopsy and frozen serum was stored locally at −80 °C for 63–811 d (C. 0003439; https://biobancs.isiii.es) and then shipped on dry ice to Proteomedix (Zurich-Schlieren, Switzerland). Processing of serum samples, the ELISA kit, and calculation of the risk score by laboratory technicians were performed blind before any clinical information was available. THBS1 and CTSD were measured using a Proclarix kit (Proteomedix, Zurich-Schlieren, Switzerland) according to the kit instructions [15]. Serum total PSA and free PSA were reanalysed for all samples using a Roche Cobas immunoassay system (Roche Diagnostics, Rotkreuz, Switzerland). Proclarix risk calculation was performed according to the instructions and the results ranged from 0% to 100%. PSAD was calculated from the prostate volume measured on MRI and the total PSA value from the Proclarix test. The ERSPC MRI risk of high-grade PCa was calculated for every man using the Prostate Cancer Research Foundation (Reeuwijk, The Netherlands) web application at www.prostatecancer-riskcalculator.com. The ERSPC MRI risk calculator includes PSA (0.5–50 ng/ml), repeat biopsy (yes/no), DRE (normal/abnormal), prostate volume (10–110 cm3, which can now be obtained from MRI), age (50–75 years), and PI-RADS version 1 score [14]. We introduced the MRI-based prostate volume and the PI-RADS version 2 categories. When the observed values were not within the acceptable range, we entered the minimum or maximum acceptable value, whichever was closer to the observed value.
2.3. Outcome measurements
The main outcome measured was the rate of csPCa detection. csPCa was defined as International Society of Urological Pathology grade group ≥2 [16]. The rate of prostate biopsies avoided and the rate of iPCa overdetection were secondary outcome measurements.
2.4. Statistical analysis
Quantitative variables are presented as the median and interquartile range (IQR). Qualitative variables are presented as the frequency and proportion. Associations between variables were analysed using the Mann-Whitney U test and the Kruskal-Wallis test. Associations between variables were also analysed with the χ2 test. The odds ratio and 95% confidence interval (CI) were estimated. Receiver operating characteristic (ROC) curves were constructed and areas under the ROC curve (AUCs) were estimated and compared with the DeLong test. The PSAD, Proclarix, and ERSPC MRI model thresholds were selected to analyse the optimal sensitivity for csPCa. Sensitivity, specificity, positive and negative predictive values (PPV and NPV), accuracy, and rates of biopsies avoided, overdetection of iPCa, and misdiagnosis of csPCa were estimated. Decision curve analysis (DCA) was carried out to assess the net benefits. Clinical utility curves (CUCs) were used to check the correlation of rates of csPCa misdiagnosis and biopsies avoided regarding the thresholds on a continuous basis. A p value of <5% was considered significant. SPSS version 25 (IBM Corp., Armonk, NY, USA) and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses.
3. Results
3.1. Efficacy of Proclarix, PSAD, and the ERSPC MRI predictive model for csPCa detection across the PI-RADS categories
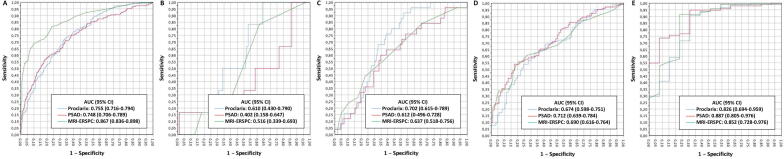
The distribution of PCa, csPCa, and iPCa among the 567 participants by PI-RADS category is presented in Table 1. csPCa was detected in 6% of men with PI-RADS <3, 14.8% of men with PI-RADS 3, 54.7% of men with PI-RADS 4, and 88% of men with PI-RADS 5 findings (p < 0.001). Fig. 1 shows the efficacy of Proclarix, PSAD, and the ERSPC MRI predictive model for the overall population and by PI-RADS category.
Table 1.
Distribution of men with suspected PCa by PI-RADS category and the corresponding rates of PCa, csPCa, and iPCa
| PI-RADS category | Men, n (%) | PCa, n (%) | csPCa, n (%) | iPCa, n (%) |
|---|---|---|---|---|
| 1 | 77 (13.6) | 10 (13.0) | 4 (5.2) | 6 (7.8) |
| 2 | 23 (4.1) | 5 (21.7) | 2 (8.7) | 3 (13.0) |
| 3 | 169 (29.8) | 53 (31.4) | 25 (14.8) | 28 (16.6) |
| 4 | 190 (33.5) | 129 (67.9) | 94 (54.7) | 25 (13.2) |
| 5 | 108 (19.0) | 99 (91.7) | 95 (88.0) | 4 (3.7) |
| All | 567 (100) | 296 (52.2) | 230 (40.6) | 66 (11.6) |
PI-RADS = Prostate Imaging-Reporting and Data System; PCa = prostate cancer; csPCa = clinically significant PCa; iPCa = insignificant PCa.
Fig. 1.
Efficacy of Proclarix, PSAD, and the ERSPC MRI predictive model for detection of clinically significant prostate cancer in the overall population and by PI-RADS category. Receiver operating characteristic curves and area under the curve (AUC) for (A) the overall population, (B) men with PI-RADS <3, (C) men with PI-RADS 3, (D) men with PI-RADS 4, and (E) men with PI-RADS 5 findings. PSAD = prostate-specific antigen density; ERSPC = European Randomized Study of Screening for Prostate Cancer; MRI = magnetic resonance imaging; PI-RADS = Prostate Imaging-Reporting and Data System; CI = confidence interval.
3.2. Characteristics of the cohort of men with PI-RADS 3 findings
Men with a PI-RADS 3 lesion represented 29.8% of all men with suspected PCa for whom prebiopsy mpMRI data were available (Table 1). The median age for this group was 66 yr and the median serum PSA was 6.0 ng/ml (Table 2). The rate of abnormal DRE was 7.1%, the rate of PCa family history was 6.5%, and the rate of men with prior negative biopsy results was 28.4%. Prostate biopsy showed benign tissue in 116 men (68.6%) and PCa in 53 men (31.4%), of whom 25 (47.2%) had csPCa and 28 (52.8%) had iPCa. The csPCa and iPCa detection rates were 14.8% and 16.6%, respectively (p = 0.573). csPCa was detected in both guided and systematic biopsies in 16 men (64%), and exclusively in five systematic biopsies (20%) and four guided biopsies (16%; p = 0.236).
Table 2.
Characteristics of men in PI-RADS category 3
| Characteristic | Result |
|---|---|
| Number of cases | 169 |
| Median age, yr (IQR) | 66 (60–72) |
| Median total PSA, ng/ml (IQR) | 6.0 (3.6–10.2) |
| Abnormal digital rectal examination, n (%) | 12 (7.1) |
| Median free PSA, ng/ml (IQR) | 1.1 (0.7–1.6) |
| Median prostate volume, ml (IQR) | 66 (45–85) |
| Median percentage free PSA, % (IQR) | 16.4 (11.5–20.7) |
| Median PSA density, ng/ml/cm3 (IQR) | 0.11 (0.07–0.16) |
| Repeat biopsy, n (%) | 48 (28.4) |
| Family history of PCa, n (%) | 11 (6.5) |
| Overall PCa detection, n (%) | 53 (31.4) |
| csPCa detection, n (%) | 25 (14.8) |
| iPCa detection, n (%) | 28 (16.6) |
PI-RADS = Prostate Imaging-Reporting and Data System; IQR = interquartile range; PSA = prostate-specific antigen; PCa = prostate cancer; csPCa = clinically significant PCa; iPCa = insignificant PCa.
3.3. Efficacy, net benefit, and clinical utility of Proclarix, PSAD and MRI-ERSPC predictive model for csPCa detection in PI-RADS 3
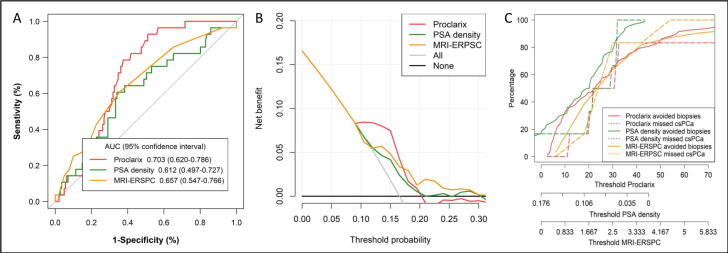
ROC curves for csPCa detection according to Proclarix, PSAD, and the ERSPC MRI model are presented in Fig. 2A. The AUC was 0.703 (95% CI 0.621–0.768) for Proclarix, 0.612 (95% CI 0.497–0.727) for PSAD, and 0. 657 (95% CI 0.547–0.766) for the ERSPC MRI model (p = 0.027). DCA showed a net benefit for Proclarix versus PSAD and the ERSPC MRI model at low thresholds within 0.09% versus 0.17% and 0.2%, respectively (Fig. 2B). CUCs showing the rates of csPCa missed and biopsies avoided in relation to the thresholds for the three tools are presented in Fig. 2C. Analysis of Proclarix scores, PSAD values, and ERSPC MRI likelihood values showed that the thresholds with the highest sensitivity for csPCa were 10%, 0.07 ng/ml/cm3, and 1.5%, which yielded sensitivity of 100% (25/25), 84% (21/25), and 96% (24/25), respectively. The corresponding specificity was 5% (36/144) for Proclarix, 28.5% (41/144) for PSAD, and 7.6% (11/144) for the ERSPC MRI model. The NPV and PPV were 100% (36/36) and 19.8% (25/133) for Proclarix, 91.1% (41/45) and 16% (21/124) for PSAD, and 91.7% (11/12) and 15.3% (24/157) for the ERSPC MRI model, respectively. The diagnostic accuracy was 36.1% (61/169) with Proclarix, 36.7% (62/169) with PSAD, and 20.7% (35/169) with the ERSPC MRI model. In terms of clinical efficacy, Proclarix would avoid 21.3% (36/169) of prostate biopsies and reduce overdetection of iPCa from 16.6% to 11.2% (19/169) without misdiagnosing csPCa. PSAD would avoid 26.2% (45/169) of prostate biopsies, reduce overdetection of iPCa from 16.6% to 11.2% (19/169), but misdiagnose 16% (four out of 25) of csPCa cases. The ERSPC MRI predictive model would avoid only 7.1% (12/169) of prostate biopsies, reduce overdetection of iPCa from 16.6% to 15.3% (26/169), and misdiagnose 4% (two out of 25) of csPCa cases, as shown in Table 3. The performance of Proclarix, PSAD, and the ERSPC MRI model according to biopsy status (biopsy-naïve vs repeat biopsy) is presented in Table 4. Proclarix outperformed PSAD and the ERSPC MRI model, detecting all csPCa cases in both subsets and avoiding 24.8% of prostate biopsies in the biopsy-naïve group and 12.5% in the repeat biopsy group.
Fig. 2.
Analysis of the efficacy, net benefit, and clinical utility of Proclarix, PSA density, and the ERSPC MRI predictive model for detection of clinically significant prostate cancer in men with a PI-RADS 3 lesion. (A) Receiver operating characteristic curves and area under the (AUC), (B) decision curve analysis, and (C) clinical utility curves. PSA = prostate-specific antigen; ERSPC = European Randomized Study of Screening for Prostate Cancer; MRI = magnetic resonance imaging; PI-RADS = Prostate Imaging-Reporting and Data System.
Table 3.
Performance of Proclarix, PSAD, and the ERSPC MRI predictive model for csPCa detection in men in the PI-RADS 3 category using the most sensitive thresholds
| Parameter | csPCs detection, n/N (%) |
||
|---|---|---|---|
| Proclarix (cutoff = 10%) | PSAD (cutoff = 0.07 ng/ml/cm3) | ERSPC MRI RC (cutoff = 1.5%) | |
| Sensitivity | 25/25 (100) | 21/25 (84.0) | 24/25 (96.0) |
| Specificity | 36/144 (25.0) | 41/144 (28.5) | 11/144 (7.6) |
| Negative predictive value | 36/36 (100) | 41/45 (91.1) | 11/12 (91.7) |
| Positive predictive value | 25/133 (19.8) | 21/124 (16.0) | 24/157 (15.3) |
| Accuracy | 61/169 (36.1) | 62/169 (36.7) | 35/169 (20.7) |
| Prostate biopsies avoided | 36/169 (21.3) | 45/169 (26.2) | 12/169 (7.1) |
| Decrease in iPCa overdetection | 9/169 (5.3) | 9/169 (5.3) | 2/169 (1.2) |
| Misdiagnosis of csPCa | 0/25 (0) | 4/25 (16.0) | 1/25 (4.0) |
PI-RADS = Prostate Imaging-Reporting and Data System; PCa = prostate cancer; iPCa = insignificant PCa; csPCa = clinically significant PCa; PSAD =prostate-specific antigen density; ERSPC = European Randomized Study of Screening for Prostate Cancer; MRI = magnetic resonance imaging; RC = risk calculator.
Table 4.
Performance of Proclarix, PSAD, and the ERSPC MRI model for csPCa detection using the most sensitive threshold in biopsy-naïve men and men undergoing repeat biopsy
| Parameter | csPCs detection, n/N (%) |
|||||
|---|---|---|---|---|---|---|
| Proclarix (cutoff = 10%) |
PSAD (cutoff = 0.07 ng/ml/cm3) |
ERSPC MRI RC (cutoff = 1.5%) |
||||
| Initial Bx | Repeat Bx | Initial Bx | Repeat Bx | Initial Bx | Repeat Bx | |
| Sensitivity | 18/18 (100) | 7/7 (100) | 15/18 (83.3) | 6/7 (85.7 | 17/18 (94.4) | 7/7 (100) |
| Specificity | 30/103 (29.1) | 6/41 (14.6) | 33/103(32.0) | 8/41 (19.5) | 6/103 (5.8) | 5/41 (12.2) |
| Negative predictive value | 30/30 (100) | 6/6 (100 | 33/36 (91.7) | 8/9 (88.9) | 6/7 (85.7) | 5/5 (100) |
| Positive predictive value | 18/91 (19.8) | 7/42 (16.7) | 15/85 (17.6) | 6/39 (15.4) | 17/114 (14.9) | 7/43 (16.3) |
| Accuracy | 48/121 (39.7) | 13/48 (27.1) | 48/121(39.7) | 14/48 (29.2 | 23/121 (19.0) | 13/48 (27.1) |
| Prostate biopsies avoided | 30/121 (24.8) | 6/48 (12.5) | 36/121 (29.8) | 9/48 (18.8) | 7/121 (5.8) | 5/48 (10.4 |
| Decrease in iPCa overdetection | 7/121 (5.8) | 2/48 (4.2) | 7/121 (5.8) | 2/48 (4.2) | 1/121 (0.8) | 1/48 (2.1) |
| Misdiagnosis of csPCa | 0/18 (0) | 0/7 (0) | 3/18 (16.7) | 1/7 (14.3) | 1/8 (5.6) | 0/7 (0) |
PSAD = prostate-specific antigen density; PCa = prostate cancer; csPCa = clinically significant PCa; iPCa = insignificant PCa; Bx = biopsy; ESPRC = European Randomized Study of Screening for Prostate Cancer; MRI = magnetic resonance imaging; RC = risk calculator.
4. Discussion
The efficacy of diagnostic tools changes in relation to the incidence of the disease in question. This is why we observed differences in utility for the three tools analysed across the PI-RADS categories. We noted a progressive increase in the csPCa detection rate across the PI-RADS categories, as well as differences in efficacy. Currently, most clinicians accept that prostate biopsies can be avoided for men with negative mpMRI because of its high NPV [4.5]. In the present study, the NPV of 94% observed represents an overall csPCa misdiagnosis rate of 2.6% and a 17.6% reduction in prostate biopsies, so it is acceptable to avoid prostate biopsies in these men. By contrast, many clinicians would not accept a test that does not guarantee 100% sensitivity for csPCa in men with PI-RADS >3 lesions. Thus, it makes sense to focus our attention on men with the recognised challenging PI-RADS 3 category [7], [8].
The incidence of PI-RADS 3 findings in our series of patients with suspected PCa was 29.8%, which is within the range of 14–46% reported in the literature. This incidence mainly depends on the proportions of biopsy-naïve men and men with a prior negative biopsy, which is approximately 30% in mixed samples [7]. The incidence of csPCa detected among the 169 men with PI-RADS 3 findings (14.8%) is also within the wide range (5–30%) reported in the literature [8]. We observed a net benefit of Proclarix over PSAD and the ERSPC MRI predictive model for determining the likelihood of csPCa, especially at low thresholds, for which high sensitivity is observed. This finding is consistent with the ROC curves, according to which Proclarix outperformed PSAD and the ERSPC MRI predictive model, showing 100% sensitivity, although its AUC of 0.703 seems suboptimal. This is positive for clinicians, given that Proclarix can detect all csPCa cases while avoiding 21.3% of all prostate biopsies (24.8% in biopsy-naïve men and 12.5% in those scheduled for repeat biopsy). Although PSAD seems slightly more specific than Proclarix, avoiding 26.3% of biopsies, it misdiagnoses 16% of csPCa cases, which is hardly acceptable. This PSAD performance is similar to previous findings [11], [12], [13]. The accuracy of the ERSPC MRI predictive model was notably lower compared to Proclarix and PSAD, except at high thresholds, at which the sensitivity is very low. Finally, the reduction in overdetection of iPCa was 5.3% for Proclarix and PSAD (decrease from 16.6% to 11.2%) and 1.2% for the ERSPC MRI model.
Modern markers have been analysed in the context of the current pathway for csPCa diagnosis and are intended to avoid mpMRI scans and subsequent prostate biopsies or to select appropriate candidates for prostate biopsy after mpMRI [17]. Some of these markers are combined with clinical independent predictors in predictive models [15], [18]. The Prostate Health Index (PHI) and PCA3 [19], PHI [20], [21], 4K [22], [23], [24], [25], and the Stockholm 3 test [26] have been analysed, although their specific behaviours regarding PI-RADS categories has never been reported as a main research objective. Data for PHI and SelectMDx could be extracted from analyses of overall series published in the literature [18], [27], [28] and one specific series of men with PI-RADS 3 findings [29]. Supplementary Table 1 summarises the clinical utility of PHI [27] and SelectMDx [18], [28], [29] for csPCa detection in men with suspected PCa and a PI-RADS 3 lesion in comparison to Proclarix in the present study. Fan et al. [27] analysed the PHI performance for a group of 56 men and observed a rate of avoidable biopsies of 67.9% while misdiagnosing one out of 16 men (6.3%) men with detected csPCa using a cutoff point of 50. Maggi et al. [18] reported that SelectMDx with a threshold of 13% misdiagnosed 12/14 men (85.7%) diagnosed with csPCa among a sample of 54, avoiding 33.3% of prostate biopsies. Hendriks et al. [28] found that SelectMDx with a threshold of 13% misdiagnosed 7/9 men (77.8%) diagnosed with csPCa among a sample of 38 men, avoiding 40.2% of prostate biopsies. We recently observed that SelectMDx with a threshold of 13% misdiagnosed four out of six men (66.7%) with csPCa in a sample of 62 men, avoiding 40.6% of prostate biopsies [29]. The present study shows that Proclarix is very sensitive for csPCa, making it reliable enough to reassure clinicians. PHI seems more sensitive than SelectMDx but less sensitive than Proclarix. Multicentre validation studies should be performed to confirm the effectiveness of any marker and cost-benefit studies regarding the quality-adjusted life years gained are desirable [30].
The present study was carried out on the largest published sample of men with suspected PCa and PI-RADS 3 findings; however, the sample size may still be a limitation because of the low incidence of csPCa. Unfortunately, we found no way to estimate the appropriate cohort size to assess the efficacy of certain tools owing to the lack of previous data. Although all studies use the same definition of csPCa, we understand that the true incidence of csPCa observed in surgical specimens may be overestimated by prostate biopsies. In the era of MRI and guided biopsies it seems important to analyse the efficacy of any tool for improving the detection of csPCa regarding PI-RADS categories. Results for the overall efficacy, net benefits, and clinical utility may result in confusion for clinicians. The overall analyses are important, but they do not guarantee the same effectiveness across the PI-RADS categories [12], [13], [28].
5. Conclusions
The efficacy of tools for the appropriate selection of candidates for prostate biopsy varies regarding PI-RADS categories. Proclarix performed better than PSAD and the ERSPC MRI predictive model in the challenging scenario of PI-RADS category 3. Proclarix was able to reach 100% detection of csPCa, avoiding almost a quarter of unnecessary prostate biopsies and reducing iPCa overdetection from 16.6% to 11.2%.
Author contributions: Juan Morote had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Morote.
Acquisition of data: Celma, Regis, Planas, Semidey, de Torres, Mast.
Analysis and interpretation of data: Morote, Campistol, Triquell.
Drafting of the manuscript: Morote, Campistol, Triquell.
Critical revision of the manuscript for important intellectual content: Trilla, Santamaria.
Statistical analysis: Morote.
Obtaining funding: Morote.
Administrative, technical, or material support: None.
Supervision: Morote, Trilla.
Other: None.
Financial disclosures: Juan Morote certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was financed in part by Instituto de Salud Carlos III (ES) (PI20/01666). Proteomedix provided Proclarix evaluations. The sponsors played no direct role in the study.
Acknowledgments: The likelihood of csPCa according to the ERSPC MRI risk calculator was assessed using the Prostate Cancer Research Foundation (Reeuwijk, The Netherlands) web application at www.prostatecancer-riskcalculator.com.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2021.12.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fan et al is ref 27. Maggi et al is ref 18 Please change to references, Fan et al is reference 27. Maggi et al is reference 18
References
- 1.Hugosson J., Roobol M.J., Månsson M., et al. A 16-yr follow-up of the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2019;76:43–51. doi: 10.1016/j.eururo.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mottet N., van den Bergh R.C.N., Briers E., et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force, Grossman D.C., Curry S.J., et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901–1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 4.Moldovan P.C., Van den Broeck T., Sylvester R., et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Sathianathen N.J., Omer A., Harriss E., et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the Prostate Imaging Reporting and Data System era: a systematic review and meta-analysis. Eur Urol. 2020;78:402–414. doi: 10.1016/j.eururo.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 6.Mazzone E, Stabile A, Pellegrino F, et al. Positive predictive value of Prostate Imaging Reporting and Data System version 2 for the detection of clinically significant prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol. In press. 10.1016/j.eururo.2021.07.027. [DOI] [PubMed]
- 7.Schoots I.G. MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions. Transl Androl Urol. 2018;7:70–82. doi: 10.21037/tau.2017.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Görtz M., Radtke J.P., Hatiboglu G., et al. The value of prostate-specific antigen density for Prostate Imaging-Reporting and Data System 3 lesions on multiparametric magnetic resonance imaging: a strategy to avoid unnecessary prostate biopsies. Eur Urol Focus. 2021;7:325–331. doi: 10.1016/j.euf.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Klocker H., Golding B., Weber S., et al. Development and validation of a novel multivariate risk score to guide biopsy decision for the diagnosis of clinically significant prostate cancer. BJUI Compass. 2020;1:15–20. doi: 10.1002/bco2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steuber T, Heidegger I, Kafka M, et al. PROPOSe: a real-life prospective study of Proclarix, a novel blood-based test to support challenging biopsy decision-making in prostate cancer. Eur Urol Oncol. In press. 10.1016/j.euo.2020.12.003. [DOI] [PubMed]
- 11.Boesen L., Nørgaard N., Løgager V., et al. Prebiopsy biparametric magnetic resonance imaging combined with prostate-specific antigen density in detecting and ruling out Gleason 7–10 prostate cancer in biopsy-naïve men. Eur Urol Oncol. 2019;2:311–319. doi: 10.1016/j.euo.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Morote J., Celma A., Planas J. Re: Lars Boesen, Nis Nørgaard, Vibeke Løgager, et al. Prebiopsy biparametric magnetic resonance imaging combined with prostate-specific antigen density in detecting and ruling out Gleason 7–10 prostate cancer in biopsy-naïve men. Eur Urol Oncol. 2019;2:311–319. doi: 10.1016/j.euo.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Morote J., Celma A., Díaz F., et al. Prostatic-specific antigen density behavior according to multiparametric magnetic resonance imaging result. Urol Oncol. 2020;38:410–417. doi: 10.1016/j.urolonc.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Alberts A.R., Roobol M.J., Verbeek J.F.M., et al. Prediction of high-grade prostate cancer following multiparametric magnetic resonance imaging: improving the Rotterdam European Randomized Study of Screening for Prostate Cancer risk calculators. Eur Urol. 2019;75:310–318. doi: 10.1016/j.eururo.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Macagno A., Athanasiou A., Wittig A., et al. Analytical performance of thrombospondin-1 and cathepsin D immunoassays part of a novel CE-IVD marked test as an aid in the diagnosis of prostate cancer. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein J.I., Egevad L., Amin M.B., et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 17.Vickers AJ, Russo G, Lilja H, et al. How should molecular markers and magnetic resonance imaging be used in the early detection of prostate cancer. Eur Urol Oncol. In press. 10.1016/j.euo.2021.01.010. [DOI] [PubMed]
- 18.Fan Y.H., Pan P.H., Cheng W.M., et al. The Prostate Health Index aids multi-parametric MRI in diagnosing significant prostate cancer. Sci Rep. 2021;11:1286. doi: 10.1038/s41598-020-78428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porpiglia F., Russo F., Manfredi M., et al. The roles of multiparametric MRI, PCA3, and PHI: which is the best predictor of prostate cancer after a negative biopsy? Results of a prospective study. J Urol. 2014;192:60–66. doi: 10.1016/j.juro.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Roobol M.J., Vedder M.M., Nieboer D., et al. Comparison of two prostate cancer risk calculators that include the Prostate Health Index. Eur Urol Focus. 2015;1:185–190. doi: 10.1016/j.euf.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Gnanapragasam V.J., Burling K., George A., et al. The Prostate Health Index adds predictive value to multi-parametric MRI in detecting significant prostate cancers in a repeat biopsy population. Sci Rep. 2016;6:35364. doi: 10.1038/srep35364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punnen S., Nahar B., Soodana-Prakash N., et al. Optimizing patient’s selection for prostate biopsy: a single institution experience with multi-parametric MRI and the 4Kscore test for the detection of aggressive prostate cancer. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falagario U.G., Martini A., Wajswol E., et al. Avoiding unnecessary magnetic resonance imaging (MRI) and biopsies: negative and positive predictive value of MRI according to prostate-specific antigen density, 4Kscore and risk calculators. Eur Urol Oncol. 2020;3:700–704. doi: 10.1016/j.euo.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Marzouk K., Ehdaie B., Vertosick E., Zappala S., Vickers A. Developing an effective strategy to improve the detection of significant prostate cancer by combining the 4Kscore and multiparametric MRI. Urol Oncol. 2019;37:672–677. doi: 10.1016/j.urolonc.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbeek J.F.M., Bangma C.H., Kweldam C.F., et al. Reducing unnecessary biopsies while detecting clinically significant prostate cancer including cribriform growth with the ERSPC Rotterdam risk calculator and 4Kscore. Urol Oncol. 2019;37:138–144. doi: 10.1016/j.urolonc.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Grönberg H., Eklund M., Picker W., et al. Prostate cancer diagnostics using a combination of the Stockholm3 blood test and multiparametric magnetic resonance imaging. Eur Urol. 2018;74:722–728. doi: 10.1016/j.eururo.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Maggi M., Del Giudice F., Falagario U.G., et al. SelectMDx and multiparametric magnetic resonance imaging of the prostate for men undergoing primary prostate biopsy: a prospective assessment in a multi-institutional study. Cancers. 2021;13:2047. doi: 10.3390/cancers13092047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendriks R.J., van der Leest M.M.G., Israël B., et al. Clinical use of the SelectMDx urinary-biomarker test with or without mpMRI in prostate cancer diagnosis: a prospective, multicenter study in biopsy-naïve men. Prostate Cancer Prostat Dis. 2021;24:1110–1119. doi: 10.1038/s41391-021-00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morote J, Díaz F, Celma A, et al. Behaviour of SelectMDx and prostate-specific antigen density in the challenging scenario of Prostate Imaging-Reporting and Data System category 3 lesions. Eur Urol. In press. 10.1016/j.eururo.2021.09.019. [DOI] [PubMed]
- 30.Govers T.M., Hessels D., Vlaeminck-Guillem V., et al. Cost-effectiveness of SelectMDx for prostate cancer in four European countries: a comparative modeling study. Prostate Cancer Prostat Dis. 2018;22:101–109. doi: 10.1038/s41391-018-0076-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.