Abstract
The first step in the conversion of the isoprenoid intermediate, farnesyl diphosphate (FDP), to sesquiterpene phytoalexins in cotton (Gossypium barbadense) plants is catalyzed by δ-cadinene (CDN) synthase. CDN is the precursor of desoxyhemigossypol and hemigossypol defense sesquiterpenes. In this paper we have studied the mechanism for the cyclization of FDP and the putative intermediate, nerolidyl diphosphate, to CDN. A purified recombinant CDN synthase (CDN1-C1) expressed in Escherichia coli from CDN1-C1 cDNA isolated from Gossypium arboreum cyclizes (1RS)-[1-2H](E, E)-FDP to >98% [5-2H]and [11-2H]CDN. Enzyme reaction mixtures cyclize (3RS)-[4,4,13,13,13-2H5]-nerolidyl diphosphate to 62.1% [8,8,15,15,15-2H5]-CDN, 15.8% [6,6,15,15,15-2H5]-α-bisabolol, 8.1% [6,6,15,15,15-2H5]-(β)-bisabolene, 9.8% [4,4,13,13-2H4]-(E)-β-farnesene, and 4.2% unknowns. Competitive studies show that (3R)-nerolidyl diphosphate is the active enantiomer of (3RS)-nerolidyl diphosphate that cyclized to CDN. The kcat/Km values demonstrate that the synthase uses (E,E)-FDP as effectively as (3R)-nerolidyl diphosphate in the formation of CDN. Cyclization studies with (3R)-nerolidyl diphosphate show that the formation of CDN, (E)-β-farnesene, and β-bisabolene are enzyme dependent, but the formation of α-bisabolol in the reaction mixtures was a Mg2+-dependent solvolysis of nerolidyl diphosphate. Enzyme mechanisms are proposed for the formation of CDN from (E,E)-FDP and for the formation of CDN, (E)-β-farnesene, and β-bisabolene from (3RS)-nerolidyl diphosphate. The primary structures of cotton CDN synthase and tobacco epi-aristolochene synthase show 48% identity, suggesting similar three-dimensional structures. We used the SWISS-MODEL to test this. The two enzymes have the same overall structure consisting of two α-helical domains and epi-aristolochene synthase is a good model for the structure of CDN synthase. Several amino acids in the primary structures of both synthases superimpose. The amino acids having catalytic roles in epi-aristochene synthase are substituted in the CDN synthase and may be related to differences in catalytic properties.
Cotton (Gossypium barbadense) synthesizes the defense sesquiterpenes desoxyhemigossypol (dHG) and hemigossypol (HG) in response to invasion by pathogenic fungi Verticillium dahliae and Fusarium oxysporum f. sp. vasinfectum (Bell, 1967). HG is the precursor of gossypol (G), which is the predominant terpenoid in glands in the seed and roots.
In cotton, δ-cadinene (CDN) synthase catalyzes the first step in the synthesis of dHG and HG from farnesyl diphosphate (FDP; Benedict et al., 1995; Chen et al., 1995; Davis and Essenberg, 1995; Davis et al., 1996). This reaction is induced by the infection of cotton with V. dahliae (Benedict et al., 1995; Bianchini et al., 1999). The infusion of cotton cotyledons with 3H-CDN labels dHG and HG (Davis and Essenberg, 1995). Four cDNAs of CDN synthase (cdn1-C1, cdn1-C14, cdn1-A, and cdn1-C2) from Gossypium arboreum have been isolated and the molecular weight and kinetic properties of the expressed recombinant CDN synthase (CDN1-C1) for FDP have been reported (Chen et al., 1995, 1996; Meng et al., 1999). A cDNA from cotton has been characterized that is 95% identical with cdn1-C1 and cdn1-C14 (Davis et al., 1998). Crude homogenates of cotton stems infected with V. dahliae utilized FDP and nerolidyl diphosphate (NDP) for the synthesis of CDN (Benedict et al., 1995; Alchanati et al., 1998). In this earlier research CDN was separated by HPLC and analyzed by gas chromatography-mass spectrometry (GC-MS). Other hydrocarbons generated in the crude extracts were not identified.
Using a purified CDN1-C1expressed by Escherichia coli from cdn1-C1 cDNA we now show that this CDN1-C1 is a high-fidelity enzyme forming >98% CDN from E,E-FDP. With NDP as a substrate, CDN1-C1 forms multiple sesquiterpene products. We propose a mechanism accounting for the formation of these sesquiterpenes from FDP and NDP. In addition, we have modeled the structure of CDN1-C1 using the known crystal structure of tobacco epi-aristolochene synthase (TEAS; Starks et al., 1997). We note differences in the active sites that may be related to the formation of different products by the two enzymes and the formation of different products from FDP and NDP by CDN1-C1.
RESULTS
Cyclization of FDP
The GC-MS analysis of the reaction products from the cyclization of (1RS)-[1-2H](E,E)-FDP by the purified CDN1-C1 showed one significant peak with a retention time of 19.62 min. This peak accounted for >98% of the reaction products. The major ions and the relative intensities from the mass spectrum of this hydrocarbon are given in the “Materials and Methods” and agree with the mass spectrum of authentic CDN. A previous fragmentation scheme (Benedict et al., 1995) accounts for the formation of [5-2H]and [11-2H]CDN from the (1RS)-[1-2H](E,E)-FDP by the purified CDN1-C1. These data substantiate that the recombinant enzyme is similar to the native CDN synthase present in the stem homogenates of cotton. The recombinant CDN1-C1 cyclizes E,E-FDP to a single hydrocarbon CDN, characterizing the enzyme as a single product sesquiterpene cyclase.
Cyclization of (3-RS)-NDP
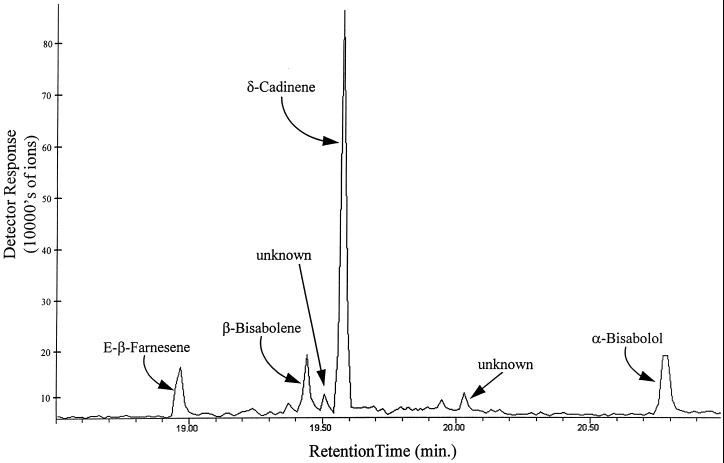
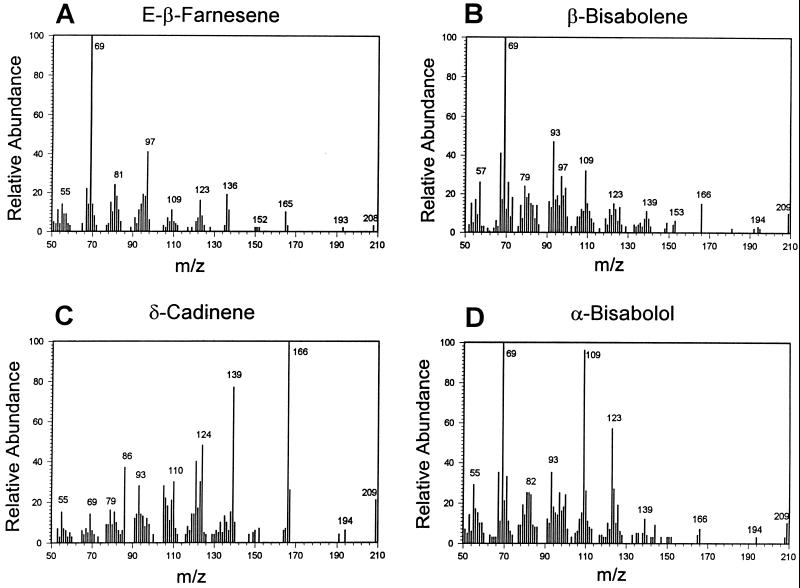
The GC-MS analysis of the products formed in the CDN1-C1 reaction mixture containing (3RS)- [4,4,13,13,13-2H5]-NDP is shown in Figures 1 and 2. The hydrocarbon fraction was separated into four major peaks with retention times of 18.96, 19.44, 19.57, and 20.77 min and two unknown peaks with retention times of 19.50 and 20.03 min (Fig. 1). The mass spectra of the major peaks are shown in Figure 2. The identity, labeling pattern, and the percentage of the products in the reaction mixture were determined by comparing these GC-MS analyses with the GC retention times and mass spectra of known amounts of authentic sesquiterpenes. These comparisons showed that the product with retention time of 18.95 min was 9.8% [4,4,13,13-2H4]-E(β)-farnesene; the product with retention time of 19.44 min was 8.1% [6,6,15,15,15-2H5]-β-bisabolene; the product with retention time of 19.57 min was 62.1% [8,8,15,15,15-2H5]-CDN; and the product with retention time of 20.77 min was 15.8% [6,6,15,15,15–2H5]-α-bisabolol.
Figure 1.
GC-MS analysis of the sesquiterpene products formed from the cyclization of (3RS)-[4,4,13,13,13-2H5]-NDP by recombinant CDN1-C1.
Figure 2.
Mass spectra of the sesquiterpenes formed from the cyclization of (3RS)-[4,4,13,13,13-2H5]-NDP by CDN1-C1. A, [4,4,13,13-2H4]-(E)-β-farnesene. B, [6,6,15,15,15-2H5]-β-bisabolene. C, [8,8,15,15,15-2H5]-CDN. D, [6,6,15,15,15-2H5]-α-bisabolol.
The Cyclization of (3R)-NDP and (3RS)-NDP
The cyclization of (3R)-NDP and (3RS)-[4-2H2]-NDP to CDN by CDN1-C1 was determined by recording the total number of ions produced by the fragmentation of CDN and deuterated CDN by GC-MS (Table I). The extraction of CDN with an m/z+ value of 204 for parent ion formed in the reaction mixture by the enzymatic cyclization of (3R)-NDP and subsequent GC-MS analysis produced 4.04 × 106 ions. In a similar manner, the GC-MS analysis of CDN with an m/z+ value of 206 formed from the cyclization of (3RS)-[4-2H2]-NDP produced 1.84 × 106 ions. The number of ions from CDN produced from the (3R)-NDP was 2.19 times greater than the number of ions from CDN produced from (3RS)-[4-2H2]-NDP. This was probably due to the fact that the concentration of the (3R)-NDP in the reaction mixture was two times the concentration of the (3R)-[4-2H2]-NDP enantiomer in the other reaction mixture. In the reaction mixture containing equal concentrations of (3R)-NDP and (3RS)-[4-2H2]-NDP, the number of ions produced from CDN formed from (3R)-NDP was over two times the number of ions from CDN formed from (3RS)-[4-2H2]-NDP. As a whole, these results demonstrate that the (3R)-NDP enantiomer of (3RS)-NDP was preferentially cyclized to CDN by CDN1-C1.
Table I.
The formation of CDN from the cyclization of (3R)-NDP and (3RS)-[4-2H2]-NDP by CND1-C1
| Reaction Mixture Contained | No. of
Ions from the GC-MS of CDNa
|
|
|---|---|---|
| Unlabeled CDN | Deuterated CDN | |
| (3R)-NDP and (3RS)-[4-2H2]-NDP | 2.7 × 106 | 7.5 × 105 |
| (3RS)-[4-2H2]-NDP | – | 1.84 × 106 |
| (3R)-NDP | 4.04 × 106 | – |
The unlabeled CDN formed from the (3R)-NDP and the deuterated CDN formed from (3RS)-[4-2H2]-NDP were chromatographically separated on the capillary column making possible a determination of the total ions of CDN and [2H2]-CDN by the GC-MS analysis. Equimolar amounts of (3R)-NDP and (3RS)-[4-2H2]-NDP were used in these experiments.
The Sesquiterpene Products Produced from (3R)-NDP
The data in Table II show the total ions resulting from the fragmentation of (E)-β-farnesene, β-bisabolene, CDN, and α-bisabolol produced from (3R)-NDP in reaction mixtures of CDN1-C1. It is seen that the conversion of (3R)-NDP to (E)-β-farnesene, β-bisabolene, and CDN was enzyme dependent, but the formation of α-bisabolol from (3R)-NDP was not. In separate experiments we have shown that the formation of α-bisabolol from (3R)-NDP was dependent on the concentration of Mg2+ in the reaction mixture. Thus, the α-bisabolol was produced by a Mg2+-dependent solvolysis of the (3R)-NDP in a reaction similar to the metal ion dependent solvolysis of an allylic pyrophosphate (Brems and Rilling, 1977) and the nonenzyme solvolysis of neryl and geranyl pyrophosphate (Chayet et al., 1984).
Table II.
The sesquiterpene products formed from (3R)-NDP
| Sesquiterpene Product | No. of ions from GC-MS analysis
|
|
|---|---|---|
| Plus CDN1-C1 | Minus CDN1-C1 | |
| (E)-β-Farnesene | 5.4 × 105 | None |
| β-Bisabolene | 5.9 × 105 | None |
| CDN | 4.7 × 106 | None |
| α-Bisabolol | 1.1 × 106 | 1.4 × 106 |
The Kinetic Properties of CDN Synthase
The kinetic properties of the purified recombinant CDN1-C1 are shown in Table III. The Km and kcat values for FDP are similar to these kinetic values reported for His-tag recombinant CDN1-C1 synthase (Chen et al., 1995). The kcat and Km values of the enzyme for (3R)-NDP were one-tenth of the values for E,E-FDP. The kcat/Km values of the enzyme for E,E-FDP and (3R)-NDP were similar, indicating an equal effectiveness of the CDN1-C1 toward both substrates.
Table III.
The kinetic properties of the purified recombinant CDN1-C1
| Substrate | Km | kcat | kcat/Km |
|---|---|---|---|
| μm | m−1 s−1 | ||
| E,E-FDP | 6.05 | 2.6 × 10−2/s | 4.3 × 10−3 |
| (3R)-NDP | 0.65 | 2.7 × 10−3/s | 4.1 × 10−3 |
The Proposed Reaction Mechanisms for the Cyclization of (E,E)-FDP and (3R)-NDP by CDN1-C1 Synthase
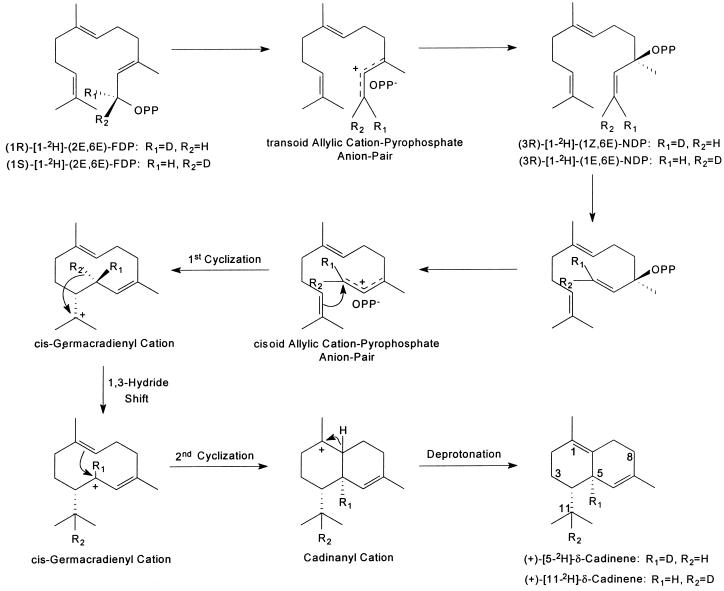
The proposed reaction mechanism for the cyclization of (1RS)-[1-2H](E,E)-FDP to CDN by CDN1-C1 is shown in Figure 3. The mechanism is consistent with the labeling pattern of the CDN from the deuterated FDP and with the demonstration that (3R)-NDP was the active enantiomer of the (3RS)-NDP mixture cyclized to CDN and the demonstration that (E,E)-FDP and (3R)-NDP were used with equal effectiveness for the formation of CDN by CDN1-C1. In the proposed reaction mechanism (Fig. 3), it has not been determined which hydrogen atom in the cis-germacradienyl cation undergoes the hydride shift. If R2 undergoes the hydride shift and it is a deuterium atom, then (+)−[11-2H]CDN is produced. If R2 is a hydrogen atom and R1 is a deuterium atom, (+)−[5-2H]CDN is produced. On the other hand if R1 undergoes the hydride shift and it is a deuterium atom, (+)−[11-2H]CDN is produced. And if R1 is a hydrogen atom and R2 is a deuterium atom, (+)−[5-2H]CDN is produced. Although we acknowledge the possibilities of the involvement of R2 and R1, in the hydride shift only the involvement of R2 is shown in Figure 3 because in a concerted reaction only the shift of R2 will result in CDN of the correct stereochemistry.
Figure 3.
Proposed reaction mechanism for the cyclization of (1RS)-[1-2H](E,E)-FDP by CDN1-C1.
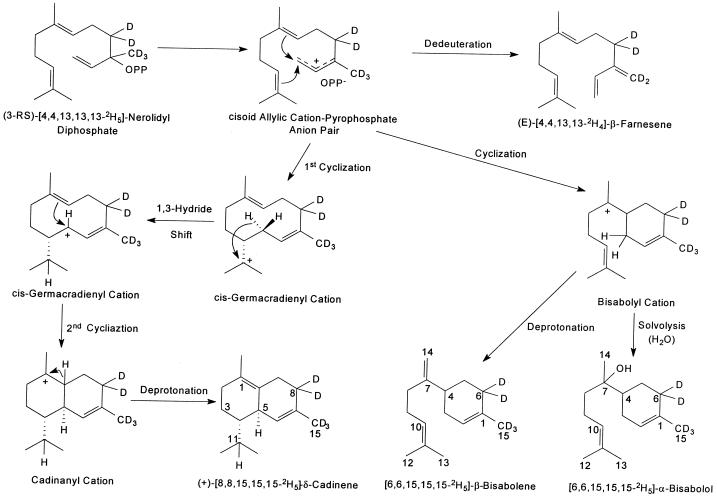
The proposed reaction mechanisms for the formation of (E)-β-farnesene, β-bisabolene, CDN, and α-bisabolol from (3R)-[4,4,13,13,13-2H5]-NDP in reaction mixtures of CDN1-C1 are shown in Figure 4. The mechanisms are consistent with the labeling patterns of (E)-β-farnesene, β-bisabolene, CDN, and α-bisabolol from the deuterated NDP; and with the demonstration that the formation of (E)-β-farnesene, β-bisabolene, and CDN from (3R)-NDP was dependent on the presence of CDN1-C1, but the formation of α-bisabolol from (3R)-NDP was a Mg2+-dependent solvolysis reaction. The volatile terpenes in cotton include CDN, γ-bisabolene, and α-bisabolol (Bell, 1986). If NDP is a substrate for CDN synthase in cotton, it is possible that the enzyme could contribute to the formation of α-bisabolol by a Mg2+-dependent solvolysis of the bisabolyl cation.
Figure 4.
Proposed reaction mechanism for the cyclization of (3RS)-[4,4,13,13,13-2H5]-NDP.
Modeling the CDN1-C1 Primary Structure and Crystal Structure of TEAS
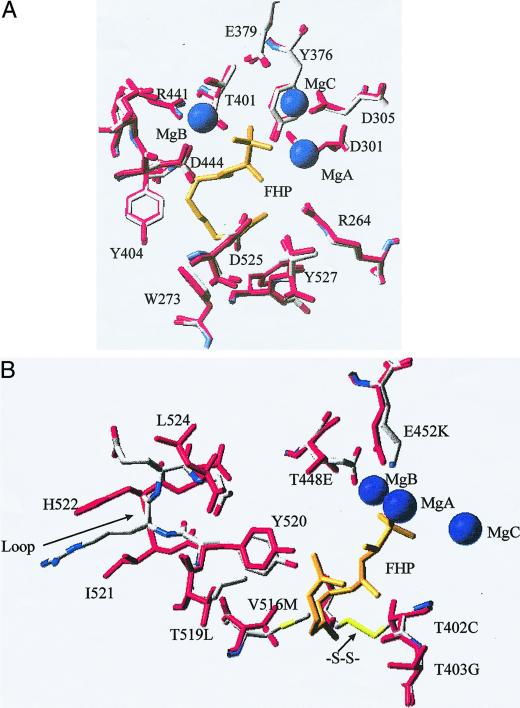
The amino acid sequence of CDN1-C1 was modeled into the crystal structure of TEAS with bound inhibitor FHP (Starks et al., 1997). The root mean square deviation for the fit of the backbone atoms was 0.035 nm. Thus, the two enzymes have the same overall structure consisting of two α -helical domains and the TEAS is a good model for the structure of CDN1-C1. The modeling approach based on the primary structure of CDN1-C1 and known crystal structure of TEAS can be used to develop a hypothesis about the active site structure of CDN1-C1.
Figure 5, A and B show two views of the active site region of the modeled structure of CDN1-C1 synthase compared with the actual structure of TEAS. Amino acid residues that are within 0.4 nm of the FHP and the three Mg(II) ions are shown. In Figure 5A, the amino acids that superimpose in the model provide a visual indication of the quality of the model for the CDN1-C1 structure that is provided by the TEAS structure. In Figure 5B, the amino acids that differ in identity or spatial location are shown. The differences occur in two regions and can be related to the differences in the enzyme mechanisms. The amino acid side chains that are involved in the coordination of Mg(II) B in TEAS are substituted in CDN1-C1, Thr-448 to Glu, and Glu-452 to Lys. Several differences are seen in the region of the active site that binds FHP. Residues 519 through 525 form a mobile loop in the TEAS structure. The amino acid residues in CDN1-C1 at the ends of the loop are superimposed on the TEAS residues. This can be seen from the positions of the backbone atoms of position 519 and the backbone and side chain atoms of position 525. However, the other positions in the loop in CDNl-Cl show only poor agreement with those of the actual TEAS structure. This is a result of deletion of the amino acid in the loop of CDN1-C1 relative to TEAS, L524Δ. One consequence of the deletion is that Tyr 520, although conserved, is modeled to a different position in CDN1-C1; the hydroxyl group is moved 0.23 nm and is 0.14 nm further from C-10 of FHP than in the TEAS structure. Two other substitutions, Val-516 to Met and Thr-519 to Leu, decrease the size of the active site cavity in CDN1-C1. The distance from the end atom of residue 516 to C5 of FHP is decreased by 0.27 nm in CDN1-C1. In CDN1-C1 Thr-402 is substituted by Cys and the modeled structure shows a disulfide bond between the new Cys side chain and the side chain of the conserved Cys-440. The sulfur atom of Cys-440 moves 0.09 nm upon formation of the disulfide bond enlarging the active site. As a whole, the change in the active site size due to these substitutions is likely to change the conformation of the bound FDP and results in different products.
Figure 5.
Active site region of CDN1-C1 modeled on the TEAS structure. Amino acids from the crystal structure of TEAS within 0.4 nm of FHP and the MG (II)n ions are shown in red and those from the modeled CDN1-C1 are shown in CPK coloring. MG (II) ions are shown as blue spheres and FHP is shown in gold. Amino acid numbers are shown for TEAS. A, Identical active site residues for TEAS and CDN1 synthase. B, Active site residues for TEAS and CDN1-C1 that differ in identity or position. The arrow labeled “loop” points to the mobile loop from TEAS residues 519 through 525. The arrow labeled “S-S” points to the disulfide bond between C402 and C440 in the CDN1-C1 model. The figure was constructed with the Swiss Pdb Viewer (Swiss Institute of Bioinformatics, Geneva).
DISCUSSION
The results in this paper demonstrate the versatility of CDN1-C1 in catalyzing the formation of a single product from FDP (the universal substrate for sesquiterpene synthases) and multiple products from NDP. The proposed mechanisms for the cyclization of E,E-FDP and (3R)-NDP include the formation of a cisoid allylic cation-pyrophosphate anion pair. With (1RS)-[1-2H](E,E)-FDP as the substrate, the formation of this intermediate involves ionization and isomerization to (3R)-NDP followed by a subsequent ionization to the ion pair and a C1-C10 cyclization to a cis-germacradienyl cation, a 1,3-hydride shift, a second cyclization to a cadinanyl cation, and deprotonation to [5-2H]and [11-2H]CDN. This mechanism is similar to the mechanism for the cyclization of E,E-FDP to the hydroxylated cadalene epicubenol by epicubenol synthase from Streptomyces sp. LL-B7 (Cane and Tandon, 1995). Since CDN has been shown to be the precursor of dHG and HG in cotton (Davis and Essenberg, 1995), the proposed mechanism for the cyclization of E,E-FDP to CDN by CDN1-C1 accounts for the formation of the carbon structure of these defense sesquiterpenes.
The cyclization of (3RS)-[4,4,13,13,13-2H5]-NDP to [8,8,15,15,15-2H5]-CDN clearly supports the mechanism involving the ionization of NDP to a cisoid allylic cation-pyrophosphate anion pair followed by a C1-C10 cyclization to a cis-germacradienyl cation, a 1,3-hydride shift, a second cyclization, and deprotonation to CDN. The labeling patterns of (E)-β-farnesene, β-bisabolene, and α-bisabolol formed from the cyclizations of (3RS)-[4,4,13,13,13-2H5]-NDP also support the mechanisms involving the ionization of NDP to a cisoid allylic cation-pyrophosphate anion pair followed by a C1-C6 cyclization to a bisabolyl cation and a deprotonation to β-bisabolene. A dedeuteration of the ion pair can also occur to form (E)-β-farnesene. The formation of α-bisabolol from NDP is a nonenzymatic reaction by a Mg2+-dependent solvolysis. The formation of a single sesquiterpene product from FDP and multiple sesquiterpene products from NDP demonstrate the versatility of the CDN1-C1.
The reaction mechanisms presented in Figures 3 and 4 postulate the intermediacy of NDP in the cyclization of FDP to CDN. However, the new findings reported in this paper show that the terpene cyclase of cotton, CDN1-C1, behaves quite differently toward free NDP offered as a substrate than toward its natural substrate FDP. Whereas FDP is converted to a single product CDN, NDP is converted by the enzyme to CDN plus (E)-β-farnesene and β-bisabolene. The Km for NDP is one-tenth the Km for FDP, suggesting that NDP may bind more tightly to the enzyme; Kcat for NDP is one-tenth the Kcat for FDP, demonstrating that the E·NDP complex formed from free NDP is converted to product much more slowly than the E·FDP complex. These observations suggest that if NDP is an intermediate in the catalysis of FDP by CDN synthase, its origin from free NDP (Fig. 4) or from enzyme-bound FDP (Fig. 3) might determine different rotational conformations about the bond between C-2 and C-3, leading to cisoid allylic cations with different conformations. The conformation arising from FDP would be favorable for cyclization to the cis-germacradienyl cation, whereas the conformation formed from free NDP would have a 62% probability of cyclizing to cis-germacradienyl cation and thence to CDN, an 8.1% probability of cyclizing to the bisabolyl cation instead (going thence to β-bisabolene), or a 9.8% probability of deprotonation to (E)-β-farnesene. It is possible that NDP is not an intermediate in the CDN1-C1 reaction, but that the transoid allylic cation (Fig. 3) formed from FDP is able to rotate to a cisoid allylic cation passing through a conformation that favors cyclization to cis-germacradienyl cation before it reaches the conformation that permits cyclization to the bisabolyl cation. When NDP is the substrate, it would form a cisoid allylic cation in a conformation with probabilities of cyclization or deprotonation to (E)-β-farnesene.
Sequence comparisons of sesquiterpene synthases (Bohlmann et al., 1998) demonstrate that regions of high similarity control common cyclization reactions (ionizations, charge stabilizations, and deprotonations) and the more variable regions may lead to differences in active sites and shapes that enforce conformation of substrates and intermediates to direct selective catalysis. To gain insight into regions of the active site of CDN1-C1 that may contribute to the proposed reaction mechanisms leading to single and multiple products, we modeled the amino acid sequence of CDN1-C1 into the crystal structure of TEAS.
The modeled CDN1-C1 structure shows that two amino acid side chains that are involved in the coordination of Mg(II) B in TEAS are substituted in CDN1-C1, Thr-448 is changed to Glu, and Glu-452 is changed to Lys. These substitutions may alter the binding of Mg(II) B in CDN1-C1. The Lys residue at position 452 may have a catalytic role in lieu of the Mg(II) ion. In any case, the electrostatic environment in this region of the active site may be different in these two enzymes. The initial steps in the reaction mechanisms postulated for sesquiterpene cyclases involve ionization of the pyrophosphate that is bound in the vicinity of Mg(II) B. The subsequent steps are different for these two enzymes. For TEAS the carbocation that is generated at C1 of FDP by the removal of pyrophosphate is attacked to give the 10-membered ring trans, trans-germacradienyl cation. In contrast, the postulated mechanism for CDN1-C1 involves ionization and isomerization of E,E-FDP to a cisoid allylic cation-pyrophosphate anion pair that is cyclized to a 10-membered ring cation cis-germacradienyl cation or a 6-membered ring cation bisabolyl cation, depending on whether the substrate is FDP or NDP. The difference in the structures around Mg(II) B are consistent with differences in the early steps in the mechanisms for the two enzymes. The sensitivity of the course of the reaction for CDN1-C1 to structure differences in this region is clearly indicated by the different products that are obtained from FDP and NDP.
There are several differences in the substrate binding pocket. Tyr-520 is modeled to a different position in the loop in the CDN1-C1. In the TEAS structure with bound FHP, the residues in the loop have higher temperature factors than the residues at the ends of the loop. Thus, this loop appears to be a flexible region. Differences in the flexibility of this loop may be reflected in the synthesis of multiple products from NDP and a single product from FDP by CDN1-C1 and differences in products from FDP by TEAS and CDN1-C1. The change in the active site due to substitution of Val-516 with Met and Thr-519 with Leu and the substitution of Thr-402 by Cys with a disulfide bond between the new Cys side chain and the side chain of the conserved Cys-440 may collectively change the conformation of the bound FDP and result in different products by TEAS and CDN1-C1. In the mechanism proposed for TEAS (Starks et al., 1997), electric dipole moments from the peptide carbonyls of residues 401 and 402 and from the hydroxyl group of Thr-403 are oriented toward the positive charge that is delocalized over C1, C2, and C3 of the allylic carbocation that is formed by removal of the diphosphate from FDP. The dipoles orient the cationic end of the bound farnesyl chain for attack on C10. In CDN1-C1 Thr-403 is substituted by Gly, which eliminates one of the dipoles. The peptide carbonyls at position 401 and 402 are, of course, retained in CDN1-C1. However, addition of the modeled disulfide bond between Cys-402 and Cys-440 is expected to alter the electrostatic environment and influence the fate of the carbocation intermediates.
MATERIALS AND METHODS
Chemicals
[1-3H](E,E)-FDP (832.5 Gbq nmol−1) was purchased from New England Nuclear (Boston). The ammonium salt of FDP was purchased from Sigma (St. Louis). The (1RS)-[1-2H](E,E)-FDP was synthesized by the procedure described by Benedict et al. (1995). The (3RS)-nerolidyl diphosphate and the (3RS)-[4,4,13,13,13-2H5]-nerolidyl diphosphate were synthesized by the procedures described by Alchanati et al. (1998).
Instrumentation
1H (300 MHz), 13C (75 MHz), and 31P (121 MHz) nuclear magnetic resonance (NMR) spectra were recorded on a Bruker ARX-300 instrument. GC-MS analyses were performed using a Hewlett-Packard 5989B GC-Mass Spectrometer (Palo Alto, CA) equipped with a split/splitless injector and a BP-1 fused-silica capillary column (25 m × 0.22 mm I.D., 0.25-μm film thickness; Scientific Glass Engineering, Berkeley, CA). Helium flow was 1.0 mL min −1. A 1.0-μL sample of the hexane-EtOAc extract was injected automatically. The GC column oven was programmed as follows: 40°C, hold for 9 min; heat to 280°C at 15 min and hold for 5 min; purge was initially off and turned on 1 min after injection. Source was at 260°C, quadruple 100°C, injection 280°C, and transfer line 280°C.
The analytical GC was performed on a Hewlett-Packard HP 6890 capillary instrument equipped with an HP7683 injector, flame ionization detector, and HP6890 integrating recorder, with helium carrier gas at 20 psi head pressure using 1-μL injection volumes in a splitless mode. A 25 m × 0.25 mm ID BPI column (Scientific Glass Engineering) was used with a temperature program of 40°C for 9 min, then to 300°C at 15° min.
Synthesis of (3R)-(6E)-3,7,11-Trimethyl-1,6,10-Dodecatrien-3-yl Triammonium Diphosphate [(3R)-Nerolidyl Diphosphate, (3R)-NDP]
(3R)-Nerolidol was prepared from E,E-farnesol according to the procedure of Donath and Boland (1995). (3R)-Nerolidol is pyrophosphorylated according to the method of Popjak et al. (1962) to give (3R)-nerolidyl diphosphate. The procedure of Holloway and Popjak (1967) was used to purify the (3R)-nerolidyl diphosphate. The 1H NMR (D2O-ND4OD, pH 8.5, DOH signal is taken as 4.84 ppm) was measured as follows: δ 6.12 (1H, dd, J1,2(trans) = 17.4 Hz, J1,2(cis) = 10.9 Hz, H2), 5.26 (1H, dd, J1,2(trans) = 17.4 Hz, Jgem = 1.0 Hz, H1 (trans)), 5.14 ∼ 5.26 (2H, m, H6, H10), 5.14 (1H, dd, J1,2(cis) = 10.9 Hz, Jgem = 1.0 Hz, H1 (cis)), 2.00 ∼ 2.19 (6H, m, H5, H8, H9), 1.70 ∼ 1.88 (2H, m, H4), 1.71 (3H, s, H12), 1.64 (6H, s, H14, H15), and 1.58 (3H, s, H13). Assignments of carbon numbers to specific chemical shifts are based on APT experiments, deterium labeling, and correlations with that of nerolidol (Alchanati, et al., 1998). The 13C NMR (D2O-ND4OD, pH 8, CD3OD [internal] signal is taken as 49.0 ppm) was measured as follows: δ 142.68 (C2), 136.46 (C7), 134.47 (C11), 124.41 (C6/C10), 124.31 (C10/C6), 113.23 (C1), 82.15 (C3), 41.18 (C4), 38.73 (C8), 25.69 (C9), 24.77 (C12), 23.68 (C13), 22.37 (C5), 16.88 (C15), 15.12 (C14). 31P NMR (D2O, H3PO4 as external standard, 0.0 ppm), δ-7.30 (d, Jp,p = 19.4 Hz, external P), −14.76 (d, internal P).
Synthesis of (3RS)-[4,4-2H2]-(6E)-3,7,11-Trimethyl-1,6,10-Dodecatrien-3-yl Triammonium Diphosphate [(3RS)-[4,4-2H2]-Nerolidyl Diphosphate, (3RS)-[4,4-2H2]-NDP]
(3RS)-[4,4-2H2]-Nerolidol was synthesized in seven steps starting from dimethyl melonate with an overall yield of 10%. (3RS)-[4,4-2H2]-nerolidol was pyrophosphorylated according to the method of Popjak et al. (1962) to give (3RS)-[4,4-2H2]-nerolidyl diphosphate. The procedure of Holloway and Popjak (1967) was used to purify the (3RS)-[4,4-2H2]-NDP. The 1H NMR (D2O-ND4OD, pH 8.5, DOH signal is taken as 4.84 ppm) was comparable with the spectrum of (3R)-nerolidol diphosphate except for the disappearance of a multiplet at δ 1.70 to 1.88. The 13C NMR (D2O-ND4OD, pH 8, CD3OD [internal] signal is taken as 49.0 ppm) was comparable with the spectrum of (3-R)-nerolidol diphosphate except for the disappearance of the peak at δ 41.2. 31P NMR (D2O, H3PO4 as external standard, 0.0 ppm), δ −5.40 (d, Jp,p = 20.3 Hz, external P), −13.06 (d, internal P).
Construction of Expression Plasmid
A pair of primers, 97L01 (AGCTGAGGATCCATGGCTCACAAGTTTCTC) and 97L02 (GTACCTCTCGAGTTTC- AAAGTGCAATTGGTTCC), were designed for PCR reaction to add a BamHI site (underlined in primer 97L01) immediately upstream of the start codon of cdn1-C1 cDNA (a gift from Dr. Peter Heinstein, Purdue University, West Lafayette, Indiana) and a XhoI site (underlined in primer 97L02) downstream of the coding sequence. The PCR with cdn1-C1 plasmid DNA as the template was performed in 10 μm Tris-HCl, pH 8.3, 50 μm KCl, 1.5 μm MgCl2, 200 μm dNTP, 1.0 μm for both primers, and 2.5 units of Ampli-Tag DNA polymerase in a final volume of 100 μL. The polymerase reaction involved denaturing at 94°C for 1 min, annealing at 49°C for 2 min, and extension at 72°C for 3 min for a total of 30 cycles. The PCR product was digested with BamHI and XhoI and ligated into corresponding sites of plasmid pGEX-4T-1 (Amersham Pharmacia Biotech, Uppsala), which contains a glutathione-S-transferase (GST) tag upstream from the cloning sites. The ligation product was transformed into Escherichia coli strain XL1-Blue and BL21 according to a standard CaCl2 transformation procedure. The clones were screened for CDN synthase activity in the crude cell lysate after induction with isopropylthio-β-galactoside, since the GST-CDN1-C1 fusion protein shows CDN synthase activity. A positive clone BL21/pGEXC26 was selected for further investigation. The cDNA insert in pGEXC26 was sequenced with the dideoxynucleotide chain-termination method. The sequence of the insert was the same as the sequence of the cdn1-C1 cDNA (Chen et al., 1995) except one nucleotide changed from G to A at position 1,150 and resulted in a change of amino acid at 384 from Val to Met. This position corresponds to Val-378 in TEAS, which is a surface residue.
Bacterial Expression and Purification of Recombinant CDN1-C1 Synthase
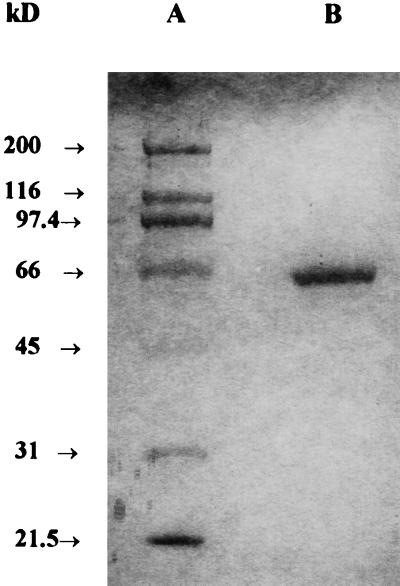
BL21/pGEXC26 was used for in vivo protein expression. A single colony was inoculated in Luria-Bertani medium with ampicillin (100 μg/mL) and was grown overnight at 37°C. The following morning, 10 mL of this suspension was added to 1 L of fresh medium containing antibiotics. After 3 h of growth (A600 was about 0.6) isopropylthio-β-galactoside was added to a final concentration of 0.5 μm. After 3 h of induction, the cells were harvested by centrifugation at 5,000g for 15 min. The pellet was resuspended in phosphate-buffered saline (1× PBS), the cells were broken in a French pressure cell (SLM Aminco, Urbana, IL), and the homogenate was centrifuged at 15,000g at 4°C for 20 min. The resulting supernatant fraction containing the GST-CDN1-C1 fusion protein was assayed for CAD synthase activity and was subsequently purified by affinity chromatography. The supernatant fraction was loaded on a Glutathione-Sepharose-4B column that had been pre-equilibrated with 1× PBS. The unbound proteins were eluted from the column with 10 bed volumes of 1× PBS. The GST-CDN1-C1 fusion protein was eluted with two bed volumes of elution buffer (10 μm glutathione in 50 μm Tris-HCl, pH 8.0). SDS-PAGE of the eluate showed a single band with a molecular mass of 92 kD. The intact CDN1-C1 with two extra amino acids (Gly·Ser) left on the N-terminal end was released from the fusion protein with thrombin digestion. There were no extra amino acids at the C terminus. Incubating 1 mg of GST-CDN1-C1 synthase fusion protein per milliliter of 50 μm Tris-HCl, pH 8.0, containing 1 unit of thrombin per 100 μg of fusion protein for 60 min at 20°C resulted in splitting the GST-CDN1-C1 fusion protein into a CDN1-C1 band with a molecular mass of 64 kD and a GST protein molecular mass of 28 kD on SDS-PAGE. At the end of the reaction, phenylmethylsulfonyl fluoride was added to the reaction mixture to a final concentration of 2 μm to inactivate the thrombin and the mixture was immediately added to a fast flow Q-Sepharose anion-exchange column. The proteins were eluted from the column with a NaCl gradient of 0 to 0.6 m in 50 μm Tris-HCl, pH 8.0. The protein eluate was monitored at 280 nm. The elution profile showed two protein peaks. The first peak was the GST protein. The second peak was active in cyclizing FDP to CDN and gave a single band of 64 kD on SDS-PAGE gel (Fig. 6). The purified CDN1-C1 with the GST protein removed was used in all of the subsequent experiments.
Figure 6.
SDS-PAGE of purified recombinant CDN1-C1. A, Protein markers. B, CDN1-C1 synthase after the removal of the GST protein from the GST-CDN1-C1 fusion protein with thrombin and the separation of the CDN1-C1 free of the GST protein by chromatography on a fast-flow Q-Sepharose anion-exchange column.
Assay of CDN Synthase Activity
The reaction mixture for the assay of CDN synthase activity contained 3 μg of purified CDN1-C1, 5 μL of 0.1 m GSH in 1 m Tris-HCl, pH 7.5, 10 μL of 0.1 m MgCl2, 20 μL of (1RS)-[1-3H](E,E)-FDP containing 0.45 nmol, and 22.6 × 106 cpm of radioactivity in a total volume of 100 μL. The reaction mixture was incubated at 30°C for 15 min and the reaction was stopped by extracting the reaction mixture with 2.0 mL of hexane-EtOAc (3:1). A 200-μL aliquot of the hexane-EtOAc extract was assayed for radioactivity in a liquid scintillation spectrometer counter (Beckman Instruments, Fullerton, CA).
The Cyclization of E,E-FDP and (3RS)-NDP by CDN1-C1 Synthase
The reaction mixtures for determining the cyclization reactions contained: 20 μg of purified CDN1-C1, 50 μL of 0.1 m GSH in 1 m Tris-HCl, pH 7.5, 100 μL of 0.1 m MgCl2, and 50 nmol of (1RS)-[1-2H](E,E)-FDP or 50 nmol (3RS)-[4,4,13,13,13,-2H5]-NDP in a total volume of 1 mL. The reaction mixture was incubated at 30°C for 15 min. The reaction products were extracted from the aqueous phase with 150 μL of hexane-EtOAc (3:1). An aliquot of the total extracted [2H]hydrocarbons was analyzed directly by GC-MS. The mass spectra of the [2H]CDN formed from the enzymatic cyclization of (1RS)-[2H](E,E)-FDP by the recombinant CDN1-C1 show ions at m/z(%): 206(15), 205(84), 190(29), 163(21), 162(100), 161(90), 160(10), 159(12), 135(58), 134(58), 133(15), 131(11), 129(11), 120(48), 119(51), 118(13), and 117(13).
The mass spectra of the [2H]β-bisabolene, [2H]α-bisabolol, [2H]CDN, and [2H](E)-β-farnesene formed from the cyclization of [4,4,13,13,13-2H5]-NDP show ions at m/z(%): 209(16), 194(20), 193(20), 189(6), 167(17), 166(19), 139(14), 138(9), 124(14), 123(17), 109(31), 99(28), 93(51), 79(22), 69(100), and 55(23) for [2H]β-bisabolene; m/z(%): 209(16), 194(20), 166(17), 139(14), 123(50), 109(94), 108(15), 93(23), 71(29), 79(11), 69(100), and 55(22) for [2H]α-bisabolol; m/z(%): 209(44), 208(1), 194(8), 167(23), 166(100), 165(8), 139(60), 124(38), 121(34), 120(11), and 119(9) for [2H]CAD; and m/z(%): 208(3), 193(2), 166(3), 165(10), 137(11), 136(19), 128(8), 123(16), 122(7), 109(11), 107(7), 98(6), 97(41), 96(18), 95(19), 94(14), 93(11), 91(7), 83(11), 82(18), 81(24), 80(10), 79(15), 71(8), 70(14), 69(100), 68(14), 67(22), 57(9), 56(9), 55(14), and 53(11) for [2H](E)-β-farnesene.
Authentic Sesquiterpenes
CDN
Naturally occurring CDN was isolated from fresh cotton (Gossypium barbadense) leaves by pentane extraction. The GC retention time was 19.57 min and the MS m/z(%) was 205(13), 204(51), 189(20), 162(27), 161(100, 157(14), 145(11), 134(59), 133(16), 129(10), 128(11), 120(10), 119(55), 115(13), and 105(49).
α-Bisabolol
The α-bisabolol that was purchased from Fluka Chemical Company (Milwaukee, WI) had a GC retention time of 20.77 min and MS m/z(%) of 205(2), 204(12), 189(3), 161(11), 147(5), 139(6), 135(5), 134(10), 133(8), 121(23), 119(72), 95(27), 93(57), 91(19), 81(19), 69(100), 67(42), and 55(34).
E-(β)-Farnesene and β-Bisabolene
E-(β)-Farnesene was prepared by the dehydration of E-nerolidol using phosphorous oxychloride in pyridine and had a GC retention time of 18.96 min and MS m/z(%) of 204(4), 189(4), 161(17), 148(8), 147(5), 134(8), 133(37), 121(8), 120(28), 119(8), 109(8), 109(6), 107(14), 106(6), 105(8), 95(5), 94(8), 93(64), 92(9), 91(17), 82(5), 81(20), 79(27), 77(13), 69(100), 67(24), 65(7), 55(16), and 53(14). The 1H-NMR and 13C-NMR [(CDCl3): 146.1 (s), 139.0 (d), 135.4 (s), 131.3 (s), 124.3 (d), 124.0 (d), 115.7 (t), 113.0 (t), 39.7 (t), 31.4 (t), 26.7 (t), 26.6 (t), 25.7 (q), 17.7 (q), 16.0 (q) δ] are in good agreement with those previously published by Brimble et al. (1996). The β-bisabolene was prepared by the dehydration of α-bisabolol using phosphorous oxychloride in pyridine and had a GC retention time of 19.44 min and MS m/z(%) of 205(3), 204(29), 161(33), 147(5), 135(12), 134(15), 133(12), 109(33), 107(13), 106(11), 105(24), 94(43), 93(100), 98(18), 91(34), 79(37), 67(43), and 55(16). The 1H-NMR agreed with that reported by Rocca et al. (1992). The 13C-NMR (CDCl3) was determined as follows: 154.3 (s), 133.7 (s), 131.5 (s), 124.3 (d), 120.8 (d), 107.1 (t), 39.8 (d), 34.9 (t), 31.4 (t), 30.8 (t), 28.4 (t), 26.9 (t), 25.7 (q), 23.4 (q), 17.7 (q), δ.
Enzyme Kinetics
The reaction mixtures were the same as described above for the cyclization studies except different concentrations of E,E-FDP, (3RS)-NDP, or (3R)-NDP replaced the deuterated substrates. The reaction products were extracted from the enzymatic mixtures with 150 μL of hexane-EtOAc (3:1) and 1.0 μL of the organic phase was analyzed by GC-flame ionization detector. CDN had a retention time of 7.65 min. The areas under the peak for CDN were compared with a standard curve of area versus nanomoles of authentic sample. The kinetic data were analyzed by direct, nonlinear least-squares fitting to a Michaelis-Menten equation using Kaleida Graph simulation software (Synergy Software, Reading, PA).
Cyclization of (3R)-NDP
The enzymatic and nonenzymatic cyclizations of (3R)-NDP were carried out to determine if the sesquiterpenes (E)-β-farnesene, β-bisabolene, CDN, or α-bisabolol were produced from the cyclization of (3R)-NDP by CDN1-C1. The reaction mixtures contained 50 μL of 0.1 m GSH in 1.0 m Tris-HCl buffer, pH 7.5, 100 μL of 0.1 m MgC12, and 80 nmol of (3R)-NDP with and without 20 μg of purified CDN1-C1 in a total volume of 1.0 mL. The reactions were incubated 15 min at 30°C. The sesquiterpene products were extracted with 150 μL of hexane-EtOAc (3:1). A 1-μL aliquot of the extracted hydrocarbon fraction was analyzed by GC-MS. Retention times of 18.96, 19.44, 19.57, and 20.77 min and the fragmentation patterns of these peaks agreed with authentic samples of (E)-β-farnesene, β-bisabolene, CDN, and α-bisabolol, respectively.
Cyclization of (3R)-NDP and (3RS)-[4-2H2]-NDP
The comparative cyclizations of (3R)-NDP and (3RS)-[4-2H2]-NDP were carried out to determine the active enantiomer of the (3RS)-NDP that was cyclized to CDN by CDN1-C1. The reaction mixtures contained 20 μg of purified CDN1-C1, 50 μL of 0.1 m GSH in 1.0 m Tris-HCl, pH 7.5, 100 μL of 0.1 m MgCl2, and 80 nmol of (3R)-NDP, 80 nmol of (3RS)-[4-2H2]-NDP, or a mixture of 80 nmol of (3R)-NDP and 80 nmol of (3RS)-[4-2H2]-NDP in a total volume of 1.0 mL. The reaction mixtures were incubated at 30°C for 15 min and the reaction products were extracted with 150 μL of hexane-EtOAc (3:1). A 1-μL aliquot of the extracted hydrocarbons was analyzed by GC-MS. The fragmentation of the hydrocarbon and the retention time of 19.57 min agreed with that of authentic CDN, and the fragmentation of the hydrocarbon with a retention time of 19.53 agreed with that of [2H2]-CDN (see above for the m/z values of CDN).
Modeling of CDN1-C1 Synthase Primary Structure into Crystal Structure of TEAS
Comparison of the primary structures of CDN1-C1 and TEAS shows 66% similarity with 45% identity. The high degree of amino acid similarity strongly suggests that these enzymes have similar three-dimensional structures. We used the SWISS-MODEL automated homology modeling server of the Swiss Institute of Bioinformatics (http://www.expasy.ch/) to test this hypothesis (Geux and Peitsch, 1997). The amino acid sequence of CDN1-C1 from Gossypium arboreum (amino acids 24–554 of accession no. Q39761) was modeled into the crystal structure of TEAS with the bound inhibitor farnesyl hydroxyphosphonate (amino acids 17–548 of 5EAT.PDB).
ACKNOWLEDGMENT
We wish to thank Dr. Peter Heinstein of Purdue University for the gift of cDNA of δ-cadinene synthase.
Footnotes
This work was supported in part by the Texas A&M Agricultural Experiment Station, by Cotton Incorporated, by the Texas Advanced Technology Program, and by the U.S. Department of Agriculture.
LITERATURE CITED
- Alchanati I, Patel JA, Liu J, Benedict CR, Stipanovic RD, Bell AA, Cui Y, Magill CW. The enzymatic cyclization of nerolidyl diphosphate by δ-cadinene synthase from cotton stele tissue infected with Verticillium dahliae. Phytochemistry. 1998;47:961–967. [Google Scholar]
- Bell AA. Formation of gossypol in infected or chemically irritated tissues of Gossypium spp. Phytopathology. 1967;57:759–764. [Google Scholar]
- Bell AA. Physiology of secondary products. In: Mauney JR, McD Stewart J, editors. Cotton Physiology. Memphis, TN: The Cotton Foundation; 1986. pp. 597–622. [Google Scholar]
- Benedict CR, Alchanati I, Harvey PJ, Liu J, Stipanovic RD, Bell AA. The enzymatic formation of δ-cadinene from farnesyl diphosphate in extracts of cotton. Phytochemistry. 1995;39:327–331. [Google Scholar]
- Bianchini GM, Stipanovic RD, Bell AA. Induction of δ-cadinene synthase and sesquiterpenoid phytoalexins in cotton by Verticillium dahliae. J Agric Food Chem. 1999;47:4403–4406. doi: 10.1021/jf990195y. [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA. 1998;95:4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brems DN, Rilling HC. On the mechanism of the prenyltransferase reaction: metal ion dependent solvolysis of an allylic pyrophosphate. J Am Chem Soc. 1977;99:831–832. doi: 10.1021/ja00467a055. [DOI] [PubMed] [Google Scholar]
- Brimble MA, Rowan DD, Spicer JA. Synthesis of chiral hydroxylated farnesene derivatives. Synthesis. 1996;1996:116–122. [Google Scholar]
- Cane DE, Tandon M. Epicubenol synthase and the stereochemistry of the enzymatic cyclization of farnesyl and nerolidyl diphosphate. J Am Chem Soc. 1995;117:5602–5603. [Google Scholar]
- Chayet L, Rojas MC, Cori O. Complexes of bivalent cations with neryl and geranyl pyrophosphate: their role in terpene biosynthesis. Bioorganic Chem. 1984;12:329–338. [Google Scholar]
- Chen XY, Chen Y, Heinstein P. Cloning, expression, and characterization of (+)-δ-cadinene synthase: a catalyst for cotton phytoalexin biosynthesis. Arch Biochem Biophys. 1995;324:255–266. doi: 10.1006/abbi.1995.0038. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wang M, Chen Y, Davisson VJ, Heinstein P. Cloning and heterologous expression of a second (+)-δ-cadinene synthase from Gossypium arboreum. J Nat Prod. 1996;59:944–951. doi: 10.1021/np960344w. [DOI] [PubMed] [Google Scholar]
- Davis EM, Chen YS, Essenberg M, Pierce ML. cDNA sequence of a (+)-δ-cadinene synthase gene (accession no. U88318) induced in Gossypium hirsutum L. by bacterial infection. Plant Physiol. 1998;116:1192. [Google Scholar]
- Davis EM, Tsuji J, Davis GD, Pierce ML, Essenberg M. Purification of (+)-δ-cadinene synthase, a sesquiterpene cyclase from bacteria-inoculated cotton foliar tissue. Phytochemistry. 1996;41:1047–1055. doi: 10.1016/0031-9422(95)00771-7. [DOI] [PubMed] [Google Scholar]
- Davis GD, Essenberg M. (+)-δ-Cadinene is a product of sesquiterpene cyclase activity in cotton. Phytochemistry. 1995;39:553–567. [Google Scholar]
- Donath J, Boland W. Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute configuration of the nerolidol precursor. Phytochemistry. 1995;39:785–790. [Google Scholar]
- Geux N, Peitsch MC. SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Holloway PW, Popjak G. The purification of 3,3-dimethylallyl- and geranyl-transferase and of isopentenyl pyrophosphate isomerase from pig liver. Biochem J. 1967;104:57–70. doi: 10.1042/bj1040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng YL, Jia JW, Liu CJ, Liang WQ, Heinstein P, Chen XY. Coordinated accumulation of (+)-δ-cadinene synthase mRNAs and gossypol in developing seeds of Gossypium hirsutum and a new member of the cad1 family from G. arboreum. J Nat Prod. 1999;62:248–252. doi: 10.1021/np980314o. [DOI] [PubMed] [Google Scholar]
- Popjak G, Cornforth JW, Cornforth RH, Ryhage R, Goodman DW. Studies on the biosynthesis of cholesterol: XVI. Chemical synthesis of 1-H23-2-14C and 1-D2-2-14C-trans-trans-farnesyl pyrophosphate and their utilization in squalene biosynthesis. J Biol Chem. 1962;237:56–61. [PubMed] [Google Scholar]
- Rocca JR, Nation JL, Strekowski L, Battiste MA. Comparison of volatiles emitted by male Caribbean and Mexican fruit flies. J Chem Ecol. 1992;18:223–244. doi: 10.1007/BF00993755. [DOI] [PubMed] [Google Scholar]
- Starks CM, Back K, Chappell J, Noel JP. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science. 1997;277:1815–1824. doi: 10.1126/science.277.5333.1815. [DOI] [PubMed] [Google Scholar]