Abstract
Rete mirabile (RM), an arterial network normally existing in some vertebrate animals interconnecting the extracranial and intracranial arterial circulation, can rarely be found in humans whether asymptomatic or presenting with cerebral ischaemia or haemorrhage. Encompassing diverse angiographic characteristics and similarities with other arterial malformations, proper diagnosis and differential diagnosis is challenging. We hereby describe an unusual RM case variant, presenting to us with lethal subarachnoid haemorrhage owing to a ruptured small aneurysm associated with the RM network. Angiography disclosed an absent P1 segment of the posterior cerebral artery (PCA) and an RM network anastomosing the basilar apex with the normal distal PCA. Brain death was confirmed on the fifth day after admission and attributed to the severity of subarachnoid haemorrhage (SAH). This is an exceedingly rare case representing an intradural-to-intradural RM anastomosis in a patient presenting with lethal SAH. RM epidemiology, pathophysiology, presentation, angiographic findings and prognosis are reviewed.
Keywords: neurology, neuroimaging, neuro ITU
Background
In animals, rete mirabile (RM) is a normal transdural arterial anastomosis interconnecting the internal carotid artery (ICA) with the external carotid artery. It is considered to serve thermoleguratory,1 pressure absorption2 or blood flow regulation purposes.2 It is normally found in lower mammals, such as pigs, cows and sheep.3
When identified in humans, RM encompasses a congenital malformation involving a collateral, usually transdural, network between two arteries. The most commonly implicated intracranial vessels are the petrous or cavernous ICA,4 5 receiving blood flow from extracranial arteries such as the maxillary artery6 7 or the ascending pharyngeal artery.7 The term carotid RM (CRM) has been used when ICA is implicated in the anastomosis. When the vertebral artery is also implicated, the term carotid and vertebral RM has been used.3 An abundance of cases with bilateral RM has been reported.3 8–10 RM of the posterior circulation is an even more rare variant, with only a few cases reported over the years.3 11 Most of the RM vessels tend to occur extradurally providing a connection with a vessel across the dura.4 12 Nevertheless, the term RM has been used quite liberally in the literature to describe an abnormal, usually reticular-like, artery-to-artery anastomosis, since there has been no strict angiographic definition or criteria developed thus far. We identified, including our case, multiple cases of intradural-to-intradural anastomosis3 8–11 13–17 and also a single extradural-to-extradural anastomosis case.18 Finally, a single acquired CRM case has been reported.19
Case presentation
We present a middle-aged patient who was admitted with ‘worst headache of life’. Initial Glasgow Coma Scale (GCS) was 12 but the patient quickly deteriorated to GCS 4. An initial non-contrast CT scan of the head showed diffuse subarachnoid haemorrhage (SAH) with intraventricular extension, complicated by acute hydrocephalus (figure 1). SAH was assessed as grade 5 on Hunt and Hess Scale.
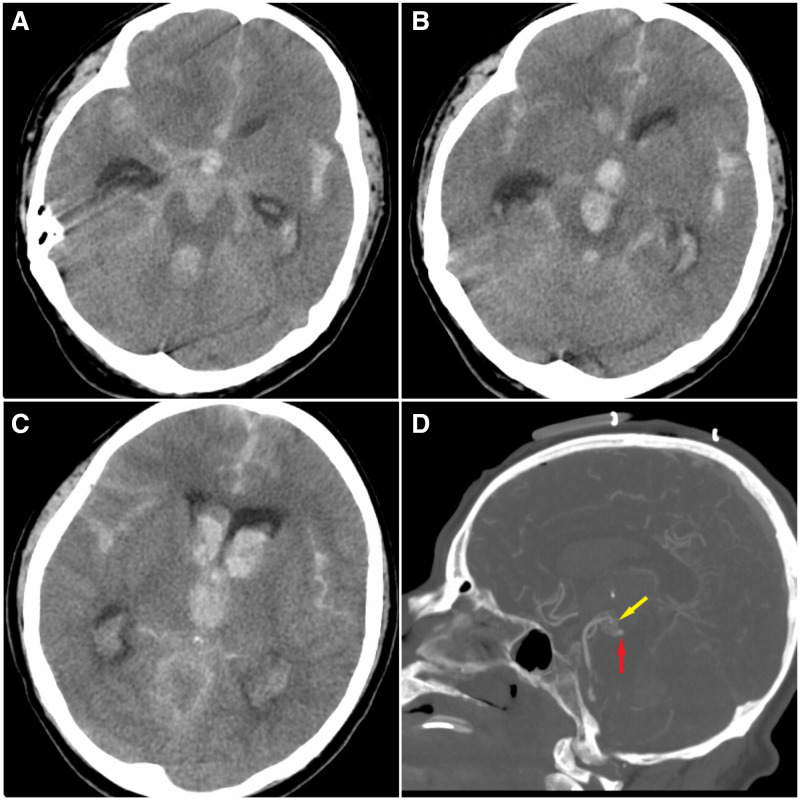
Figure 1.
(A) Non-enhanced CT scan revealing severe acute cisternal subarachnoid haemorrhage, especially in the interhemispheric and sylvian compartments, as well as acute brainstem intraparenchymal haemorrhage. (B, C) More rostral slices of the same CT scan demonstrating intraventricular haemorrhage in the third ventricle and frontal horn of the lateral ventricles. (D) CT angiography demonstrating an abnormal vascular network at the tip of the basilar artery and possibly a small aneurysm.
Treatment and outcome
The patient was intubated and a frontal external ventricular drain (EVD) was placed. Angiography revealed complete absence of the proximal part of the P1 segment of the right posterior cerebral artery (PCA). An RM network was identified interconnecting the basilar terminus with the existing P1 part of the PCA (figures 2–4). Posterior circulation angiography (figure 3) and microcatheter-based angiography with the microcatheter tip at the basilar terminus (figure 4) are available in 3D reconstruction. Bilateral thalamic and midbrain perforators emerged from this anastomosing small calibre RM network (figure 4). A small pseudoaneurysm was detected at the dorsal part of the network, measuring 2.2×1.9×8 mm. The patient was deemed non-amenable to endovascular or surgical treatment and hence was placed on aminocaproic acid. In another angiogram performed 2 days later, the aneurysm was found to be smaller, measuring 1.63×1.33 mm. An additional EVD system was placed on the second day of hospitalisation. Electroencephalogram showed diffuse low-voltage (<20 μV) waves with continuous diffuse severe slowing, and coma.
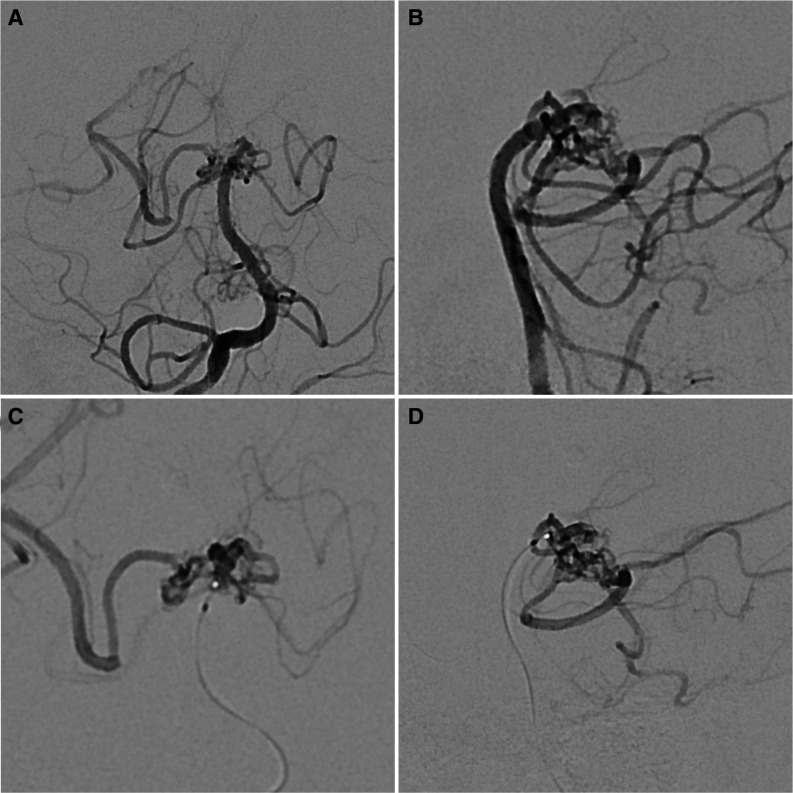
Figure 2.
Posterior circulation angiography demonstrating the RM network in anteroposterior (A) and lateral (B) views. Microcatheter angiography of the RM network, with the tip of the microcatheter being at the basilar artery tip. Anteroposterior (C) and lateral (D) views showing no definite normal connection between the basilar artery and the right posterior cerebral artery. RM, rete mirabile.
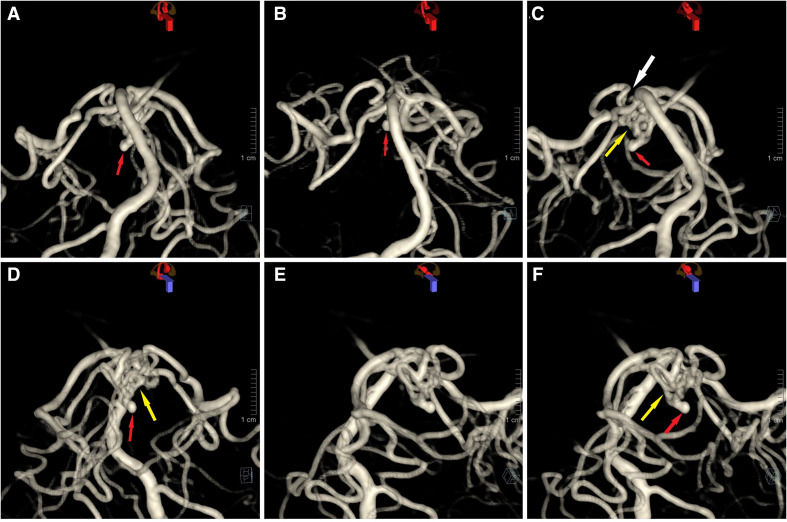
Figure 3.
3D reconstruction of the posterior circulation in anteroposterior (A, B, D) and lateral (C, E, F) views. Left-sided posterior cerebral artery (PCA) has an embryologic origin. On the right side, there is no existing normal connection between the PCA and the basilar tip (C, white arrow). Instead, there is a rete mirabile (RM) network (C, D, F, yellow arrow) interconnecting these vessels. The right PCA demonstrated delayed filling. The aneurysm (red arrow) is associated with the RM network and protrudes caudally and anteriorly.
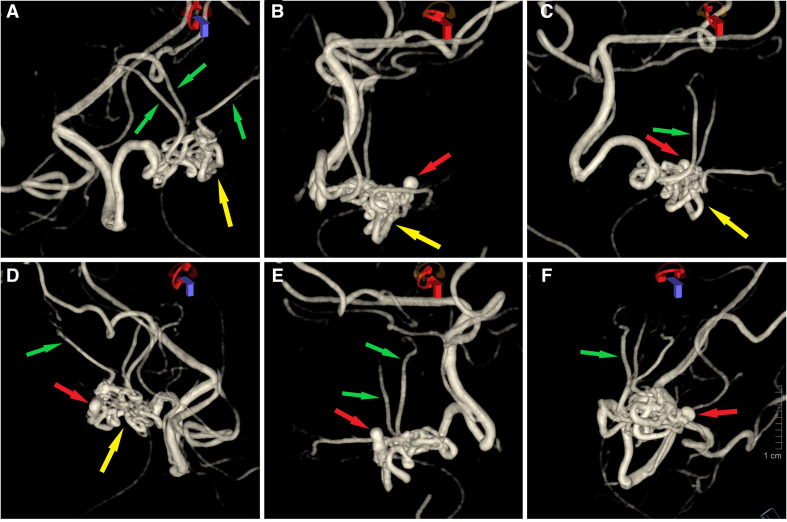
Figure 4.
3D reconstruction of microcatheter-based angiography demonstrating the RM network. Findings are the same as in figure 3. Normal thalamoperforators (A, C–F) are seen emerging form the RM network (green arrows). Aneurysm (red arrow) and its relationship with the RM network (yellow arrow) is demonstrated (B–F). RM, rete mirabile.
Outcome and follow-up
The patient had developed decerebrate rigidity by the second day of hospitalisation and progressively deteriorated developing intracranial hypertension, cerebral oedema and brain herniation. On the fifth day of hospitalisation, he met brain death criteria and was pronounced deceased.
Discussion
The first RM case was reported in 1966.20 RM is usually associated with ICA12 or vertebral3 hypoplasia/aplasia/segmental occlusion, with the RM being responsible for supplying the areas lacking normal supply by the absent vessels.6 Hypothetically hypoplasia/aplasia promotes primordial vessels to create new ‘salvage’ anastomosing networks to irrigate ischaemic regions, during embryogenesis.9 The PCA’s proximal segment derives from ICA’s posterior division, while the cortical branches stem from the part of the anterior choroidal artery lying next to lateral geniculate body.21 A defect during embryogenesis could explain the RM formation in our case. These events are likely promoted by ischaemia due to the occlusion.11 RM appearance has been described in prior ipsilateral ICA ligation.19
The angiographic prevalence of RM was reported as 0.01% within a single institution, until 1997.22 However, prevalence has been reported higher in the Asian population,9 being as high as 0.67% in angiographies.23 However, true prevalence and what percentage becomes symptomatic are currently unknown. RM has been identified in patients with haemorrhagic or ischaemic manifestations.24 25 In a 2015 review, 34% of the identified RM cases (12/35) presented with SAH,24 the first of which was reported in 1967.26 SAH in patients with RM is thought to be caused not only by ruptured aneurysms3 7 24 but also by haemodynamic stress22 or rupture of the anastomosing vessels,12 since an aneurysm was identified only in a small subset of patients. Haemodynamic changes related to the abnormal anastomotic pattern may lead to mechanical stress on vessels, thus promoting aneurysm formation.3 In a case described by Henkes et al, RM was associated with fibromuscular dysplasia, as shown by biopsy, and the presence of a ruptured aneurysm.27
Diverse RM angiographic features have been reported as follows: ICA hypoplasia/agenesis,12 transdural anastomotic arterial plexus between cavernous ICA and maxillary artery (usually small calibre27), ophthalmic-to-maxillary artery anastomosis, ophthalmic artery and RM plexus supplying a normal supraclinoid ICA, bilateral RM and RM network resembling intradural moyamoya disease. Another study classified RM as moyamoya type or nidus type, depending on whether the vessels resembled stages 3 and 4 of moyamoya disease or the nidus of an arteriovenous malformation, respectively.22 Moyamoya type anastomotic vessels (ethmoidal pattern) have been found in a case of ICA occlusion.5 RM may at times be confused with an arteriovenous malformation or fistula.11 RM constitutes an artery-to-artery anastomosis. Selective angiography could exclude these diagnoses by showing the absence of a draining vein.11 Differential diagnosis from moyamoya is challenging. The authors considered this case to be an RM one and not moyamoya disease, since the abnormal network seemed to be reconstituting the gap between the PCA and basilar artery while it did not resemble the small and thin ‘puff of smoke’-like vessels of moyamoya.
Prognosis of patients with RM is usually good, based on the available reports from the literature. Notably, there has been another lethal SAH case in a patient with RM,19 like ours. Lethality in our case is related to the severe SAH presentation (Hunt and Hess grade 5). Different management approaches have been endeavoured in patients with RM who presented with SAH, including aneurysm clipping.24
Learning points.
Rete mirabile is an artery-to-artery anastomosis, found normally in vertebrate animals, but rarely found in humans, associated with arterial agenesis/hypoplasia/severe stenosis.
Most common pattern is an extradural-to-intradural anastomosis between the internal carotid artery and the anterior part of circle of Willis.
Diverse angiographic features can be demonstrated including resemblance to arteriovenous malformations (AVMs) or moyamoya vessels.
Angiographic prevalence is 0.01%, most of the patients are asymptomatic, but haemorrhagic or ischaemic phenomena might ensue.
Experience is required for proper diagnosis and differential diagnosis and the therapeutic approach should be tailored on a case-by-case basis depending on the type of presentation.
Footnotes
Contributors: The case was managed by TS (senior author) and JTF who also helped with final edits and images selection. The case report was drafted and brought to a final form by SM (lead author).
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Jessen C, Pongratz H. Air humidity and carotid rete function in thermoregulation of the goat. J Physiol 1979;292:469–79. 10.1113/jphysiol.1979.sp012865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelman NH, Epstein P, Cherniack NS, et al. Control of cerebral blood flow in the goat; role of the carotid rete. Am J Physiol 1972;223:615–9. 10.1152/ajplegacy.1972.223.3.615 [DOI] [PubMed] [Google Scholar]
- 3.Nagahata M, Kondo R, Mouri W, et al. Bilateral carotid and vertebral rete mirabile presenting with subarachnoid hemorrhage caused by the rupture of spinal artery aneurysm. Tohoku J Exp Med 2013;230:205–9. 10.1620/tjem.230.205 [DOI] [PubMed] [Google Scholar]
- 4.Mikami T, Takahashi A, Houkin K. Carotid rete mirabile associated with subarachnoid hemorrhage. Neurol Med Chir 2005;45:201–4. 10.2176/nmc.45.201 [DOI] [PubMed] [Google Scholar]
- 5.Ito M, Sato K, Tsuji O, et al. Multiple aneurysms associated with bilateral carotid occlusion and venous angioma: surgical management risk-case report. J Clin Neurosci 1994;1:62–8. 10.1016/0967-5868(94)90013-2 [DOI] [PubMed] [Google Scholar]
- 6.Karasawa J, Touho H, Ohnishi H, et al. Rete mirabile in humans--case report. Neurol Med Chir 1997;37:188–92. 10.2176/nmc.37.188 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Masuzawa T, Kitano I, et al. [Hypoplasia of the left internal carotid artery associated with anterior communicating aneurysm, intercarotid anastomosis and left rete carotidis]. No To Shinkei 1996;48:170–4. [PubMed] [Google Scholar]
- 8.Hyogo T, Nakagawara J, Nakamura J, et al. Multiple segmental agenesis of the cerebral arteries: case report. Neuroradiology 1996;38:433–6. 10.1007/BF00607267 [DOI] [PubMed] [Google Scholar]
- 9.Mahadevan J, Batista L, Alvarez H, et al. Bilateral segmental regression of the carotid and vertebral arteries with rete compensation in a Western patient. Neuroradiology 2004;46:444–9. 10.1007/s00234-003-1086-x [DOI] [PubMed] [Google Scholar]
- 10.Herwadkar A. A case of carotid rete mirabile associated with basilar tip aneurysm. Interv Neuroradiol 2006;12:161–4. 10.1177/159101990601200211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng L, Metcalfe R, Bhattacharya JJ. A rete mirabile of the posterior cerebral artery. embryonic and phylogenetic remnant of choroidal artery origin. Interv Neuroradiol 2008;14:195–202. 10.1177/159101990801400212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kino T, Fuwa I, Kitano I, et al. Carotid rete mirabile presenting subarachnoid haemorrhage. Report of two cases. Acta Neurochir 1999;141:1183–6. 10.1007/s007010050416 [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Liu J, Wang L, et al. Bilateral segmental agenesis of carotid and vertebral arteries with rete mirabile and the prominent anterior and posterior spinal arteries as compensations. Interv Neuroradiol 2014;20:13–19. 10.15274/INR-2014-10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin Y, Lee KM, San Lee J, et al. Isolated trochlear nerve palsy in a patient with superior cerebellar rete mirabile. World Neurosurg 2019;130:546–9. 10.1016/j.wneu.2019.05.244 [DOI] [PubMed] [Google Scholar]
- 15.Giragani S, Pavunesan SK, Balasubramaniam A, et al. Rete mirabile of posterior inferior cerebellar artery: a rare cause of subarachnoid hemorrhage. Interv Neuroradiol 2018;24:662–5. 10.1177/1591019918782147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Hou K, Wang X, et al. Spontaneous recession of a posterior cerebral artery aneurysm concurrent with carotid rete mirabile and moyamoya-pattern collateral vessels: a case report. BMC Neurol 2019;19:51. 10.1186/s12883-019-1277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baek JH, Kim BM. Stenting for symptomatic vertebral artery stenosis associated with bilateral carotid rete mirabile: the long-term clinical and angiographic outcome. Korean J Radiol 2015;16:678–81. 10.3348/kjr.2015.16.3.678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aburto-Murrieta Y, Dulce B-D. Asymptomatic carotid rete mirabile and contralateral carotid agenesis: a case report. Vasc Endovascular Surg 2011;45:361–4. 10.1177/1538574411401760 [DOI] [PubMed] [Google Scholar]
- 19.Rockett JF, Johnson TH. Bilateral rete mirabile intracranial (vascular) anastomosis in man. A case report. Radiology 1968;90:46–8. 10.1148/90.1.46 [DOI] [PubMed] [Google Scholar]
- 20.Minagi H, Newton TH. Carotid rete mirabile in man. Radiology 1966;86:100–2. 10.1148/86.1.100 [DOI] [PubMed] [Google Scholar]
- 21.Abbie AA. The morphology of the Fore-Brain arteries, with especial reference to the evolution of the basal ganglia. J Anat 1934;68:433–70. [PMC free article] [PubMed] [Google Scholar]
- 22.Nakaoka T, Kanma H, Matsuura H. [A case of rete mirabile with congenital dysplasia of bilateral internal carotid arteries]. No Shinkei Geka 2000;28:161–6. [PubMed] [Google Scholar]
- 23.Cho K-C, Kim J-J, Jang C-K, et al. Rete middle cerebral artery anomalies: a unifying name, case series, and literature review. J Neurosurg 2018;131:453–61. 10.3171/2018.2.JNS1832 [DOI] [PubMed] [Google Scholar]
- 24.Paschoal EHA, Yamaki VN, Júnior FMP, et al. Carotid rete mirabile associated with subarachnoid hemorrhage from intracranial aneurysm: a case report and systematic review. Interv Neuroradiol 2015;21:55–60. 10.15274/INR-2014-10107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mondel PK, Saraf R, Limaye US. Rete mirabile associated with pial arteriovenous fistula: imaging features with literature review. J Neurointerv Surg 2017;9:e36. 10.1136/neurintsurg-2016-012939.rep [DOI] [PubMed] [Google Scholar]
- 26.Hawkins TD, Scott WC. Bilateral rete carotidis in man. Clin Radiol 1967;18:163–5. 10.1016/S0009-9260(67)80011-4 [DOI] [PubMed] [Google Scholar]
- 27.Henkes H, Reinartz J, Fischer S, et al. [Rete compensation in agenesis of the internal carotid artery]. Nervenarzt 2007;78:948–53. 10.1007/s00115-007-2260-x [DOI] [PubMed] [Google Scholar]