Abstract
Objectives
To systematically assess the prevalence and risk factors for senile pruritus (SP) in the elderly (≥60 years of age).
Design
A meta-analysis was used to pool the prevalence and risk factors for SP estimated from individual studies. Four subgroup analyses were conducted to explore the prevalence for SP in different age, sex, research sites and region.
Setting, participants and measures
SP reduces quality of life in the elderly, yet the worldwide prevalence is unclear. Moreover, the risk factors for SP are controversial. Data from cross-sectional studies, case–control studies, longitudinal studies and cohort studies that reported the prevalence or the risk factors for SP were collected by searching nine electronic databases up to October 2020, including Web of Science, PubMed, Embase, Cochrane Library, CINAHL, CBM, CNKI, Wanfang and VIP. Two reviewers independently screened studies according to the inclusion and exclusion criteria, extracted data and assessed methodological quality. Data analysis was performed using Stata V.15.1 software.
Results
Seventeen studies involving 28 666 participants were included. The overall pooled prevalence of SP was 21.04% (95% CI 11.37% to 32.72%). In addition, the results showed that smoking, excessive drinking and monophagism were possible risk factors for SP, with pooled ORs of 1.26 (95% CI 1.14 to 1.40), 25.03 (95% CI 18.28 to 34.25) and 1.22 (95% CI 1.12 to 1.33), respectively.
Conclusions
The overall prevalence of SP was high. Smoking, excessive drinking and monophagism were possible risk factors for SP.
PROSPERO registration number
CRD42019143295.
Keywords: geriatric dermatology, dermatological epidemiology, perinatology
Strengths and limitations of this study.
To our knowledge, this study is the first systematic review and meta-analysis providing comprehensive assessment on the prevalence of senile pruritus (SP) in worldwide.
The risk factors of SPs are evaluated.
This systematic review and meta-analysis, covering five different countries, was composed of 17 studies, with 28 666 participants were included.
The definitions of SP differed across the included studies.
Introduction
The geriatric population (≥60 years of age) has been growing steadily worldwide in recent decades. It is estimated that the geriatric population will account for 20% of the world’s population by the middle of this century.1–3 Ageing results in numerous adverse changes in the structure and function of multiple human organs, including the skin.4 Senile pruritus (SP) is defined as generalised pruritus in patients without primary skin lesions.5–8 Pruritus is the most common skin disorder in the geriatric population.9 It can lead to an unpleasant cutaneous sensation, which provokes the desire to scratch (itchiness) and is accompanied by skin lesions, pain and infection.10 Furthermore, it can lead to adverse consequences for patients’ psychological health and quality of life, including anxiety, depression, disruption of normal sleep patterns and poor daytime concentration.10 11 Therefore, investigating the prevalence of SP is essential for informing policymakers, clinicians, and the general population.
The prevalence of SP has been reported around the world, ranging from 41% in Thailand,12 40.6% in America,13 18.9% in Italy14 and 14.2% in China.15 However, these studies were limited by sample size and regional differences, and therefore do not represent the prevalence of SP worldwide. Furthermore, several studies conducted surveys on inpatients or outpatients to report the prevalence of SP.1 11 16 17 Inpatients or outpatients do not represent the whole elderly population, making the results less representative of the actual prevalence of SP in the community. For these reasons, the precise prevalence and characteristics of the population are unknown worldwide. Furthermore, the risk factors for SP have been reported extensively, but with controversial conclusions.18 19 For example, Yang et al indicated that smoking was associated with SP (OR 2.23, 95% CI 1.35 to 17.40).18 However, Chen et al reported that smoking was not associated with SP (OR 1.25, 95% CI 0.99 to 1.35).19
In this study, we conducted a systematic review and meta-analysis to synthesise the prevalence of SP in different ages, sexes and regions based on the general population and to evaluate the risk factors for SP.
Materials and methods
Protocol registration
This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.20
Search strategy
Nine databases were searched in this study, including Web of Science, PubMed, Embase, Cochrane Library, CINAHL, CBM, CNKI, Wanfang and VIP. The following strategy was used in the searches: (Pruritus OR Itching) AND (Senile OR Aging OR Aged OR Geriatrics) AND (Incidence OR Epidemiology OR Prevalence OR Risk factors). Complete details of the search strategy are available in online supplemental table S1. All of the databases were searched from their inception dates to the 24 October 2020. Additional relevant literature was included following a manual search of the included studies reference lists.
bmjopen-2021-051694supp001.pdf (44.4KB, pdf)
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) the study design was either cross-sectional study, case–control study, cohort study or longitudinal study; (2) participants were greater than or equal to 60 years of age; (3) exact diagnostic criteria for SP were provided and (4) prevalence or risk factors for SP were reported. The exclusion criteria were as follows: (1) the study populations were inpatients and outpatients; (2) the prevalence or risk factor effect value (mainly referred to as OR in this study) of SP was not clearly reported in the original study, and the data provided by the original study couldn't calculate the prevalence or risk factor effect value of SP; (3) republished literature and (4) or studies published in a language other than Chinese or English.
Quality of the studies
Two independent reviewers assessed the quality of the included studies according to 11 criteria recommended by the American Agency for Healthcare Research and Quality. The criteria included assessment of selection bias, performance bias, attrition bias, detection bias and publication bias. An item would be scored ‘1’ if it was answered ‘YES’, and if it was answered ‘NO’ or ‘UNCLEAR’, then the item scored ‘0’,21 providing a maximum score of 11.
Data extraction
Study selection and data extraction were independently conducted by two reviewers. Any disagreement was resolved by discussion the two reviewers or a third reviewer. The articles were first screened by the title and abstract, and then full-text documents were reviewed for inclusion if they reported the prevalence and risk factors for SP. By using a standardised and pilot-tested form, two reviewers independently extracted data from eligible studies, including the title, first author name, publication year, study location, age, sample size, diagnostic criteria, prevalence and risk factors for SP.
Data analysis
Double arcsine transformation was used to convert the prevalence of SP so that the data can follow an approximately normal distribution.22 The ORs with their corresponding 95% CIs were selected to assess the effect size of risk factors for SP. Heterogeneity among studies was tested by Cochrane’s Q and I2 statistics. Heterogeneity was recognised as significant when I2 >50%. A fixed-effect model (Mantel and Haenszel method) was used if I2 ≤50%, otherwise a random-effects model (DerSimonian and Laird method) was used.23 Forest plots were constructed for a visual display of the pooled results if necessary. Four subgroup analyses were conducted to explore the prevalence for SP in different age, sex, research sites and region. Sensitivity analysis were assessed by excluding single studies. Publication bias was assessed by using Begg’s and Egger’s tests.24 Tests of publication bias and sensitivity analysis were not conducted in the risk factor analysis section due to the limited number of studies included. Statistical analyses were conducted using STATA V.15.1 (Stata).
Results
Study description
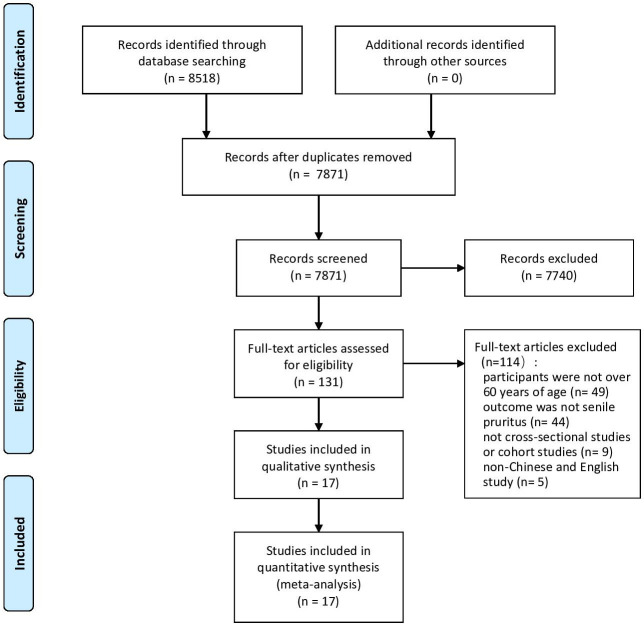
A total of 8518 records were identified from the 9 databases, of which 647 were duplicates. After screening titles and abstracts, 7740 records were excluded with reasons of age, outcome, study design. Full-text documents of 131 records were screened, and 114 studies were excluded with reasons listed as follows: participants were not ≥60 years of age (n=49), outcome was not SP (n=44), not cross-sectional, case–control, cohort or longitudinal study (n=9), non-Chinese and English study (n=5), duplicate publication (n=7). In summary, 17 studies were eligible and included in the meta-analysis finally (figure 1).
Figure 1.
Flow chart of the study selection process.
Characteristics of the included studies
The characteristics of the 17 studies are summarised in table 1. Eleven articles were written in Chinese,18 19 25–33 and six were written in English.34–39 Thirteen studies were conducted in Asia18 19 25–33 35 37 and four in Europe.34 36 38 39 Sample sizes ranged from 4539 to 8252.36 Four of the 17 studies reported the risk factors for SP.18 19 26 33
Table 1.
Characteristics of included studies
| Authors | Publication years | Study area | Diagnostic criteria | Sample size | Prevalence (%) |
Risk factors |
| Dalgard et al34 | 2004 | Norway | ① | 3876 | 6.91 | NA |
| Li et al25 | 2000 | China | NA | 534 | 12.36 | NA |
| Xue27 | 2008 | China | NA | 311 | 19.29 | NA |
| Ni et al28 | 2012 | China | ④ | 426 | 5.63 | NA |
| Zhang29 | 2012 | China | NA | 1283 | 9.90 | NA |
| Li et al30 | 2014 | China | ⑤ | 500 | 33.40 | NA |
| Wu and Zhang31 | 2014 | China | ⑤ | 1286 | 42.38 | NA |
| Yang et al32 | 2014 | China | NA | 5000 | 33.84 | NA |
| Tseng et al35 | 2014 | China | NA | 313 | 7.35 | NA |
| Miller et al36 | 2016 | Denmark | ① | 8252 | 6.31 | NA |
| Kara et al37 | 2017 | Turkey | NA | 105 | 19.05 | NA |
| Cowdell et al38 | 2017 | Britain | ⑥ | 1116 | 9.32 | NA |
| Dyhre-Petersen and Gazerani39 | 2019 | Denmark | ⑦ | 45 | 28.89 | NA |
| Ge et al26 | 2006 | China | ② | 1236 | 66.91 | Age, xerosis, astriction |
| Yang et al18 | 2009 | China | ③ | 3785 | 61.98 | Less water intake; bathing with soap; baths too much; smoking; malignant tumour. |
| Chen et al19 | 2015 | China | ④ | 200 | 10.50 | Bathing with soap; smoking; chronic illness; excessive drinking; monophagism; Insomnia; contact with animal |
| Hou and Zhang33 | 2016 | China | ④ | 398 | 18.09 | Smoking; chronic illness; excessive drinking; monophagism; insomnia; contact with animal |
Diagnostic criteria: ① self-reported skin complaints scale; ② participants ≥60 years, an itch lasting more than 2 weeks, pruritus of whole body or multiple parts, no primary rash, no other pruritic skin disease, no obvious liver and kidney damage, diabetes and mental disease; ③ dermatology and venereology; ④ clinical dermatology; ⑤dermatovenerolog; ⑥ self-report skin diseases scale;38 ⑦ self-report skin diseases scale.
NA, not available.
Risk of bias assessment
Results of the risk of bias assessment are listed in online supplemental table S2. Higher scores indicative of less bias and more quality. Article quality was assessed as follows: 0–3 indicates a low quality, 4–7 indicates a moderate quality and 8–11 indicates a high quality.40 Study quality was found to be moderate in 11 studies and high in the other six studies.
bmjopen-2021-051694supp002.pdf (78.8KB, pdf)
Prevalence of SP
Seventeen studies, involving 28 666 participants reported the prevalence of SP, ranging from 5.63% to 66.91%. A random-effects model-based meta-analysis showed that the pooled prevalence of SP was 21.04% (95% CI 11.37% to 32.72%). Subgroup analyses indicated that the pooled prevalence of SP for people aged 60–69, 70–79, 80–89 and ≥90 years old were 11.98% (95% CI 3.91% to 23.62%), 26.79% (95% CI 8.71% to 50.36%), 51.31% (95% CI 47.20% to 96.33%) and 57.53% (95% CI 8.18% to n98.09%), respectively. The pooled prevalence of SP was 8.26% (95% CI 5.88% to 11.00%) in females and 18.65% (95% CI 0.83% to 51.61%) in males. The pooled prevalence of SP in health examination centres, nursing homes and communities was 43.83% (95% CI 19.39% to 69.94%), 16.26% (95% CI (4.55% to 33.29%) and 12.21% (95% CI 3.46% to 25.34%), respectively. The pooled prevalence of SP in Turkey and China was 24.34% (95% CI 14.03% to 36.38%). The pooled prevalence of SP in Norway, Denmark and Britain was 8.23% (95% CI 6.36% to 10.35%). The results of subgroup analyses of age, sex, research site and region are shown in table 2.
Table 2.
Subgroup analyses by age, sex, research sites and region
| Subgroup | Prevalence (%) | 95% CI (%) | Heterogeneity | |
| I2 (%) | P value | |||
| Age | ||||
| 60–69 | 11.98 | 3.91 to 23.62 | 98.1 | 0.000 |
| 70–79 | 26.79 | 8.71 to 50.36 | 99.7 | 0.000 |
| 80–89 | 51.31 | 47.20 to 96.33 | 99.6 | 0.000 |
| ≥90 | 57.53 | 8.18 to 98.09 | 99.0 | 0.000 |
| Sex | ||||
| Females | 8.26 | 5.88 to 11.00 | 87.4 | 0.000 |
| Males | 18.65 | 0.83 to 51.61 | 99.9 | 0.000 |
| Research sites | ||||
| Health examination centre | 43.83 | 19.39 to 69.94 | 99.8 | 0.000 |
| Nursing homes | 16.26 | 4.55 to 33.29 | 93.2 | 0.000 |
| Community | 12.21 | 3.46 to 25.34 | 99.8 | 0.000 |
| Region | ||||
| Turkey, China | 24.34 | 14.03 to 36.38 | 99.6 | 0.000 |
| Norway, Denmark, Britain | 8.23 | 6.36 to 10.35 | 90.2 | 0.000 |
Risk factors
Four studies, including 5619 participants, reported the risk factors for SP.18 19 26 33 There were three studies,18 19 33 including 4383 participants, that reported the association of smoking and SP. Meta-analyses showed smoking was associated with SP (pooled OR of 1.26 (95% CI 1.14 to 1.40), I2=0%). The results of two studies,19 33 involving 598 participants, suggested that excessive drinking increased the occurrence of SP (pooled OR of 25.03 (95% CI 18.28 to 34.25), I2=0%). Two studies19 33 involving 589 participants reported the association of monophagism and SP (pooled OR of 1.22 (95% CI 1.12 to 1.33), I2=0%) (table 3).
Table 3.
Pooled risk factors of senile pruritus
| No. | Risk factors | OR | 95% CI | P value | Heterogeneity | |
| I2 (%) | P value | |||||
| 1 | Smoking | 1.26 | 1.14 to 1.40 | 0.000 | 0% | 0.673 |
| 2 | Excessive drinking | 25.03 | 18.28 to 34.25 | 0.000 | 0% | 0.980 |
| 3 | Monophagism | 1.22 | 1.12 to 1.33 | 0.000 | 0% | 0.926 |
Sensitivity analysis
Sensitivity analysis was performed by excluding a single study and showed that the results of the meta-analysis were stable (18.61%–22.23%). Sensitivity analysis was not conducted for the risk factor analysis due to the limited number of studies.
Publication bias
Publication bias was assessed by using Begg’s and Egger’s tests. Begg’s (Z=0.70, p=0.484) and Egger’s test (t=0.26, p=0.796) results showed that the possibility of publication bias was less in the overall prevalence pooled analysis. Publication bias was not assessed in the risk factor analysis due to the limited number of studies.
Discussion
In this study, 17 studies involving 28 666 participants were included encompassing Norway, China, Denmark, Turkey and Britain. Subgroup analyses found that the difference in the prevalence of SP based on epidemiological factors. Subgroup analyses indicated that a steadily increasing prevalence of SP was associated with increasing age. Xerosis is related to ageing and is reported as the most common cause of SP.41–43 One of the skin’s most important functions is to retain water. skin surface lipids and sebum on the skin helps retain water.44 As skin ages, there is a decrease in lipids and sebum on the skin, leading to suboptimal moisture retention.42 It was reported that pruritus can also be secondary to diabetes, kidney disease, liver disease, etc.44 45 Furthermore, pruritus is commonly listed as a medication complication,46 including ACE inhibitors, salicylates, chloroquine and calcium channel blockers.44 However, elderly people have more basic diseases and complex medication, which also contributed to the higher incidence of pruritus. Another view is that SP is probably a subclinical neuropathy, degenerative change in peripheral nerve endings may be attributable to age. This age alteration can cause pruritus without specific stimuli.7 In addition, immunosenescence occurs with ageing and also produces a higher incidence of pruritus.42 Moreover, decreases in androgen, oestrogen and glucocorticoid in aged people can contribute to SP.47 48 All these factors will increase the prevalence of SP with age.
The results of subregional analyses found that individuals who were living in Turkey and China were associated with a higher prevalence of SP. Pruritus is influenced by multiple factors, such as genetic, biological, psychological, social, environmental and cultural factors.49 Different countries vary in society, culture and environment, genetic, biological and psychological factors also differ among populations in different countries. Therefore, the different prevalence among countries is related to the above factors.
The results of subsex found that the prevalence of male is higher. Subresearch sites analyses found that the highest prevalence of SP was found in health examination centres. The reasons for these results are unclear based on the current scientific knowledge available on SP prevalence. Further studies would be helpful in further exploring these phenomena. In conclusion, the prevalence of SP varies among different populations. However, the reasons underlying the differences in prevalence observed in the current study remain unclear. It is suggested that further studies of the prevalence of SP in different populations be conducted in the future.
Meta-analyses showed that smoking, excessive drinking, and monophagism were possible risk factors for SP. It has been shown that smoking can cause nutrient and oxygen deprivation in cutaneous tissues, decreases collagen and elastin fibres in the dermis, and increases keratinocyte dysplasia.50 These changes reduce skin lipids, sebum and moisture retention, leading to dryness and pruritus of skin.51 52 Therefore, smoking is a potential risk factor for SP. This study also identified drinking alcohol as a potential risk factor for SP. Studies have demonstrated that alcohol consumption could reduce the concentration of carotenoids in the skin.53 Carotenoids can neutralise free radicals, delay premature skin ageing and skin diseases caused by free radicals.53–55 It could be proposed that alcohol consumption may lead to skin diseases by affecting the concentration of carotenoids. The human body cannot synthesise carotenoids in sufficient amounts without relying on a nutrient rich diet including fruit and vegetables. Therefore, monophagism could contribute to reducing the concentration of carotenoids and it could be considered a risk factors for skin diseases. Regrettably, the specific types of monophagism wasn't pointed out in the included study, which prevented further analysis and discussion. We expect that follow-up studies will explore and investigate this. In addition, point out the participants' dietary structure and specific types of monophagism.
Although this study indicated smoking, excessive drinking and monophagism were associated with an increased risk of SP, all the studies included in the meta-analysis were cross-sectional. Consequently, It is not possible to infer on the causality between exposure and outcomes. Further studies are needed to confirm these findings. In addition to the three risk factors identified through the meta-analyses, the included studies also showed that the risk factors for SP also include age, xerosis, astriction, less water intake, bathing with soap, bathing too frequently, malignant tumour, chronic illness, excessive drinking, insomnia and contact with animals.
To the best of our knowledge, this study is the first to provide a systematic review of SP prevalence and risk factors. However, several limitations of this study should be noted. First, The epidemiological data on SP was only from Norway, China, Turkey, Britain and Denmark, which cannot be generalised to the worldwide population. Second, the methods of identifying SP varied among the included studies, the definitions of SP may not be uniform among investigators and researchers in different countries, the study of different countries may not be unified in assessing the prevalence of SP, making it difficult to analyse the prevalence of SP using a gold standard method. These limitations make we less confident that the final estimate is close to a ‘true’ estimate. Considering these limitations, further studies will be needed to better understand the prevalence and risk factors of SP worldwide.
Conclusion
In conclusion, this study found the prevalence of SP was 21.04%. Individuals who were older, male or living in Turkey and China were associated with a higher prevalence of SP. Additionally, among health examination centres, nursing homes and communities, the highest detection rate of SP was found in the health examination centres. Smoking, excessive drinking and monophagism were possible individual risk factors for SP.
Supplementary Material
Footnotes
Contributors: SC and FZ contributed equally to this study. SC conceived and participated in the design of this review. SC and FZ performed the literature searches, study selection, data extraction and assessed the risk of bias. SC and FZ drafted the manuscript. YX helped in performing the analysis with constructive discussions. SC revised the final version. All authors read and approved the final manuscript. SC is responsible for the overall content as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1.Valdes-Rodriguez R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol 2015;95:417–21. 10.2340/00015555-1968 [DOI] [PubMed] [Google Scholar]
- 2.Hosseinpoor AR, Bergen N, Chatterji S. Socio-demographic determinants of caregiving in older adults of low- and middle-income countries. Age Ageing 2013;42:330–8. 10.1093/ageing/afs196 [DOI] [PubMed] [Google Scholar]
- 3.Darjani A, Mohtasham-Amiri Z, Mohammad Amini K, et al. Skin disorders among elder patients in a referral center in northern Iran (2011). Dermatol Res Pract 2013;2013:193205. 10.1155/2013/193205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reszke R, Pełka D, Walasek A, et al. Skin disorders in elderly subjects. Int J Dermatol 2015;54:e332–8. 10.1111/ijd.12832 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Wang HZ, Gao J. Effect of traditional Chinese medicine desire adjustment therapy on sleep quality in patients with senile skin pruritus. Chin J Nurs 2017;52:161–5. [Google Scholar]
- 6.Ständer S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International forum for the study of itch. Acta Derm Venereol 2007;87:291–4. 10.2340/00015555-0305 [DOI] [PubMed] [Google Scholar]
- 7.Clerc C-J, Misery L, Venereologica ML. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol 2017;97:433–40. 10.2340/00015555-2574 [DOI] [PubMed] [Google Scholar]
- 8.Reich A, Ständer S, Szepietowski JC. Pruritus in the elderly. Clin Dermatol 2011;29:15–23. 10.1016/j.clindermatol.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 9.Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T 2012;37:227–39. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Gao J, Shi LH. Effect of traditional Chinese medicine desire adjustment therapy on symptom and the quality of life in patients with senile skin pruritu. Chin J Geront 2016;36:4897–9. [Google Scholar]
- 11.Teoh YL, Teo RY, Yeo B. Elderly hospitalised Patients-The impact of itch and its prevalence. Ann Acad Med Singapore 2016;45:134–7. [PubMed] [Google Scholar]
- 12.Thaipisuttikul Y. Pruritic skin diseases in the elderly. J Dermatol 1998;25:153–7. 10.1111/j.1346-8138.1998.tb02371.x [DOI] [PubMed] [Google Scholar]
- 13.Caretti KL, Mehregan DR, Mehregan DA. A survey of self-reported skin disease in the elderly African-American population. Int J Dermatol 2015;54:1034–8. 10.1111/ijd.12520 [DOI] [PubMed] [Google Scholar]
- 14.Rubegni P, Poggiali S, Nami N, et al. Skin diseases in geriatric patients: our experience from a public skin outpatient clinic in Siena. G Ital Dermatol Venereol 2012;147:631–6. [PubMed] [Google Scholar]
- 15.Liao YH, Chen KH, Tseng MP, et al. Pattern of skin diseases in a geriatric patient group in Taiwan: a 7-year survey from the outpatient clinic of a university medical center. Dermatology 2001;203:308–13. 10.1159/000051778 [DOI] [PubMed] [Google Scholar]
- 16.Reszke R, Pełka D, Walasek A, et al. Skin disorders in elderly subjects. Int J Dermatol 2015;54:e332–8. 10.1111/ijd.12832 [DOI] [PubMed] [Google Scholar]
- 17.Reszke R, Białynicki-Birula R, Lindner K, et al. Itch in elderly people: a cross-sectional study. Acta Derm Venereol 2019;99:1016–21. 10.2340/00015555-3271 [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Zhao H, Zhou Y. A case-control study on risk of 410 male senile pruritus. Mod Prevent Med 2009;36:1820–2. [Google Scholar]
- 19.Chen ZH, HX M, Xian JF. The study on the morbidity of common senile dermatosis in Junjing community in Guangzhou City. J Jinan Univ 2015;36:73–6. [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng XT, Liu H, Chen X. Meta analysis series 4: quality assessment tools for observational studies. Chin J Evid Based Cardiovasc Med 2012;4:297–9. [Google Scholar]
- 22.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39. 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barili F, Parolari A, Kappetein PA. Statistical primer: heterogeneity. random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg 2018;27:317–21. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann D, Sinnett P, Holmes J, et al. Statistical controversies in clinical research: publication bias evaluations are not routinely conducted in clinical oncology systematic reviews. Ann Oncol 2017;28:931–7. 10.1093/annonc/mdw691 [DOI] [PubMed] [Google Scholar]
- 25.XS L, BX H, Yang XQ. Investigation of dermatosis in cadres above division level in Changchun. Shenyang Budui Yiyao 2000;13:39. [Google Scholar]
- 26.NL G, Xie ZH, Zhang CL. A case-control study on risk of 122 simple senile pruritus. Chin J Epidemiol 2006;27:627–9. [Google Scholar]
- 27.Xue SQ. Survey on elderly skin disorders of workers in Taigang factory. Chin J Dermatovenereol 2008;22:409–10. [Google Scholar]
- 28.HL N, XY K, Qian MQ. Analysis of prevalence of elderly itch skin disease in two pension institutions in Shanghai. Chin J Dermato Venerol Integ Trad W Med 2012;11:157–8. [Google Scholar]
- 29.Zhang L. The dermatopathic study of Middle—age and elderly Cadres in TaiYuan. Taiyuan, Shanxi, China: Shanxi Medical University, 2012. [Google Scholar]
- 30.Li L, Wang T, Liu XZ. Analysis of risk factors and health guidance for senile pruritus. J Mili Surg in Southwest Chin 2014:591–2. [Google Scholar]
- 31.Wu N, Zhang DL. Investigation and etiological analysis of senile pruritus. Chin J Convalescent Med 2014;23:368–9. [Google Scholar]
- 32.Yang J, Dou N, SH M. Influencing factors of senile pruritus in Tangshan City. Chin J Geront 2014;34:5205–7. [Google Scholar]
- 33.Hou XF, Zhang LJ. Investigation and analysis of 196 influencing factors of elderly dermatosis. Shaanxi Med 2016;45:1559–60. [Google Scholar]
- 34.Dalgard F, Svensson A, Holm J Ø, et al. Self-Reported skin morbidity in Oslo. associations with sociodemographic factors among adults in a cross-sectional study. Br J Dermatol 2004;151:452–7. 10.1111/j.1365-2133.2004.06058.x [DOI] [PubMed] [Google Scholar]
- 35.Tseng H-W, Lam H-C, Ger L-P, et al. A survey of dermatological diseases among older male adults of a Veterans home in southern Taiwan. Aging Clin Exp Res 2015;27:227–33. 10.1007/s40520-014-0260-9 [DOI] [PubMed] [Google Scholar]
- 36.Miller IM, Zarchi K, Ellervik C, et al. Self-Reported skin morbidity in Denmark: a population-based cross-sectional study. Eur J Dermatol 2016;26:281–6. 10.1684/ejd.2016.2766 [DOI] [PubMed] [Google Scholar]
- 37.Kara PA, Alatas ET, Doğan G. Prevalence of skin diseases among elderly residing in nursing homes in Mugla. Turk J Geriatr 2017;20:23–9. [Google Scholar]
- 38.Cowdell F, Dyson J, Long J, et al. Self-Reported skin concerns: an epidemiological study of community-dwelling older people. Int J Older People Nurs 2018;13:12195. 10.1111/opn.12195 [DOI] [PubMed] [Google Scholar]
- 39.Dyhre-Petersen N, Gazerani P. Presence and characteristics of senile pruritus among Danish elderly living in nursing homes. Future Sci OA 2019;5:Fso399. 10.2144/fsoa-2019-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong F, Lai YQ, JX T. Epidemiological characteristics of sleep disorders in the Chinese elderly: a meta-analysis. Chin J Evid-Based Med 2020;19:398–403. [Google Scholar]
- 41.Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging 2015;32:201–15. 10.1007/s40266-015-0246-0 [DOI] [PubMed] [Google Scholar]
- 42.Patel T, Yosipovitch G. The management of chronic pruritus in the elderly. Skin Therapy Lett 2010;15:5–9. [PubMed] [Google Scholar]
- 43.Paul C, Maumus-Robert S, Mazereeuw-Hautier J, et al. Prevalence and risk factors for xerosis in the elderly: a cross-sectional epidemiological study in primary care. Dermatology 2011;223:260–5. 10.1159/000334631 [DOI] [PubMed] [Google Scholar]
- 44.Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA 2013;310:2443–50. 10.1001/jama.2013.282023 [DOI] [PubMed] [Google Scholar]
- 45.Ko M-J, Yang J-Y, Wu H-Y, et al. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: a randomized controlled trial. Br J Dermatol 2011;165:633–9. 10.1111/j.1365-2133.2011.10448.x [DOI] [PubMed] [Google Scholar]
- 46.Reich A, Ständer S, Szepietowski JC. Drug-Induced pruritus: a review. Acta Derm Venereol 2009;89:236–44. 10.2340/00015555-0650 [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZJ, Zhu J, Me H. Advances in the pathogenesis of senile pruritus. Chin J Geront 2018;38:4598–600. [Google Scholar]
- 48.Liu YS. Endocrine system characteristics and diseases of the elderly. Chin J Geriatr 2005;24:637–9. [Google Scholar]
- 49.Tey HL, Yosipovitch G. Itch in ethnic populations. Acta Derm Venereol 2010;90:227–34. 10.2340/00015555-0867 [DOI] [PubMed] [Google Scholar]
- 50.Leow YH, Maibach HI. Cigarette smoking, cutaneous vasculature, and tissue oxygen. Clin Dermatol 1998;16:579–84. 10.1016/s0738-081x(98)00042-x [DOI] [PubMed] [Google Scholar]
- 51.Farage MA, Miller KW, Elsner P, et al. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci 2008;30:87–95. 10.1111/j.1468-2494.2007.00415.x [DOI] [PubMed] [Google Scholar]
- 52.Friedman O. Changes associated with the aging face. Facial Plast Surg Clin North Am 2005;13:371–80. 10.1016/j.fsc.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 53.Lademann J, Meinke MC, Sterry W, et al. Carotenoids in human skin. Exp Dermatol 2011;20:377–82. 10.1111/j.1600-0625.2010.01189.x [DOI] [PubMed] [Google Scholar]
- 54.Berson DS. Natural antioxidants. J Drugs Dermatol 2008;7:s7–12. [PubMed] [Google Scholar]
- 55.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta 2005;1740:101–7. 10.1016/j.bbadis.2004.12.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-051694supp001.pdf (44.4KB, pdf)
bmjopen-2021-051694supp002.pdf (78.8KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as online supplemental information.