Abstract
There is an increasing heavy disease burden of major depressive disorder (MDD) globally. Both high diagnostic heterogeneity and complicated pathological mechanisms of MDD pose significant challenges. There is much evidence to support anhedonia as a core feature of MDD. In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, anhedonia is further emphasised as a key item in the diagnosis of major depression with melancholic features. Anhedonia is a multifaceted symptom that includes deficits in various aspects of reward processing, such as anticipatory anhedonia, consummatory anhedonia, and decision-making anhedonia. Anhedonia is expected to become an important clinicopathological sign for predicting the treatment outcome of MDD and assisting clinical decision making. However, the precise neurobiological mechanisms of anhedonia in MDD are not clearly understood. In this paper, we reviewed (1) the current understanding of the link between anhedonia and MDD; (2) the biological basis of the pathological mechanism of anhedonia in MDD; and (3) challenges in research on the pathological mechanisms of anhedonia in MDD. A more in-depth understanding of anhedonia associated with MDD will improve the diagnosis, prediction, and treatment of patients with MDD in the future.
Keywords: depressive disorder, major
Introduction
Major depressive disorder (MDD) is a highly debilitating disease in China and worldwide.1–3 However, currently the diagnosis and treatment of MDD face many serious difficulties. Diagnosis is mainly based on phenomenological evaluation, and treatment is based on empirical judgements, resulting in high rates of misdiagnosis and missed diagnosis,1 low treatment efficacy, and low recovery rate.3 4 According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), MDD diagnosis requires five or more symptoms in the diagnostic criteria to be present for at least two weeks, with evident distress or functional impairment to the patient. Individuals can present with at least 256 unique symptom profiles according to these criteria.5 Therefore, MDD as a single disease has high diagnostic heterogeneity.6 There are at least two reasons for the discrepancies in MDD diagnosis, one of which is the heterogeneity of clinical features. In addition to the above-mentioned 256 unique symptoms, each episode also manifests heterogeneity in the onset form of the disease (single episode, recurrence, seasonality, etc.), severity (mild, moderate, or severe), age of onset (early onset, late onset, or postpartum onset), characteristics of comorbidities (comorbidity with other mental disorders or various physical diseases), course of disease (acute or chronic), and treatment outcome (refractory, complete remission, or partial remission), and so on. The second is the complicated pathological mechanism of MDD. Previously, MDD was considered a ‘functional disease’; however, in recent decades, with the wide application of various research techniques and analysis methods in MDD research, many different hypotheses have been proposed, including the genetic and epigenetic anomaly hypothesis, the monoamine hypothesis, the inflammatory hypothesis, the hypothalamic-pituitary-adrenal (HPA) axis dysfunction hypothesis, the neuroplasticity hypothesis, structural and functional brain changes hypothesis, and social psychological hypothesis.2 7 8 These hypotheses do not exist in isolation, but are closely related and interact with each other. Therefore, reconsidering the consequences of the heterogeneity of depression diagnosis, identifying new biological subtypes of depression, and uncovering objective markers for the early diagnosis and prediction of treatment response have become important research topics and directions in the field of depression in recent years.
Based on the diagnostic heterogeneity of depression and its causes, using functional neuroimaging data-driven strategies, Drysdale et al established associations between different patterns of brain functional connectivity and distinct MDD-symptom/behaviour profiles, and identified MDD-symptom/behaviour domains of anhedonia and anxiety as highly related to biological mechanisms, thus subdividing MDD into four subtypes.9 Anhedonia has been supported as an important symptom or behavioural domain of MDD by many studies and is expected to become an important clinicopathological sign for predicting the treatment outcome of MDD and assisting clinical decision making.
Anhedonia and MDD
In ancient Greek, anhedonia (an=‘without’, hēdonē=‘pleasure’) mainly describes the inability to experience any pleasure from usually pleasant activities, hobbies, sexual activities, or social interactions. In a broad sense, it refers to the reduced ability to experience pleasure or the lack of appropriate emotional responses to rewards or pleasant stimuli.10 11 Since the 1970s, anhedonia has been regarded as the first observable sign of the initial onset or recurrence of endogenous depression.3 In the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, text revision, anhedonia is defined as diminished interest or pleasure in response to stimuli that were previously perceived as rewarding during a premorbid state and is considered one of the core symptoms (depressed mood, loss of interest, and anhedonia) of MDD, along with depressed mood.12 In the DSM-5, anhedonia is further emphasised as a key item in the diagnosis of the melancholic subtype of major depression.5 In recent years, clinical studies have also found that about 70% of patients with MDD showed clinically obvious features of anhedonia,13 which was an important clinicopathological sign for differentiating MDD from other mental diseases such as anxiety,14 schizophrenia,15and others. In addition, anhedonia has been shown to be related to the severity of MDD16 17 and a prolonged disease course,18 and often it indicates a worse long-term prognosis and a higher suicide rate.5 6 Thus, anhedonia has become an important predictor of disease progression and treatment outcome in MDD.18
Anhedonia is related to impaired reward processing in the brain. A series of neuropsychological and neurobiological studies have found that reward processing comprises multiple aspects, including desire, effort/motivation/decision, anticipation pleasure, and consummatory pleasure.19 Berridge and Robinson20 delineated the dissociable psychological components of reward as: ‘liking’ (hedonic impact), ‘wanting’ (incentive salience), and learning (predictive associations and cognitions). Anhedonia can be manifested in abnormalities in various aspects of the reward processing, such as anticipatory anhedonia, consummatory anhedonia, and decision-making anhedonia.19 21–23 Individuals with anhedonia can present with loss of desire for previously pleasant rewards (diminished interest), lack of pleasure/satisfaction after receiving rewards (loss of pleasant experience and depressed mood), or both (diminished interest, loss of pleasant experience, and depressed mood). In addition, individuals cannot feel any enjoyment from activities that were previously perceived as pleasurable and may also manifest social withdrawal, lack of motivation, and reduced activities. Some patients experience a significant decline in sexual interest and desire, abnormal reward learning (cognitive impairment), and so on. The above-mentioned manifestations are typical clinical features commonly identified in patients with depression. These features are supported by the results of many current multidimensional big data analysis.6
The biological basis of the pathological mechanism of anhedonia in MDD
Anhedonia is related to the dysfunction of the reward circuit in the brain. The brain’s reward circuit is a neural network with a dense distribution of dopamine (DA)24 that mainly comprises several cortical regions, including the orbitofrontal cortex, ventromedial prefrontal cortex, and anterior cingulate cortex, and subcortical regions, such as the nucleus accumbens, ventral tegmental area (VTA), and amygdala. Among them, the DA pathway of limbic midbrain system, including the VTA, ventral striatum, and the prefrontal cortex, plays a key role in reward processing.25 The midbrain DA system is composed primarily of neurons in the VTA of the midbrain that project to the ventral striatum, regulating the reward processing by changing the sensitivity of the medium spiny neurons of the striatum to cortical and subcortical glutamatergic afferents26 27 (figure 1). Various primary and secondary reward stimuli, including food, sex, and drugs, can increase the release of DA while inducing the reward processing.28 Genome-wide association studies (GWAS) and other genetic studies have found that genetic polymorphisms of DA synthesis, metabolism, and functional activity regulator proteins were related to the functional activities of brain regions associated with reward processing29 30 and were further significantly related to the clinical features of anhedonia in patients with MDD. Polygenic risk scores (PRS) analysis using GWAS data and anhedonia scores showed that the PRS of anhedonia were significantly associated with a decrease in volume of the reward-related brain regions, including the orbitofrontal cortex, nucleus accumbens, and putamen.31 Notably, increasing evidence indicates that the aberrant activity of the lateral habenula (LHb), as the brain’s ‘antireward centre’, is associated with depressive symptoms such as anhedonia and helplessness32 33 (figure 1). The LHb plays an important role in the coding of negative reward signals and in the control of motivated behaviours. Therefore, the development of LHb-specific targeting of drugs, antibodies, or antisense oligonucleotides for MDD is promising.34
Figure 1.
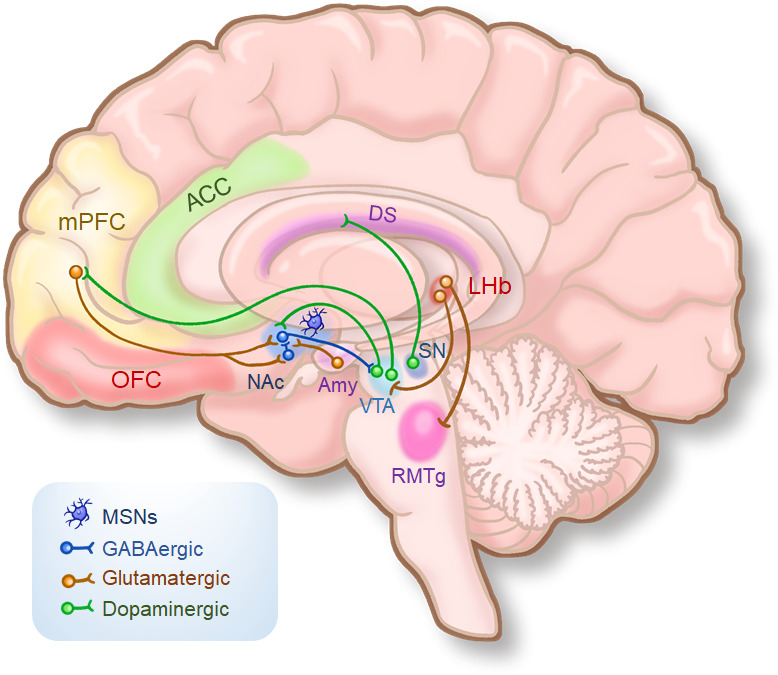
A simplified schematic of the reward circuit in the human brain. ACC, anterior cingulate cortex; Amy, amygdala; DS, dorsal striatum; LHb, lateral habenula; mPFC, medial prefrontal cortex; MSNs, medium spiny neurons; NAc, nucleus accumbens; OFC, orbitofrontal cortex; RMTg, rostromedial tegmental nucleus; SN, substantia nigra; VTA, ventral tegmental area.
It was further found that the levels of plasma inflammatory factors (such as inflammatory-6 and C reaction protein (CRP)) in patients with MDD with symptoms of anhedonia were significantly increased,35 and patients with anhedonia and high levels of inflammatory factors also showed immunometabolic disturbances and reductions in the tyrosine metabolism pathway.36 37 These results were confirmed in both basic and clinical studies. One study found that provision of exogenous inflammatory stimulation to humans and experimental animals or a state of chronic endogenous inflammation leads to a lack of motivation in subjects, subsequently leading to anhedonia.38 Imaging studies further revealed that the elevated plasma CRP level in patients with MDD was significantly related to the decreased functional connectivity and elevated glutamate level in the brain’s reward circuit, as well as the symptoms of anhedonia.39 A recent study found that increased levels of inflammatory factors in the central nervous system were positively correlated with the increased levels of inflammatory factors in plasma (r=0.855), as well as the symptoms of anhedonia in patients with MDD.40 At present, it is believed that the immune-inflammatory responses have varying degrees of impact on the HPA axis, neurotransmitter system, secretion of the neurotrophic factors, synaptic plasticity, and so on, which disrupts the reward circuit and leads to anhedonia.41 Moreover, through the interactions with the DA and glutamate systems, the immune-inflammatory mechanism might be more involved in the pathological process of abnormal reward motivation, anticipation pleasure, and reinforcement learning, while consummatory anhedonia was more involved with the central opioid pathway.42
In recent years, resting-state and task imaging studies have revealed significant data on the abnormal reward circuit in depression involving various brain regions of the reward circuit. For reward liking and wanting, striatal hypoactivation was observed, alongside hypoactivation and hyperactivation across frontal regions. For reward learning, blunted frontostriatal sensitivity to positive feedback was observed.43 Specifically, the reward prediction error signals of the striatum were significantly decreased, and the VTA-striatum functional connectivity was reduced, which were significantly related to the impaired reward learning ability of the patients with MDD.44–46
These results also provided new strategies for the determination of the clinical features of anhedonia and its treatment in patients with depression. In terms of treatment, the current common antidepressants, such as selective serotonin reuptake inhibitors, are more effective at reducing negative emotions, rather than enhancing ‘pleasure ability’ (i.e., increasing positive emotions, improving insufficient reward feedback, and motivation); thus, it is difficult to alleviate the symptoms of anhedonia.47 48 Some clinical experts have called for the development of a treatment strategy to specifically target the symptoms of anhedonia in patients49 to improve the poor treatment response and clinical outcomes. Several studies have so far evaluated the efficacy of dopaminergic drugs in the treatment for anhedonia in MDD, such as the DA transporter inhibitor bupropion. These studies, including basic studies,50 comparative studies with standard antidepressant monotherapy,51 and synergistic treatment studies,52 revealed that the synergistic therapeutic effect of bupropion could help improve the positive affective dimension (energy, motivation, and enjoyment) of patients with MDD, but a controlled study comparing bupropion with the standard antidepressant monotherapy did not show its superiority over escitalopram. Other dopaminergic modulators are second-generation antipsychotics, of which aripiprazole has attracted more attention. The Canadian Biomarker Integration Network in Depression has just completed a study of sequential treatment with an evaluation of anhedonia and found that 61% of patients with MDD who had no response to escitalopram treatment were relieved after combining treatment with the dopaminergic drug aripiprazole, resulting in significant improvements in the patients’ anhedonia symptoms, reward processing evaluated by imaging, and brain activities.53
Challenges in research on the pathological mechanisms of anhedonia in MDD
Although anhedonia is defined as the core symptom of MDD in the DSM-5, research on the mechanisms of anhedonia in MDD still faces great challenges (table 1). First, anhedonia is a transdiagnostic psychopathological sign; that is, in addition to MDD, it is also seen in other mental disorders, including schizophrenia. In the Research Domain Criteria, human function/behaviour is divided into six research domains, of which the positive valence system is related to the abnormality of the reward system. In terms of the connotation of the concept, there are differences in anhedonia between the two diseases. In the schizophrenia spectrum, anhedonia is a subordinate construct, which is narrowly defined as ‘the decreased ability to experience pleasure from positive stimuli or a degradation in the recollection of pleasure previously experienced’ (DSM-5, p. 88), and is included in the domain of negative symptoms. Other negative symptoms include alogia, avolition, asociality, and diminished emotional expression. Conversely, in MDD, anhedonia is defined as a supraordinate construct. As a general criterion, it covers the description of different clinical features of MDD, including feeling less interested in hobbies (‘not caring anymore’) or not feeling any enjoyment in activities that were previously considered pleasurable (DSM-5, p. 163).25 Therefore, the term ‘anhedonia’ in MDD is more analogous to the term ‘negative symptoms’ in schizophrenia. Confusion is easily caused in the literature due to the competing definitions for anhedonia. A refined definition of anhedonia that identifies deficits in different aspects of reward processing should be introduced in future versions of diagnostic systems. Computational approaches are needed to provide objective methods of assessing different profiles within the heterogeneous symptom domain.25 Second, although anhedonia is the core symptom of MDD, it is not equal to depression, and there are individual differences in the presentation of anhedonia in depressed patients. Existing evidence supports the causal relationship between reward processing abnormalities and depression, but the temporal correlation and operability are weak.54 In the future, it will be necessary to improve the evaluation of reward processing and depression and to optimise study design to solve the current dilemma. Therefore, the final challenge is the lack of specific assessment techniques for anhedonia in MDD. Currently, the commonly used assessment tools for anhedonia include the Snaith-Hamilton Pleasure Scale (SHAPS), Fawcett-Clark Pleasure Capacity Scale (FCPS), Chapman Social Anhedonia Scale (CSAS), Temporal Experience of Pleasure Scale (TEPS), and Dimensional Anhedonia Rating Scale (DARS).19 Among these, SHAPS does not have a limitation on diagnoses. FCPS and CSAS are culturally specific and have limited evaluation domains. Only the DARS is dedicated to assessing the multidimensional features of anhedonia in patients with depression, and it can be used to evaluate the anticipation pleasure, consummatory pleasure, and decision-making pleasure for natural stimuli, food, and social stimuli, reflecting the features of anhedonia in patients with MDD more comprehensively. Currently, there is only a Chinese version,55 a Spanish version56 and an English version;57 therefore, it needs to be optimised in the future for a wider population.
Table 1.
Current challenges in research on the pathological mechanisms of anhedonia in major depressive disorder (MDD)
| Challenges | Potential solutions |
| Competing definitions for anhedonia in schizophrenia and MDD | New clinical terminology should be introduced in future versions of the diagnostic systems to facilitate identifying deficits in different aspects of reward processing in the anhedonia domain of MDD. |
| The weak temporal correlation and operability between reward processing and MDD | Improving the evaluation of reward processing in MDD and optimising study design to solve the problem are necessary. |
| Lack of specific assessment tools for anhedonia in MDD | The current commonly used anhedonia assessment tools have several limitations. The recently developed Dimensional Anhedonia Rating Scale can comprehensively reflect anhedonia features in patients with MDD; as an ideal tool, it needs to be optimised in the future for a wider population. |
Conclusion
MDD is currently a highly debilitating disease worldwide, and the diagnostic heterogeneity is a key factor in the current difficulties in its diagnosis and treatment.2 The diagnostic heterogeneity of MDD stems from the complex heterogeneity of its clinical features, aetiology, and pathology. Big data model analysis suggests that anhedonia may be an important clinical subtype of MDD. Anhedonia has gradually been regarded as a core symptom of MDD since DSM-III, and especially after DSM-5 has emphasised further its importance in the diagnosis of MDD. Furthermore, in recent years, basic, genetic, molecular biology, and imaging studies based on MDD populations all support the importance of anhedonia in the diagnosis and treatment of depression. However, research on the mechanisms of anhedonia in MDD still faces a series of challenges. Anhedonia is a transdiagnostic pathological sign, and the study design needs to be optimised in the future to further investigate the characteristic symptoms, signs, and biological mechanisms of anhedonia in MDD. The optimisation of specific assessment techniques is warranted to deepen the understanding of anhedonia associated with depression and to improve the diagnosis, prediction, and treatment of patients with MDD with anhedonia.
Acknowledgments
We thank Chenchen Zhang for her excellent drawing of the schematic reward circuit in the review.
Biography
Dr Yun-Ai Su completed her PhD in psychiatry from Peking University in 2007. She successively received funding from the China Scholarship Council to be a visiting scholar at Max Planck Institute of Psychiatry, Germany (12 months) and University of Wollongong, Australia (6 months). She currently is an investigator and a supervisor of doctoral students at the Department of Psychopharmacology, Peking University Sixth Hospital. She is also a member of basic and clinical branch of psychiatry, Chinese neuroscience society. Her main research interests include basic and clinical psychopharmacology, biomarkers of affective disorders. As the principal investigator, she got five funds support from National Natural Science Foundation of China and Beijing Municipal Science and Technology Commission. With the financial support of these grants, she has made some important and interesting scientific discoveries and published more than 60 scientific papers in international peer-reviewed journals.

Footnotes
Contributors: Y-AS: writing original draft. TS: conceptualization, writing—review, funding acquisition.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81630031), National Key Technology R&D Program (2015BAI13B01).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1.Zhong B-liang, Chen H-hui, Zhang J-fang, et al. Prevalence, correlates and recognition of depression among inpatients of general hospitals in Wuhan, China. Gen Hosp Psychiatry 2010;32:268–75. 10.1016/j.genhosppsych.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 2.Malhi GS, Mann JJ. Depression. Lancet 2018;392:2299–312. 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- 3.Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers 2016;2:16065. 10.1038/nrdp.2016.65 [DOI] [PubMed] [Google Scholar]
- 4.Leuchter AF, Cook IA, Hamilton SP, et al. Biomarkers to predict antidepressant response. Curr Psychiatry Rep 2010;12:553–62. 10.1007/s11920-010-0160-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th edn. Washington, DC: APA Publishing, 2013. [Google Scholar]
- 6.Lynch CJ, Gunning FM, Liston C. Causes and consequences of diagnostic heterogeneity in depression: paths to discovering novel biological depression subtypes. Biol Psychiatry 2020;88:83–94. 10.1016/j.biopsych.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 7.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011;73:114–26. 10.1097/PSY.0b013e31820ad12b [DOI] [PubMed] [Google Scholar]
- 8.Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolic depression. Biol Psychiatry 2020;88:369–80. 10.1016/j.biopsych.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 9.Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 2017;23:28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribot T. La Psychologie des Sentiment [The Psychology of Feelings]. Paris: Felix Alcan, 1896. [Google Scholar]
- 11.Klein DF. Endogenomorphic depression. A conceptual and Terminological revision. Arch Gen Psychiatry 1974;31:447–54. 10.1001/archpsyc.1974.01760160005001 [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edn. Washington, DC: APA Publishing, 2000. [Google Scholar]
- 13.Shankman SA, Katz AC, DeLizza AA, et al. The different facets of anhedonia and their associations with different psychopathologies. In: Anhedonia: a comprehensive handbook volume I: conceptual issues and neurobiological advances. Springer, 2014: 3–22. [Google Scholar]
- 14.Watson D, Clark LA, Weber K, et al. Testing a tripartite model: II. exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol 1995;104:15–25. 10.1037/0021-843X.104.1.15 [DOI] [PubMed] [Google Scholar]
- 15.Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model's prediction of anhedonia's specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Res 2003;119:243–50. 10.1016/S0165-1781(03)00131-8 [DOI] [PubMed] [Google Scholar]
- 16.Gong L, Yin Y, He C, et al. Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res 2017;84:9–17. 10.1016/j.jpsychires.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 17.Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry 2009;8:22. 10.1186/1744-859X-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spijker J, Bijl RV, de Graaf R, et al. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands mental health survey and incidence study (nemesis). Acta Psychiatr Scand 2001;103:122–30. 10.1034/j.1600-0447.2001.103002122.x [DOI] [PubMed] [Google Scholar]
- 19.Rizvi SJ, Pizzagalli DA, Sproule BA, et al. Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev 2016;65:21–35. 10.1016/j.neubiorev.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci 2003;26:507–13. 10.1016/S0166-2236(03)00233-9 [DOI] [PubMed] [Google Scholar]
- 21.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 2011;35:537–55. 10.1016/j.neubiorev.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rømer Thomsen K, Whybrow PC, Kringelbach ML. Reconceptualizing anhedonia: novel perspectives on balancing the Pleasure networks in the human brain. Front Behav Neurosci 2015;9:49. 10.3389/fnbeh.2015.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keren H, O'Callaghan G, Vidal-Ribas P, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry 2018;175:1111–20. 10.1176/appi.ajp.2018.17101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010;35:4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper JA, Arulpragasam AR, Treadway MT. Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci 2018;22:128–35. 10.1016/j.cobeha.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soder HE, Cooper JA, Lopez-Gamundi P, et al. Dose-Response effects of d-amphetamine on effort-based decision-making and reinforcement learning. Neuropsychopharmacology 2021;46:1078–85. 10.1038/s41386-020-0779-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westbrook A, van den Bosch R, Määttä JI, et al. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science 2020;367:1362–6. 10.1126/science.aaz5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egerton A, Mehta MA, Montgomery AJ, et al. The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev 2009;33:1109–32. 10.1016/j.neubiorev.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung YS, Barch DM. Frontal-striatum dysfunction during reward processing: relationships to amotivation in schizophrenia. J Abnorm Psychol 2016;125:453–69. 10.1037/abn0000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren H, Fabbri C, Uher R, et al. Genes associated with anhedonia: a new analysis in a large clinical trial (GENDEP). Transl Psychiatry 2018;8:150. 10.1038/s41398-018-0198-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guffanti G, Kumar P, Admon R, et al. Depression genetic risk score is associated with anhedonia-related markers across units of analysis. Transl Psychiatry 2019;9:236. 10.1038/s41398-019-0566-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Cui Y, Sang K, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018;554:317–22. 10.1038/nature25509 [DOI] [PubMed] [Google Scholar]
- 33.Cui Y, Yang Y, Ni Z, et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018;554:323–7. 10.1038/nature25752 [DOI] [PubMed] [Google Scholar]
- 34.Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci 2020;21:277–95. 10.1038/s41583-020-0292-4 [DOI] [PubMed] [Google Scholar]
- 35.Tang W, Liu H, Chen L, et al. Inflammatory cytokines, complement factor H and anhedonia in drug-naïve major depressive disorder. Brain Behav Immun 2021;95:238–44. 10.1016/j.bbi.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 36.Bekhbat M, Treadway MT, Goldsmith DR, et al. Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub-type of depression with high CRP and anhedonia. Brain Behav Immun 2020;88:161–5. 10.1016/j.bbi.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Ding L, Shen T, et al. Hmgb1 involved in stress-induced depression and its neuroinflammatory priming role: a systematic review. Gen Psychiatr 2019;32:e100084. 10.1136/gpsych-2019-100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucido MJ, Bekhbat M, Goldsmith DR, et al. Aiding and abetting anhedonia: impact of inflammation on the brain and pharmacological implications. Pharmacol Rev 2021;73:1084–117. 10.1124/pharmrev.120.000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horn SR, Long MM, Nelson BW, et al. Replication and reproducibility issues in the relationship between C-reactive protein and depression: a systematic review and focused meta-analysis. Brain Behav Immun 2018;73:85–114. 10.1016/j.bbi.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felger JC, Haroon E, Patel TA, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry 2020;25:1301–11. 10.1038/s41380-018-0096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016;16:22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron 2015;86:646–64. 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borsini A, Wallis ASJ, Zunszain P, et al. Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn Affect Behav Neurosci 2020;20:816–41. 10.3758/s13415-020-00804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar P, Goer F, Murray L, et al. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology 2018;43:1581–8. 10.1038/s41386-018-0032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutledge RB, Moutoussis M, Smittenaar P, et al. Association of neural and emotional impacts of reward prediction errors with major depression. JAMA Psychiatry 2017;74:790–7. 10.1001/jamapsychiatry.2017.1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reilly EE, Whitton AE, Pizzagalli DA, et al. Diagnostic and dimensional evaluation of implicit reward learning in social anxiety disorder and major depression. Depress Anxiety 2020;37:1221–30. 10.1002/da.23081 [DOI] [PubMed] [Google Scholar]
- 47.Argyropoulos SV, Nutt DJ. Anhedonia revisited: is there a role for dopamine-targeting drugs for depression? J Psychopharmacol 2013;27:869–77. 10.1177/0269881113494104 [DOI] [PubMed] [Google Scholar]
- 48.Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry 2009;195:211–7. 10.1192/bjp.bp.108.051110 [DOI] [PubMed] [Google Scholar]
- 49.Krystal AD, Pizzagalli DA, Mathew SJ, et al. The first implementation of the NIMH FAST-FAIL approach to psychiatric drug development. Nat Rev Drug Discov 2018;18:82–4. 10.1038/nrd.2018.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randall PA, Lee CA, Podurgiel SJ, et al. Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. Int J Neuropsychopharmacol 2014;18. 10.1093/ijnp/pyu017. [Epub ahead of print: 31 Oct 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clayton AH, Croft HA, Horrigan JP, et al. Bupropion extended release compared with escitalopram: effects on sexual functioning and antidepressant efficacy in 2 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry 2006;67:736–46. 10.4088/jcp.v67n0507 [DOI] [PubMed] [Google Scholar]
- 52.Patel K, Allen S, Haque MN, et al. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol 2016;6:99–144. 10.1177/2045125316629071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennedy SH, Lam RW, Rotzinger S, et al. Symptomatic and functional outcomes and early prediction of response to escitalopram monotherapy and sequential adjunctive aripiprazole therapy in patients with major depressive disorder: a CAN-BIND-1 report. J Clin Psychiatry 2019;80. 10.4088/JCP.18m12202. [Epub ahead of print: 05 Feb 2019]. [DOI] [PubMed] [Google Scholar]
- 54.Nielson DM, Keren H, O'Callaghan G, et al. Great expectations: a critical review of and suggestions for the study of reward processing as a cause and predictor of depression. Biol Psychiatry 2021;89:134–43. 10.1016/j.biopsych.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia H, Wang L, An J. Reliability and validity of the Chinese version of dimensional anhedonia rating scale of depression in China. Chin J Psych 2020;53:216–20. [Google Scholar]
- 56.Arrua-Duarte E, Migoya-Borja M, Barrigón ML, et al. Spanish adaptation of the dimensional anhedonia rating scale (DARS). J Affect Disord 2019;245:702–7. 10.1016/j.jad.2018.11.040 [DOI] [PubMed] [Google Scholar]
- 57.Rizvi SJ, Quilty LC, Sproule BA, et al. Development and validation of the dimensional anhedonia rating scale (DARS) in a community sample and individuals with major depression. Psychiatry Res 2015;229:109–19. 10.1016/j.psychres.2015.07.062 [DOI] [PubMed] [Google Scholar]


