Abstract
Background:
As a result of progress in diagnosis and treatment, there is a growing prevalence of metastatic breast cancer (MBC) with isolated CNS metastases. This study describes the largest-to-date real-life cohort of this clinical setting and compares it to other clinical presentations.
Methods:
We retrospectively analysed the French Epidemiological Strategy and Medical Economics (ESME) MBC database including patients who initiated treatment for MBC between 2008 and 2016. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method. Descriptive statistics and multivariate Cox model were used.
Results:
Of 22,266 patients, 647 (2.9%) and 929 (4.2%) patients had isolated first-site CNS metastases or combined with extra-CNS metastases, with longer OS for the group with isolated CNS metastases (16.9 versus 13.9 months, adjusted HR = 1.69 (95% CI: 1.50–1.91), p < 0.001). Among the 541 (2.4%) patients with isolated CNS metastases and no intrathecal therapy (excluding leptomeningeal metastases), HER2+ cases were preponderant over TN or HR+ /HER2− cases (41.6% versus 26.1% versus 28.5%, respectively, p < 0.01). The treatment strategy consisted of a combination of local treatment and systemic therapy (49.2%), local treatment only (35.5%) or systemic therapy only (11.4%), or symptomatic therapy only (3.9%). Median PFS was 6.1 months (95% CI: 5.7–6.8). Median OS was 20.7 months (95% CI: 17.3–24.3), reaching 37.9 months (95% CI: 25.9–47.6) in the HR+ /HER2+ subgroup. Older age, TN subtype, MBC-free interval of 6–12 months, lower performance status, and WBRT were associated with poorer survival. Patients who received systemic therapy within 3 months from MBC diagnosis had longer OS (24.1 versus 16.1 months, p = 0.031), but this was not significant on multivariate analysis [HR = 1.0 (95% CI: 0.7–1.3), p = 0.806].
Conclusions:
Patients with isolated CNS metastases at MBC diagnosis represent a distinct population for which the role of systemic therapy needs to be further investigated in prospective studies.
Keywords: breast cancer, CNS metastases, real-world data
Introduction
Breast cancer (BC) is the most commonly diagnosed tumour and the leading cause of cancer death in women, representing a growing health concern worldwide despite the therapeutic progress achieved in the past two decades. 1
Central nervous system (CNS) metastases represent very poor outcomes, associating debilitating symptoms, impaired quality of life and poor survival,2,3 and BC is the second most common primary tumour associated with CNS metastases.2,4 Although routine screening for CNS metastases is not recommended in the absence of CNS symptoms, recent reviews have reported an incidence exceeding 20% in the overall metastatic BC (MBC) population,2,5 reaching 30–40% in the presence of human epidermal growth factor receptor 2 overexpression (HER2+) or triple-negative BC (TNBC). 6 Some authors have also reported an increasing incidence of BC-related CNS metastases over the last two decades, likely due to progress in imaging techniques and systemic therapies for MBC. 7 Pharmacokinetic hypotheses such as poor diffusion of trastuzumab across the brain-blood barrier (BBB) could explain the high incidence of CNS metastases observed in HER2+ MBC patients, especially those with long-term control of extracranial disease.8,9 Some papers have even reported a higher incidence of CNS metastases as the first site of recurrence after adjuvant trastuzumab therapy, 10 which could be due to the poor CNS diffusion of trastuzumab in the presence of a supposedly intact BBB that fails to effectively prevent growth of CNS micro-metastases, while more effectively controlling extracranial micro-metastatic disease. 2
Patients with CNS metastases as the first and isolated metastases from BC are rare and have been reported in small retrospective series.11–14 Most published studies, as well as current guidelines, refer to CNS metastases occurring at any time during the course of BC,15,16 which is of limited value to draw conclusions about specific strategies, whether CNS metastases are isolated or combined with extracranial disease. However, several authors have stressed the potentially different clinicopathological characteristics and prognosis of isolated CNS metastases, requiring a different approach11,12 compared to the more common situation in which extracranial disease is also present and drives the choice of systemic therapy.5,17,18 Except for HER2+ disease, for which maintenance of targeted anti-HER2 systemic therapy is recommended whether or not CNS metastases are isolated, there is no strong recommendation for the use of additional systemic therapy for isolated CNS disease following local treatment.16,19 Real-world data from large populations may contribute to a better understanding of the prognostic factors and management of this specific clinical entity of MBC with isolated CNS metastases.
Materials and methods
Study design
Based on the large Epidemiological Strategy and Medical Economics (ESME) MBC data platform, this study was designed to provide an in-depth analysis of the clinical features of patients with isolated CNS metastases as first metastatic site compared to patients with both CNS and extra-CNS metastases and patients with extra-CNS metastases only.
The ESME MBC database (NCT03275311) is a national multicentre retrospective observational programme that collects individual data from all consecutive patients, aged ⩾18 years, who have initiated treatment for MBC at one of the 18 French Comprehensive Cancer Centres belonging to the UNICANCER network, as from 2008. Data are updated annually and include the main patient and tumour characteristics, outcome and treatment patterns.
In line with French regulations, the ESME MBC database has been approved by the French data protection authority (Registration ID 1704113 and authorization No. DE-2013-117). In compliance with the applicable European regulations, a complementary authorization was obtained on 14 October 2019 regarding the ESME research data warehouse. The present analysis was approved by an independent Ethics Committee (Comité de Protection des Personnes Sud-Est II-2015-79). No specific formal informed consent was required for this study, but all patients had approved the re-use of their electronically recorded data. The ESME Research Programme is managed by UNICANCER according to best practice guidelines, and the ESME Scientific Committee approved the present study.
Study objectives
The primary objective was to describe the outcome of patients with isolated CNS as first metastatic site, using median overall survival (OS) and progression-free survival (PFS) as primary endpoints. Secondary objectives were (1) to describe and compare characteristics and outcomes of MBC cases according to the first site of metastases (isolated CNS metastases, both CNS and extra-CNS metastases, or no CNS metastases); (2) report local treatment and first-line systemic therapy modalities; and (3) report time to CNS progression (TTCNS) and time to extra-CNS progression (TTexCNS) in patients with isolated CNS metastases.
Study population and data collected
All female patients available in the ESME MBC database (included in the database between 1 January 2008 and 31 December 2016) were included in the comparative analysis. This in-depth analysis was based on patients with isolated and exclusive intraparenchymal brain metastases (BM) at MBC diagnosis, excluding patients treated with intrathecal (IT) chemotherapy as a proxy for leptomeningeal metastases. Only local treatment and systemic therapy initiated during the first 3 months after the diagnosis of MBC with isolated CNS disease were documented. According to hormone receptor (HR) and HER2 statuses, tumours were classified into four subtypes: HR+ /HER2−, HR+ /HER2+, HR−/HER2+, and triple-negative (TN).
Statistical analysis
Clinicopathological characteristics and demographic data were assessed using descriptive statistics.
OS was defined as the time between the date of MBC diagnosis and the date of death from any cause, and PFS was defined as the time between the date of MBC diagnosis and the date of progression or death. TTCNS was defined as the time between the date of MBC diagnosis and the date of CNS progression or death, while TTexCNS was defined as the time between the date of MBC diagnosis and the date of extra-CNS progression or death. Patients not experiencing an event were censored at the date of last news in the centre. MBC diagnosis was based on imaging exams, and pathological confirmation was not mandatory. The time to MBC was defined as the time between the diagnosis of primary cancer and the diagnosis of MBC.
Survival distribution was estimated by the Kaplan–Meier method, reported as the median and 95% confidence interval (CI) and compared between groups using the log-rank test. Univariate and multivariable Cox proportional hazards regression models were performed to assess significant prognostic factors for patients with isolated CNS metastases. Comparisons of survival hazard ratios (HR) between groups were reported with point estimates and 95% CI. For all tests, a two-sided p value <0.05 was considered statistically significant. All statistical analyses were performed using R software, version 3.6.1.
Further details on the study population, data collection and statistical analysis are reported in Supplementary Materials and Methods.
Results
Patient characteristics according to the first metastatic site
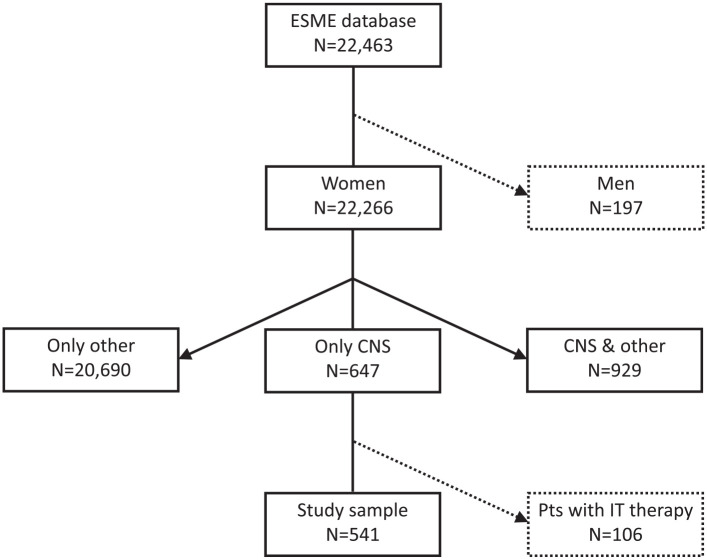
Of 22,266 MBC female patients included in the ESME database between 2008 and 2016, 20,690 (92.9%) had only extra-CNS metastases, 929 (4.2%) had CNS and extra-CNS metastases, and 647 (2.9%) had isolated CNS metastases as first metastatic site (Figure 1). Among these 647 patients, 541 (2.4% of 22,266 patients) were considered to have exclusively intraparenchymal BM, after excluding 106 patients treated with IT chemotherapy, suggesting the presence of leptomeningeal metastases.
Figure 1.
Flowchart of MBC patients in the ESME MBC cohort according to the first metastatic site.
CNS, central nervous system; ESME, Epidemiological Strategy and Medical Economics; IT, intrathecal; Pts, patients.
Some of the main patient characteristics according to the distribution of metastases are reported in Supplementary Table 1.
Patients with isolated CNS metastases more commonly had a HER2+ tumour compared to those with both CNS and extra-CNS metastases, or extra-CNS metastases only (37.1% versus 23.3% versus 17.2%, p < 0.001), and less commonly had a HR+ /HER2− tumour (29.5% versus 42.6% versus 63.6%, p < 0.001). The distribution of TNBC was not different between patients with isolated CNS metastases or CNS metastases together with extra-CNS metastases, but TNBC was more common in these patients compared to those with no CNS metastases (28.4% and 28.1% versus 12.3%, respectively, p < 0.001). Higher rates of CNS metastases were observed in HER2+ and TN subtypes compared to HR+ /HER2− subtype (11.4% and 14.9% versus 4.3%, p < 0.001) and this was also true for isolated CNS metastases (6% and 6.2% versus 1.4%, respectively, p < 0.001).
Patients with CNS metastases (isolated or combined with extra-CNS metastases) had more commonly received adjuvant or neoadjuvant chemotherapy (excluding patients with de novo MBC, 89.6% and 82.6% versus 69.2%), presented a histological grade III tumour (46.5% and 40.9% versus 28.0%), were young (median age at MBC diagnosis of 56 years and 56 years versus 61 years) and had a poor ECOG PS (PS = 2–4) compared with those with no CNS metastasis (15.9% and 21.3% versus 9.7%), all p < 0.001.
The shortest median time to MBC (estimated only for recurrent MBC) diagnosis was observed in patients with isolated CNS metastases compared to those with both CNS and extra-CNS metastases or those with extra-CNS metastases only [24.3 months (IQR 15.3–45.5) versus 39.3 months (IQR: 20.8–89.6) versus 71.1 months (IQR: 33.2–136), respectively, p < 0.001].
More patients with isolated CNS metastases did not receive any systemic therapy during the first 3 months following the diagnosis of MBC, as opposed to patients with both CNS and extra-CNS metastases or patients with extra-CNS metastases only (32.6% versus 9.4% versus 3.6%, respectively, p < 0.0001).
Survival according to the first metastatic site
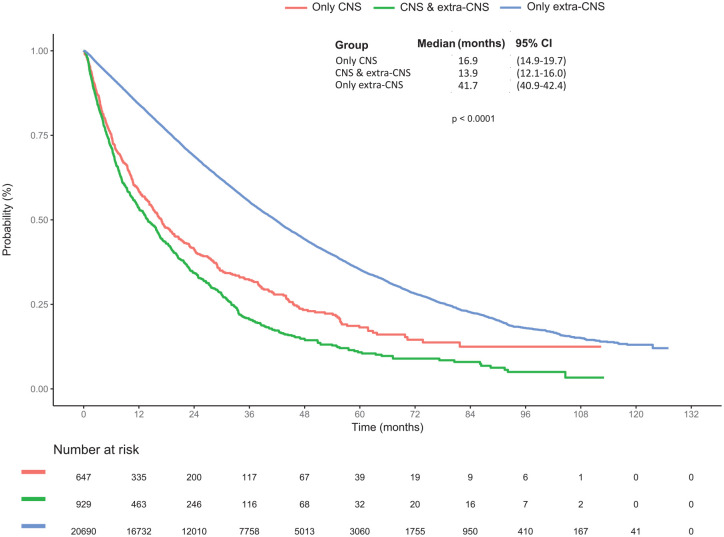
Patients with CNS metastases had a poorer survival than those without CNS metastases, irrespective of tumour subtype (p < 0.001, data not shown). Median OS ranged from 16.9 months (95% CI: 14.9–19.7) in patients with isolated CNS metastases, 13.9 months (95% CI: 12.1–16.0) in those with both CNS and extra-CNS metastases, and 41.7 months (95% CI: 40.9–42.4) in those with only extra-CNS metastases, p < 0.001 (Figure 2). In multivariable analysis, patients with both CNS and extra-CNS metastases had significantly poorer OS than those with isolated CNS metastases [HR = 1.69 (95% CI: 1.50–1.91), p < 0.001]. On the other hand, patients with only extra-CNS metastases had better OS than those with isolated CNS metastases [HR = 0.76 (95% CI: 0.69–0.84), p < 0.001; Supplementary Table 2].
Figure 2.
Kaplan–Meier curves for overall survival of patients according to the first site(s) of metastasis: only CNS metastases versus CNS and extra-CNS metastases versus only extra-CNS metastases (log-rank p < 0.0001).
CNS, central nervous system; OS, overall survival.
Characteristics of patients with isolated CNS metastases
Our main analysis was based on 541 patients with exclusive and isolated parenchymatous brain metastases, after the exclusion of 106 patients who received IT chemotherapy, in order to control for heterogeneity, as reflected by the very different outcomes following adjustment for subtypes: median OS of 20.7 months (95% CI: 7.3–24.3) versus 7.4 months (95% CI: 4.9–9.7) depending on the use of IT therapy, respectively [HR = 1.99 (95% CI: 1.56–2.53), p < 0.001; data not shown]. Compared with patients with no IT therapy, those receiving IT therapy also more often had poor PS (2–4) (28.3% versus 13.5%), TNBC (40.6% versus 26.1%), HR+/HER2− subtype (34.9% versus 28.5%) or lobular histology (23.1% versus 10.4%) and less often had HER2+ disease (16% versus 41.4%), all p < 0.01 (data not shown).
In our population of interest (N = 541), the median age at MBC diagnosis was 57 years (IQR: 47–67) and the median time to MBC diagnosis (after excluding patients with de novo MBC) was 24.4 months (IQR: 16.3–45.5). De novo MBC (defined as MBC diagnosed within 6 months from primary cancer diagnosis) was diagnosed in 46 patients, while 492 patients had recurrent MBC. The majority of patients had HER2+ tumours (41.4%). Also, the majority of patients was symptomatic at MBC diagnosis (Table 1).
Table 1.
Patient characteristics in our population of interest.
| Characteristic | N = 541 | % |
|---|---|---|
| Tumour grade | ||
| Grade I/II | 204 | 37.7 |
| Grade III | 252 | 46.6 |
| NA | 85 | 15.7 |
| Histological type | ||
| Ductal | 410 | 75.8 |
| Lobular | 56 | 10.4 |
| Other | 64 | 11.8 |
| NA | 11 | 2.0 |
| (Neo-)adjuvant chemotherapy a | ||
| No | 55 | 10.2 |
| Yes | 437 | 80.8 |
| NA | 49 | 9.0 |
| Age at MBC diagnosis | ||
| <55 years | 236 | 43.6 |
| ⩾55 years | 305 | 56.4 |
| Time to MBC diagnosis b | ||
| <6 months | 46 | 8.6 |
| 6–11 months | 75 | 13.9 |
| 12–23 months | 164 | 30.5 |
| 24–59 months | 155 | 28.8 |
| ⩾60 months | 98 | 18.2 |
| NA | 3 | 0.6 |
| Symptoms at MBC diagnosis | ||
| Yes | 411 | 76 |
| No | 117 | 21.6 |
| NA | 13 | 2.4 |
| BC Subtype | ||
| HR+/HER2− | 154 | 28.5 |
| HR+/HER2+ | 117 | 21.6 |
| HR−/HER2+ | 107 | 19.8 |
| TN | 141 | 26.1 |
| NA | 22 | 4.1 |
| Performance status | ||
| PS 0 | 65 | 12 |
| PS 1 | 81 | 15 |
| PS 2–4 | 73 | 13.5 |
| NA | 322 | 59.5 |
| Treatment strategy c | ||
| Local and systemic | 266 | 49.2 |
| Only local | 192 | 35.5 |
| Only systemic | 62 | 11.4 |
| No specific therapy | 21 | 3.9 |
| Surgical resection (+/-systemic therapy) c ? | ||
| Surgical resection and systemic therapy | 46 | 8.5 |
| Surgical resection without systemic therapy | 42 | 7.8 |
| No surgical resection | 453 | 83.7 |
HER2, human epidermal growth factor receptor 2; HR, hormone receptor; MBC, metastatic breast cancer; NA, not available; PS, performance status; TN, triple-negative.
The category ‘NA’ included patients with de novo MBC.
Defined as the time between the diagnosis of primary cancer and the diagnosis of MBC.
Within the first 3 months after CNS metastases diagnosis.
Treatment characteristics of patients with isolated CNS metastases
In our main population (N = 541), 266 (49.2%) patients received both local treatment and first-line systemic therapy, 192 (35.5%) received local treatment only, 62 (11.4%) received systemic therapy only, and 21 (3.9%) received no specific treatment in the first 3 months after MBC diagnosis (Table 1).
Details on the modalities of local treatment and systemic therapy and the agents most commonly used in each subgroup are reported in Supplementary Tables 3 and 4.
Local treatment consisted of surgical resection in 88 patients (16.3%) either alone (n = 19) or followed by stereotactic radiosurgery (SRS; n = 34) or whole-brain radiotherapy (WBRT; n = 35), exclusive SRS in 94 patients (17.4%), or exclusive WBRT in 276 patients (51.0%), while 83 patients (15.3%) did not receive any local treatment.
Systemic therapy was administered within the first 3 months after MBC diagnosis in 328 patients (60.6%) and in 266 (58.1%) of the 458 patients with local treatment, with no significant difference according to the type of local treatment administered (p = 0.49). The prevalence of systemic therapy was associated with BC subtype: 74.7% of HR+ /HER2− cases, 82.9% of HR+ /HER2+ cases, 54.2% of HR−/HER2+ cases, and 31.9% of TNBC cases received systemic therapy (p < 0.001). The type of systemic therapy varied according to subtype: 50% of HR+ /HER2− BC patients received endocrine therapy (ET) alone while 22.7% received chemotherapy (either alone or in combination with ET or bevacizumab). The majority of patients with HER2+ tumours (50.4%) received anti-HER2 therapy either alone or in combination with chemotherapy or ET, while TNBC patients more commonly received chemotherapy alone (27.7%) or in combination with bevacizumab (4.3%).
Survival of patients with isolated CNS metastases
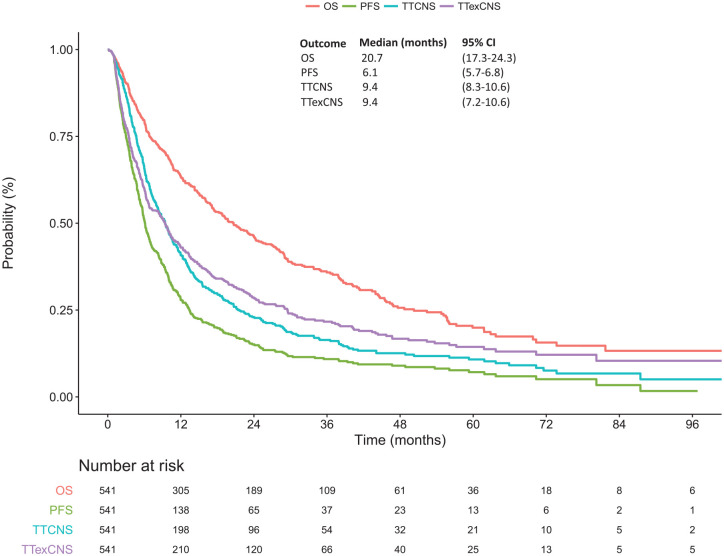
With a median follow-up of 43.3 months (range: 0.8–112.4 months, 95% CI: 36.8–50.7) 352/541 patients have died. Median OS, PFS, TTCNS, and TTexCNS were 20.7 months (95% CI: 17.3–24.3), 6.1 months (95% CI: 5.7–6.8), 9.4 months (95% CI: 8.3–10.6), and 9.4 months (95% CI: 7.2–10.6), respectively (Figure 3). The first site of subsequent progression was CNS in 37.7% of patients and extra-CNS in 28.8% of patients, while 14.2% of patients did not experience progression. Prognostic factors associated with significantly poorer OS on multivariable analysis (Table 2) were age at MBC ⩾55 years [HR = 1.8 (95% CI: 1.5–2.3), p < 0.001], TNBC subtype [HR = 1.5 (95% CI: 1.1–2.0), p = 0.011], time to metastatic disease from initial diagnosis of 6–12 months [HR = 2.0 (95% CI: 1.2–3.1), p = 0.001], and PS 2–4 (HR = 1.7 (95% CI: 1.1–2.8), p = 0.001). However, the presence of symptoms at MBC diagnosis versus none did not significantly influenced OS [HR = 1.1 (95% CI: 0.9–1.4), p = 0.436] and PFS [HR = 1.1 (95% CI: 0.9–1.3), p = 0.537].
Figure 3.
Kaplan–Meier curves for overall survival (OS), progression-free survival (PFS), time to CNS progression (TTCNS), and time to extra-CNS progression (TTexCNS) in our population of interest.
CNS, central nervous system; MBC, metastatic breast cancer.
Table 2.
Cox univariate and multivariable analysis for OS and PFS in patients with isolated CNS metastases who received or did not receive systemic therapy.
| Categories | N | Cox univariate analysis |
Cox multivariable analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | PFS | OS | PFS | ||||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | ||
| Type of local treatment | |||||||||||||
| WBRT | 275 | 1 | <0.001 | 1 | 0.001 | 1 | <0.001 | 1 | 0.001 | ||||
| Surgical resection | 88 | 0.3 | (0.2–0.5) | 0.6 | (0.5–0.8) | 0.4 | (0.2–0.5) | 0.6 | (0.5–0.8) | ||||
| SRS | 93 | 0.7 | (0.5–0.9) | 0.7 | (0.6–0.9) | 0.6 | (0.4–0.8) | 0.7 | (0.5–0.9) | ||||
| No local treatment | 82 | 0.8 | (0.6–1.0) | 0.8 | (0.6–1.0) | 0.7 | (0.5–1.0) | 0.8 | (0.6–1.1) | ||||
| Systemic therapy | |||||||||||||
| No systemic therapy within the first 3 months | 212 | 1 | 0.031 | 1 | 0.201 | 1 | 0.806 | 1 | 0.957 | ||||
| At least one systemic therapy | 326 | 0.8 | (0.6–1.0) | 0.9 | (0.7–1.1) | 1.0 | (0.7–1.3) | 1.0 | (0.8–1.2) | ||||
| Age at MBC diagnosis | |||||||||||||
| <55 years | 236 | 1 | < 0.001 | 1 | 0.041 | 1 | < 0.001 | 1 | 0.048 | ||||
| ⩾55 years | 302 | 1.7 | (1.4–2.1) | 1.2 | (1.0–1.5) | 1.8 | (1.5–2.3) | 1.2 | (1.0–1.5) | ||||
| Time to MBC | |||||||||||||
| <6 months | 46 | 1 | < 0.001 | 1 | 0.03 | 1 | 0.001 | 1 | 0.033 | ||||
| 6–12 months | 75 | 1.7 | (1.1–2.6) | 1.7 | (1.1–2.6) | 2.0 | (1.2–3.1) | 1.9 | (1.2–2.9) | ||||
| 12–24 months | 164 | 0.9 | (0.6–1.3) | 1.1 | (0.8–1.6) | 1.0 | (0.7–1.5) | 1.2 | (0.8–1.8) | ||||
| 24–60 months | 155 | 0.8 | (0.5–1.2) | 1.1 | (0.8–1.6) | 0.9 | (0.6–1.4) | 1.3 | (0.9–1.8) | ||||
| ⩾60 months | 98 | 0.8 | (0.5–1.2) | 1.1 | (0.8–1.7) | 0.8 | (0.5–1.3) | 1.3 | (0.9–2.0) | ||||
| Symptoms at MBC diagnosis a | |||||||||||||
| No | 115 | 1 | 0.436 | 1 | 0.537 | Not included | |||||||
| Yes | 410 | 1.1 | (0.9–1.4) | 1.1 | (0.9–1.3) | ||||||||
| BC subtype | |||||||||||||
| HR+ /HER2− | 153 | 1 | < 0.001 | 1 | 0.007 | 1 | 0.011 | 1 | 0.036 | ||||
| HR+ /HER2+ | 116 | 0.8 | (0.6–1.0) | 1.0 | (0.8–1.3) | 0.8 | (0.6–1.1) | 1.0 | (0.8–1.3) | ||||
| HR−/HER2+ | 107 | 1.1 | (0.8–1.5) | 0.9 | (0.7–1.2) | 0.9 | (0.7–1.3) | 0.9 | (0.7–1.2) | ||||
| TN | 141 | 1.8 | (1.4–2.4) | 1.5 | (1.1–1.9) | 1.5 | (1.1–2.0) | 1.4 | (1.0–1.9) | ||||
| NA | 21 | 0.9 | (0.5–1.8) | 1.0 | (0.5–1.7) | 0.9 | (0.4–1.7) | 0.9 | (0.5–1.6) | ||||
| Performance status | |||||||||||||
| PS 0 | 64 | 1 | < 0.001 | 1 | 0.031 | 1 | 0.001 | 1 | 0.183 | ||||
| PS 1 | 81 | 1.2 | (0.7–1.8) | 1.3 | (0.9–1.9) | 0.9 | (0.6–1.5) | 1.2 | (0.8–1.7) | ||||
| PS 2–4 | 72 | 2.1 | (1.3–3.3) | 1.8 | (1.2–2.5) | 1.7 | (1.1–2.8) | 1.5 | (1.0–2.2) | ||||
| PS NA | 321 | 1.8 | (1.3–2.6) | 1.3 | (1.0–1.8) | 1.6 | (1.1–2.2) | 1.2 | (0.9–1.7) | ||||
CI, confidence interval; CNS, central nervous system; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; HR, hormone receptor; MBC, metastatic breast cancer; NA, not available; OS, overall survival; PFS, progression-free survival; PS, performance status; SRS, stereotactic radiosurgery; TN, triple-negative; WBRT, whole-brain radiotherapy.
Not included in the multivariable analysis as it did not reach statistical significance in univariate analysis.
Outcomes differed across subtypes: OS decreased from 37.9 months (95% CI: 25.9–47.6) in HR+ /HER2+ cases to 22.9 months (95% CI: 17.1–31.9) in HR+ /HER2− cases, 19.2 months (95% CI: 14.3–28.9) in HR−/HER2+ cases, and 11.5 months (95% CI: 9.6–15.4) in the TNBC group (p < 0.001). A similar pattern was observed for PFS: 7.1 months (95% CI: 5.9–10.1), 6.8 months (95% CI: 5.7–9.4), 6.1 months (95% CI: 5.4–8.7), and 4.8 months (95% CI: 3.6–6.1), respectively, p = 0.0033 (Supplementary Figure 1). Between de novo and recurrent MBC, no significant difference in OS (19.2 months (95% CI: 12.7–37.8) versus 21.1 months (95% CI: 17.5–25.9), p = 0.59) or PFS (6.3 months (95% CI: 3.9–12.1) versus 6.1 months (95% CI: 5.7–6.9), p = 0.31) was noted (Supplementary Figure 2).
Survival of patients with isolated CNS metastases, according to treatment
Compared with patients with no early systemic therapy, those who had received systemic therapy during the first 3 months after MBC diagnosis had longer median OS: 24.1 months (95% CI: 19.7–29) versus 16.1 months (95% CI: 11.7–21.6), HR = 0.8 (95% CI: 0.6–1.0), p = 0.031. This advantage was not confirmed by multivariable analysis after adjustment for local treatment and the previously identified prognostic factors [adjusted HR = 1.0 (95% CI: 0.7–1.3), p = 0.806]. Systemic therapy in the first 3 months did not influence PFS (median PFS 6.2 months versus 5.8 months) on univariate or multivariable analysis (Table 2, Supplementary Figure 3).
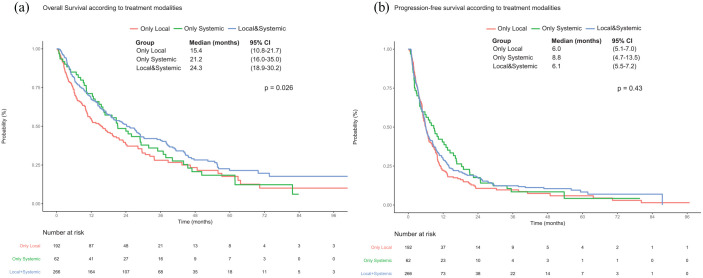
Among patients who received at least one treatment modality during the first 3 months after MBC diagnosis, the use of systemic therapy conferred an OS benefit compared with local treatment alone [24.3 months (95% CI: 18.9–30.2) versus 15.4 months (95% CI: 10.8–21.7), p = 0.026], similar to the exclusive use of systemic therapy [median OS of 21.2 months (95% CI: 16.0–35.0); Figure 4]. This advantage on univariate analysis [HR = 0.7 (95% CI: 0.6–0.9), p = 0.009] was no longer statistically significant on multivariable analysis [HR = 0.85 (95% CI: 0.6–1.1), p = 0.26] and was not observed for PFS (p = 0.43; data not shown).
Figure 4.
Kaplan–Meier curves for (a) overall survival and (b) progression-free survival in patients who received local treatment or systemic therapy in our population of interest, according to treatment modalities (local treatment only versus systemic therapy only versus local treatment and systemic therapy) (log-rank p = 0.026 and 0.43, respectively).
CI, confidence interval; OS, overall survival; PFS, progression-free survival.
In HR+ /HER2− BC patients with local treatment, the use of either chemotherapy, ET alone or no systemic therapy was not associated with a statistically significant difference in terms of OS [27.5 months (95% CI: 7.1–NA) versus 23.3 months (95% CI: 16.0–44.6) versus 18.6 months (95% CI: 6.2–NA), p = 0.68] or PFS [6.2 months (95% CI: 5.1–11.9) versus 6.7 months (95% CI: 5.4–12.3) versus 5.7 months (95% CI: 3.7–23.2), p = 0.58; Supplementary Figure 4].
Survival of patients with isolated CNS metastases according to local treatment
Patients treated with exclusive WBRT had a significantly poorer outcome compared to patients treated by surgical resection, SRS, or no local treatment (Supplementary Figure 5). This was consistent across BC subtypes (Supplementary Figure 6). On multivariable analysis, compared with WBRT as reference [median OS of 14.3 months (95% CI: 11.2–17.4), median PFS of 5.4 months (95% CI: 4.8–6.1)], surgical resection was associated with the best outcome [median OS: 59.9 months, adjusted HR = 0.4 (95% CI: 0.2–0.5), p < 0.001, median PFS: 8.3 months, adjusted HR = 0.6 (95% CI: 0.5–0.8), p = 0.001], followed by SRS [25.5 months (adjusted HR = 0.6 (95% CI: 0.4–0.8), p < 0.001] and 6.9 months [adjusted HR = 0.7 (95% CI: 0.5–0.9), p = 0.001, respectively]. No significant interaction was noted between WBRT and BC subtypes in the multivariable analysis (pinteraction = 0.73). Median OS and PFS were also longer in patients with no local treatment (21.2 and 7.4 months), but this difference was not statistically significant (Table 2, Supplementary Figure 5).
Discussion
This is the largest study to extensively describe the clinical features of isolated CNS metastases as first site of metastatic disease, highlighting potential prognostic factors that could be useful in treatment decision-making.
Based on more than 20,000 cases of MBC observed over nearly a decade, we found 2.9% of cases of isolated CNS metastases and 4.17% of CNS metastases concurrent with other distant metastatic sites, as first sites of metastatic disease. The incidence of CNS metastases as first site of recurrence following management of early-stage BC per year of follow-up ranges from 0.1–0.2 to more than 3% in aggressive subtypes (HER2+, TNBC, or inflammatory BC) in the literature.6,20–22 The rate of isolated CNS metastases among MBC patients is even more variable, ranging from 1.5% to 14%, depending on subtypes.20–24 In the present study, we confirm this variability with a higher propensity for CNS metastases among patients with HER2+ tumours or TNBC.
Risk factors for CNS metastases usually include ER−21,22 and HER2+ 20,22 tumours, higher tumour grade,22,25 larger size, and axillary node involvement.20,22 However, isolated CNS relapse has rarely been reported in the literature, and many questions remain unanswered concerning the specific prognosis and management of isolated CNS metastases. Although several large population-based studies (such as those based on the Surveillance, Epidemiology, and End Results database) have highlighted the prognostic impact of brain metastases compared to isolated extracranial metastatic disease3,26 or inversely the presence of extracranial metastases in addition to brain metastases,18,26 these studies did not extensively describe patients with isolated CNS metastases as a distinct and specific population nor did they compare this population with the other two MBC presentations described here.
To the best of our knowledge, this is the first large-scale study comparing the three clinical settings of MBC patients with either isolated CNS metastases, both CNS and extra-CNS metastases, or only extra-CNS metastases. In our series, patients with CNS metastases (with or without extracranial metastases) presented distinct characteristics to those with only extracranial metastases, as consistently reported in the literature (i.e. more commonly younger age, grade III, HER2 + tumours or TNBC, neoadjuvant or adjuvant chemotherapy, low PS and short time to MBC diagnosis). More importantly, they had significantly poorer survival, confirming the prognostic impact of the first metastatic site and the negative impact of CNS involvement.3,23,24,26
Interestingly, these patients also presented different characteristics depending on whether CNS metastases occurred alone or with concurrent extra-CNS disease: patients with isolated CNS metastases more often had HER2 + tumours, had less frequently received systemic therapy, had a shorter time to MBC diagnosis, and significantly better OS, suggesting a different clinical entity and stressing the need for further research.12,25 We also confirm the negative prognostic impact of the presence of extracranial metastases in addition to CNS metastases, as already observed in the general MBC population and as supported in an updated breast-Graded Prognostic Assessment (GPA).5,17,18,26
In our study, the population with isolated CNS metastases had a better survival than that reported in other less strictly selected series (10.5 to 14 months),11,13,25 likely reflecting the inclusion of patients with leptomeningeal metastases in some series or differences in management over time. For example, Sperduto et al. 17 reported an improvement of OS in MBC patients with CNS metastases (alone or combined with extracranial metastases) from 11 months between 1985 and 2007 to 16–23 months in more recent cohorts. A limitation of our study was the fact that parenchymal and leptomeningeal metastases were not directly differentiated, so the use of intrathecal chemotherapy had to be used as a proxy for leptomeningeal disease. However, intrathecal chemotherapy is recommended by the current EANO-ESMO guidelines as part of the therapeutic approach for the grand majority of leptomeningeal metastases (LM) 27 and, despite the still existing heterogeneity in the use of different treatment modalities, intrathecal chemotherapy is reported as being commonly used in this setting.28,29 Our results, showing significantly inferior outcomes for patients with CNS metastases treated with intrathecal chemotherapy versus those without intrathecal therapy, confirm that they represent two different subgroups of patients.
Of note, compared with other analyses conducted in the general ESME MBC population, patients included in this study had shorter survival, 30 although longer than that of all unselected patients with CNS metastases. 31 We also found a longer time to CNS progression than in other studies, 13 but subsequent progression more often occurred in the CNS, consistent with previous reports,11,13,14 suggesting that, despite the potential for long-term survival, long CNS response is a rare phenomenon.
In the population with isolated CNS MBC, we confirm the impact of well-known prognostic factors for CNS metastases, such as BC subtype and age, now included in the modified or updated breast-GPA,17,32 as well as time to MBC diagnosis. In our study, an interval of 6 to 12 months to MBC diagnosis was found prognostic for worse outcome than de novo MBC, in line with other studies that report worse survival in patients with ‘early relapse’ than in those with de novo MBC or with ‘later relapse’.30,33 HR+ /HER2+ BC patients had the longest survival. This result is in line with the prognosis of all HR+ /HER2+ MBC patients with brain metastasis in the ESME database, which was significantly better even than that of HR+ /HER2− patients. 31 Also, Sperduto et al. 17 reported significantly better prognosis for patients with ‘luminal B’ MBC with brain metastasis than all other subtypes. As reported in other studies,5,13,18,31 TNBC was correlated with poorer outcome on multivariable analysis.
In our cohort, 84.7% of patients received local treatment, a considerably higher proportion than in the overall MBC population with CNS metastases in the ESME database and with higher rates of ‘focal’ treatment. 34 In agreement with previous studies,13,17,35 surgical resection and SRS were associated with significantly longer survival compared to exclusive WBRT on multivariable analysis, irrespective of the use of systemic therapy. This outcome was probably in large part the expression of the impact on survival of the intracranial disease burden, that also influenced the choice of treatment, but an important limitation of our work is the missing data for the number and size of brain lesions for all patients and PS for nearly one-half of patients. Patients treated with WBRT, as a proxy for a high burden of intracranial disease, had poorer outcomes than the rest of patients consistently across all BC subtypes.
Despite growing evidence of the beneficial impact of systemic agents on the course of MBC with CNS involvement, 36 no systemic therapy has yet been specifically approved for the treatment of brain metastases. 16 In the literature, a significant proportion (50–67%) of BC patients receive systemic therapy as part of their first-line treatment strategy in the presence of isolated CNS metastases,11,13,14 as found in our series. Despite the reported positive impact of systemic therapy on outcome, the addition of systemic therapy to the management of patients with isolated CNS metastases remains controversial and many confounding and limiting factors have been identified, such as small sample sizes, retrospective design, and patient selection.11,13,14,37 Our study contributes to the previous literature by providing a detailed description of real-life patterns of care in a large cohort. Administration of systemic therapy was associated with prolonged OS only on univariate analysis. Interestingly, patients who did not receive local treatment also had a similar, relatively good outcome, suggesting that local treatment could be delayed when an effective systemic therapy is available, as previously reported in the case of WBRT, which has a deleterious effect on cognitive function. 38
In the absence of specific recommendations, a large number of HR + BC patients in our cohort received ET, possibly due in part to continuing adjuvant therapy. Although the literature is limited to case reports 39 and retrospective analyses, 40 ET has also been shown to have a good CNS distribution,36,40 making it an attractive modality for HR + BC patients with isolated CNS metastases13,14 not exposed to prior lines of palliative ET, with a lower risk of endocrine resistance. As the impact of ET on survival has been shown to be not significantly different from that of chemotherapy, ET could represent a good alternative to chemotherapy with fewer side effects.
Finally, the lack of significant impact on multivariable analysis of early systemic therapy could be explained by several limitations: (1) most patients who did not undergo surgical resection for BM received systemic therapy according to the primary subtype, while subtype switching has been described between timepoints;34,41 (2) the 3-month cut-off was arbitrarily defined and some patients may have started systemic therapy just after 3 months; (3) we could not assess whether the ET used for adjuvant therapy was continued following CNS relapse; (4) effective targeted agents such as CDK4/6 inhibitors, inhibitors of the PI3K/AKT/mTOR axis, pertuzumab or T-DM1 have been gradually introduced and may have mitigated or diluted the impact of first-line treatment on survival; and (5) these data are not randomized, with all the usual confounding factors, the use of real-world data is associated with a potential selection bias that may have influenced treatment strategies. These limitations support randomized studies designed to specifically address the value of adding systemic therapy for each subtype. Another important question to be addressed would be whether local treatment with significant toxicity can be delayed or avoided in certain cases, with the use of effective systemic therapies such as CDK4/6 inhibitors, newer generations of anti-HER2 agents, or other novel therapeutic agents already demonstrated to be active on CNS metastases or currently investigated in ongoing clinical trials. 36
Conclusion
The first metastatic site influences survival in MBC patients. Patients who initiate their metastatic disease with isolated CNS involvement represent a specific population, with a potential for better outcome than patients with both CNS and extra-CNS metastases. In the absence of specific guidelines, we found a great variety of real-life management strategies. Although patients who received systemic therapy in addition to local treatment had longer median survival, systemic therapy was not an independent prognostic factor on multivariable analysis. Efforts should be pursued to further refine specific management. Inclusion of more patients with CNS metastases, even in the absence of extracranial disease, in prospective studies evaluating systemic therapies should therefore be encouraged.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221077082 for Clinicopathological characteristics and prognosis of breast cancer patients with isolated central nervous system metastases in the multicentre ESME database by Marcela Carausu, Matthieu Carton, Luc Cabel, Anne Patsouris, Christelle Levy, Benjamin Verret, David Pasquier, Marc Debled, Anthony Gonçalves, Isabelle Desmoulins, Isabelle Lecouillard, Thomas Bachelot, Jean-Marc Ferrero, Jean-Christophe Eymard, Marie-Ange Mouret-Reynier, Michaël Chevrot, Eleonora De Maio, Lionel Uwer, Jean-Sébastien Frenel, Marianne Leheurteur, Thierry Petit, Amélie Darlix and Laurence Bozec in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank the 18 French Comprehensive Cancer Centres for providing the data and each ESME local coordinator for managing the project at the local level. Moreover, we thank the ESME Scientific Group and Strategic Committee for their ongoing support.
18 participating French Comprehensive Cancer Centres (FCCC): Institut Curie, Paris/Saint-Cloud, Gustave Roussy, Villejuif, Institut de Cancérologie de l’Ouest, Angers/Nantes, Centre F. Baclesse, Caen, ICM Montpellier, Centre L. Bérard, Lyon, Centre G-F Leclerc, Dijon, Centre H. Becquerel, Rouen; Institut C. Regaud, Toulouse; Centre A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; Centre E. Marquis, Rennes; Institut Paoli-Calmettes, Marseille; Centre J. Perrin, Clermont Ferrand; Institut Bergonié, Bordeaux; Centre P. Strauss, Strasbourg; Institut J. Godinot, Reims; Centre O. Lambret, Lille.
Programme director: Anne-Laure Martin. Data Management team: Coralie Courtinard, Emilie Nguyen, Olivier Payen, Irwin Piot, Dominique Schwob and Olivier Villacroux. Operational team: Nathalie Bouyer, Michaël Chevrot, Daniel Couch, Patricia D’Agostino, Pascale Danglot, Levent Dinc, Tahar Guesmia, Elodie Kupfer, Harmonie Oulie, Frédéric Roy, Gaëtane Simon and Toihiri Said. Software design team: Matou Diop, Blaise Fulpin, Fréja Messo, José Paredes and Alexandre Vanni.
ESME local coordinators: Thomas Bachelot, Delphine Berchery, Etienne Brain, Mathias Breton, Loïc Campion, Emmanuel Chamorey, Sandrine Dabakuyo, Valérie Dejean, Stéphanie Delaine, Anne-Valérie Guizard, Anne Jaffré, Lilian Laborde, Carine Laurent, Marie-Paule Lebitasy, Agnès Loeb, Damien Parent, Geneviève Perrocheau, Marie-Ange Mouret-Reynier, Michel Velten.
Footnotes
Author contributions: Marcela Carausu: Conceptualization; Writing – original draft.
Matthieu Carton: Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Luc Cabel: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Anne Patsouris: Investigation; Writing – review & editing.
Christelle Levy: Investigation; Writing – review & editing.
Benjamin Verret: Investigation; Writing – review & editing.
David Pasquier: Investigation; Writing – review & editing.
Marc Debled: Investigation; Writing – review & editing.
Anthony Gonçalves: Investigation; Writing – review & editing.
Isabelle Desmoulins: Investigation; Writing – review & editing.
Isabelle Lecouillard: Investigation; Writing – review & editing.
Thomas Bachelot: Investigation; Writing – review & editing.
Jean-Marc Ferrero: Investigation; Writing – review & editing.
Jean-Christophe Eymard: Investigation; Writing – review & editing.
Marie-Ange Mouret-Reynier: Investigation; Writing – review & editing.
Michaël Chevrot: Data curation; Writing – review & editing.
Eleonora De Maio: Investigation; Writing – review & editing.
Lionel Uwer: Investigation; Writing – review & editing.
Jean-Sébastien Frenel: Investigation; Writing – review & editing.
Marianne Leheurteur: Investigation; Writing – review & editing.
Thierry Petit: Investigation; Writing – review & editing.
Amélie Darlix: Investigation; Writing – review & editing.
Laurence Bozec: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JSF reports consulting fees from Novartis, Pfizer, AstraZeneca, Lilly, Roche, Biocad, Daiichi, Tesaro, GSK, Pierre Fabre. TB reports honoraria from Novartis, Roche, Pfizer, AstraZeneca and Seattle Genetics. The other authors report no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Epidemiological Strategy and Medical Economics (ESME) metastatic breast cancer database receives financial support from an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai and Daiichi Sankyo). Data collection, analysis and publication are managed entirely by UNICANCER independently of the industrial consortium.
Previous presentation: Data of this manuscript were presented at the ESMO Virtual Congress 2020, September 19–21, Mini Oral Session.
ORCID iDs: Marcela Carausu  https://orcid.org/0000-0002-2414-2500
https://orcid.org/0000-0002-2414-2500
Matthieu Carton  https://orcid.org/0000-0002-6793-3273
https://orcid.org/0000-0002-6793-3273
Jean-Sébastien Frenel  https://orcid.org/0000-0002-5273-0561
https://orcid.org/0000-0002-5273-0561
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Marcela Carausu, Department of Medical Oncology, Institut Curie, Saint-Cloud, France.
Matthieu Carton, Department of Biostatistics, Institut Curie, Saint-Cloud, France.
Luc Cabel, Department of Medical Oncology, Institut Curie, Saint-Cloud, France.
Anne Patsouris, Department of Medical Oncology, Institut de Cancérologie de l’Ouest, Angers, France.
Christelle Levy, Department of Medical Oncology, Centre François Baclesse, Caen, France.
Benjamin Verret, Department of Medical Oncology, Institut Gustave Roussy, Villejuif, France.
David Pasquier, Department of Radiation Oncology, Centre Oscar Lambret, CRIStAL UMR CNRS 9189, Lille University, Lille, France.
Marc Debled, Department of Medical Oncology, Institut Bergonié, Bordeaux, France.
Anthony Gonçalves, Department of Medical Oncology, Institut Paoli-Calmettes, Marseille, France.
Isabelle Desmoulins, Department of Medical Oncology, Centre Georges-François Leclerc, Dijon, France.
Isabelle Lecouillard, Department of Radiation Oncology, Centre Eugène Marquis, Rennes, France.
Thomas Bachelot, Department of Medical Oncology, Centre Léon Bérard, Lyon, France.
Jean-Marc Ferrero, Department of Medical Oncology, Centre Antoine Lacassagne, Nice, France.
Jean-Christophe Eymard, Department of Medical Oncology, Institut de Cancérologie Jean-Godinot, Reims, France.
Marie-Ange Mouret-Reynier, Department of Medical Oncology, Centre Jean Perrin, Clermont-Ferrand, France.
Michaël Chevrot, Unicancer, Paris, France.
Eleonora De Maio, Department of Medical Oncology, Institut Claudius Regaud – IUCT Oncopole, Toulouse, France.
Lionel Uwer, Department of Medical Oncology, Institut de Cancérologie de Lorraine, Vandœuvre-lès-Nancy, France.
Jean-Sébastien Frenel, Department of Medical Oncology, Institut de Cancérologie de l’Ouest–René Gauducheau, Saint-Herblain, France.
Marianne Leheurteur, Department of Medical Oncology, Centre Henri Becquerel, Rouen, France.
Thierry Petit, Department of Medical Oncology, Centre Paul Strauss, Strasbourg, France.
Amélie Darlix, Department of Medical Oncology, Institut régional du Cancer de Montpellier (ICM), Institut de Génomique Fonctionnelle, INSERM U1191-CNRS UMR 5203, Université de Montpellier, Montpellier, France.
Laurence Bozec, Department of Medical Oncology, Institut Curie, 35 rue Dailly, 92210 Saint-Cloud, France.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Wilcox JA, DeAngelis LM. Epidemiology and socioeconomic impact of CNS metastases. In: Ramakrishna R, Magge RS, Baaj AA, et al. (eds) Central nervous system metastases: diagnosis and treatment. Cham: Springer International Publishing, 2020, pp. 3–18. [Google Scholar]
- 3. Wang R, Zhu Y, Liu X, et al. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019; 19: 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berghoff AS, Schur S, Füreder LM, et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open 2016; 1: e000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rostami R, Mittal S, Rostami P, et al. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol 2016; 127: 407–414. [DOI] [PubMed] [Google Scholar]
- 6. Komorowski AS, Warner E, MacKay HJ, et al. Incidence of brain metastases in nonmetastatic and metastatic breast cancer: is there a role for screening? Clin Breast Cancer 2020; 20: e54–e64. [DOI] [PubMed] [Google Scholar]
- 7. Tabouret E, Chinot O, Metellus P, et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res 2012; 32: 4655–4662. [PubMed] [Google Scholar]
- 8. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003; 97: 2972–2977. [DOI] [PubMed] [Google Scholar]
- 9. Le Scodan R, Jouanneau L, Massard C, et al. Brain metastases from breast cancer: prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC Cancer 2011; 11: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olson EM, Abdel-Rasoul M, Maly J, et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol 2013; 24: 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niwin´ska A. Brain metastases as site of first and isolated recurrence of breast cancer: the role of systemic therapy after local treatment. Clin Exp Metastasis 2016; 33: 677–685. [DOI] [PubMed] [Google Scholar]
- 12. Berghoff AS, Bago-Horvath Z, Ilhan-Mutlu A, et al. Brain-only metastatic breast cancer is a distinct clinical entity characterised by favourable median overall survival time and a high rate of long-term survivors. Br J Cancer 2012; 107: 1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nieder C, Oehlke O, Hintz M, et al. The challenge of durable brain control in patients with brain-only metastases from breast cancer. SpringerPlus 2015; 4: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hulsbergen AFC, Cho LD, Mammi M, et al. Systemic therapy following craniotomy in patients with a solitary breast cancer brain metastasis. Breast Cancer Res Treat 2020; 180: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Comprehensive Cancer Network. Central nervous system cancers, version 3, 2020, https://www.nccn.org/professionals/physician_gls/pdf/cns_blocks.pdf (2020, accessed 5 July 2020). [DOI] [PubMed]
- 16. Soffietti R, Abacioglu U, Baumert B, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol 2017; 19: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sperduto PW, Mesko S, Li J, et al. Beyond an updated graded prognostic assessment (breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys 2020; 107: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim Y-J, Kim J-S, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol 2018; 144: 1803–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020; 31: 1623–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laakmann E, Witzel I, Fasching PA, et al. Development of central nervous system metastases as a first site of metastatic disease in breast cancer patients treated in the neoadjuvant trials GeparQuinto and GeparSixto. Breast Cancer Res 2019; 21: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berman AT, Thukral AD, Hwang W-T, et al. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer 2013; 13: 88–94. [DOI] [PubMed] [Google Scholar]
- 22. Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 2006; 17: 935–944. [DOI] [PubMed] [Google Scholar]
- 23. Ording AG, Heide-Jørgensen U, Christiansen CF, et al. Site of metastasis and breast cancer mortality: a Danish nationwide registry-based cohort study. Clin Exp Metastasis 2017; 34: 93–101. [DOI] [PubMed] [Google Scholar]
- 24. Gerratana L, Fanotto V, Bonotto M, et al. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis 2015; 32: 125–133. [DOI] [PubMed] [Google Scholar]
- 25. Saip P, Cicin I, Eralp Y, et al. Identification of patients who may benefit from the prophylactic cranial radiotherapy among breast cancer patients with brain metastasis. J Neurooncol 2009; 93: 243–251. [DOI] [PubMed] [Google Scholar]
- 26. Xiong Y, Cao H, Zhang Y, et al. Nomogram-predicted survival of breast cancer brain metastasis: a SEER-based population study. World Neurosurg 2019; 128: e823–e834. [DOI] [PubMed] [Google Scholar]
- 27. Le Rhun E, Weller M, Brandsma D, et al. EANO–ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017; 28: iv84–iv99. [DOI] [PubMed] [Google Scholar]
- 28. Le Rhun E, Rudà R, Devos P, et al. Diagnosis and treatment patterns for patients with leptomeningeal metastasis from solid tumors across Europe. J Neurooncol 2017; 133: 419–427. [DOI] [PubMed] [Google Scholar]
- 29. Scott BJ, Oberheim-Bush NA, Kesari S. Leptomeningeal metastasis in breast cancer – a systematic review. Oncotarget 2015; 7: 3740–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deluche E, Antoine A, Bachelot T, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer 2020; 129: 60–70. [DOI] [PubMed] [Google Scholar]
- 31. Darlix A, Louvel G, Fraisse J, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer 2019; 121: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Subbiah IM, Lei X, Weinberg JS, et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol 2015; 33: 2239–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lobbezoo DJA, van Kampen RJW, Voogd AC, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer 2015; 112: 1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasquier D, Darlix A, Louvel G, et al. Treatment and outcomes in patients with central nervous system metastases from breast cancer in the real-life ESME MBC cohort. Eur J Cancer 2020; 125: 22–30. [DOI] [PubMed] [Google Scholar]
- 35. Niikura N, Hayashi N, Masuda N, et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat 2014; 147: 103–112. [DOI] [PubMed] [Google Scholar]
- 36. Bailleux C, Eberst L, Bachelot T. Treatment strategies for breast cancer brain metastases. Br J Cancer 2021; 124: 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soomro ZA, Alhalabi O, Sun R, et al. Systemic therapy for patients with breast cancer and one to three brain metastases (BM). J Clin Oncol 2020; 38: 1090–1090. [Google Scholar]
- 38. Boogerd W, Groenveld F, Linn S, et al. Chemotherapy as primary treatment for brain metastases from breast cancer: analysis of 115 one-year survivors. J Cancer Res Clin Oncol 2012; 138: 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Q, Sun B, Liu C, et al. Brain metastases from breast cancer may respond to endocrine therapy: report of two cases. Onco Targets Ther 2019; 12: 1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bergen ES, Berghoff AS, Medjedovic M, et al. Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res 2019; 25: 2737–2744. [DOI] [PubMed] [Google Scholar]
- 41. Hulsbergen AFC, Claes A, Kavouridis VK, et al. Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro Oncol 2020; 22: 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221077082 for Clinicopathological characteristics and prognosis of breast cancer patients with isolated central nervous system metastases in the multicentre ESME database by Marcela Carausu, Matthieu Carton, Luc Cabel, Anne Patsouris, Christelle Levy, Benjamin Verret, David Pasquier, Marc Debled, Anthony Gonçalves, Isabelle Desmoulins, Isabelle Lecouillard, Thomas Bachelot, Jean-Marc Ferrero, Jean-Christophe Eymard, Marie-Ange Mouret-Reynier, Michaël Chevrot, Eleonora De Maio, Lionel Uwer, Jean-Sébastien Frenel, Marianne Leheurteur, Thierry Petit, Amélie Darlix and Laurence Bozec in Therapeutic Advances in Medical Oncology