Abstract
We describe a case of IgA vasculitis without typical skin rash concomitated with c-ANCA positivity in a 6-year-old boy who presented with persistent severe generalized colicky abdominal pain, recurrent episodes of vomiting, non-pitting edema of both hands and feet, both knees and ankles arthritis with no associated skin rash following a history of an upper respiratory tract infection 2 weeks before presentation. Initially, he had normal laboratory findings apart from sub-nephrotic range proteinuria and microscopic hematuria in his urine analysis. Two weeks later, he started to have hypertension, gross hematuria, nephrotic range proteinuria, marked elevation of serum urea and creatinine associated with positive serum C-ANCA. Renal biopsy revealed heavy IgA mesangial deposition with marked crescent formation involving more than 89% of the glomeruli (grade V). Aggressive therapeutic measures were initiated including IV pulsed steroid therapy and IV pulsed cyclophosphamide for 5 cycles followed by oral steroid and mycophenolate with close monitoring of the patient who showed marked improvement. Up to our knowledge, this is the first reported case of IgA-vasculitis-associated nephritis with bowel angina symptoms, arthritis, and edema but without typical skin rashes.
Keywords: Henoch-Schönlein Purpura, a-typical, child, rapidly progressive glomerulonephritis
Introduction
Henoch-Schönlein Purpura (HSP) is the most common systemic vasculitis in childhood. The annual incidence is 13 to 20 per 100 000 children, it is characterized by nonthrombocytopenic palpable purpura, in almost 100% of cases and it can be accompanied by arthritis, bowel angina, and hematuria/proteinuria. 1
The course of HSP is usually benign and self-limited. Joint involvement occurs in 60% to 84% of cases and appears in association with palpable purpura in almost 100% of cases as the initial presentation. However, progressive kidney damage might affect 1% and 33% in children and adults respectively. 2
The gastrointestinal system is affected in up to 76% of patients varying from abdominal pain, nausea, and vomiting to intestinal hemorrhage and perforation, intestinal obstruction, intussusception, pancreatitis, and hydrops of the gallbladder. Neurological, pulmonary, and cardiac involvements are extremely rare. It is known that HSP is associated with an immune system dysfunction and/or infections preceding its onset, especially upper respiratory infections caused by streptococcus, parainfluenza virus, “1” or parvovirus B19. 1
Even in the absence of a typical HSP rash, if gastrointestinal symptoms, arthritis and renal impairment are observed IgA-vasculitis-associated nephritis (IgA-VN) should be considered and, comprehensive renal investigations should be initiated. We herein report a case of IgA-VN with bowel angina symptoms, arthritis and edema with positive c-ANCA, but without typical skin rashes.
Case Report
We herein report, a 6-year-old Saudi boy not known to have any chronic medical conditions, who presented with a one-week history of persistent diffuse colicky abdominal pain, recurrent episodes of vomiting, pain, edema of hands and feet, swelling, and limitation of movements of both ankles and knees without any other associations following a history of upper respiratory tract infection 2 weeks before presentation.
His past medical and family histories were non-significant. Physical examinations revealed bilateral hands, wrists, ankles, feet, and knees non-pitting edema with tenderness and limitation of joints mobility.
His laboratory works up revealed normal complete blood count, serum (s.) urea, s. creatinine, s. electrolytes, s. albumin, acid-base, erythrocyte sedimentation rate with microscopic hematuria and non-nephrotic range proteinuria (urine albumin/creatinine ratio was 150 mg/mmol) on spot morning sample, and his stool analysis was positive for occult blood.
After a few days, the patient’s gastrointestinal symptoms, edema, and both knee joint arthritis resolved with only supportive therapy, and he was discharged home without a definite diagnosis and given a close follow-up appointment.
Two weeks later, the patient started to have dark cola-colored urine, oliguria, puffy eyes, hypertension (blood pressure of 149/95 mmHg) with pallor, and feet and hands pitting edema with associated moderate ascites, the patient was re-hospitalized and thouroughly investigated.
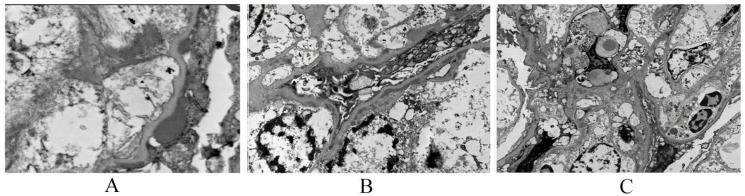
His complete blood count revealed; hemoglobin 7 g/dL, mean corpuscular volume 87 fl, corrected reticulocytes 1.5%, white blood cells 11.4 × 109/L, platelets 401 × 109/L. Erythrocyte sedimentation rate was 87 mm/hour. Blood urea nitrogen was 45 mmol/L, creatinine was 198 mmol/L, and the urine analysis showed nephrotic range proteinuria (urine protein/creatinine was 5436 mg/mmol), urine red blood cells was over 100 cells/high power field. The serum electrolyte showed sodium 132 mmol/L, potassium 4.2 mmol/L, chloride 94 mmol/L, bicarbonate 14.2 mmol/L, normal C3, the cytoplasmic antineutrophil cytoplasmic antibody was positive (c ANCA), antinuclear antibody (ANA) was negative, and stool analysis was positive for occult blood. His renal ultrasound reported enlarged slightly echogenic both kidneys. A decision of kidney biopsy was agreed which revealed a picture of rapidly progressive crescent glomerulonephritis (Figure 1), with extensive areas of glomerular proliferation with the crescent formation in more than 89%, immunofluorescence microscopy showed Ig A mesangial deposition confirming HSP crescentic glomerulonephritis (Figure 1).
Figure 1.
Electron microscopy showing (A) occasional subepithelial deposits, (B) subendothelial deposits and endocapillary hypercellularity, and (C) mesangium and capillary loops.
Immediate intravenous (IV) pulse methylprednisolone 30 mg/kg was commenced every other day for 6 doses followed by oral steroids in association with IV pulse cyclophosphamide. Antihypertensive medications were started and the patient received packed red blood cells transfusion.
The patient started to improve dramatically both clinically and biochemically until he attained complete remission. He was discharged with monthly IV cyclophosphamide for another additional 4 cycles and oral steroids with close follow-up. Figure 2 summarizes serum creatinine values and urine protein creatinine ratio throughout the disease course.
Figure 2.
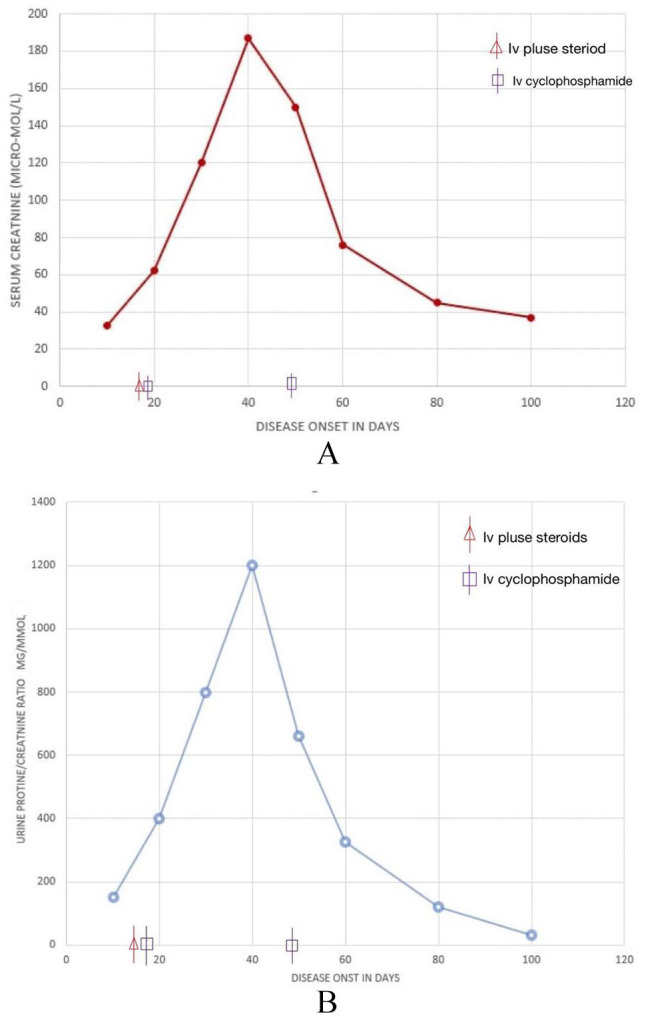
(A) Serum creatinine values throughout the disease course and (B) urine protein creatinine ratio throughout the disease course.
Kidney biopsy report
Light microscopy
Sections stained for Hematoxylin, Eosin, Periodic acid-Schiff, Trichrome, and Jones show one long of renal cortex with medulla along with 2 cores of renal cortex that include up to 9 glomeruli, all of them perfused (Figure 1). Seven of the 9 glomeruli show endocapillary hypercellularity with active cellular crescents; one shows a fibro cellular crescent. There is an increase in mesangial matrix and cellularity. Thrombi are not seen. There is diffuse acute tubular injury and focal mononuclear cell tubulitis. tubules focally contain hyaline and RBC casts. There is no tubular atrophy. The cortical and medullary Interstitium is diffusely edematous and contains moderate numbers of mononuclear cells, including plasma cells. Polymorphonuclear leukocytes, eosinophils are not seen. There is peritubular capillaritis in the medulla. Arteries are not included.
Immunofluorescence microscopy
Frozen section stained for albumin, Fibrinogen, IgG, IgM, IgA, C3, C1q, C4d kappa and lambda light chains include cortex and medulla including 2 perfused glomeruli, there is mesangial and segmental capillary wall positivity for IgA (2+), C3 (2+), lgG (2+), kappa (traces) and lambda (2+). Ig M IgG, C1q, Alhumin and Fibrinogen are negative.
Electron microscopy
Three glomeruli were available for assessment. The podocytes show microvillation with marked cytoplasmic vacuolation. About 75% of the podocyte foot processes are effaced. Occasional subepithelial deposits are noted. There is segmental widening with the clearing of subendothelial space with numerous electron-dense deposits. Numerous electron densities are seen in mesangial areas with an increase in the mesangial matrix. Occasional endothelial cells are swollen, Segmental endocapillary hypercellularity is seen. Tubuloreticular inclusions are not detected. Apical vacuoles are seen in proximal tubular epithelial cells probably representing lipid/protein reabsorption droplets and lysosomal profiles. Peritubular capillaries show no lesions. Small arteries are not sampled. Glomerular basement membrane measurements (in nm) are Mean 203, Standard Deviation 38, Maximum 301, and Minimum 151. These are within the normal reference range for the patient’s age according to the laboratory where the sample was processed, and measurements were made.
Renal biopsy interpretation
Ig A positive crescent glomerulonephritis consistent with IgA-VN, active crescent score 89%, glomerulosclerosis 0%. Oxford classification score: E1 S0 T0 C2 3
Discussion
HSP is a self-limited, systemic, non-granulomatous, autoimmune complex, small vessel vasculitis, with multiorgan involvement. Its etiology is unclear probably polygenic due to abnormal Ig A as it is associated with infections (bacterial, viral, parasitic), medications, and vaccination. HSP is the most common cutaneous vasculitis in children. 4
Antigen-antibody complexes deposit in the small vessel walls and activate the alternate complement pathway which leads to neutrophil accumulation resulting in inflammation and vasculitis without a granulomatous reaction. This can involve multiple systems including skin, gastrointestinal tract (GIT), kidneys, and joints but it can involve any organ system. Vasculitis causes extravasation of blood and its components into the interstitial spaces resulting in edema and hemorrhage. 4
Renal involvement is the most serious long-term complication and usually develops within the first 4 weeks of the illness. Manifestations include isolated microhematuria or gross hematuria. Mild transient proteinuria to nephrotic syndrome, rapidly progressive glomerulonephritis (RPGN) can occur but they are rare. In general, most of the HSP cases are self-limited, with a good prognosis. One-third of the patients have relapses, which are milder and shorter in duration, usually within 4 months, and involving the same organs. The prognosis depends on the age of onset, the extent of renal involvement and the extent of skin involvement, and neurological involvement. 5
Our case had severe abdominal pain, vomiting, polyarthritis and renal involvement. Significant laboratory findings were elevated serum urea, creatinine, nephrotic range proteinuria, hematuria, positive cytoplasmic antineutrophil cytoplasmic antibody (c-ANCA) and positive occult blood in the stool. Extensive mesangial immunoglobin A (IgA) glomerular deposition with the extensive crescent formation in renal biopsy. However, there was a complete absence of skin rash.
The diagnosis of HSP was made by the American College of Rheumatology-1990 and European League Against Rheumatism-2006 criteria, The patient is said to have HSP if at 2 out of the following criteria are present (Palpable purpura, gastrointestinal, renal involvement, and Any biopsy showing predominant IgA deposition or Arthritis or arthralgia).6,7 In our case, there were gastrointestinal, renal involvement and polyarthritis, fulfilling the diagnostic criteria of HSP without skin rashes.
The reported patient differs from previously reported cases in literature as he had an initial presentation of joint, gut, and renal involvement without typical skin rash. The nephritis associated with HSP usually follows the onset of the rash, often presenting weeks or even months after the initial presentation of the disease. Nephritis can be manifested at the initial presentation, but only rarely before the onset of rash. Purpura is the presenting symptom in more than 95% of patients, preceding other symptoms by a mean of 4 days in 1 series. 8
One important issue is that we started IV pulsed steroids and IV cyclophosphamide, immediately after the kidney biopsy which might mask the appearance of typical skin rash. 4
Our patient had positive (c)-ANCA. C-ANCA are a group of autoantibodies directed against proteins in the granules of neutrophils and peroxidase-positive lysosomes of peripheral blood monocytes. They have been detected in HSP patients with unclear clinical significance. (c)-ANCA and perinuclear (p)-ANCA are characteristic for pauci-immune small vessel vasculitis, whereas the presence of ANCA in immune-complex-mediated vasculitis, especially HSP, remains controversial.9,10
Differential diagnoses of ANCA-associated-GN include: (1) Necrotizing granulomatous inflammation which usually involves the upper and lower respiratory tract, and necrotizing vasculitis affecting predominantly small-to-medium vessels (eg, capillaries, venules, arterioles, arteries, and veins). Necrotizing GN is common. (2) Microscopic polyangiitis which is a necrotizing vasculitis with few or no immune deposits, predominantly affecting small vessels (ie, capillaries, venules, or arterioles). Necrotizing arteritis involving small and medium arteries may be present. Pulmonary capillaritis often occurs while granulomatous inflammation is absent. (3) Eosinophilic granulomatosis with polyangiitis (Churg–Strauss) which is an eosinophil-rich necrotizing granulomatous inflammation often involving the respiratory tract, and necrotizing vasculitis predominantly affecting small-to-medium vessels and is associated with asthma and eosinophilia. ANCA is more frequent when GN is present. 11
In our patient absence of respiratory, ocular, neurological manifestations, and eosinophilia with the above-mentioned kidney biopsy findings can exclude these causes of ANCA-associated GN.
IgA -C-ANCA can be detected during the acute phase of HSP in children with high sensitivity and was proposed to be a good marker for confirming an HSP diagnosis despite having a low specificity. 12
The presence of c-ANCA in HSP is very unusual, 13 it was suggested that the presence of c-ANCA in patients diagnosed with HSP might be related to the release of tumor necrosis factor alfa and defective T-regulatory cells. It has been reported that ANCA of the IgG isotype may be associated with rapid progressive glomerulonephritis. However, the clinical and pathological significance of IgG-c-ANCA in the setting of HSP and whether these antibodies could be used as potential markers for aggressive HSP needs to be further studied.9,10,13 However, our case report might reinforce this postulation.
Conclusions
Even in the absence of typical skin rash, if HSP is suspected, aggressive diagnostic work up and therputic interventions must be considered especially to detect and follow-up renal and GIT involvement.
As HSP nephritis is increasingly seen as an important cause of chronic kidney disease among pediatric patients, early diagnosis and differentiation of HSP nephritis play an important role in avoiding or delaying the occurrence of end-stage renal disease.
Also, detection of c-ANCA in patients with HSP, which is very unusual, may be a marker of rapidly progressive glomerulonephritis, and hence aggressive management is indicated.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MA: Diagnosed the patient, set the idea of the study, and designed the study. AS, NK: reviewed literature, drafted the manuscript, critically analyzed the data. MA, WA, SA: collected data. All authors reviewed and approved the manuscript for final publication.
Availability of Data and Materials: All data and materials related to the study are included in the current manuscript.
Ethical Approval and Consent to participate: The study was approved by the local research and ethical committee. The parents of the re signed written informed consents for the participation of their children in the current study.
Consent for Publication: All parents of enrolled children signed written informed consents for publication of the current study.
ORCID iD: Naglaa M Kamal  https://orcid.org/0000-0002-8535-3838
https://orcid.org/0000-0002-8535-3838
References
- 1. Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014;13:355-358. [DOI] [PubMed] [Google Scholar]
- 2. Park CW, Lim IS, Yun SW, Chae SA, Lee NM, Yi DY. Henoch-Schönlein purpura without typical lesions, presenting with gastrointestinal manifestations and kidney involvement following influenza - a case report. J Pak Med Assoc. 2016;66:1339-1342. [PubMed] [Google Scholar]
- 3. Trimarchi H, Barratt J, Cattran DC, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy classification working group. Kidney Int. 2017;91:1014-1021. [DOI] [PubMed] [Google Scholar]
- 4. Tizard EJ, Hamilton-Ayres MJ. Henoch Schonlein purpura. Arch Dis Child Educ Pract Ed. 2008;93:1-8. [DOI] [PubMed] [Google Scholar]
- 5. Chen KR, Carlson JA. Clinical approach to cutaneous vasculitis. Am J Clin Dermatol. 2008;9:71-92. [DOI] [PubMed] [Google Scholar]
- 6. Mills JA, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum. 1990;33:1114-1121. [DOI] [PubMed] [Google Scholar]
- 7. Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jauhola O, Ronkainen J, Koskimies O, et al. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: a 6-month prospective study. Arch Dis Child. 2010;95:871-876. [DOI] [PubMed] [Google Scholar]
- 9. Boulis E, Majithia V, McMurray R. Adult-onset Henoch-Schonlein purpura with positive c-ANCA (anti-proteinase 3): case report and review of literature. Rheumatol Int. 2013;33:493-496. [DOI] [PubMed] [Google Scholar]
- 10. Kallenberg CG, Brouwer E, Weening JJ, Tervaert JW. Antineutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1-15. [DOI] [PubMed] [Google Scholar]
- 11. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1-11. [DOI] [PubMed] [Google Scholar]
- 12. Ozaltin F, Bakkaloglu A, Ozen S, et al. The significance of IgA class of antineutrophil cytoplasmic antibodies (ANCA) in childhood Henoch-Schönlein purpura. Clin Rheumatol. 2004;23:426-429. [DOI] [PubMed] [Google Scholar]
- 13. Kim JE, Shin JI. Positive c-ANCA in Henoch-Schonlein purpura: what is the mechanism? Comment on: adult-onset Henoch-Schonlein purpura with positive c-ANCA (anti-proteinase 3): case report and review of literature (Rheumatol Int. 2013 Feb; 33(2):493-496). Rheumatol Int. 2014;34:1017. [DOI] [PubMed] [Google Scholar]