Abstract
Objective
This study analyzed the characteristics and tendencies of resistance to common antibiotics for Klebsiella pneumoniae to provide a basis for clinical treatment and prevention.
Methods
A total of 71,743 isolates were collected from hospital clinical specimens following standard procedures from 2006 to 2020. Statistical analyses were conducted on laboratory test results.
Results
A total of 3054 isolates of K. pneumoniae were mainly isolated from sputum (53.77%), urine (14.70%), and blood (8.42%). Isolation rates of strains in the AIDS, hepatology, and intensive care wards were 9.72%, 12.52%, and 16.45%, respectively. Resistance rates of imipenem, cefazolin, gentamicin, tobramycin, ciprofloxacin, and ceftazidime respectively increased from 2.33%, 27.91%, 16.28%, 13.95%, 18.60%, and 9.30% to 12.83%, 40.82%, 21.57%, 25.07%, 44.61%, and 17.78%, while piperacillin–tazobactam resistance decreased from 13.95% to 13.70%. Differences in resistance rates to seven antibiotics were significant among specimen types. Detection rates of carbapenem-resistant K. pneumoniae were significantly different among blood, sputum, and urine specimens, and between wards.
Conclusions
The prevalence and drug resistance of K. pneumoniae showed an upward trend over time, and resistance varied according to ward and specimen source. The prevention of nosocomial infections and rational drug use must be emphasized to reduce antimicrobial resistance.
Keywords: Klebsiella pneumoniae, antimicrobial resistance, tertiary hospital, prevalence, clinical specimen, nosocomial infection
Introduction
Klebsiella pneumoniae (K. pneumoniae), as a common opportunistic pathogen, is a main cause of nosocomial infections, which affect the lung, bloodstream, and urinary tract. 1 K. pneumoniae is second only to Escherichia coli for the highest detection rate among Gram-negative bacteria in China. 2 E. coli was the most isolated bacterium from blood and urine samples in major hospitals in China during 2018, and showed serious drug resistance, 3 while that of K. pneumoniae is also increasing in severity.
Hu et al. 3 found that the resistance rates of K. pneumoniae to imipenem and meropenem increased more than eight-fold from 2005 to 2018 (from 3.0%–25% and from 2.9%–26.3%, respectively), while those to ceftazidime and ciprofloxacin increased from 19.7% to 21.7% and from 17.3% to 22.4%, respectively, from 2014 to 2019. 2 The resistance mechanism of K. pneumoniae mainly includes the production of β-lactamase, the lack of membrane porin proteins, and the active efflux of antibacterial drugs. Extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae has a high degree of drug resistance, which can simultaneously present with multiple resistance mechanisms, often resulting in multidrug resistance. 4
Carbapenem antibiotics are commonly used in the clinic for the ESBL-producing K. pneumoniae. However, their overuse has led to significant increases in K. pneumoniae resistance rates in recent years. For example, the China Antimicrobial Resistance Surveillance Network (CHINET) showed that the resistance rate of K. pneumoniae to carbapenems was over 20% in 2018. 3 Carbapenem-resistant K. pneumoniae (CRKP) causes nosocomial infections with high morbidity, high mortality, and requiring extensive hospital stays; this has become a global problem with a high socioeconomic burden. 5 To understand the prevalence of K. pneumoniae infection in Hangzhou, China, and to provide a basis for clinical treatment and nosocomial infection control, this retrospective study investigated and analyzed the prevalence and resistance trends of K. pneumoniae in Hangzhou Xixi Hospital Affiliated to Zhejiang Chinese Medical University from 2006 to 2020, which specializes in treating liver disease and AIDS.
Materials and methods
Specimen sources
A total of 3054 routine clinical specimens, including sputum, urine, and blood, were taken from patients infected with K. pneumoniae who had been admitted to different wards of Hangzhou Xixi Hospital Affiliated to Zhejiang Chinese Medical University, a tertiary infectious diseases hospital, from 2006 to 2020. Informed consent was not required because the samples were obtained for a previous study. Patients were divided into two groups: the older group (≥65 years old, n = 1196), and the younger group (<65 years old, n = 1858). Strains of K. pneumoniae were isolated from the samples using standard microbiology laboratory procedures, and identified using the VITEK 2 compact system (bioMérieux, Craponne, France). Duplicate strains, defined as the same bacterial species from the same specimen source from the same patient, were excluded from analysis. This study was approved by the local medical ethics committee of Hangzhou Xixi Hospital.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing with the VITEK 2 compact system was performed using the VITEK 2 AST-GN13 card (bioMérieux). Based on the suggested groupings of antimicrobial agents that should be considered for testing and reporting on non-fastidious organisms by the Clinical and Laboratory Standards Institute (CLSI) 6 , we selected group A antibiotics for analysis. This included antimicrobial agents considered appropriate for inclusion in a routine, primary testing panel, as well as for the routine reporting of results for the specific organism group. Ultimately, we assessed resistance to seven antibiotics: imipenem, cefazolin, gentamicin, tobramycin, ciprofloxacin, piperacillin–tazobactam, and ceftazidime. E. coli ATCC 25922 was used as a quality control.
Statistical analyses
Statistical analysis was performed with WHONET5.6 7 and IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA). Categorical variables were described as counts and percentages, and compared using the chi-square test (χ2). P-values <0.05 were considered statistically significant.
Results
Distribution of K. pneumoniae
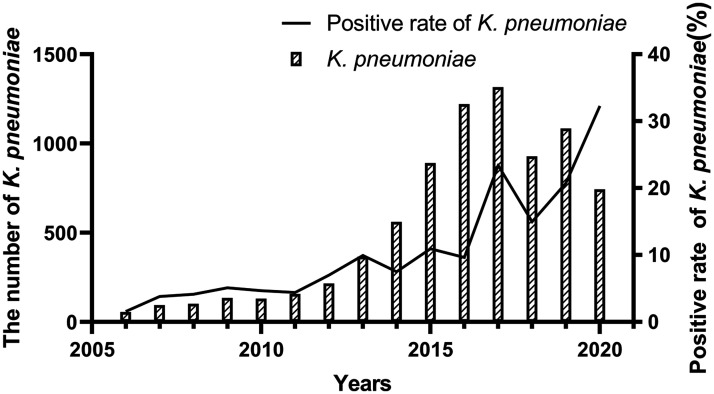
A total of 71,743 K. pneumoniae isolates were collected from clinical specimens between 2006–2020. Annual isolate numbers ranged from 55 to 1316. The largest number of isolates was detected in 2017 (n = 1316), and the detection rate peaked in 2020, accounting for up to 32.30% of the total isolates (Figure 1).
Figure 1.
The number and positive rate of K. pneumoniae infections from 2006 to 2020.
Characteristics of K. pneumoniae
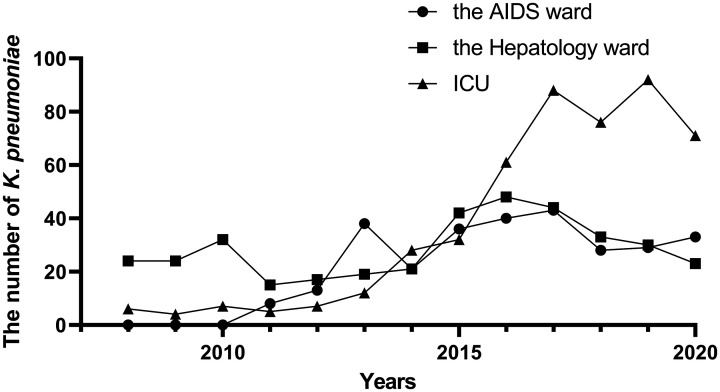
Between 2008 and 2020, detection rates of K. pneumoniae were 9.72%, 12.52%, and 16.45% in the AIDS ward, hepatology ward, and intensive care unit (ICU), respectively, and 61.31% in other wards. There was no detailed division of wards in 2006 to 2007, so this analysis was excluded. Figure 2 shows that the numbers of K. pneumoniae detected in the ICU and AIDS ward increased over time, especially in the ICU between 2015 and 2017, while numbers in the hepatology ward remained relatively stable.
Figure 2.
The number of K. pneumoniae infections in different hospital wards. Because there was no detailed division of wards in 2006–2007, this analysis was not included.
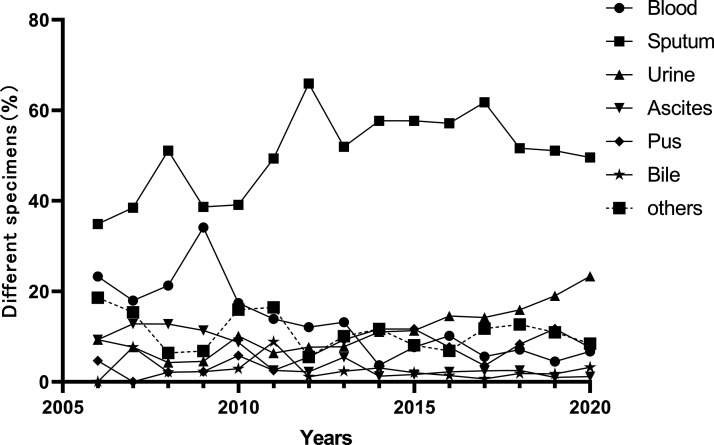
K. pneumoniae strains were mostly isolated from sputum (53.77%) and urine (14.70%). Strains were also isolated from blood (8.42%), pus (7.89%), ascites (2.69%), bile (2.10%), and other specimens (10.45%). The proportion of K. pneumoniae isolated from sputum was consistently highest. The number of K. pneumoniae isolated from urine, but not blood, increased over time. K. pneumoniae isolated from bile and ascites occupied a relatively small percentage of the total bacterial population (Figure 3).
Figure 3.
Percentage of different specimens with positive K. pneumoniae isolates from 2006 to 2020.
Antibiotic resistance of K. pneumoniae
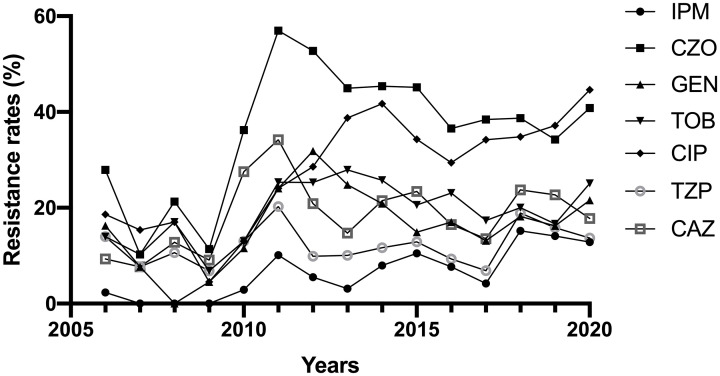
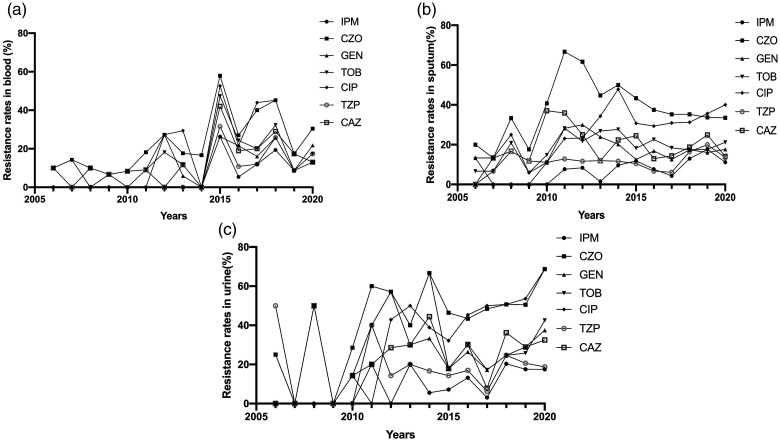
K. pneumoniae resistance rates to six of the antibiotics increased in varying degrees from 2006 to 2020, while that to piperacillin–tazobactam was relatively stable (Figure 4). Increases in resistance rates were significant for imipenem (which increased from 2.33% to 12.83% from 2006–2020; P < 0.05), and ciprofloxacin (which increased from 18.60% to 44.61% from 2006–2020; P < 0.05). Resistance rates were not significantly different for the other five antibiotics (Table 1).
Figure 4.
Resistance rates of K. pneumoniae to different antibiotics from 2006 to 2020
IPM, imipenem; CZO, cefazolin; GEN, gentamicin; TOB, tobramycin; CIP, ciprofloxacin; TZP, piperacillin–tazobactam; CAZ, ceftazidime.
Table 1.
Comparison of K. pneumoniae drug resistance.
| Antibiotic | Resistance rate in 2006 (%) | Resistance rate in 2020 (%) |
|---|---|---|
| IPM | 2.33 (1/43) | 12.83 (44/343)* |
| CZO | 27.91 (12/43) | 40.82 (140/343) |
| GEN | 16.28 (7/43) | 21.57 (74/343) |
| TOB | 13.95 (6/43) | 20.07 (86/343) |
| CIP | 18.60 (8/43) | 44.61 (153/343)* |
| TZP | 13.95 (6/43) | 13.70 (47/343) |
| CAZ | 9.30 (4/43) | 17.78 (61/343) |
*P < 0.05. IPM, imipenem; CZO, cefazolin; GEN, gentamicin; TOB, tobramycin; CIP, ciprofloxacin; TZP, piperacillin–tazobactam; CAZ, ceftazidime.
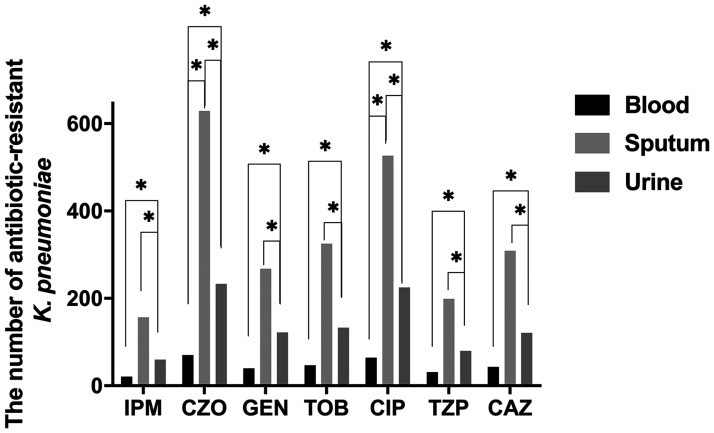
Considering resistance trends of K. pneumoniae isolated from blood, sputum, and urine samples, we detected the highest resistance rate to cefazolin, regardless of the specimen analyzed (Figure 5). K. pneumoniae resistance rates to different antibiotics were then compared between different sample types. Only cefazolin and ciprofloxacin resistance rates showed significant differences between blood and sputum specimens (P < 0.05; Figure 6). Significant differences in the resistance rates of all seven antibiotics were identified between blood and urine specimens and between sputum and urine specimens (P < 0.05; Figure 6).
Figure 5.
Resistance of K. pneumoniae to different antibiotics among specimens.
IPM, imipenem; CZO, cefazolin; GEN, gentamicin; TOB, tobramycin; CIP, ciprofloxacin; TZP, piperacillin–tazobactam; CAZ, ceftazidime.
Figure 6.
The number of antibiotic-resistant K. pneumoniae among different specimens. *P < 0.05.
IPM, imipenem; CZO, cefazolin; GEN, gentamicin; TOB, tobramycin; CIP, ciprofloxacin; TZP, piperacillin–tazobactam; CAZ, ceftazidime.
Isolated CRKP were then compared with respect to specimen type, patient age, and hospital ward. The detection rate of CRKP in older patients (15.55%) was significantly higher than in younger patients (5.49%) (P < 0.05). Detection rates of CRKP among blood (8.17%), sputum (9.56%), and urine (13.36%) specimens were also significant (P < 0.05). Similarly, data analyzed from 2011 to 2020 showed that CRKP detection rates differed significantly among the hepatology ward (1.37%), ICU (21.19%), and AIDS ward (5.88%) (Table 2).
Table 2.
CRKP analysis between 2006 and 2020.
| Characteristic | Total K. pneumoniae cases, n (%) | CRKP cases, n (positivity %) | P-value |
|---|---|---|---|
| Patient age (years) | |||
| ≥65 | 1196 (39.16%) | 186 (15.55%) | <0.05 |
| <65 | 1858 (60.84%) | 102 (5.49%) | |
| Specimen | |||
| Blood | 257 (8.42%) | 21 (8.17%) | <0.05 |
| Sputum | 1642 (53.77%) | 157 (9.56%) | |
| Urine | 449 (14.70%) | 60 (13.36%) | |
| Ward | |||
| Hepatology ward | 292 (10.38%)† | 4 (1.37%) | <0.05 |
| ICU | 472 (16.79%)† | 100 (21.19%) | |
| AIDS ward | 289 (10.28%)† | 17 (5.88%) | |
†Data from 2011 to 2020.
Discussion
As an important conditional pathogen of hospitals, K. pneumoniae can cause multiple systemic infections such as pneumonia, urinary tract infections, and bloodstream infections. 1 K. pneumoniae is the second most common pathogen of nosocomial infections in China. 3 This study analyzed data from Hangzhou Xixi Hospital Affiliated to Zhejiang Chinese Medical University, a tertiary that takes site-directed admissions of infectious disease cases in Zhejiang Province, China. Between 2006 and 2020, the isolation rate of K. pneumoniae has progressively grown from 1.57% to 32.30%, and now exceeds the national average. This suggests that K. pneumoniae is becoming increasingly widespread and dominating in this area.2,8
The treatment of AIDS and liver disease is a feature of our hospital, and ICU patients are prone to opportunistic infections because they are immunocompromised. Accordingly, we observed the highest detection rate of K. pneumoniae in the ICU; the rate increased rapidly between 2015 and 2017, and remained at a high level since then. A moderate increase was observed in the AIDS ward, while the rate was relatively stable in the hepatology ward. A possible explanation for this is that the ICU primarily admits patients with severe disease, including critically ill patients with infections and those with multiple diseases. Moreover, cross-infection can occur through a variety of channels, including treatment devices and equipment. Conversely, patients in the AIDS and hepatology wards have usually received some treatment and developed a level of immunity. Patients in the ICU may also be at a higher risk of developing ventilator-associated pneumonia (VAP) because invasive mechanical ventilation damages the respiratory tract’s local mucosal barrier. Indeed, in Taiwan, Thailand, and Singapore, K. pneumoniae is one of the most prevalent bacteria causing hospital-acquired pneumonia and VAP.9,10 We recommend that increased attention be paid to the management of hospital departments to prevent and control the spread and cross-infection of K. pneumoniae.
With respect to K. pneumoniae detection among different specimens, we observed the highest rate in sputum samples, followed by samples of urine and blood, which is consistent with nationwide findings in China. 3 This suggests that K. pneumoniae is one of the main pathogenic bacteria of respiratory tract infections, which should be taken into account during diagnosis. Several studies have analyzed K. pneumoniae isolated from blood or urine specimens.11 –14 Zanichelli et al. showed that the isolation frequency of K. pneumoniae from urine samples in Switzerland between 2009 and 2016 was secondary only to that of E. coli, and the drug resistance trend was increasingly serious, which was consistent with the findings of Ding et al. in Chongqing, China. Most pathogens associated with urinary tract infections were previously shown to be Enterobacteriaceae, while 5% to 10% were K. pneumoniae.15,16 Moreover, K. pneumoniae was reported to be the second most prevalent Gram-negative bacilli pathogen of bacteremia in hospitals and communities, after E. coli, and to have a high mortality rate. 17
Increasing resistance rates of K. pneumoniae to imipenem, resulting in CRKP, pose a huge challenge to clinical treatment. In our study, CRKP detection rates were highest in the ICU, which is consistent with previous findings. 5 The main risk factors of CRKP infection were reported to be sputum suction 18 and admission to the ICU.19,20 We observed significant differences in the resistance rates of K. pneumoniae to seven antibiotics among blood, urine, and sputum specimens, indicating that different antibiotic treatment schemes should be adopted for patients with different infection sites.
CHINET data from 2018 revealed increased resistance rates of K. pneumoniae to imipenem and meropenem,3 while the resistance rate of K. pneumoniae to carbapenem similarly increased from 2% to 12% in Singapore, 21 and showed steady increases in Vietnam, Thailand, Malaysia, the Philippines, and Italy.22,23 As one of the most important treatments for ESBL-producing bacteria, carbapenem antibiotics are widely used to treat serious infections.22,24 However, the overuse of antibiotics has increased the spread of CRKP, and even resulted in the emergence of extensively drug-resistant bacteria, which has caused challenges for clinical treatment. 11
Carbapenemase production is the main cause of K. pneumoniae resistance to carbapenems. The first K. pneumoniae carbapenemase-1-producing K. pneumoniae strain was reported in the USA in 2001 by Yigit et al. 25 It caused CRKP nosocomial outbreaks in many countries and regions because of the easy transmission of genes encoding KPC-1 enzymes.26 –29 In China, the first KPC-2-producing K. pneumoniae was reported in Zhejiang Province in 2007; 30 KPC-2 strains are currently the most common isolates in the clinic.5,31
The present study has some limitations. Because of its long time span, the antibiotics used for susceptibility testing at the start of the study differed from those used more recently. Accordingly, we did not analyze the resistance of K. pneumoniae to all antibiotics, but only seven. Additionally, this was a single-center study of K. pneumoniae drug resistance trends, which does not necessarily reflect the entire region. Future work will analyze the underlying molecular biology of the CRKP resistance mechanism identified here.
Conclusion
We observed a significant increase in the K. pneumoniae detection rate at our hospital between 2006 and 2020, particularly in the ICU. We also detected significant differences in K. pneumoniae resistance to seven antibiotics among different types of specimens. Although the detection rates of CRKP observed in this study were lower than those reported by CHINET, the rising trend is still of concern. We suggest that increased efforts should focus on the prevention and control of hospital infections, and the management of antimicrobial clinical applications to maximize bacterial resistance surveillance.
Acknowledgement
We are grateful to Shibo Liu for proofreading the article.
Footnotes
Ethical approval: This study was approved by the local medical ethics committee of Hangzhou Xixi Hospital.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Project from the Health Commission of Zhejiang Province of the People’s Republic of China [2015KYB321].
ORCID iDs: Zhezhe Lin https://orcid.org/0000-0001-9816-0835
Shourong Liu https://orcid.org/0000-0002-8813-2555
References
- 1.Martin RM, Bachman MA. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol 2018; 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.China Antimicrobial Resistance Surveillance System. Antimicrobial resistance of bacteria: surveillance report from China Antimicrobial Resistance Surveillance System in 2014–2019. Chin J Infect Control 2021; 20: 15–31. (in Chinese). [Google Scholar]
- 3.Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis 2019; 38: 2275–2281. [DOI] [PubMed] [Google Scholar]
- 4.Chong Y, Shimoda S, Shimono N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Genet Evol 2018; 61: 185–188. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Liu C, Shen Z, et al. Prevalence, risk factors and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008–2018. Emerg Microbes Infect 2020; 9: 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 29th ed. CLSI supplement M100. Wayne PA: Clinical and Laboratory Standards Institute, 2019.
- 7.WHONET 5.6 software. Available at: https://whonet.org/software.html (Accessed 15 February 2022).
- 8.Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect 2016; 22 Suppl 1: S9–14. [DOI] [PubMed] [Google Scholar]
- 9.Chou CC, Shen CF, Chen SJ, et al. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect 2019; 52: 172–199. [DOI] [PubMed] [Google Scholar]
- 10.Chung DR, Song JH, Kim SH, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med 2011; 184: 1409–1417. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Zhang G, Yang Y, et al. Antimicrobial resistance comparison of Klebsiella pneumoniae pathogens isolated from intra-abdominal and urinary tract infections in different organs, hospital departments and regions of China between 2014 and 2017. J Microbiol Immunol Infect 2020; 54: 639–648. [DOI] [PubMed] [Google Scholar]
- 12.Zanichelli V, Huttner A, Harbarth S, et al. Antimicrobial resistance trends in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis urinary isolates from Switzerland: retrospective analysis of data from a national surveillance network over an 8-year period (2009-2016). Swiss Med Wkly 2019; 149: w20110. [DOI] [PubMed] [Google Scholar]
- 13.Ding Y, Wang H, Pu S, et al. Resistance trends of Klebsiella pneumoniae causing urinary tract infections in Chongqing, 2011-2019. Infect Drug Resist 2021; 14: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavriliu LC, Benea OE, Benea S. Antimicrobial resistance temporal trend of Klebsiella pneumoniae isolated from blood. J Med Life 2016; 9: 419–423. [PMC free article] [PubMed] [Google Scholar]
- 15.van Driel AA, Notermans DW, Meima A, et al. Antibiotic resistance of Escherichia coli isolated from uncomplicated UTI in general practice patients over a 10-year period. Eur J Clin Microbiol Infect Dis 2019; 38: 2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokhn ES, Salami A, El Roz A, et al. Antimicrobial susceptibilities and laboratory profiles of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates as agents of urinary tract infection in Lebanon: paving the way for better diagnostics. Med Sci (Basel) 2020; 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Zhao Y, Liu C, et al. Molecular pathogenesis of Klebsiella pneumoniae. Future Microbiol 2014; 9: 1071–1081. [DOI] [PubMed] [Google Scholar]
- 18.Zheng B, Dai Y, Liu Y, et al. Molecular epidemiology and risk factors of carbapenem-resistant Klebsiella pneumoniae infections in eastern China. Front Microbiol 2017; 8: 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saidel-Odes L, Borer A. Limiting and controlling carbapenem-resistant Klebsiella pneumoniae. Infect Drug Resist 2013; 7: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Qin RR, Huang L, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection and mortality of Klebsiella pneumoniae infection. Chin Med J (Engl) 2018; 131: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teo JQ, Cai Y, Lim TP, et al. Carbapenem resistance in Gram-negative bacteria: the not-so-little problem in the little red dot. Microorganisms 2016; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu LY, Apisarnthanarak A, Khan E, et al. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in south and southeast Asia. Clin Microbiol Rev 2017; 30: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoxha A, Kärki T, Giambi C, et al. Attributable mortality of carbapenem-resistant Klebsiella pneumoniae infections in a prospective matched cohort study in Italy, 2012-2013. J Hosp Infect 2016; 92: 61–66. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Zou M, Dou Q, et al. Characterization of clinical extensively drug-resistant Pseudomonas aeruginosa in the Hunan province of China. Ann Clin Microbiol Antimicrob 2016; 15: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45: 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aires-de-Sousa M, Ortiz de la Rosa JM, Gonçalves ML, et al. Epidemiology of carbapenemase-producing Klebsiella pneumoniae in a hospital, Portugal. Emerg Infect Dis 2019; 25: 1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis 2018; 18: 37–46. [DOI] [PubMed] [Google Scholar]
- 28.Lau MY, Teng FE, Chua KH, et al. Molecular characterization of carbapenem resistant Klebsiella pneumoniae in Malaysia hospital. Pathogens 2021; 10: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migliorini LB, de Sales RO, Koga PCM, et al. Prevalence of bla(KPC-2), bla(KPC-3) and bla(KPC-30)-carrying plasmids in Klebsiella pneumoniae isolated in a Brazilian hospital. Pathogens 2021; 10: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei ZQ, Du XX, Yu YS, et al. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother 2007; 51: 763–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin X, Wu S, Hao M, et al. The colonization of carbapenem-resistant Klebsiella pneumoniae: epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J Infect Dis 2020; 221: S206–s214. [DOI] [PubMed] [Google Scholar]