Abstract
Objectives:
The aim of this study was to assess the safety and efficacy of complete restoration of respiratory muscle function in subjects with spinal cord injury.
Methods:
This was an interventional study investigating three subjects maintained on a diaphragm pacing system who were implanted with the spinal cord stimulation system to restore cough. Peak expiratory airflow and airway pressure generation were the primary physiologic outcome measures; an assessment of the degree of difficulty in raising secretions was the primary clinical outcome measure.
Results:
Mean peak expiratory airflow and airway pressure generation during spontaneous efforts were 1.7 ± 0.2 L/s and 31 ± 7 cmH2O, respectively. When spinal cord stimulation was applied after pacing volume associated with the subjecťs maximum inspiratory effort and synchronized with the subject's maximum expiratory effort, peak expiratory airflow and airway pressure generation were 9.0 ± 1.9 L/s and 90 ± 6 cmH2O, respectively (P < 0.05). Moreover, each subject experienced much greater ease in raising secretions and marked improvement in the ease in raising secretions compared with other methods.
Conclusions:
Complete restoration of respiratory muscle function can be safely and effectively achieved in the same individuals with spinal cord injury. Spinal cord stimulation results in peak expiratory airflow and airway pressure generation characteristic of a normal cough, whereas diaphragm pacing was successful in maintaining patients off mechanical ventilation.
Keywords: Spinal Cord Injuries, Tetraplegia, Respiratory Muscles, Cough, Functional Electrical Stimulation, Rehabilitation
Electrical stimulation techniques have been used successfully to restore skeletal muscle function in a wide variety of motor systems in subjects with spinal cord injury (SCI).1 With regard to respiratory muscle function, Glenn et al.2,3 achieved restoration of inspiratory muscle function in patients with chronic respiratory failure secondary to cervical SCI more than 5 decades ago. This technique involves electrical stimulation of the phrenic nerves via electrodes positioned directly on the phrenic nerves in the thorax. More recently, we demonstrated that diaphragm pacing could also be achieved via a less invasive method, that is, laparoscopically placed intramuscular diaphragm electrodes.4–6 Phrenic nerve pacing has been successful in liberating thousands of patients from mechanical ventilation.
Although restoration of inspiratory muscle function has provided significant benefits to patients with SCI, perhaps even more important is the restoration of expiratory muscle function, which is necessary to generate an effective cough.7–10 Absence of an effective cough can result in significant discomfort to patients with difficulty clearing secretions and increased risk of aspiration and the development of respiratory tract infections.7,11Respiratory tract infection remains one of the major causes of death in this patient population.12,13 In a recent clinical trial, we demonstrated that spinal cord stimulation (SCS) results in the development of large positive airway pressures and peak airflow rates characteristic of a normal cough.14–18 Use of this technique improved secretion management, reduced the incidence of respiratory tract infections, and significantly improved the quality of life of these subjects.15,18,19
In the present report, wire electrodes, which can be placed using minimally invasive techniques, were used to activate the expiratory muscles and restore cough. It should be noted that wire leads have been in clinical use for several decades for control of pain and spasticity using a minimally invasive surgical procedure allowing for significant clinical advantages over disc electrodes.14
In this report, we hypothesized that complete restoration of respiratory muscle function could be safely and effectively achieved in the same subjects with SCI, that is, inspiratory muscle function, via diaphragm pacing, and expiratory muscle function, via SCS.
METHODS
This interventional clinical trial (Clinical Trials Registry: NCT01659541) was approved by the Investigational Review Boards at MetroHealth Medical Center and the National Institute of Neurological Disorders and Stroke.
Three spinal cord injured patients who were currently being ventilated with a diaphragm pacing system were eligible for participation in this study. Each was screened and enrolled in this study. Each had an implanted intramuscular diaphragm pacing system (8.6 ± 2.2 yrs) to support ventilation. These eligible patients were part of a larger active clinical study (7 patients at this time point), which included patients who were not dependent on diaphragm pacing. Each patient met specific inclusion and exclusion criteria (Table 1). Demographic information of each subject including baseline maximal expiratory pressure (P) and peak expiratory airflow (F) is provided in Table 2. Written informed consent was obtained from each subject. All data were collected during the first 28 wks after implantation of the cough system.
TABLE 1.
Subject eligibility form
| Exclusion criteria | ||
| If the answer to any exclusion criteria is “YES,” the subject is not eligible for participation | ||
| 1. Does the subject have untreated lung, cardiovascular or brain disease? | YES | NO |
| 2. Has the subject had a minor infection at the site of implantation requiring antibiotics within the past 3 wks? | YES | NO |
| 3. Has the subject had a serious infection requiring hospitalization within the past 6 wks? | YES | NO |
| 4. Does the subject have severe scoliosis or other chest deformity? | YES | NO |
| 5. Does the subject have marked obesity? (body mass index >50) | YES | NO |
| 6. Does the subject have unmanaged hypertension or hypotension? | YES | NO |
| 7. Does the subject have low oxygenation? (SaO2 < 90% on ≤4 lpm oxygen) | YES | NO |
| 8. Is the subject pregnant or breast feeding? | YES | NO |
| Inclusion criteria | ||
| If the answer to any inclusion criteria is “NO,” the subject is not eligible for participation | ||
| 1. Does the subject? have SCI C8 level or higher? | YES | NO |
| 2. Is the subject 12 mos after injury (if AIS incomplete) or 6 mos after injury (if AIS complete)? | YES | NO |
| 3. Does the subject have expiratory muscle weakness? (maximum expiratory pressure ≤ 30% predicted normal value) | YES | NO |
| 4. Is the subject between 18 and 75 yrs of age? | YES | NO |
| 5. Has the surgical team determined that it is safe and appropriate for the subject to be taken to surgery? | YES | NO |
| 6. Does the subject have adequate oxygenation? (oxygen saturation 90% ≤ 4 lpm oxygen) | YES | NO |
AIS, American Spinal Injury Association Impairment Scale.
TABLE 2.
Clinical data of the tetraplegic subjects
| Subject | Sex | Age, y | Weight, kg | Cause of Injury | Level of Injury | ASIA | Years Since Injury | Spontaneous Vital Capacity (% Predicted), L | Peak Expiratory Airflow (% Predicted), L/s | Maximum Expiratory Pressure (% Predicted), cmH2O |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1 | M | 27 | 68 | Fall | C3/C4 | A | 5 | 0.93 (16) | 2.0 (20) | 18 (8) |
| 2 | M | 50 | 100 | MVA | C3 | B | 14 | 1.44 (28) | 1.8 (18) | 35 (15) |
| 3 | M | 28 | 63 | Fall | C2 | A | 9 | 1.51 (26) | 1.2 (11) | 41 (22) |
ASIA, American Spinal Cord Injury impairment scale; MVA, motor vehicle accidents.
Physiological Measurements to Assess Cough Efficacy
Maximum inspiratory pressure and P were measured using standard techniques with the airway occluded.20 Peak expiratory airflow was measured after release of airway occlusion.
Surgical Procedure to Place Spinal Cord Wire Electrodes to Restore Cough
Two spinal cord wire leads, each with two electrode contacts, were inserted percutaneously through a needle and positioned in parallel. Each lead was advanced such that the upper electrode was positioned near the T9 spinal level on the dorsal epidural surface of the spinal cord. Given the distance between electrodes on each lead, the lower electrode was positioned near the T11 spinal level. Each lead was connected to a modified radiofrequency receiver (Finetech Medical Ltd, Welwyn Garden City, Herfordshire, United Kingdom), which was implanted anteriorly in a subcutaneous pocket over the upper chest wall. There were no postoperative complications.
Functional Electrical Stimulation System to Restore Cough
Electrical stimulation was applied by activating a small portable external transmitter connected to an antenna, which was secured to the skin directly over the implanted receiver with tape (Fig. 1). The system was designed to provide either monopolar stimulation at the T9 spinal level or bipolar stimulation at the T9–T11 levels. The transmitter could be activated by depression of a small button on the device.
FIGURE 1.
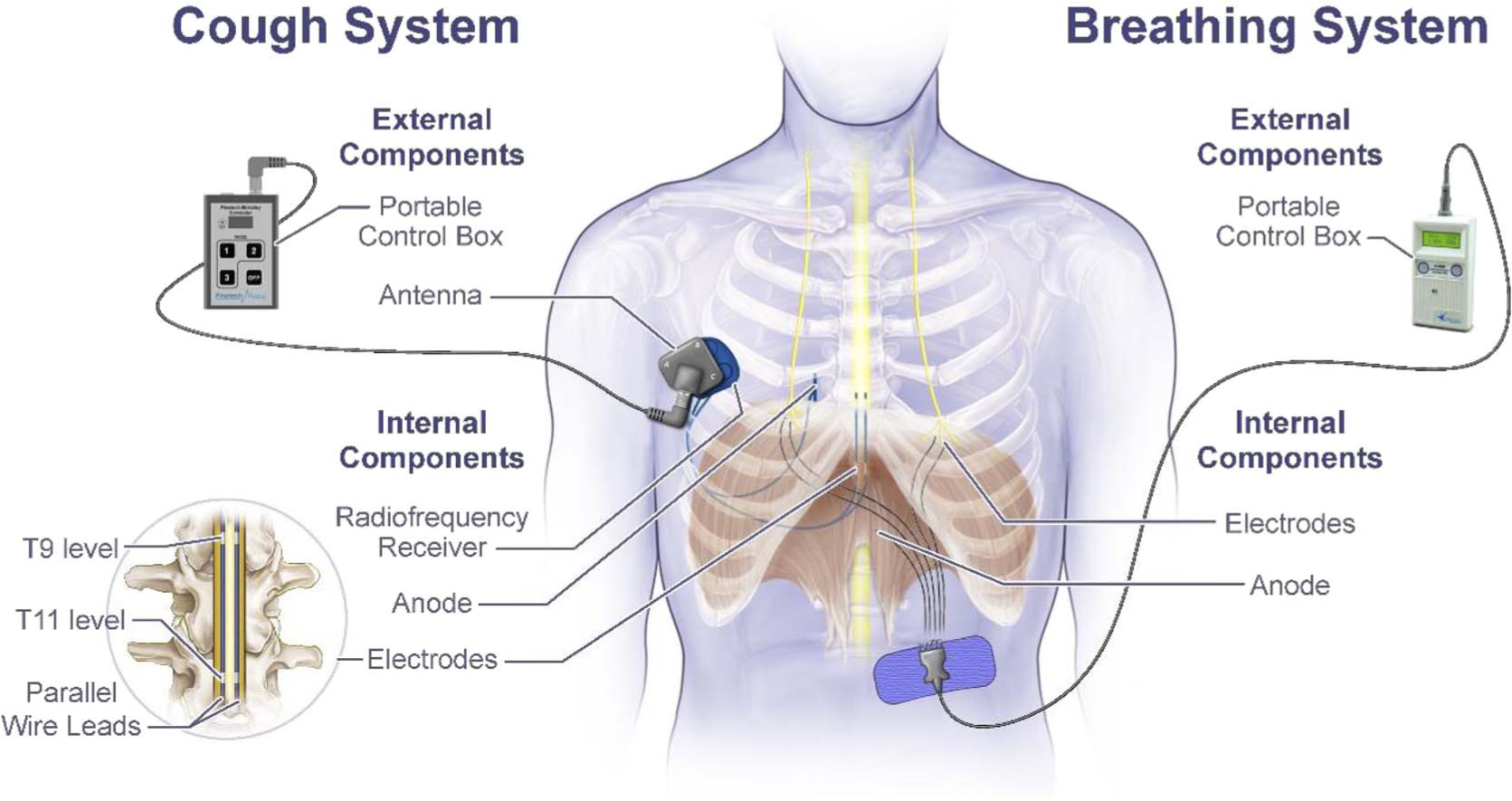
Schematic representation of internal and external components of the diaphragm pacing system to restore breathing and spinal cord stimulation system to activate the expiratory muscles to restore cough.
Muscle Reconditioning
Because the expiratory muscles were significantly atrophied secondary to disuse, a period of repeated muscle stimulation was necessary to restore muscle strength. After an initial evaluation session, subjects were instructed to apply with caregiver assistance, SCS every 30 seconds for 5–10 minutes, 2 or 3 times/ day, in the out-patient setting. Stimulus parameters were set at values resulting in maximal positive airway pressure generation. Subjects were also instructed to use the device for evacuation of secretions or airway clearance, as needed.
Measurements
A BIOPAC Data Acquisition and Analysis System with AcqKnowledge software, MP150 system with TSD 160C pressure transducer and TSD117 pneumotach airflow transducer interfaces with the DA 100C transducer amplifiers (Biopac Systems Inc, 42 Aero Camino, CA) was used to monitor online F and P. Measurements were made through the tracheostomy tube in the seated posture.
During the initial phase of stimulation, vital signs were closely monitored. If absolute systolic blood pressure exceeded 140 mm Hg systolic or 90 mm Hg diastolic, stimulation was withheld until values returned to baseline or less than 140 systolic and 90 diastolic. Stimulation was then applied at less frequent intervals. Because monopolar stimulation generally resulted in less pressure generation, all subjects were instructed to use only bipolar SCS. All the results presented therefore are limited to bipolar SCS.
Repeat physiological measurements of peak airflow and pressure generation were made during out-patient visits every 4–5 wks for an approximately 6-month period postimplantation. The inspiratory capacity was assessed at each patient visit as an index of resting lung volume.
Clinical Outcomes
Each subject completed a short questionnaire before and at the 20, 24, and 28 weeks after implantation of the device. They were asked to (a) rate their difficulty in raising secretions and (b) rate their ease in raising secretions with use of the cough system compared with other secretion management methods, which included airway suctioning and use of the mechanical insufflation-exsufflation device. To rate their difficulty in raising secretions, they were asked, “How much difficulty have you had with managing your airway secretions?” To rate their ease in raising secretions with use of the cough system, they were asked, “Indicate any change in ease with which you are able to manage your airway secretions on a typical day using the expiratory muscle stimulator as compared with other methods.”
RESULTS
Baseline demographics of each subject are provided in Table 2. Each subject had experienced a cervical SCI 5–14 years before the study and were on artificial ventilatory support via a diaphragm pacing system (Synapse Biomedical, Inc, Oberlin, OH).
Baseline respiratory parameters during diaphragm pacing for each subject are provided in Tables 2 and 3. Each subject had evidence of significant expiratory muscle weakness as evidenced by marked reductions in F and P. Unassisted inspired volumes during diaphragm pacing averaged 0.8 ± 0.1 L. When subjects assisted the pacing system by making a maximal inspiratory effort synchronized with the paced breath, inspired volumes increased to 1.5 ± 0.1 L. When subjects made maximum inspiratory and expiratory efforts synchronized with diaphragm pacing, mean F and P were 2.2 ± 0.2 L/s and 39 ± 6 cmH2O, respectively.
TABLE 3.
Respiratory parameters during diaphragm pacing
| Pacing Volume and Subject Maximal Spontaneous Inspiratory and Expiratory Effort |
||||
|---|---|---|---|---|
| Subject | Pacing Volume, L | Pacing Volume and Maximum Inspiratory Effort, L | F, L/s | P, cmH2O |
|
| ||||
| 1 | 0.876 | 1.579 | 2.3 | 27 |
| 2 | 0.886 | 1.323 | 1.8 | 42 |
| 3 | 0.711 | 1.455 | 2.5 | 49 |
| Mean ± SE | 0.8 ± 0.1 | 1.5 ± 0.1 | 2.2 ± 0.2 | 39 ± 6 |
P, airway pressure.
Physiologic Parameters Associated With SCS
Because the tracheostomy tubes were uncuffed, a small degree of air leakage was unavoidable, which likely underestimated F and P measurements. In instances of glottic closure or obvious large mask leakage, data were discarded. Results are presented after achievement of a plateau in peak airflow and pressure generation for at least a 2-week period. This occurred at a mean of 16.0 ± 5.9 wks after initiation of SCS.
The effects of SCS on F and P are shown for one subject in Figure 2. Spontaneous F and P while this subject made a maximum inspiratory and expiratory effort synchronized with the paced breath were 2.3 L/s and 27 cmH2O, respectively. Spinal cord stimulation after inspired volume provided by the pacing system without patient effort (maneuver #1) F and P increased to 3.0 L/s and 50 cmH2O(P <0.05), respectively. When SCS was applied after pacing volume in addition to subject maximum inspiratory effort (maneuver #2), P increased to 9.4 L/s and 83 cmH2O, respectively (P < 0.05). When SCS was applied after pacing volume in addition to subject maximum inspiratory effort and in association with a maximal expiratory effort (maneuver #3), F and P were 11.8 L/s and 98 cmH2O, respectively.
FIGURE 2.
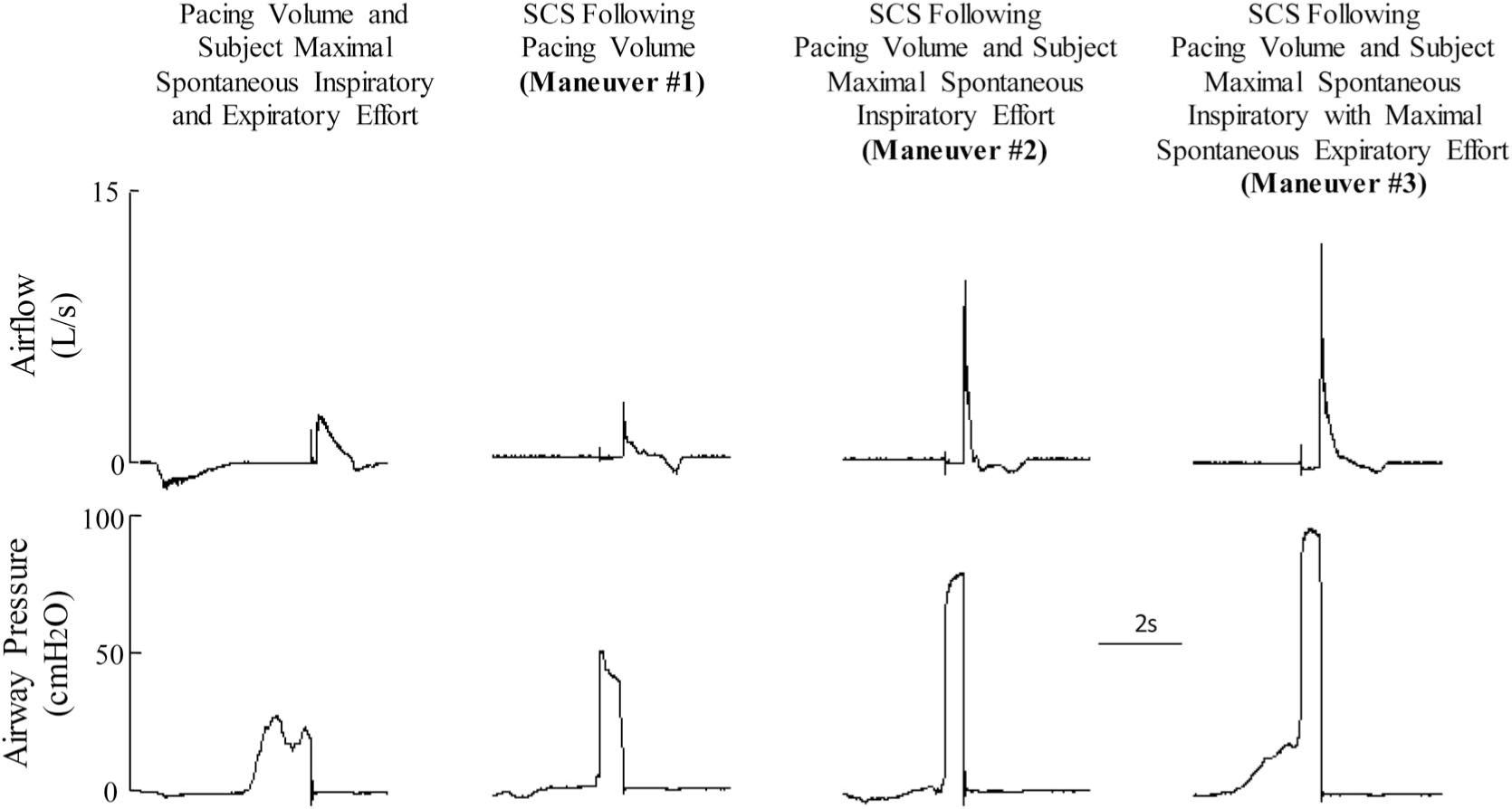
Representative tracings of expiratory airflow and airway pressure in one subject. Spontaneous maximum expiratory effort after a paced breath (far left panel), SCS after a paced breath (maneuver #1), SCS after a maximum inspiratory effort during a paced breath (maneuver #2), and SCS after a maximum inspiratory effort during a paced breath and subsequent maximum expiratory effort in conjunction with SCS (maneuver #3). See text for further explanation.
The results for each subject and mean data are provided in Table 4. Mean results are also displayed graphically in Figure 3. With maneuver #1, F and P were 3.7 ± 0.4 L/s and 56 ± 3 cmH2O respectively (P < 0.05 for both when compared with unassisted efforts). With maneuver #2, F and P were 7.5 ± 1.5 L/s and 75±4 cmH2O respectively(P < 0.05 for both when compared with maneuver #1). With maneuver #3, F and P were 9.0 ± 1.9 L/s and 90 ± 6 cmH2O respectively (P < 0.05 when compared with maneuvers #1 and #2).
TABLE 4.
Effects of SCS on peak expiratory flow and maximum expiratory pressure
| SCS After Pacing Volume (Maneuver #1) |
SCS After Pacing Volume and Subject Maximal Spontaneous Inspiratory Effort (Maneuver #2) |
SCS After Pacing Volume and Subject Maximal Spontaneous Inspiratory with Maximal Spontaneous Expiratory Effort (Maneuver #3) |
||||
|---|---|---|---|---|---|---|
| Subject | F, L/s | P, cmH2O | F, L/s | P, cmH2O | F, L/s | P, cmH2O |
|
| ||||||
| 1 | 3 | 50 | 9.4 | 83 | 11.8 | 98 |
| 2 | 3.9 | 57 | 8.6 | 70 | 9.8 | 78 |
| 3 | 4.3 | 61 | 4.6 | 73 | 5.3 | 93 |
| Mean ± SE | 3.7 ± 0.4 | 56 ± 3 | 7.5 ± 1.5 | 75 ± 4 | 9.0 ± 1.9 | 90 ± 6 |
P, airway pressure.
FIGURE 3.
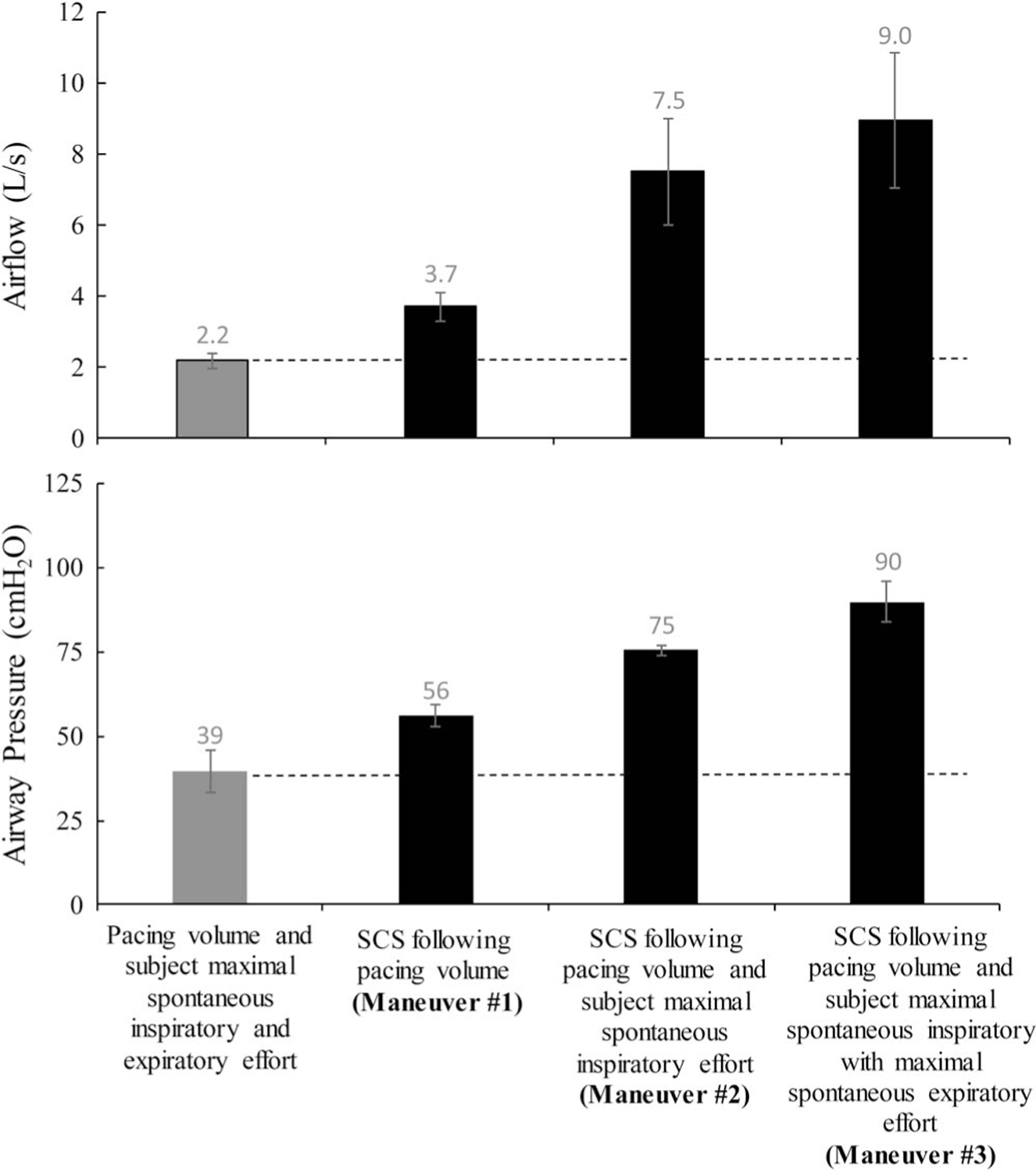
Mean peak airflow rate (upper panel) and mean airway pressure (lower panel) during pacing volume and subjects maximal spontaneous inspiratory and expiratory effort (gray bars) and also during synchronized SCS (black bars).
Clinical Outcomes Associated With SCS
With regard to difficulty in raising secretions, each subject had moderate to marked difficulty before application of SCS to restore cough system (Fig. 4). When assessed at the 20-, 24-, and 28-week time points after implantation, each subject reported substantial improvement reporting none to only mild difficulty. With regard to ease in raising secretions with use of the cough system compared with other methods, which included the cough assist maneuver, suctioning, and use of the insufflator/exsufflator device, there was also marked improvement in each subject at each of the three time points.
FIGURE 4.
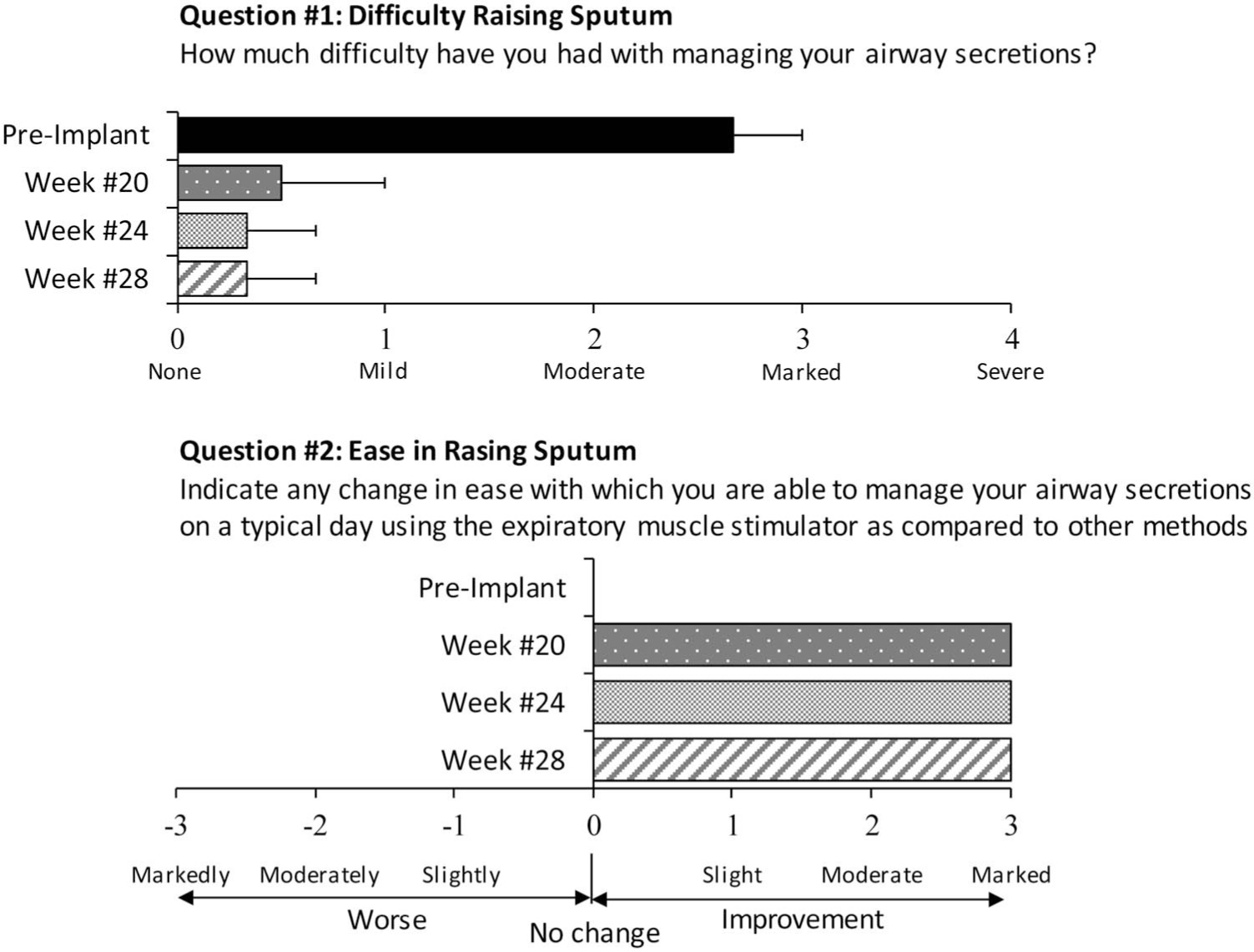
Subject responses when asked to numerically rate their difficulty in raising secretions according to a scale between none and severe (upper panel). Compared with preimplant values, there was marked improvement in their difficulty in raising sputum. When asked to numerically rate the degree of improvement with regard to their ease in raising sputum compared with other methods, there was marked improvement postimplant (lower panel).
Side Effects Associated With SCS
As with our previous investigations to restore cough, two of the three subjects in this study developed signs of autonomic dysfunction, that is, increases in blood pressure and reductions in heart rate during the conditioning phase of the study. Importantly, these hemodynamic changes were asymptomatic in each instance. When hemodynamic changes were observed, the frequency of SCS was reduced to allow cardiovascular variables to return to baseline values, which usually occurred within 10 mins. During our initial subject evaluation of SCS, these parameters were closely monitored to determine the appropriate safe interval of SCS. After several weeks of daily stimulation, however, there were no observable hemodynamic changes associated with SCS.
No other side effects were noted including unwanted body movements or inadvertent bowel movements. Although subjects had urinary catheter in place, there was no subjective increase in urinary flow associated with SCS.
DISCUSSION
Although restoration of inspiratory muscle function is a clinically accepted modality to liberate spinal cord injured patients from mechanical ventilation, restoration of expiratory muscle function is a relatively new innovation. Using electrical stimulation techniques in patients with SCI, previous investigations have demonstrated restoration of breathing via diaphragm stimulation and restoration of cough via SCS, in separate patient groups.14–19 This report however represents the first demonstration of successful restoration of both inspiratory and expiratory muscle function, that is, complete restoration of respiratory muscle function, in the same individuals with SCI. Consistent with our previous reports of lower thoracic SCS to restore cough using disc electrodes,15–18 each subject in this study also described much greater ease in raising secretions and use of other methods of secretion removal were either markedly reduced or completely eliminated.
A potential concern with the implantation of multiple electrical systems is the potential for adverse electrical interaction. This is of particular concern given the high stimulus intensities required to activate each muscle group. With regard to diaphragm activation, two electrodes are positioned in each hemidiaphragm in the vicinity of the phrenic nerve motor points.4–6,21 The delivery of approximately 25 mA to each electrode is required to achieve sufficient diaphragm activation to maintain ventilatory support. Previous studies have demonstrated that the implantation of diaphragm pacing systems could be safely performed in patients with cardiac pacemakers.22–24 More specifically, the activation of implanted wire electrodes in the body of the left hemidiaphragm does not impact cardiac pacing systems. In addition, cardiac pacing does not impact diaphragm pacing system. Intraoperative evaluations during implantation of diaphragm pacing systems (>300 subjects) demonstrated no device-to-device interactions when checked using the most sensitive cardiac pacemaker settings and highest diaphragm pacing settings.24 With regard to SCS to restore cough, approximately 30–40 V is required to achieve sufficient activation of the expiratory muscles to generate an effective cough.14–18 Electrical stimulation was applied after activation of the diaphragm pacing system, that is, after the breath was delivered, because cough efforts are not typically made during inspiration. In our patient group, the application of SCS never resulted in activation of the diaphragm pacing system. Moreover, the diaphragm pacing system never triggered activation of the cough system. This is not entirely surprising because each system has separate ground electrodes in place, which limits current flow to each specific muscle group.
Effects of Lung Volume on Expiratory Muscle Function
Spontaneous cough efforts typically vary in magnitude based on clinical need. Given the length-tension relationship of the expiratory muscles, positive airway pressure generation is greatest when applied at high lung volumes because these muscles are increasingly stretched as lung volume increases. Therefore, mild cough efforts as with throat clearing may be associated with only a small inspiration, whereas stronger cough efforts as may be required to expectorate thick mucus or foreign body are typically associated with larger inspirations. Because the magnitude of inspired volume generation is severely restricted in these individuals, the magnitude of airway pressure generation is also limited. As shown in our results, airway pressure generation during SCS was smallest when inspiration was achieved by passive diaphragm pacing alone. Because inspired volume was significantly higher when subjects made inspiratory efforts in conjunction with the paced breaths, it was not surprising that maximum expiratory pressures were also significantly higher in tandem. Because these subjects were able to generate positive expiratory pressures, albeit small, even greater pressure generation occurred when subjects both assisted the inspiratory effort and also mimicked a normal cough by making a strong expiratory effort during SCS.
Mechanism of Expiratory Muscle Activation Via SCS
Based on previous animal studies, dorsal epidural SCS over the lower thoracic and upper lumbar spinal cord resulted in maximum expiratory muscle activation via direct motor root stimulation in addition to dorsal column stimulation to active more caudal motor roots.25–29 These data formed the basis of our previous clinical trial in which we demonstrated that monopolar SCS of any two of three sites (T9, T11, L1) on the dorsal epidural surface of the spinal cord resulted in maximum airway pressure generation.15–18 Because stimulation at the L1 level often resulted in unwanted leg movement, stimulation was usually limited to the T9 and T11 levels. Use of SCS to restore cough led to significant clinical benefit including reduction in the incidence of respiratory tract infections, reduced need for caregiver support, and much greater ease in raising secretions.15,18,19
Use of disc electrode technology however required an invasive procedure involving laminotomies and a long surgical procedure (~5–6 hrs). In more recent animal studies, we found that wire electrodes, which can be placed using minimally invasive technology, also resulted in substantial airway pressure generation, approximately 80% of that achieved with disc electrodes.29 The advantage of these types of electrodes lies in the fact that they can be placed via minimally invasive techniques resulting in much smaller incisions, shorter surgical times, and lower overall risk. Moreover, we also demonstrated in previous animal studies that bipolar stimulation between T9 and T11 (which restricts current flow to these spinal cord regions) resulted in similar pressure generation compared with monopolar stimulation at these levels.29 These results formed the basis of the application of bipolar stimulation using wire electrode technology in our current clinical trial.
Maximal airway pressure generation in the present investigation is somewhat smaller compared with our previous study with disc electrodes (77%) because of several factors including smaller total lung capacity, smaller degree of expiratory muscle activation with wire electrodes, and tracheostomy leakage.14–18 Use of bipolar stimulation in this region however eliminated the previously observed leg movements observed with unipolar stimulation.
Clinical Benefits of Complete Restoration of Respiratory Muscle Function
The clinical benefits of restoration of inspiratory muscle function via diaphragm pacing have been reviewed exten-sively in previous reports.4–6,11 Briefly, this technique results in improved quality of life, improved speech and olfactory sensation, increased mobility, reduced anxiety, and embarrassment and reduced costs. There is also some evidence that this method may reduce the incidence of respiratory tract infection and prolong life, compared with mechanical ventilation.7 Restoration of expiratory muscle function via lower thoracic SCS also has substantial clinical benefits and appear additive to those achieved with diaphragm pacing.15,18,19 In the small study sample and relatively short follow-up period of the present investigation, there was demonstrable improvement in secretion management and much greater ease in raising secretions compared with other more cumbersome methods. The commonly used insufflator/exsufflator device, for example, is labor intensive, requires the presence of trained personnel, specialized equipment, provider-patent coordination, and may be uncomfortable. Some studies have shown some clinical usefulness of this device. However, a recent benefit/risk analysis was assessed in a systematic review and concluded that the current scientific evidence does not support the use of mechanical insufflation-exsufflation devices for cough augmentation in patients with neuromuscular disorders.30 In contrast to other methods of secretion management, the SCS system is a portable device whereby untrained personnel can activate the system and generate an effective cough by depressing a button. Of note, two subjects in the present study relayed that they felt more comfortable travelling alone, had greater mobility, and only rarely needed suctioning.
Given the fact that airway pressure and peak airflow generation are in the same range as that achieved in our previous report (using the disc electrodes), in which subjects were followed for a much longer period, we would expect the subjects in the present investigation to achieve the same additional clinical benefits described previously.
Study Limitations
Although this study strongly suggests that restoration of cough can be achieved in patients also being ventilated using diaphragm pacing, our study population was small. Therefore, to fully assess potential benefits and risks of this technique, our results need to be replicated in a larger study sample. The duration of this study was also relatively short. A longer study period is necessary to assess the long-term impact of restoration of cough in this population including morbidity, for example, incidence of respiratory infections and quality of life and, ultimately, Mortality.
SUMMARY
This study represents the first report of complete restoration of respiratory muscle function in tetraplegic patients. Our results suggest that SCS to restore cough can be used safely and effectively in conjunction with diaphragm pacing to restore inspiratory muscle function. In this subject group, SCS using wire electrodes, which can be placed using minimally invasive techniques, results in similar clinical benefits as that achieved with disc electrodes in our previous investigation. In addition to the cost savings achieved with diaphragm pacing, it is likely that additional cost savings can be achieved by restoration of an effective cough in this subject population.
Acknowledgments
This study was supported by the NIH-NINDS (U01 NS083696) and CTSA (UL1TR000439). This investigation was approved by the Institutional Review Board of MetroHealth Medical Center (IRB15-00014).
Footnotes
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
AFD holds two United States Patents for technology related to the content of this article: method and apparatus for electrical activation of the expiratory muscles to restore cough (5,999,855) and bipolar spinal cord stimulation to activate the expiratory muscles to restore cough (8,751,004).
Presented as a poster at the Annual Conference of the Academy of Spinal Cord Injury Professionals, Denver, CO, September 3–6, 2017.
Clinical Trials Registry: NCT01659541; FDA IDE: G980267.
Contributor Information
Anthony F. DiMarco, Department of Physical Medicine and Rehabilitation, Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio; Department of MetroHealth Research Institute, Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio.
Robert T. Geertman, Department of Neurosurgery, Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio.
Kutaiba Tabbaa, Department of Anesthesiology, Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio.
Krzysztof E. Kowalski, Department of Medicine, Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio; Department of MetroHealth Research Institute, Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio; Research Service, Louis Stokes Cleveland VA Medical Center, Cleveland, Ohio.
REFERENCES
- 1.Creasey GH, Ho CH, Triolo RJ, et al. : Clinical applications of electrical stimulation after spinal cord injury. J Spinal Cord Med 2004;27:365–75 [DOI] [PubMed] [Google Scholar]
- 2.Glenn WW: The treatment of respiratory paralysis by diaphragm pacing. Ann Thorac Surg 1980;30:106–9 [DOI] [PubMed] [Google Scholar]
- 3.Glenn WW, Phelps ML, Elefteriades JA, et al. : Twenty years of experience in phrenic nerve stimulation to pace the diaphragm. Pacing Clin Electrophysiol 1986;9:780–4 [DOI] [PubMed] [Google Scholar]
- 4.DiMarco AF, Onders RP, Ignagni A, et al. : Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 2005;127:671–8 [DOI] [PubMed] [Google Scholar]
- 5.DiMarcoAF Onders RP, Ignagni A, et al. : Inspiratory muscle pacing in spinal cord injury: case report and clinical commentary. J Spinal Cord Med 2006;29:95–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMarco AF, Onders RP, Kowalski KE, et al. : Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med 2002;166:1604–6 [DOI] [PubMed] [Google Scholar]
- 7.Brown R, DiMarco AF, Hoit JD, et al. : Respiratory dysfunction and management in spinal cord injury. Respir Care 2006;51:853–68 [PMC free article] [PubMed] [Google Scholar]
- 8.De Troyer A, Estenne M, Heilporn A: Mechanism of active expiration in tetraplegic subjects. N Engl J Med 1986;314:740–4 [DOI] [PubMed] [Google Scholar]
- 9.Estenne M, De Troyer A: Cough in tetraplegic subjects: an active process. Ann Intern Med 1990;112:22–8 [DOI] [PubMed] [Google Scholar]
- 10.Kelly BJ, Luce JM: The diagnosis and management of neuromuscular diseases causing respiratory failure. Chest 1991;99:1485–94 [DOI] [PubMed] [Google Scholar]
- 11.Meyers AR, Feltin M, Master RJ, et al. : Rehospitalization and spinal cord injury: cross-sectional survey of adults living independently. Arch Phys Med Rehabil 1985;66:704–8 [PubMed] [Google Scholar]
- 12.DeVivo MJ, Krause JS, Lammertse DP: Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil 1999;80:1411–9 [DOI] [PubMed] [Google Scholar]
- 13.Soden RJ, Walsh J, Middleton JW, et al. : Causes of death after spinal cord injury. Spinal Cord 2000;38:604–10 [DOI] [PubMed] [Google Scholar]
- 14.DiMarco AF, Geertman RT, Tabbaa K, et al. : Minimally invasive method to activate the expiratory muscles to restore cough. J Spinal Cord Med 2018;41:562–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMarco AF, Kowalski KE, Geertman RT, et al. : Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part II: clinical outcomes. Arch Phys Med Rehabil 2009;90:726–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMarco AF, Kowalski KE, Geertman RT, et al. : Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part I: methodology and effectiveness of expiratory muscle activation. Arch Phys Med Rehabil 2009;90:717–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMarco AF, Kowalski KE, Geertman RT, et al. : Spinal cord stimulation: a new method to produce an effective cough in patients with spinal cord injury. Am J Respir Crit Care Med 2006;173:1386–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiMarco AF, Kowalski KE, Hromyak DR, et al. : Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury. J Spinal Cord Med 2014;37:380–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiMarco AF, Geertman RT, Tabbaa K, et al. : Economic consequences of an implanted neuroprosthesis in subjects with spinal cord injury for restoration of an effective cough. Top Spinal Cord Inj Rehabil 2017;23:271–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black L, Hyatt R: Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 1969;99:696–702 [DOI] [PubMed] [Google Scholar]
- 21.Onders RP, DiMarco AF, Ignagni AR, et al. : Mapping the phrenicnerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series. Surgery 2004;136:819–26 [DOI] [PubMed] [Google Scholar]
- 22.Kolb C, Eicken A, Zrenner B, et al. : Cardiac pacing in a patient with diaphragm pacing for congenital central hypoventilation syndrome (Ondine's curse). J Cardiovasc Electrophysiol 2006;17:789–91 [DOI] [PubMed] [Google Scholar]
- 23.Krishnan U, Ramrakha PS, Money-Kyrle A: A rare instance of 'cardio-respiratory pacing': permanent pacemaker insertion for symptomatic bradycardia in a quadriplegic man dependent on diaphragmatic pacing by phrenic nerve stimulators. Cardiology 2010; 116:98–100 [DOI] [PubMed] [Google Scholar]
- 24.Onders RP, Khansarinia S, Weiser T, et al. : Multicenter analysis of diaphragm pacing in tetraplegics with cardiac pacemakers: positive implications for ventilator weaning in intensive care units. Surgery 2010;148:893–7 [DOI] [PubMed] [Google Scholar]
- 25.DiMarco AF, Kowalski KE, Supinski G, et al. : Mechanism of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol (1985) 2002;92:2341–6 [DOI] [PubMed] [Google Scholar]
- 26.DiMarco AF, Romaniuk JR, Kowalski KE, et al. : Mechanical contribution of expiratory muscles to pressure generation during spinal cord stimulation. J Appl Physiol (1985) 1999;87:1433–9 [DOI] [PubMed] [Google Scholar]
- 27.DiMarco AF, Romaniuk JR, Kowalski KE, et al. : Pattern of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol (1985) 1999;86:1881–9 [DOI] [PubMed] [Google Scholar]
- 28.DiMarco AF, Romaniuk JR, Supinski GS: Electrical activation of the expiratory muscles to restore cough. Am J Respir Crit Care Med 1995;151:1466–71 [DOI] [PubMed] [Google Scholar]
- 29.Kowalski KE, DiMarco AF: Comparison of wire and disc leads to activate the expiratory muscles in dogs. J Spinal Cord Med 2011;34:600–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auger C, Hernando V, Galmiche H: Use of mechanical insufflation-exsufflation devices for airway clearance in subjects with neuromuscular disease. Respir Care 2017;62:236–45 [DOI] [PubMed] [Google Scholar]


