Abstract
Background
IgE bound on the surface of mast cells contributes to the pathogenesis of chronic spontaneous urticaria (CSU). Atopy is a predisposing factor for CSU, where omalizumab is a widely used monoclonal antibody to control urticaria symptoms via capturing serum free IgE. However, the role of serum free IgE is not clarified in CSU. The present study evaluated the clinical relevance of serum free IgE in patients with CSU.
Methods
Eighty-eight patients with CSU and 76 healthy controls (HCs) were enrolled in this study. Serum total and Dermatophagoides pteronyssinus (Der p)-specific IgE levels were measured by ImmunoCAPs. The serum free IgE levels were measured by ELISA using a novel IgETRAP, and their associations with clinical parameters, including urticaria activity score (UAS), were evaluated. Changes in serum free and total IgE levels after omalizumab treatment were observed in 23 CSU patients in comparison between responders (≥50% reduction in UAS) and non-responders (<50% reduction).
Results
Significantly higher serum free/total IgE levels were noted in CSU patients than in HCs with a positive correlation between the 2 values (rho = 0.87, P < 0.001). Among CSU patients, atopics had significantly higher serum free IgE levels than non-atopics, while no associations were noted with UAS, urticaria duration, or the results of serum ANA or autologous serum skin tests. In addition, there were no significant changes in serum free IgE levels during 12 months of omalizumab treatment. No significant differences were noted in serum free/total IgE levels or clinical parameters between responders and non-responders, while responders have higher serum Der p-specific IgE level and its ratio to serum free/total IgE level than non-responders (P < 0.05, respectively).
Conclusions
These findings suggest that increased serum free IgE may be involved in the development of CSU by activating mast cells, especially in atopics. High Der p-specific IgE level and its ratio to serum free IgE level may be a potential biomarker for predicting favorable responses to omalizumab in CSU.
Keywords: Chronic urticaria, IgE, Atopy, Anti-IgE antibody, Autoimmunity, Treatment, House dust mite
Abbreviations: CSU, Chronic spontaneous urticaria; UAS, Urticaria activity score; ANA, Antinuclear antibody; ASST, Autologous serum skin test; ELISA, Enzyme-linked immunosorbent assay
Introduction
Chronic spontaneous urticaria (CSU) is a skin itching disease which accompanies wheal or flare without any specific causes, but sustains for at least 6 weeks. CSU has commonly non-serious and self-limited disease characteristics. The global prevalence of CSU is reported to range between 0.02% and 5.0%.1 Recurrent and unpredictable characteristics substantially impact quality of life and decrease work productivity or daily activities. Recent international guidelines recommend non-sedating, second-generation H1-antihistamines as a first-line treatment for patients with CSU.2,3 Over 50% of patients who are refractory to H1-antihistamines at approved doses can use anti-IgE antibody (omalizumab), which is a widely used monoclonal antibody to control symptoms via capturing serum free IgE.
Serum IgE is an essential mediator in the development and maintenance of allergic inflammation. The high-affinity receptor for IgE (FcεRI) is expressed on tissue mast cells, blood basophils, airway smooth muscle cells, and antigen-presenting cells.4 Therefore, the measurement of serum IgE level is widely used to evaluate atopic status. Increased serum total IgE level, a marker of CSU, could induce activation of skin mast cells and basophils, contributing to the pathogenesis of CSU.5
The measurement of serum IgE levels has some limitation to the management of CSU patients. Current detection methods (including ImmunoCAP) could not distinguish circulating complex forms of IgE from circulating free IgE.6 Furthermore, the measurement of serum total IgE level is not sensitive to differentiate between atopic and non-atopic responders, and does not correlate with clinical characteristics including disease activity in patients with CSU.7 Omalizumab is beneficial to control symptoms in patients with severe CSU refractory to antihistamines and known to be effective to capture serum circulating free IgE antibody. Therefore, there is a need to develop a reliable method for detecting serum free IgE in order to apply in the clinical practice of CSU, especially anti-IgE antibody therapy.
A recent study using an ELISA-based assay by a novel IgETRAP protein demonstrated that the serum free IgE level has better predictive value than total IgE level for predicting atopic status and the phenotype of type 2 asthma in adult asthmatics.8 The present study measured serum free IgE level using a novel IgETRAP protein to evaluate its clinical relevance in patients with CSU. The changes in serum free IgE levels were observed after omalizumab treatment.
Materials and methods
Study design and subjects
The present study used 2 cohorts of CSU patients as well as healthy control subjects (HCs) in a real clinical practice setting: one (composing 88 CSU patients and 76 HCs) was enrolled to measure serum free IgE levels in association with clinical parameters and the other included 23 patients with CSU, in which changes in serum free and total IgE levels were compared during the omalizumab treatment. CSU patients were clinically diagnosed as those whose symptoms persisted over 6 weeks without any specific causes. HCs had neither acute nor chronic allergic disease like CSU. Twenty-three patients with CSU who were inadequately controlled with a first-line treatment for CSU (including multiple antihistamines and leukotriene modifiers) and added omalizumab treatment were enrolled in the second cohort. Regarding the dose of omalizumab, all the patients started with 150 mg of omalizumab via the subcutaneous route, and if the patients did not respond after 2 weeks (UAS <50% reduction), additional 150 mg was administered (total 300 mg per month). After 4 weeks, changes in UAS were used to classify responders and non-responders to omalizumab. With applying the cutoff value of 50% UAS compared with baseline, responders (≥50% reduction) and non-responders (<50% reduction) were categorized. Antihistamines were taken if needed. All study subjects were over 18 years old and never exposed to IgE-related biologics. All participants agreed to participate voluntarily in the study and provided written informed consent.
Evaluation of clinical parameters and data collection
The demographic and clinical characteristics of participants were obtained by investigators. Concomitant allergic disease and atopic status were documented. The atopic status was defined by a skin prick test result and/or elevated serum specific IgE level (≥0.35 IU/mL) to at least 1 common environmental allergen including Dermatophagoides pteronyssinus (Der p). The ratio of specific IgE to serum free and total IgE levels were calculated following formula and analyzed. Before analyzing the ratio, the unit of serum free IgE (ng/mL) was converted to kU/L by dividing 2.4.
Autoimmunity, which is a well-known factor in CSU, was evaluated by the presence of serum anti-nuclear antibodies (ANA) or the result of autologous serum skin test (ASST). The subjects who received ASST had an intradermal injection of 50 μL of their own sera on their arm. Wheal and flare responses were measured after 30 min compared with the response of normal saline and histamine as negative and positive controls.9 Thyroid autoimmunity were screened by the history of thyroid disease and thyroid stimulating hormone (TSH) level in all the patients. Thyroid autoantibodies including anti-thyroglobulin (TG) or anti-thyroid peroxidase (TPO) were checked in the patients who had positive ASST results or abnormal TSH levels. Eosinophil cationic protein (ECP) levels in serum were evaluated by the ImmunoCAP® system (ThermoFisher Scientific, Waltham, MA, USA). Other laboratory tests included complete blood counts including eosinophil, basophil, and platelet counts.
The severity of CSU was evaluated on the basis of disease duration, current medication requirements, and urticaria activity score (UAS) modified from UAS6.3 Weekly UAS was measured before and after the omalizumab treatment. The scoring tool for disease activity is based on the degree of pruritus and the number/size/distribution/duration of wheal in each study subject. Patients were classified as follows: 0, symptom-free; 1 to 5, mild urticaria; 6 to 10, moderate urticaria; and 11 to 15, severe urticaria.
Measurement of serum free and total IgE levels
The level of serum free IgE was measured using homemade IgE ELISA by using a novel IgETRAP and compared with clinical parameters. The IgETRAP protein (YH35324, Yuhan, Seoul, South Korea) is a human FcεRIα extracellular domain combined with a human IgG4 Fc domain, which has a high affinity for serum free IgE. IgE ELISA for detecting free IgE were performed as previously described.8 ELISA plates with 0.1μg/well coated with IgETRAP protein was incubated overnight at 4 °C and washed 3 times with 0.05% phosphate-buffered saline (PBS)-Tween. Then, they were blocked with 300 μL of blocking buffer (PBS containing 1% BSA) for 30 min. Sera from each CSU patient or HCs were diluted to 1:10, incubated for 1 h in IgETRAP protein-coated wells at room temperature, and washed 3 times. After that, each well was incubated with antihuman IgE antibody (1:2000; ThermoFisher Scientific) for 1 h and washed 5 times. Finally, it was incubated with HRP-conjugated anti-rabbit IgG secondary antibody (1:2000; Novusbio, Littleton, CO, USA) for 1 h, followed by incubation with TMB substrate (BD Biosciences, Franklin Lakes, NJ, USA) for 10 min. The absorbance value was read by using an ELISA reader (BioTek, Winooski, VT, USA) at 450 nm to obtain the absolute levels of serum free IgE in each serum after stopping the reaction with 2 N H2SO4. Serum total and Der p-specific IgE levels were measured by ImmunoCAPs (ThermoFisher Scientific).
Statistical analysis
For comparative analyses of parametric and non-parametric continuous variables, the t-test and the Mann-Whitney test were used. Hypothetical median values in non-parametric distribution were compared using the Wilcoxon signed-rank test. If the variables had a log-normal distribution, log-transformations were performed. The Wilcoxon matched paired signed-rank test compared paired variables before and after omalizumab treatment. Comparisons among over 2 groups were analyzed by ANOVA, and if standard deviations were not equal, Welch's ANOVA test was used. Bonferroni and Dunnett were applied as post hoc tests, respectively. Bland-Altman analysis was used to compare each method for the serum free IgE and serum total IgE levels. Correlations were analyzed with the Pearson correlation coefficient. Receiver operating characteristic (ROC) curve analysis evaluated the predictive value of serum free and total IgE levels for assessing atopic status in CSU patients, and Der p-specific IgE and its ratio to serum free or total IgE for assessing omalizumab responses. The second model of ROC curve was assessed using Wilcoxon-Mann-Whitney test in order to evaluate the null hypothesis (the AUC is equal to 0.5) and their probability values. All statistical analyses were performed, and graphs were created using R 4.1.0 (R Core Team, 2021), GraphPad Prism Version 9.2.0 (GraphPad Software, San Diego, CA, USA). In all tests, statistical significance was set at P less than 0.05.
Results
Comparison of serum free and total IgE levels between CSU patients and HCs
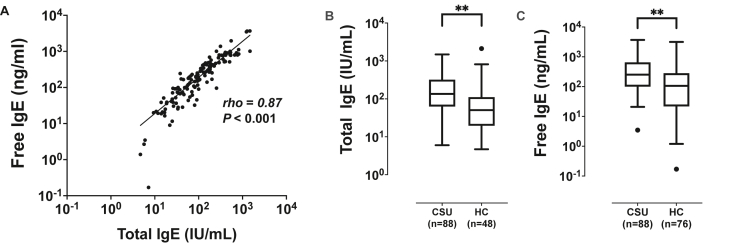
Eighty-eight patients with CSU (CSU group) and 76 HCs (HC group) were enrolled in the first cohort. No differences were found in sex or atopic status between the 2 study groups, except that the control group was found to be older than the CSU group (42.7 ± 12.4 vs. 37.8 ± 12.8 years, P = 0.02). Other demographic characteristics of CSU patients were viewed in Supplementary Table S1. Fig. 1 shows the correlation analysis of serum free and total IgE levels between the 2 groups. A strong positive correlation was noted between serum free and total IgE levels (rho = 0.87, P < 0.001, Fig. 1A). However, Bland-Altman plot showed higher heterogeneity of serum total IgE level at the level of 2, which was logarithmic transformed from 100 IU/mL (Supplementary Fig. S3). The correlation coefficients between serum free and total IgE levels were higher in the high serum IgE group (IgE >100 IU/mL, rho = 0.85, P < 0.001) than in the low serum IgE group (IgE <100 IU/mL, rho = 0.38, P = 0.08). The CSU group showed significantly higher serum free and total IgE levels than the control group (P < 0.0001, respectively, Fig. 1B and C).
Fig. 1.
Free and total IgE levels in sera of the study subjects. (A) Correlation analysis between serum free and total IgE levels. (B, C) Comparisons of serum free and total IgE levels in patients with chronic spontaneous urticaria (CSU) and healthy control subjects (HCs). Values are displayed in log-transformed scales. ∗∗P < 0.0001
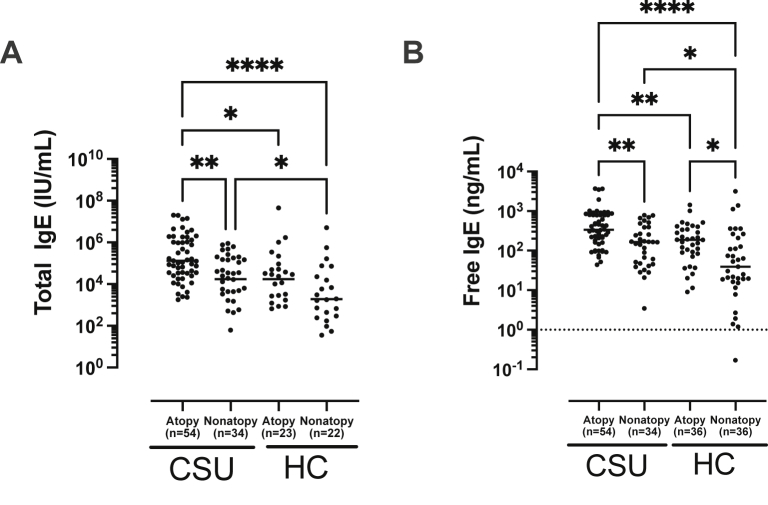
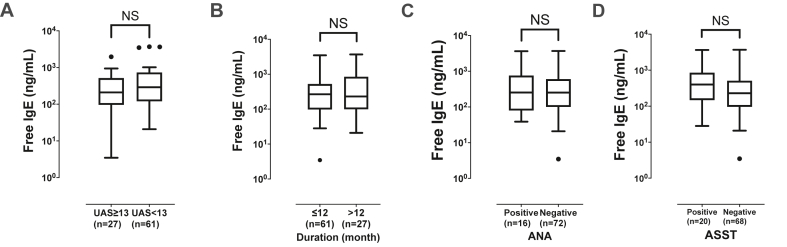
In CSU patients, atopics showed significantly higher serum free and total IgE levels than non-atopics (P < 0.01 for each, Fig. 2A and B). Among HCs, serum free IgE levels are significantly higher in atopics than in non-atopics (P = 0.05), while total IgE levels tended to be higher in atopics than in non-atopics (P = 0.07). In addition, even non-atopic patients in the CSU group had significantly higher serum free and total IgE levels than those in the control group (P < 0.05 for each). The ratio of Der p-specific IgE levels to serum free/total IgE levels were higher in atopic patients with CSU than in non-atopic patients with CSU (the ratio of Der p-specific IgE to serum free IgE, 1.04 [0.18; 5.29] vs. 0.07 [0.02; 0.25]; the ratio of Der p-specific IgE to serum total IgE, 0.87 [0.12; 4.31] vs. 0.06 [0.03; 0.12]; P < 0.001 respectively; data not shown). Among atopic CSU patients, serum free IgE level showed a positive correlation with peripheral basophil counts (rho = 0.32, P = 0.02, Supplementary Fig. S1B). In addition, the predictive value of serum free and total IgE levels for predicting atopic status was evaluated by receiver operating characteristic curves (ROC) analysis. The ROC showed that the values of the area under the curve (AUC) with optimal sensitivity and specificity of serum free and total IgE levels for identifying atopic status in CSU patients were 0.691 and 0.730 with 33.3% vs. 72.7% sensitivity; 64.7% vs. 100.0% specificity without statistically significant differences. The optimal cutoff value of serum free and total IgE levels for ROC analysis were 201.38 ng/mL and 390.50 IU/mL, respectively. When serum free IgE levels were compared according to symptom severity and autoimmunity, no significant associations were noted between serum free IgE levels and UAS, disease duration, TSH levels, the presence of thyroid diseases, and the results of ANA or ASST (P > 0.05, for each) (see Fig. 3). Peripheral basophil counts showed a weak positive correlation with serum free and total IgE levels in the CSU group (free IgE, rho = 0.28, P < 0.01; total IgE, rho = 0.22, P = 0.04, Supplementary Fig. S1A).
Fig. 2.
Comparisons of total (A) and free IgE (B) levels according to atopic status in patients with chronic spontaneous urticaria. Values are displayed in log-transformed scales. P values were evaluated by ANOVA. Asterisks (∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001) indicate results of post hoc test (Bonferroni and Dunnett). CSU, chronic spontaneous urticaria; HCs, healthy control subjects
Fig. 3.
Comparisons of serum free IgE levels according to the urticaria activity score (A), duration of urticaria (B), ANA (C), and ASST (D) positivity. Values are displayed by using Tukey method and transformed in log-scales. UAS, urticaria activity score; ANA, antinuclear antibody; ASST, autologous serum skin test; NS, no significant difference
Changes in serum total and free IgE after the omalizumab treatment
Twenty-three CSU patients who took omalizumab were enrolled in the second cohort. Their baseline characteristics are summarized in Supplementary Table S2. Their mean age was 40.2 ± 9.1 years, females had a higher proportion than males (52.20% vs. 47.80%), and atopic status was predominant than non-atopics (72.7% vs. 27.3%) (Supplementary Table S2). Disease characteristics and laboratory markers were indicated in the Supplementary Table S2. Mean disease duration was 92.7 ± 53.8 months, and involvement with angioedema was observed in a high proportion of the patients (69.6%). The mean levels of serum free and total IgE were 776.6 ± 955.4 ng/mL and 385.5 ± 427.7 kU/L respectively.
Clinical and laboratory findings comparing between responders and non-responders are as shown in the Supplementary Table S3. There were no significant differences in mean age, sex, or clinical/laboratory parameters including basophil counts between responders and non-responders. In addition, no differences were found in serum free or total IgE levels between responders and non-responders.
Serum Der p-specific IgE levels were significantly higher in responders than in non-responders; as shown in the Supplementary Table S3 (P = 0.04), and higher positive rate of serum Der p-specific IgE (≥0.35 kU/L) was shown in responders than in non-responders as in the Supplementary Table S4 (P = 0.02), while no differences were found in urticaria duration, the prevalence of angioedema or results of ANA/ASST between the 2 responder groups. In addition, significantly higher ratio of Der p-specific IgE to serum free IgE and total IgE were found in responders compared to in non-responders (the ratio to free IgE; 0.81 [0.40; 2.31] vs. 0.13 [0.05; 0.38], P = 0.03; the ratio to total IgE 0.49 [0.29; 1.51] vs. 0.08 [0.02; 0.44], P = 0.04).
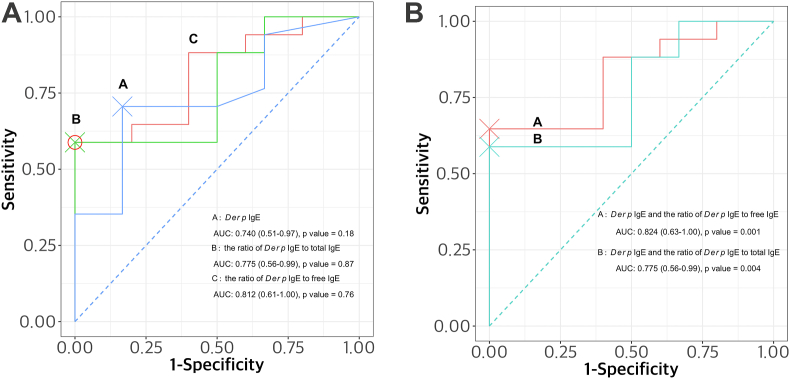
In addition, the predictive value of the ratio of Der p-specific IgE to serum free or total IgE for omalizumab response in CSU patients was evaluated by ROC analysis. The AUC values of Der p-specific serum IgE and its ratio to free IgE and total IgE for identifying favorable responders were 0.740, 0.812, and 0.775, with 70.6%, 58.8%, and 58.8% sensitivity; 83.3%, 100% and 100% specificity with no statistically significances (Fig. 4A). However, when they were combined and analyzed by the Wilcoxon-Mann-Whitney's test, combinations of Der p-specific IgE and its ratio to serum free and total IgE could significantly predict omalizumab responses as shown in Fig. 4B.
Fig. 4.
Two models of receiver operating characteristic curves (ROC) for estimating the predictive values of serum Der p-specific IgE and its ratio to serum free or total IgE levels. The area under the curve (AUC) and 95% CI were shown for each ROC curve. The first model showed of individual AUC values of serum Der p-specific IgE (0.740) and its ratio to free IgE (0.812) or total IgE (0.775) (A), and the second ROC curves of combined values of serum Der p-specific IgE and its ratio to free or total IgE using the Wilcoxon and Mann-Whitney U tests as shown in Fig. 4B. The optimal cutoff points for sensitivity and specificity were indicated as cross and circle on the figures. CSU, Chronic spontaneous urticaria; AUC, area under the curve; ROC, receiver operating characteristic curves; Der p, Dermatophagoides pteronyssinus
Changes in serum free and total IgE levels during omalizumab treatment are shown (Supplementary Fig. S2). The levels of serum total IgE tended to increase 1 month after treatment (P = 0.06), and decline progressively during the twelve-month treatment period, with no significant difference. However, the total IgE level after 6 months shows significantly higher level than the baseline level (P < 0.001; Supplementary Fig. S2A), indicating that serum total IgE level could increase after omalizumab treatment. Serum free IgE levels showed insignificant changes during the twelve-month treatment period (Supplementary Fig. S2B).
Discussion
Mast cells are major effector cells in the pathogenesis of CSU and could be activated by both IgE- and non-IgE-mediated mechanisms. Atopy is noted as a predisposing factor for CSU.10,11 Anti-IgE antibody, omalizumab, which captures circulating serum free IgE, is widely prescribed in the management of severe CSU. After omalizumab treatment, reductions in FcεRI expression on mast cells and basophils were observed, indicating that IgE-mediated mast cell activation is the major mechanism to induce CSU.12, 13, 14, 15 There have been several studies suggesting that serum free and total IgE levels may be potential serum biomarkers for predicting or monitoring omalizumab treatment in adult asthmatic patients.5,8,16 A recent study demonstrated that serum free IgE level, as measured using novel IgETRAP protein, is a reliable predictive biomarker for type 2 airway inflammation in adult asthmatics.8 The present study demonstrated significantly higher serum free IgE levels in CSU patients compared to HCs, with applying the same detection method. Moreover, among CSU patients, atopics showed significantly higher serum free IgE levels than non-atopics. Even among non-atopics, higher serum free IgE levels were noted in CSU patients than in HCs, indicating that serum free IgE levels may contribute to the pathogenesis of CSU, regardless of atopic status. Furthermore, responders to omalizumab had higher serum Der p-specific IgE and its ratio to serum free IgE, indicating that sensitization to house dust mite may be a predisposing factor for IgE-mediated mast cell activation in CSU patients. Serum free IgE level may be a potential serum biomarker for atopic status, contributing to the pathogenesis of CSU.
IgE, binding with a high-affinity IgE receptor (FcεRI) expressed on skin mast cells and basophils, regulates mast cell activation, and releases histamine, kinins, lipid mediators and cytokines, which contributes to the pathogenesis and severity of CSU.17,18 Higher serum total IgE levels were reported in CSU patients, especially in atopics,19,20 which is comparable to the results of the present study. Although there have been a few studies suggesting significant association between high serum total IgE levels and disease duration or severity of CSU,5, 6, 7,21 the present study showed no significant associations between the serum total IgE level and urticaria-related clinical parameters including disease duration, angioedema or UAS. In addition, previous studies demonstrated that serum total IgE levels increased after omalizumab treatment,16,22 which are comparable to our finding (significantly higher serum total IgE level after 3 months of treatment). Serum total IgE levels measured by current available methods could not represent serum free IgE levels.23, 24, 25, 26 In the present study, Bland-Altman analysis (comparing between serum free and total IgE levels) showed higher diversity in patients with low serum IgE levels than in those with high serum IgE levels, suggesting that patients with low serum IgE (<100 IU/mL) may present higher serum total IgE levels than serum free IgE levels as shown in Supplementary Fig. S3). Taken together, serum total IgE levels may not be a useful serum biomarker representing clinical severity or predicting omalizumab responses in CSU patients. Alternative methods for the measurement of serum free IgE levels are required in the management of CSU.8,18,26,27 Although a Japanese study has demonstrated that serum free IgE levels decreased after omalizumab treatment with clinical responses in patients with severe allergic asthma,28 that detection method has not been replicated in other cohorts of asthma or CSU as far as we know. Recently, a new method was reported for detecting serum free IgE in adult asthmatics using type 2 markers.8 The present study is the first one demonstrating high serum free IgE levels in the sera of CSU patients (especially in atopics). The detection method is reproducible and easy to set up; therefore, it can be applicable in clinical practice for better management of CSU.
Omalizumab is approved for use in allergic asthma and CSU refractory to conventional treatment. The doses of omalizumab in allergic asthma are determined on the basis of serum total IgE level and body weight, while a fixed dose is recommended in CSU.29,30 These differences may be attributed to the fact that both IgE- and non-IgE-mediated mechanisms (including autoimmunity) are involved in mast cell activation in CSU. It is suggested that circulating autoreactive IgE antibodies to self-antigens (autoallergy), and IgG/M/A autoantibodies to FcεRIα contribute to autoimmunity (up to 40%) of CSU,31, 32, 33 which are not currently applicable in clinical practice. Autoimmune-related CSU presents more severe disease activity and poor responses to omalizumab.34 Conventional methods to evaluate autoimmunity for CSU include measurement of serum ANA and ASST in real practice. The presence of ANA may be related to poor response to omalizumab.35 A higher rate of positive ASST was associated with serum autoreactivity and poor responses to omalizumab.34 ASST has been suggested to examine autoantibodies including IgG against FcεRIα or against IgE, and adopted as the gold-standard test to define autoimmune status in CSU patients.9,36, 37, 38 However, there have been conflicting comments on the relationship between ASST positivity and disease severity.9 Patients with positive ASST had more severe urticaria, more prolonged duration, and more frequent attacks of urticaria and gastrointestinal symptoms than negative ASST patients.39 On the other hand, there was no significant difference between ASST-positive and ASST-negative patients with CSU regarding total duration and frequency of disease.40 A recent study in Korea reported that the predicted positivity of ASST decreased with increasing of serum total IgE level and disease duration with a bimodal distribution of two peaks for twenties and sixties.41 Another result based on the multicenter, multinational study of CSU reported that low serum total IgE levels were associated with autoimmune CSU of which diagnosis was assisted by using ASST results.42 The present study showed no association between autoimmunity and responses to omalizumab, suggesting that presence of ANA or the positivity to ASST may not be a useful marker for predicting unfavorable responses to omalizumab in real-world practice. Altogether, we speculated that ASST-positivity is not solely linked to autoimmunity, but higher serum free IgE levels have a few associations with this autoreactivity, therefore, ASST tests may have a limited role in CSU with high serum free IgE levels, reflecting atopic status.
Basophil, a key effector cell in CSU, is considered a marker for disease activity. FcεRI expressed on the surfaces of basophils are involved in IgE-mediated activation and degranulation. When activated, basophils are recruited from blood vessels to skin lesions and induce basopenia, which is related to disease severity.43 The present study demonstrated weak positive correlations of serum free and total IgE levels with basophil counts in patients with CSU. Further investigations are needed to evaluate the relationship between free IgE level and basophils.
There have been a few studies showing progressive decline in serum free IgE levels and FcεRIα expressions on mast cells after omalizumab treatment,13,27 suggesting that serum free IgE level may be a potential marker predicting or monitoring the response to omalizumab. In the present study, when serum free and total IgE levels were monitored during 12 months of omalizumab treatment, the total IgE level increased after 1 month, and then progressively tended to decrease during the treatment period, which is consistent with the findings of the previous studies.5,16,44,45 However, serum free IgE levels showed variable responses with no statistical significances.
Serum free IgE has shorter half-life by only 2-3 days than other forms which bound to the high-affinity receptor FcεRI on mast cells or basophils for several weeks.46 Due to its shorter half-life and higher affinity for receptors, most IgE antibodies exist as bound forms attached to cells, and a low proportion of IgE antibodies exist free in the plasma.47 Omalizumab mainly binds to serum free IgE and develops the IgG-IgE complex, preventing serum IgE from binding to FcεRI and CD23.48 Consequently, a vacant part of FcεRI on the cell surface leads to internalization and degradation, which results in decreased FcεRI levels on mast cells and basophils.13,14 Moreover, omalizumab has a half-life of 26 days and slow clearance rate, which makes omalizumab persist in serum longer than serum free IgE. In the present study, we used omalizumab at variable doses ranging from 150 to 300 mg. Further investigations are needed to clarify the clinical relevance of serial changes in serum free and total IgE levels after omalizumab treatment in a lager cohort of CSU patients.
Serum IgE levels have been reported in CSU as biomarkers for predicting responses to omalizumab treatment in CSU patients.49,50 In a recent systematic review, low serum total IgE level was suggested to predict decreased response to omalizumab.49 Lower serum total IgE levels may predict the reduced efficacy of omalizumab and the shorter time to relapse after discontinuation in CSU patients.22,51 The assessment of pre- and post-treatment IgE levels and their ratios were helpful in classifying patients with CSU who are suitable for omalizumab treatment.16 The present study demonstrated higher serum free and total IgE levels in responders than in non-responders, but did not showed a significant difference. Instead, responders, as evaluated at 3 months (early responses), had higher serum Der p-specific IgE levels (representing atopic status) and the higher ratio of Der p-specific IgE to serum free IgE levels than non-responders. The higher ratio of specific IgE to serum IgE might indicates the higher levels of allergen-specific IgE antibodies on the effector cells (including mast cells and basophils), and the higher proportion of attachment to the effector cells in CSU.52 Considering that Der p-specific IgE is a major inhalant causative allergen in this country, high serum Der p-specific IgE indicates high atopic status, suggesting that CSU patients at high atopic status may present more favorable responses to omalizumab.
There are some limitations to the present study. First, this is not a controlled trial of omalizumab treatment in CSU patients, because the study subjects used concurrent medications such as antihistamines and leukotriene modifiers, and the doses of omalizumab were not consistent. Secondly, the number of the study subjects is not large enough to validate the predictive function of allergen-specific IgE or the ratio of allergen-specific IgE to serum free IgE, and serial changes in serum free IgE levels during the omalizumab treatment. Thirdly, functional tests for mast cell activation and autoimmunity, such as basophil activation test or circulating IgG/IgE to FcεRI, were not performed. Despite these limitations, this is a validation study on serum free IgE levels, the ratio of allergen-specific IgE to serum free IgE levels, and serial changes in serum free IgE levels in CSU patients in response to omalizumab treatment in a real clinical setting.
In conclusion, increased serum free IgE is involved in the development of CSU by activating mast cells, especially in atopic patients. High Der p-specific IgE level and the ratio of Der p-specific IgE to serum free IgE may be a potential biomarker for predicting favorable responses to omalizumab.
Funding
This study was supported by a grant of the Korea Health Technology R&D Project (HR16C0001) through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author contributions
JH Jang has coordinated the study, collected patient data, was involved in statistical analysis, and drafted the manuscript. EM Yang, JY Moon and MS Ryu performed laboratory tests and was involved in the proof-reading of the manuscript. YS Lee and YM Ye were involved in manuscript preparation and proof-reading of the manuscript. HS Park was the overall study coordinator and was involved in manuscript preparation and the proof-reading of the manuscript.
Authors’ consent for publication
All the authors reviewed the final draft and provided consent for publication.
Ethics approval
This study was approved by the Institutional Review Board of Ajou University Hospital. (AJIRB-BMR-KSP-20-435, AJIRB-MED-OBS-11-300).
Declaration of competing interest
There are no financial or other issues that might lead to conflict of interest.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100629.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Song W.J., Choi M., Lee D.H., et al. The KAAACI/KDA evidence-based practice guidelines for chronic spontaneous urticaria in Korean adults and children: Part 1. Definition, methodology and first-line management. Allergy Asthma Immunol Res. 2020;12(4):563–578. doi: 10.4168/aair.2020.12.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi J.-H., Lee D.H., Song W.-J., et al. The KAAACI/KDA evidence-based practice guidelines for chronic spontaneous urticaria in Korean adults and children: Part 2. Management of H1-antihistamine-refractory chronic urticaria. Allergy Asthma Immunol Res. 2020;12(5):750–770. doi: 10.4168/aair.2020.12.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuberbier T., Aberer W., Asero R., et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 4.Sutton B.J., Davies A.M., Bax H.J., Karagiannis S.N. IgE antibodies: from structure to function and clinical translation. Antibodies. 2019;8(1):19. doi: 10.3390/antib8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altrichter S., Fok J.S., Jiao Q., et al. Total IgE as a marker for chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2021;13(2):206–218. doi: 10.4168/aair.2021.13.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gergen P.J., Arbes S.J., Calatroni A., Mitchell H.E., Zeldin D.C. Total IgE levels and asthma prevalence in the US population: results from the national Health and nutrition examination survey 2005-2006. J Allergy Clin Immunol. 2009;124(3):447–453. doi: 10.1016/j.jaci.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek Y.S., Jeon J., Kim J.H., Oh C.H. Severity of acute and chronic urticaria correlates with D-dimer level, but not C-reactive protein or total IgE. Clin Exp Dermatol. 2014;39(7):795–800. doi: 10.1111/ced.12413. [DOI] [PubMed] [Google Scholar]
- 8.Woo S.D., Yang E.M., Jang J., et al. Serum-free immunoglobulin E: a useful biomarker of atopy and type 2 asthma in adults with asthma. Ann Allergy Asthma Immunol. 2021;127(1):109–115 e101. doi: 10.1016/j.anai.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinou G.N., Asero R., Maurer M., Sabroe R.A., Schmid-Grendelmeier P., Grattan C.E.H. EAACI/GA2LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009;64(9):1256–1268. doi: 10.1111/j.1398-9995.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 10.Ban G.-Y., Kim M.-Y., Yoo H.-S., et al. Clinical features of elderly chronic urticaria. Korean J Intern Med (Engl Ed) 2014;29(6):800–806. doi: 10.3904/kjim.2014.29.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asero R., Ferrucci S., Casazza G., Marzano A.V., Cugno M. Total IgE and atopic status in patients with severe chronic spontaneous urticaria unresponsive to omalizumab treatment. Allergy. 2019;74(8):1561–1563. doi: 10.1111/all.13754. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan A.P., Giménez-Arnau A.M., Saini S.S. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72(4):519–533. doi: 10.1111/all.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck L.A., Marcotte G.V., MacGlashan D., Togias A., Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J Allergy Clin Immunol. 2004;114(3):527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Saini S.S., MacGlashan D.W., Sterbinsky S.A., et al. Down-regulation of human basophil IgE and FCεRIα surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162(9):5624. [PubMed] [Google Scholar]
- 15.Logsdon S.L., Oettgen H.C. IgE antibodies: generation and function. Curr Top Microbiol. 2015;388:39–61. doi: 10.1007/978-3-319-13725-4_3. [DOI] [PubMed] [Google Scholar]
- 16.Ertas R., Ozyurt K., Atasoy M., Hawro T., Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy. 2018;73(3):705–712. doi: 10.1111/all.13345. [DOI] [PubMed] [Google Scholar]
- 17.Migalovich-Sheikhet H., Friedman S., Mankuta D., Levi-Schaffer F. Novel identified receptors on mast cells. Front Immunol. 2012;3:238. doi: 10.3389/fimmu.2012.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redegeld F.A., Yu Y., Kumari S., Charles N., Blank U. Non-IgE mediated mast cell activation. Immunol Rev. 2018;282(1) doi: 10.1111/imr.12629. [DOI] [PubMed] [Google Scholar]
- 19.Nassif A. Is chronic urticaria an atopic condition? Eur J Dermatol. 2007;17(6):545–546. doi: 10.1684/ejd.2007.0279. [DOI] [PubMed] [Google Scholar]
- 20.Montjoye Ld, Darrigade A.S., Arnau A.G., Herman A., Dumoutier L., Baeck M. Correlations between disease activity, autoimmunity and biological parameters in patients with chronic spontaneous urticaria. European Ann Allergy Clin Immunol. 2021;53(2):55. doi: 10.23822/EurAnnACI.1764-1489.132. [DOI] [PubMed] [Google Scholar]
- 21.Kessel A., Helou W., Bamberger E., et al. Elevated serum total IgE--a potential marker for severe chronic urticaria. Int Arch Allergy Immunol. 2010;153(3):288–293. doi: 10.1159/000314370. [DOI] [PubMed] [Google Scholar]
- 22.Ertas R., Ozyurt K., Ozlu E., et al. Increased IgE levels are linked to faster relapse in patients with omalizumab-discontinued chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;140(6):1749–1751. doi: 10.1016/j.jaci.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Busse W., Corren J., Lanier B.Q., et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson M., Thorell L., Sjölander A., Larsson-Faria S. Variability of total and free IgE levels and IgE receptor expression in allergic subjects in and out of pollen season. Scand J Immunol. 2015;81(4):240–248. doi: 10.1111/sji.12270. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton R.G., Marcotte G.V., Saini S.S. Immunological methods for quantifying free and total serum IgE levels in allergy patients receiving Omalizumab (Xolair) therapy. J Immunol Methods. 2005;303(1-2):81–91. doi: 10.1016/j.jim.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Qiu C., Zhong L., Huang C., et al. Cell-bound IgE and plasma IgE as a combined clinical diagnostic indicator for allergic patients. Sci Rep-uk. 2020;10(1):4700. doi: 10.1038/s41598-020-61455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gon Y., Ito R., Maruoka S., et al. Long-term course of serum total and free IgE levels in severe asthma patients treated with omalizumab. Allergol Int. 2018;67(2):283–285. doi: 10.1016/j.alit.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Tajiri T., Matsumoto H., Gon Y., et al. Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy. 2016;71(10):1472–1479. doi: 10.1111/all.12922. [DOI] [PubMed] [Google Scholar]
- 29.FDA U . 2014. Xolair (Omalizumab): US Prescribing Information; 2003. [Google Scholar]
- 30.Maurer M., Rosén K., Hsieh H.-J., et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 31.Bracken S.J., Abraham S., MacLeod A.S. Autoimmune theories of chronic spontaneous urticaria. Front Immunol. 2019;10:627. doi: 10.3389/fimmu.2019.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolkhir P., Church M.K., Weller K., Metz M., Schmetzer O., Maurer M. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139(6):1772–1781. doi: 10.1016/j.jaci.2016.08.050. e1771. [DOI] [PubMed] [Google Scholar]
- 33.Altrichter S., Zampeli V., Ellrich A., Zhang K., Church M.K., Maurer M. IgM and IgA in addition to IgG autoantibodies against FcϵRIα are frequent and associated with disease markers of chronic spontaneous urticaria. Allergy. 2020;75(12):3208–3215. doi: 10.1111/all.14412. [DOI] [PubMed] [Google Scholar]
- 34.Gericke J., Metz M., Ohanyan T., et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;139(3):1059–1061. doi: 10.1016/j.jaci.2016.07.047. e1051. [DOI] [PubMed] [Google Scholar]
- 35.Ertaş R., Hawro T., Altrichter S., et al. Antinuclear antibodies are common and linked to poor response to omalizumab treatment in patients with CSU. Allergy. 2019;75(2):468–470. doi: 10.1111/all.14033. [DOI] [PubMed] [Google Scholar]
- 36.Kolkhir P., Altrichter S., Asero R., et al. Autoimmune diseases are linked to type IIb autoimmune chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2021;13(4):545–559. doi: 10.4168/aair.2021.13.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konstantinou G.N., Asero R., Ferrer M., et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68(1):27–36. doi: 10.1111/all.12056. [DOI] [PubMed] [Google Scholar]
- 38.Zuberbier T., Latiff A.H.A., Abuzakouk M., et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2021 doi: 10.1111/all.15090. [DOI] [Google Scholar]
- 39.Patel S.G., Joshi R.R., Patel R.M. Autologous serum skin test in 250 patients of chronic spontaneous urticaria at tertiary care hospital in Gujarat. Int J Res Dermatology. 2019:466–470. [Google Scholar]
- 40.Vikramkumar A.G., Kuruvila S., Ganguly S. Autologous serum skin test as an indicator of chronic autoimmune urticaria in a tertiary care hospital in South India. Indian Dermatology Online J. 2014;5(Suppl 2):S87–S91. doi: 10.4103/2229-5178.146166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park G.-H., Choi J.-H., Kim S., Bae Y. The relation of autologous serum skin test and autologous plasma skin test result with various clinical and laboratory findings in patients with chronic spontaneous urticaria. Ann Dermatol. 2020;32(4):280–288. doi: 10.5021/ad.2020.32.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolkhir P., Kovalkova E., Chernov A., et al. Autoimmune chronic spontaneous urticaria detection with IgG anti-TPO and total IgE. J Allergy Clin Immunol Pract. 2021;9(11):4138–4146. doi: 10.1016/j.jaip.2021.07.043. [DOI] [PubMed] [Google Scholar]
- 43.Johal K.J., Chichester K.L., Oliver E.T., et al. The efficacy of omalizumab treatment in chronic spontaneous urticaria is associated with basophil phenotypes. J Allergy Clin Immunol. 2021;147(6):2271–2280. doi: 10.1016/j.jaci.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turkey DoPA, Clinical Immunology DoPAMUSoMA. Uysal P., Erge D. Effective treatment of different H1-antihistamine-refractory chronic urticaria phenotypes with omalizumab. Turkish Archives Pediatrics. 2019;53(4):250–254. doi: 10.5152/TurkPediatriArs.2016.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asero R. Are currently available biomarkers useful to discriminate CSU patients not controlled by low dose omalizumab maintenance therapy? European Ann Allergy Clin Immunol. 2020;52(6):268. doi: 10.23822/EurAnnACI.1764-1489.139. [DOI] [PubMed] [Google Scholar]
- 46.Normansell R., Walker S., Milan S.J., Walters E.H., Nair P. Omalizumab for asthma in adults and children. Cochrane Db Syst Rev. 2014;1 doi: 10.1002/14651858.CD003559.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laffer S., Lupinek C., Rauter I., et al. A high-affinity monoclonal anti-IgE antibody for depletion of IgE and IgE-bearing cells. Allergy. 2008;63(6):695–702. doi: 10.1111/j.1398-9995.2008.01664.x. [DOI] [PubMed] [Google Scholar]
- 48.Lowe P.J., Tannenbaum S., Gautier A., Jimenez P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. Br J Clin Pharmacol. 2009;68(1):61–76. doi: 10.1111/j.1365-2125.2009.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fok J.S., Kolkhir P., Church M.K., Maurer M. Predictors of treatment response in chronic spontaneous urticaria. Allergy. 2021;76(10):2965–2981. doi: 10.1111/all.14757. [DOI] [PubMed] [Google Scholar]
- 50.Asero R., Marzano A.V., Cugno M. Unresponsiveness to omalizumab in chronic spontaneous urticaria. Curr Treat Options Allergy. 2020;7(2):135–141. [Google Scholar]
- 51.Folci M., Heffler E., Canonica G.W., Furlan R., Brunetta E. Cutting edge: biomarkers for chronic spontaneous urticaria. J Immunol Res. 2018;2018:1–12. doi: 10.1155/2018/5615109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lorenzo G.D., Mansueto P., Pacor M.L., et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;123(5):1103–1110. doi: 10.1016/j.jaci.2009.02.012. e1104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.