Abstract
Breast cancer is highly heterogenous with temporal and spatial heterogeneity making it necessary for rebiopsy. DS-8201a, a new potential therapy for human epidermal growth factor receptor 2 (HER2) low expression breast cancer, had been proved that it could overcome heterogenous HER2 expression in a preclinical setting. In January 2014, a 23-year-old woman was presented with a lump in the right breast with bone metastasis, diagnosed as infiltrating ductal carcinoma, estrogen receptor (ER)+, progesterone receptor (PR)+, HER2 immunohistochemistry (IHC) 2+, and fluorescence in situ hybridization negative. The patient received a series of therapies including surgery, radiotherapy, endocrine therapy, target therapy, and chemotherapy. The longest progression-free survival was 17 months after surgery. Biopsy of liver metastasis in February 2020 showed triple negative (HER2−, ER−, PR−), which was quite different from the initial diagnosis in 2014, so retesting was performed and the results showed ER−, PR+ by 10%, HER2 IHC score of 1+, indicating heterogeneity of HER2 expression. In May 2020, DS-8201a treatment was initiated and continued for 10 cycles until November 2020. Remarkable relief in symptoms was observed after the first dose. A reduction in the metastatic lesion size (liver and brain) and improved liver function was observed during the therapy. This case indicated the heterogeneity of breast cancer, and impressive efficacy of DS-8201a in a heavily treated patient with HER2-low and HER2 heterogeneity.
Keywords: DS-8201a, heterogeneity, HER2-low expression, metastatic breast cancer
Introduction
Breast cancer (BC) is a heterogenous disorder and most commonly diagnosed cancer in women. 1 The overexpression or amplification of human epidermal growth factor receptor 2 (HER2) is observed in 15% to 20% of BCs. 2 Human epidermal growth factor receptor 2 protein expression is assessed by immunohistochemistry (IHC; measures receptors) and/or in situ hybridization (ISH; counts the number of copies of HER2 gene). An IHC score of 3+ or gene amplification on at least 1 tumor sample is considered HER2 positive, while IHC score of 1+ or 2+ with negative ISH assay is defined as HER2-low expression. 3 However, the prevailing HER2 testing guidelines consider these patients as HER2 negative.4,5 Over the past 2 decades, due to the increased availability of targeted therapies, a significant improvement in clinical outcomes has been achieved in patients with HER2-positive BC.6,7 The currently available HER2-targeted therapies have not proven effective in treatment of HER2-low expression BC and are not recommended. 8 Also, the heterogeneity of BC may increase tumor’s ability to adapt to constantly changing constraints challenging treatment diagnosis and therapies. In 2018, heterogeneity and an emerging drug resistance and metastasis formed as a consequence of heterogeneity has led to mortality of 620 000 women. 9
DS-8201a is a novel HER2-targeted monoclonal antibody and drug conjugate (ADC). 10 Modi et al 4 reported promising results with DS-8201a in treating HER2-low expression BC and controlling the disease progression. The effectiveness of DS-8201a in tumors with heterogeneity was reported by Ogitani et al 11 in a preclinical setting. Here, we present a case of a 23-year-old woman diagnosed with HER2-low and heterogenous expression metastatic breast cancer (mBC), who despite being previously heavily treated had brain and liver metastasis and she achieved improved symptoms and reduced metastasis with DS-8201a.
Case Presentation
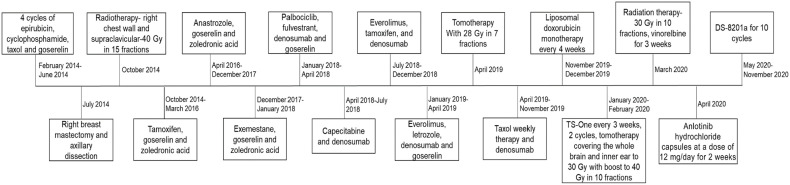
In January 2014, a 23-year-old woman was presented with a lump in the right breast measuring 10 cm. From core biopsy, the patient was found to have infiltrating ductal carcinoma grade 3, estrogen receptor (ER) positive, progesterone receptor (PR) negative, HER2 IHC score 2+, and fluorescence ISH negative. Gene test results showed BRCA1, BRCA2, and TP53 as negative that indicated no germline mutations. Bone scan and spine magnetic resonance imaging (MRI) showed metastasis at thoracic vertebra 10. The patient was initiated on chemotherapy with 4 cycles of epirubicin and cyclophosphamide followed by taxol and endocrine therapy with goserelin followed by right mastectomy of tumor (ypT2NM1) and axillary lymph node dissection. Post-surgery, 17 months of progression-free survival (PFS) was achieved with radiotherapy and endocrine therapy. Then, the disease progressed with bone metastases, for which, a series of therapies including CDK 4/6 inhibitor, endocrine therapy, target therapy, chemotherapy, and radiotherapy was given but the disease kept progressing. In November 2019, the metastasis was observed to progress to liver, lymph node, and small nasopharynx other than bones. Figure 1 represents the treatment regimens followed throughout the study.
Figure 1.
Treatment regimens followed throughout the case study.
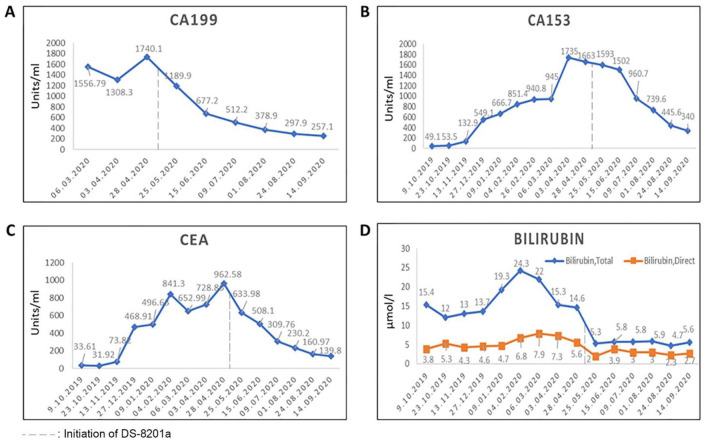
In January 2020, the patient was presented with symptoms of left ear nearly deaf and then sickness and dizziness in the following days. Increased tumor markers, CA153: 666.7 units/mL and CEA: 496.6 units/mL, and multiple brain metastases in MRI scan were observed which was followed by chemotherapy and tomotherapy was employed. Liver biopsy of metastatic carcinoma showed triple negative (HER2−, ER−, PR−), programmed death ligand-1 (PDL1) negative, while FoundationOne CDx in February 2020 showed amplification in fibroblast growth factor gene. In March 2020, MRI showed multiple metastatic nodules in liver, cervical, thoracolumbar, sacral, bilateral iliac bones, brain, and spinal cord; hence, radiation therapy was applied to the lower back 30 Gy in 10 fractions, after initiating oral vinorelbine therapy
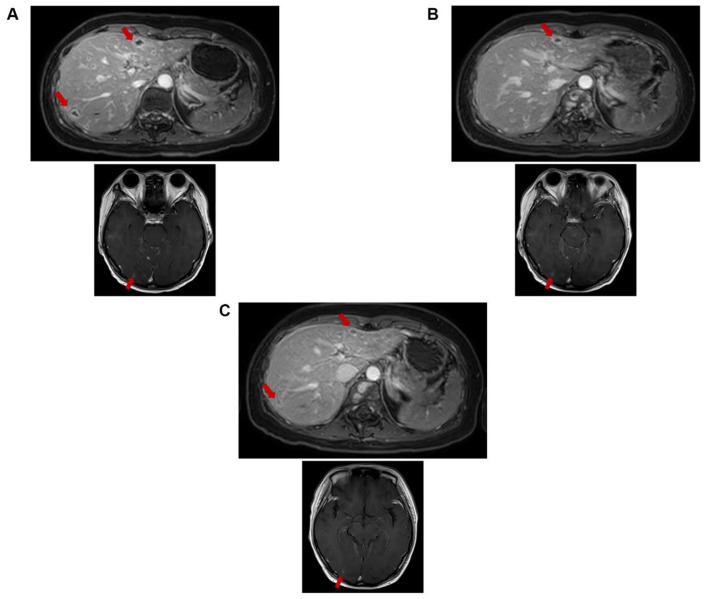
As both hormone and HER2 receptor results of liver biopsy were different from the primary sample results of 2014, the tissue collected from liver in February 2020 was retested in March 2020. The results showed ER negative, PR positive by10%, and HER2 IHC score of 1+ indicating the heterogeneity in the same sample. The CA153, CEA, and CA199 levels were 1735, 728.86, and 1308.3 units/mL, respectively, by March 2020. In April 2020, treatment with anlotinib hydrochloride capsules was initiated. On May 4, 2020, an increase in the tumor marker levels and abnormal liver function was observed with total and direct bilirubin levels of 14.6 and 5.6 µmol/L, respectively. Under multidisciplinary team (MDT) discussion and then a talk with the patient, DS-8201a was started with 1 cycle treatment by May 6, 2020. Specific symptoms such as pain disappeared; hence, pain killers were stopped and the patient was able to get up from bed and walk, 1 week after the first cycle of DS-8201a. Hence, continued with second, third, fourth, fifth, and sixth cycle of DS-8201a until May 27, June 24, July 13, August 3, and August 24, 2020, respectively. Compared with the MRI image of May 6, 2020, at the first cycle (Figure 2A), the MRI after the sixth cycle showed reduced size in lesions of intracranial (the largest one is located in the right occipital lobe, 8-6 mm), meninges, cervical thoracolumbar vertebral body spinal membrane, spinal cord, and liver multiple metastases (the largest one is located at S3, 12-9 mm) and the intrahepatic metastases were reduced and shrunk compared with MRI on June 17, 2020 (Figure 2B and C). The levels of tumor markers reduced with DS-8201a, CA153 from 1735 to 340 units/mL, CEA from 962.58 to 139.8 units/mL, and CA199 from 1740 to 257.1 units/mL (Figure 3). The liver function also improved with total bilirubin of 5.6 µmol/L and direct bilirubin of 2.7 µmol/L. Patient reported mild adverse events (grade 1 vomiting and fatigue) with DS-8201a treatment.
Figure 2.
MRI images of brain and liver. (A) MRI on May 6, 2020 (before DS8201 treatment). (B) MRI on June 17, 2020 (after 2 cycles). (C) MRI on August 3, 2020 (after 4 cycles). MRI indicates magnetic resonance imaging.
Figure 3.
Variation in tumor markers and bilirubin. (A) CA199. (B) CA153. (C) CEA. (D) Bilirubin.
After the seventh cycle of DS-8201a, the patient was presented with dizziness. Magnetic resonance imaging of September 28, 2020, revealed worsened leptomeningeal metastasis with continued shrinkage of cerebral parenchymal metastases. Under MDT discussion, radiotherapy was not suitable because whole-brain radiotherapy was conducted before. Hence, DS-8201a was continued as suggested, along with endocrine and symptomatic treatment. However, the patient’s situation worsened and treatment with DS-8201a was stopped after the 10th cycle on November 19, 2020. Subsequently, most of the treatment focused on supportive and symptomatic management. Multiple novel brain and leptomeningeal metastases were found by MRI on December 11, and the patient died in April 2021. The treatment duration of DS-8201a was 6.4 months, and overall survival since DS-8201a started was about 11 months.
Discussion
Human epidermal growth factor receptor 2 gene amplification/protein expression is considered an independent prognostic factor and a predictor of overall survival and time to disease relapse. 12 Around 40% to 50% of patients with BC have tumors with HER2-low expression. Furthermore, several studies substantiated that HER2 genetic heterogeneity occurs in BC at variable frequencies (1%-34%) and is associated with worse patient outcomes in terms of short overall survival and disease-free survival. 11 Therefore, the clinical impact of HER2 heterogeneity is currently under discussion. Moreover, medical oncologists applied a binary treatment decision-making when considering HER2, where HER2-positive patients defined by HER2 amplification or overexpression should be offered anti-HER2 agents.4,11 However, effectiveness of many drugs, such as monoclonal antibodies (trastuzumab, 8 pertuzumab, 13 and margetuximab) 14 and trastuzumab emtansine (T-DM1) explored in HER2-low expression BC, is not satisfactory. Furthermore, a randomized clinical trial reported no role of trastuzumab in treating this population. 8 This study reported a unique case of a 23-year-old mBC patient with high HER2 heterogeneity and low expression, and continued disease progression despite treatment with endocrine and chemotherapy regimens but exhibited an impressive efficacy with DS-8201a.
DS-8201a was approved by the US Food and Drug Administration in December 2019 for treatment of patients with unresectable or metastatic HER2-positive BC pretreated with 2 or more anti-HER2-based regimens in the metastatic setting. 14 This accelerated approval was based on the results of a phase 2 study DESTINY-Breast01, in which majority of the patients included in the trial had been heavily treated, where the median prior treatment line for metastatic disease was 7 (range, 2-27). However, DS-8201a showed remarkable efficacy with primary end point objective response rate (ORR) of 60.9% and median response duration of 14.8 months (95% confidence interval: 13.8, 16.9). 10 Furthermore, in a phase I trial, DS-8201a was studied in 54 patients with advanced/unresectable or metastatic HER2-low expression BC. The results indicated promising antitumor activity with an ORR of 37%, median duration of response of 10.4 months, a disease control rate of 87%, a median PFS of 11.1 months and a median overall survival of 29.4 months, and manageable safety profile in HER2-low expression patients including hormone receptor (HR) negative (–) and HER2 IHC score 1+ BC. 4 In this case, DS-8201a showed a faster onset of action with improved symptoms within a week of treatment and the patient was able to walk with mild adverse events reported. Reduced lesion size and tumor markers was also observed with DS-8201a, reaffirming the results of phase I clinical study. In this case, DS-8201a was given to the patient after too many lines of therapy. Contextually, a post hoc analysis of the DESTINY-Breast01 trial demonstrated better efficacy of DS-8201a when administered during earlier lines of therapy. 15 Thus, earlier use of DS-8201a may potentially result in even better outcomes.
Unlike monoclonal antibodies that act by direct inhibition of HER2 signaling, ADCs use the HER2 protein primarily as a means for delivery of the payload to the target cells. Compared with the previous HER2 ADC trastuzumab emtansine (T-DM1), DS-8201a has several characteristics that contribute to its remarkable efficacy, including the highly potent novel topoisomerase I inhibitor payload DXd, much higher drug-to-antibody ratio (DAR) compared with T-DM1 (8 vs 3.5), good homogeneity, and tumor selective cleavable peptide linker. 16 Unlike T-DM1, DS-8201a is internalized in HER2 expression cells upon reaching the tumor tissue, where the linker is cleaved off by the lysosomal cathepsin enzymes which are generally overexpressed in tumor cells. Consequently, the membrane-soluble payload DXd is released and penetrated by the neighboring cells, regardless of their HER2 status. This effect is considered as bystander effect, which combined with high DAR and high potency payload makes DS-8201a unique from other ADCs such as T-DM1 and may be responsible for antitumor activity of DS-8201a in HER2-low and HER2 heterogenous expression BC.8,11,17,18
Furthermore, HER2 protein overexpression could be promoted by several factors such as endocrine therapy-induced modifications, chemotherapy, and radiotherapy. 3 Hence, the patient was retested for HER2 expression in this report. Differential expression of HER2 was detected during the treatment of the present patient, and the results indicated that the tumor has temporal and spatial heterogeneity. In this case, DS-8201a was found effective even with a negative HER2 gene test but with low positive IHC protein expression, which was in accordance with the previous study by Modi et al. The study reported similar effects of DS-8201a in patients with HER2 IHC score 1+ BC and HER2 IHC score 2+ BC. 4 To the best of our knowledge, this is the first report of a Chinese patient to report effectiveness of DS-8201a in mBC with HER2 low expression and heterogeneity. Therefore, the category of HER2-low BC may constitute a subset of carcinomas with different degrees of HER2 expression and may call into question the need of a paradigm shift in the definition of HER2 status in BC, which in the next future could be based on a 3-tier system featuring HER2-positive, HER2-negative, and HER2-low BC, and taking heterogeneity into consideration, repetitive laboratory tests do assure the accurate diagnosis and selection of therapies.
In addition, in this case, the patient’s intracranial lesions were effectively controlled with DS-8201a for several months, and this curative effect of brain metastases can be considered for HER2+ phase II study. Presently, 2 phase III trials DESTINY-Breast04 and U303DESTINY-Breast06 on DS-8201a involving patients with HER2-low BC are ongoing. 19 A phase Ib trial of DS-8201a in combination with immunotherapy for patients with HER2-low BC has also been initiated.
Conclusions
To conclude, this case study showed the temporal and spatial heterogeneity of BC and impressive efficacy with DS-8201a in heavily treated patients, even with heterogeneity and HER2 low expression.
Acknowledgments
The authors would like to acknowledge Dr Satya Lavanya Jakki and Dr Anuradha Nalli (Indegene Pvt Ltd) for editorial support.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding from the Project of Doctor scientific research from Guangdong Provincial People’s Hospital (2020bq12), National Natural Science Foundation of China (81602645; 81702783; 81071851; 81001189), Natural Science Foundation of Guangdong Province (2016A030313768; 2017A030310574; 2018A030313292), and Research Funds from Guangzhou Municipal Science and Technology Project (201707010418; 201804010430).
Author Contributions: CL, CR, and NL contributed to conceptualization, methodology, writing—original draft preparation, software. CR, CL, LC, and XL contributed to data curation and writing—original draft preparation. CL, KL, CR, LW, YW, and MJ contributed to visualization and investigation. CL, XL, and NL contributed to supervision. MJ, BC, HM, JL, and WX contributed to software, validation. NL contributed to funding acquisition. CL, CR, WX, HM, JL, and NL contributed to writing—reviewing and editing.
Patient Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Ethical Approval: Our institution does not require ethical approval for reporting individual cases or case series.
ORCID iD: Ning Liao  https://orcid.org/0000-0002-1965-9425
https://orcid.org/0000-0002-1965-9425
References
- 1. Al-Thoubaity FK. Molecular classification of breast cancer: a retrospective cohort study. Ann Med Surg. 2020;49:44-48. doi: 10.1016/j.amsu.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am. 2018;27:95-120. doi: 10.1016/j.soc.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38:1951-1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 4. Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38:1887-1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NCCN Guidelines for Patients. Breast cancer invasive. https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf.
- 6. Koleva-Kolarova RG, Oktora MP, Robijn AL, et al. Increased life expectancy as a result of non-hormonal targeted therapies for HER2 or hormone receptor positive metastatic breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2017;55:16-25. doi: 10.1016/j.ctrv.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 7. Mendes D, Alves C, Afonso N, et al. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer–a systematic review. Breast Cancer Res. 2015;17:140. doi: 10.1186/s13058-015-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fehrenbacher L, Cecchini RS, Geyer CE, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and With IHC 1+ or 2. J Clin Oncol. 2020;38:444-453. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lüönd F, Tiede S, Christofori G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br J Cancer. 2021;125:164-175. doi: 10.1038/s41416-021-01328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610-621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039-1046. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 13. Schneeweiss A, Park-Simon TW, Albanell J, et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest New Drugs. 2018;36:848-859. doi: 10.1007/s10637-018-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28:855-861. doi: 10.1093/annonc/mdx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Modi S, Andre F, Krop IE, et al. Trastuzumab deruxtecan for HER2-positive metastatic breast cancer: DESTINY-Breast01 subgroup analysis. J Clin Oncol. 2020;38:1036-1036. doi: 10.1200/JCO.2020.38.15_suppl.1036. [DOI] [Google Scholar]
- 16. Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The latest research and development into the antibody–drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull. 2019;67:173-185. doi: 10.1248/cpb.c18-00744. [DOI] [PubMed] [Google Scholar]
- 17. Xu B, Hu X, Jiang Z, et al. National consensus in China on diagnosis and treatment of patients with advanced breast cancer. Ann Transl Med. 2015;3:242. doi: 10.1007/s10637-018-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a Novel DNA Topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097-5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 19. Modi S, Ohtani S, Lee CC, Wang K, Saxena K, Cameron DA. A phase III, multicenter, randomized, open label trial of [fam-] trastuzumab deruxtecan (DS-8201a) versus investigator’s choice in HER2-low breast cancer. J Clin Oncol. 2019;37:TPS1102. doi: 10.1200/JCO.2019.37.15_suppl.TPS1102. [DOI] [Google Scholar]