Abstract
People with epilepsy face serious driving restrictions, determined using retrospective studies. To relate seizure characteristics to driving impairment, we aimed to study driving behavior during seizures with a simulator. Patients in the Yale New Haven Hospital undergoing video-electroencephalographic monitoring used a laptop-based driving simulator during ictal events. Driving function was evaluated by video review and analyzed in relation to seizure type, impairment of consciousness/responsiveness, or motor impairment during seizures. Fifty-one seizures in 30 patients were studied. In terms of seizure type, we found that focal to bilateral tonic–clonic or myoclonic seizures (5/5) and focal seizures with impaired consciousness/responsiveness (11/11) always led to driving impairment; focal seizures with spared consciousness/responsiveness (0/10) and generalized nonmotor (generalized spike–wave bursts; 1/19) usually did not lead to driving impairment. Regardless of seizure type, we found that seizures with impaired consciousness (15/15) or with motor involvement (13/13) always led to impaired driving, but those with spared consciousness (0/20) or spared motor function (5/38) usually did not. These results suggest that seizure types with impaired consciousness/responsiveness and abnormal motor function contribute to impaired driving. Expanding this work in a larger cohort could further determine how results with a driving simulator may translate into real world driving safety.
Keywords: consciousness, driving simulation, EEG, epilepsy, seizure, tonic–clonic
1 |. INTRODUCTION
Society relies heavily on personal vehicles for transportation, so restriction of driving is a major concern for quality of life in people with epilepsy (PWE).1 To be allowed to drive, PWE must demonstrate seizure control, with a seizure-free period of 3–12 months or longer depending on local laws.2,3 Evidence to support driving safety decisions comes mainly from retrospective studies of motor vehicle collisions in PWE as a whole compared to healthy controls.4 Few studies have attempted to distinguish driving risk based on seizure characteristics.4–7 Furthermore, studies that prospectively examine driving in epilepsy have been limited, focusing mainly on interictal driving.8–10 Our aim for this study is to observe ictal driving behavior in PWE to relate seizure characteristics to driving impairment. Our goal is to investigate seizure characteristics with special potential relevance for driving safety, including seizure type, loss of consciousness, and motor impairment during seizures.
2 |. MATERIALS AND METHODS
2.1 |. Subjects
Subjects were patients admitted to the Yale New Haven Hospital for continuous video-electroencephalographic (EEG) monitoring. All underwent written informed consent through an approved institutional human research protocol. Eligible subjects were aged 9 years or older and able to follow instructions for the simulated driving task. Patients with nonepileptic seizures were excluded.
2.2 |. Seizure classification and localization
Video-EEG recordings were obtained with Natus or BioLogic long-term monitoring systems. Video-EEG and clinical records were reviewed by neurologists specializing in epilepsy (K.R., J.Y.Y., R.S., L.M., L.J.H., H.B.) to determine seizure onset and offset times and epilepsy diagnosis (Table S1). Seizures were classified based on clinical and electrographic features as focal, focal to bilateral tonic–clonic, myoclonic, or generalized nonmotor (generalized spike–wave bursts including slow spike-wave [GSWs]). Other seizure types were not observed. Clinical seizures of any duration were included, but clinically inapparent activity was only included for focal seizures lasting >10 s and for GSWs lasting >3 s. The 3-s cutoff was chosen for GSWs based on prior studies suggesting that longer GSWs are more likely to cause deficits,11 although it should be noted that little work has been done to investigate deficits in relation to GSW duration when atypical or slow spike–wave is included.
2.3 |. Driving task
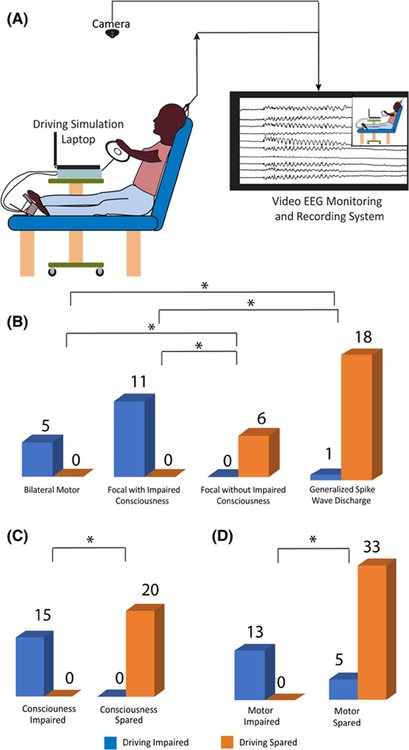
Patients were encouraged to drive as long as they could during their admission, and instructed to continue driving if a seizure occurred. A racing simulation game, rFactor (http://www.rFactor.net/; Image Space), was used that places an emphasis on realistic driving and customization. During video-EEG monitoring, patients drove a simulated standard car on a laptop computer mounted on a hospital table (Figure 1A), controlled using a steering wheel, gas, and brake pedals (Logitech MOMO Racing Force Feedback Wheel). Tracks were lined with barriers so loss of control would quickly lead to a collision, allowing detection of ictal driving impairment. Video screen capture was used to record all driving simulation on the laptop computer. In addition, patient behavior was recorded by the video-EEG monitoring camera (Figure 1A). The driving laptop was continuously synchronized to the same internet time as the computers collecting video-EEG data.
FIGURE 1.
Effects of seizure type, consciousness, and motor function on simulated driving during seizures. (A) Driving simulation setup in the video-electroencephalographic (EEG) monitoring unit. The patient participated in driving simulation using a laptop on a modified bedside table, with the appropriate gas and brake pedals and steering wheel. The video-EEG monitoring system recorded the patienťs behavior and EEG during participation in the simulated driving. (B) Relationship between driving impairment and seizure type. All generalized motor (bilateral tonic–clonic and myoclonic) seizures (5/5) and all focal seizures with impaired consciousness (11/11) resulted in driving impairment, whereas all focal seizures without impaired consciousness (6/6) and most generalized spike-wave discharges (18/19) resulted in spared driving. Focal seizures with unknown consciousness (10/27) are omitted from this analysis. Of those seizures, driving was spared in nine and impaired in one. (C) Relationship between impaired consciousness/responsiveness and driving. All seizures with impaired consciousness (15/15) resulted in driving impairment, whereas all seizures with spared consciousness (20/20) spared driving. Seizures with unknown consciousness (16/51) are excluded from this analysis. Of those seizures, driving was spared in 13 and impaired in three. (D) Relationship between motor impairment and driving. All seizures with motor impairment (13/13) led to impaired driving, whereas most seizures without motor impairment (33/38) spared driving. *p < .05, Fisher exact test with Bonferroni correction
2.4 |. Analysis of impaired driving, consciousness, and motor function
Driving, consciousness, and motor function during seizures were each evaluated by consensus of two reviewers. Evaluation of driving was done independently from evaluation of consciousness and motor function, by using separate data passes and different members of the research team. Driving, consciousness, and motor function were each rated on a binary scale as either impaired, spared, or alternatively as “unknown” if it could not be evaluated based on available data (Table S1).
Driving was evaluated during seizures based on review of the video screen capture from the driving laptop, and the external video recording of behavior by the video-EEG monitoring system. Driving was considered impaired if the driving laptop video replay showed a collision (with the border of the track or with another vehicle) or if the vehicle came to a stop on the road due to the subject not using the gas pedal; or if the external video demonstrated that the patient let go of the steering wheel, turned the steering wheel in an abnormal manner (forced version or irregular jerky movements), or looked away from the laptop screen for a sustained period during the seizure.
Impaired consciousness was defined as in previous studies based on inability to respond appropriately to external questions, commands, or other stimuli during seizures based on video review from the video-EEG monitoring.12–14 Determining consciousness was usually dependent on interactions with another person, such as answering questions appropriately, following commands, or reacting to other stimuli such as a shoulder shake or calling the patient's name. If there was no person in the room, but the individual responded appropriately to stimuli such as a ringing phone or pressing the event button to indicate that they were having a seizure, then they were considered conscious. Raters did not include driving behaviors in the evaluation of responsiveness, and if no conclusion could be drawn based on other behavioral interactions, the seizure was labeled as unknown responsiveness and was excluded from the analysis of consciousness (Table S1). We used responsiveness during seizures because of its direct relevance to driving safety, and because measures of “awareness,” such as ability to describe experiences during seizures after they had occurred, were assessed much less consistently than assessment of responsiveness (see also Section 4, Discussion).
Motor impairment was evaluated based on video review from the video-EEG monitoring demonstrating abnormal positive motor activity in the arms, legs, or head/eyes during the seizure, including tonic, dystonic, versive, myoclonic, or clonic movements. Motor function was not rated as impaired based on negative motor activity such as behavioral arrest during seizures.
2.5 |. Statistical analysis
Calculation of means and Fisher exact test were performed in Excel or Python 3.7, using a significance threshold of p < .05 with Bonferroni correction where appropriate.
3 |. RESULTS
3.1 |. Patient and seizure population
A total of 30 patients had at least one seizure while using the driving simulator. Clinical and demographic information are given in Table S1. Mean age at the time of testing was 30 (range = 9–57) years. Twenty-one patients were male (70%) and nine female (30%). There were in total 51 recorded seizures during driving, including 27 focal seizures, four focal to bilateral tonic-clonic seizures, one myoclonic seizure, and 19 GSWs meeting criteria for clinical or clinically inapparent seizures (see Section 2.2).
3.2 |. Relationship between seizure type and impaired driving
As expected, bilateral motor (including focal to bilateral tonic-clonic and myoclonic) seizures were always associated with impaired driving (5/5 seizures; Figure 1B). Similarly, focal seizures with impaired consciousness/responsiveness always resulted in impaired driving (11/11 seizures; Figure 1B). However, none of the focal seizures without impaired consciousness was associated with impaired driving (0/6 seizures), and only one GSW was associated with impaired driving (1/19; Figure 1B). The one GSW with impaired driving had a duration of 11 s. The other 18 GSWs without impaired driving occurred in six different patients, and had a median GSW duration of 14 s (range = 3–88 s; see Table S1). Significant differences in the likelihood of impaired driving function were seen between all seizure types (p < .05, Fisher exact test with Bonferroni correction) except for bilateral motor versus focal with impaired consciousness (both impaired) and focal without impaired consciousness versus GSW (both usually spared; Figure 1B). Overall, these results show a strong relationship between seizure type and impaired ictal driving, with severe impairment seen mainly in bilateral motor seizures and in focal seizures with impaired consciousness.
3.3 |. Relationship between consicousness/responsiveness, motor function, and driving
We next investigated the relationship between impaired consciousness or motor function and driving impairment regardless of seizure type. Seizures with impaired consciousness/responsiveness were invariably associated with driving impairment. All seizures with impaired consciousness resulted in impaired driving (15/15 seizures), whereas driving was not impaired in seizures without impaired consciousness (0/20 seizures; Figure 1C; p < .001, Fisher exact test). Motor impairment during seizures was also closely associated with impaired driving. All seizures with motor impairment resulted in impaired driving (13/13 seizures with motor impairment, including four focal to bilateral tonic–clonic, seven focal impaired responsiveness/consciousness, one focal unknown consciousness, and one myoclonic seizure; see also Table S1), and very few seizures without motor impairment were associated with impaired driving (5/33 seizures; Figure 1D; p < .001, Fisher exact test).
4 |. DISCUSSION
The primary aim of this study was to investigate how seizure type, impaired consciousness, and motor function affect driving behavior using a driving simulator and video-EEG monitoring. We found that bilateral motor (focal to bilateral tonic-clonic and myoclonic) seizures and focal seizures with impaired consciousness/responsiveness always led to driving impairment, whereas focal seizures with spared consciousness and GSWs were rarely associated with driving impairment. In addition, any seizures with impaired consciousness or motor involvement consistently led to impaired driving, whereas those without impaired consciousness or motor involvement usually did not.
Common sense notions about seizure characteristics currently guide decisions about driving in PWE but have not been thoroughly investigated. Short of recording EEG from PWE during actual driving, simulators provide the most objective testing approach.8,9,15 However, previous studies of ictal driving had relatively limited sample size.16,17 We examined and confirmed some common sense factors including the danger of driving with uncontrolled bilateral tonic–clonic seizures, or with seizures that impair consciousness or motor function.
The simulator we used reflected typical driving conditions, where severely impaired driving behaviors or collisions would be detected, but more subtle deficits might be missed. Methods with sudden unexpected obstacles coinciding with epileptiform activity can detect delayed reaction times or other subtle deficits in driving, which we may have missed here.8,15,17 This may be particularly true for GSWs (including slow spike–wave), where we rarely found deficits with the current approach, whereas more instantaneous testing methods are capable of uncovering deficits.8,11,15,17 It is unclear how these more subtle deficits translate into real world driving safety; this should be investigated further. More generally, it is unclear how findings with a driving simulator and including subjects who do not ordinarily drive (as in the current study) relate to real world driving safety, so these two situations should be compared directly in future work. Another future direction should be investigation of seizure types (e.g., generalized onset tonic-clonic, absence, and myoclonic seizures) that were not included or had insufficient numbers to draw conclusions from in the present work.
Our analysis of focal seizures rated impaired consciousness based on inability to respond. This approach was used because of the relevance of responsiveness to driving safety, and because other measures of conscious awareness, such as ability to describe experiences during the seizure after it has happened, were not routinely tested in this patient cohort. Although the current International League Against Epilepsy definition of focal impaired awareness seizures is based on impaired ability to recall events during seizures after they have occurred, responsiveness is also considered an important relevant factor.18 Certainly for the sake of driving safety, responsiveness should be included in the clinical features of impaired consciousness, as in other studies of epilepsy and other neurological disorders of consciousness.12–14,19
In conclusion, we found that seizures with impaired motor function and loss of consciousness lead to hazardous simulated driving. Our findings may have important implications for the current criteria denying driving licensure to PWE, reinforcing some common sense notions but also shedding light on the need for further studies. Ultimately, this information can refine criteria for safe driving, improving both public safety and quality of life for people with epilepsy.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the patients for participating in this study. This work was supported by NIH R01 NS055829, the Donaghue Medical Research Foundation, the Mark Loughridge and Michele Williams Foundation, and the Betsy and Jonathan Blattmachr family.
Funding information
R01 NS055829; Betsy and Jonathan Blattmachr family; Mark Loughridge and Michele Williams Foundation; Donaghue Medical Research Foundation
Footnotes
CONFLICT OF INTEREST
J.Y.Y. consults for Zimmer Biomet and LVIS Corporation. None of the other authors has any conflict of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Gilliam F, Kuzniecky R, Faught E, Black L, Carpenter G, Schrodt R, et al. Patient-validated content of epilepsy-specific quality-of-life measurement. Epilepsia. 1997;38:233–6. [DOI] [PubMed] [Google Scholar]
- 2.Krauss GL, Ampaw L, Krumholz A. Individual state driving restrictions for people with epilepsy in the US. Neurology. 2001;57:1780–5. [DOI] [PubMed] [Google Scholar]
- 3.State Driving Laws Database. Book state driving laws database. Bowie, MD: Epilepsy Foundation; 2020. [Google Scholar]
- 4.Chen WC, Chen EY, Gebre RZ, Johnson MR, Li N, Vitkovskiy P, et al. Epilepsy and driving: potential impact of transient impaired consciousness. Epilepsy Behav. 2014;30:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gastaut H, Zifkin BG. The risk of automobile accidents with seizures occurring while driving—relation to seizure type. Neurology. 1987;37:1613–6. [DOI] [PubMed] [Google Scholar]
- 6.Classen S, Crizzle AM, Winter SM, Silver W, Eisenschenk S. Evidence-based review on epilepsy and driving. Epilepsy Behav. 2012;23:103–12. [DOI] [PubMed] [Google Scholar]
- 7.Berg AT, Vickrey BG, Sperling MR, Langfitt JT, Bazil CW, Shinnar S, et al. Driving in adults with refractory localizationrelated epilepsy. Multi-center study of epilepsy surgery. Neurology. 2000;54:625–30. [DOI] [PubMed] [Google Scholar]
- 8.Krestel HE, Nirkko A, von Allmen A, Liechti C, Wettstein J, Mosbacher A, et al. Spike-triggered reaction-time EEG as a possible assessment tool for driving ability. Epilepsia. 2011;52:e126–9. [DOI] [PubMed] [Google Scholar]
- 9.Crizzle AM, Classen S, Winter SM, Silver W, LaFranca C, Eisenschenk S, et al. Associations between clinical tests and simulated driving performance in persons with epilepsy. Epilepsy Behav. 2012;23:241–6. [DOI] [PubMed] [Google Scholar]
- 10.Crizzle AM, Classen S, LaFranca C, Winter SM, Roper SN, Eisenschenk S, et al. Assessing the driving performance of a person with epilepsy presurgery and postsurgery. Am J Occup Ther. 2013;67:e24–9. [DOI] [PubMed] [Google Scholar]
- 11.Guo JN, Kim R, Chen YU, Negishi M, Jhun S, Weiss S, et al. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol. 2016;15:1336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englot DJ, Yang LI, Hamid H, Danielson N, Bai X, Marfeo A, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. [DOI] [PubMed] [Google Scholar]
- 14.Arthuis M, Valton L, Régis J, Chauvel P, Wendling F, Naccache L, et al. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain. 2009;132:2091–101. [DOI] [PubMed] [Google Scholar]
- 15.Nirkko AC, Bernasconi C, von Allmen A, Liechti C, Mathis J, Krestel H, et al. Virtual car accidents of epilepsy patients, interictal epileptic activity, and medication. Epilepsia. 2016;57:832–40. [DOI] [PubMed] [Google Scholar]
- 16.Yang LI, Morland TB, Schmits K, Rawson E, Narasimhan P, Motelow JE, et al. A prospective study of loss of consciousness in epilepsy using virtual reality driving simulation and other video games. Epilepsy Behav. 2010;18:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen E, Antwi P, Banz BC, Vincent P, Saha R, Arencibia CA, et al. Realistic driving simulation during generalized epileptiform discharges to identify electroencephalographic features related to motor vehicle safety: feasibility and pilot study. Epilepsia. 2020;61:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30. [DOI] [PubMed] [Google Scholar]
- 19.Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.