Abstract
Obesity is a major risk factor for lung disease development. However, little is known about the impact of chronic high-fat and high-fructose (HFHF) diet–induced obesity on lung inflammation and subsequent pulmonary fibrosis. Herein we hypothesized that dedicator of cytokinesis 2 (DOCK2) promotes a proinflammatory phenotype of lung fibroblasts (LFs) to elicit lung injury and fibrosis in chronic HFHF diet–induced obesity. An HFHF diet for 20 weeks induced lung inflammation and profibrotic changes in wild-type C57BL/6 mice. CD68 and monocyte chemoattractant protein-1 (MCP-1) expression were notably increased in the lungs of wild-type mice fed an HFHF diet. An HFHF diet further increased lung DOCK2 expression that co-localized with fibroblast-specific protein 1, suggesting a role of DOCK2 in regulating proinflammatory phenotype of LFs. Importantly, DOCK2 knockout protected mice from lung inflammation and fibrosis induced by a HFHF diet. In primary human LFs, tumor necrosis factor-α (TNF-α) and IL-1β induced DOCK2 expression concurrent with MCP-1, IL-6, and matrix metallopeptidase 2. DOCK2 knockdown suppressed TNF-α–induced expression of these molecules and activation of phosphatidylinositol 3-kinase/AKT and NF-κB signaling pathways, suggesting a mechanism of DOCK2-mediated proinflammatory and profibrotic changes in human LFs. Taken together, these findings reveal a previously unrecognized role of DOCK2 in regulating proinflammatory phenotype of LFs, potentiation of lung inflammation, and pulmonary fibrosis in chronic HFHF diet–caused obesity.
Obesity is a major heath issue worldwide, and its incidence has almost tripled globally since 1975.1 In the United States, an estimated one-third of adults are affected by obesity.2 Obesity, especially when severe (class II and III obesity, BMI 35 to 49.9 kg/m2),3,4 has been linked to poor outcomes in acute and chronic respiratory illnesses, including acute respiratory distress syndrome, asthma, chronic obstructive pulmonary disease, obstructive sleep apnea, and idiopathic pulmonary fibrosis.5, 6, 7, 8, 9
Obesity affects the respiratory system mainly through a combination of mass loading and hormonal, metabolic, inflammatory, and dietary factors.5,10, 11, 12 In obesity, adipocytes and macrophages in the adipose tissue generate proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-6 and release them into the blood, consequently inducing systemic inflammation.13, 14, 15 The increased levels of serum proinflammatory factors associated with obesity correlate with the severity of chronic conditions.15,16 In animal models, diet-induced obesity and metabolic disorders vary in severity depending on the fat content and fat composition of the diet, as well as feeding duration.10,17 For example, of the mice on 1, 3, and 6 months of a high-fat diet, only the mice on the 6-month diet had diet-induced obesity which impaired diaphragm motion, and induced restrained, low-amplitude contractions.10 A high-fat diet supplemented with high fructose has also been reported to adversely affect the ability of the liver to handle excess lipids, leading to more severe obesity.18,19 The high-fat and high-fructose (HFHF) diet model better mimics the diet in developed countries, and results in high rates of obesity.20,21 A recent report showed that mice fed with control food supplemented with 43.7% fat and 10% fructose for 16 weeks displayed increased pulmonary oxidative stress, lung neutrophilic accumulation/activation, and collagen depostion.22 However, little is known about the impact of obesity (especially severe obesity) caused by a chronic HFHF diet on the development of pulmonary inflammation and fibrotic repair.
Lung structural components, especially lung fibroblasts (LFs), play a key role in modulating inflammation/repair after lung injury23, 24, 25, 26 and contribute to lung diseases, including idiopathic pulmonary fibrosis, asthma, and chronic obstructive pulmonary disease.25,27,28 LFs are not terminally differentiated and have a broad biosynthetic repertoire.26,29 During the progression of lung injury, LFs can sense microenvironmental changes and be activated to release chemokines and cytokines [eg, monocyte chemoattractant protein-1 (MCP-1), IL-6, and IL-1β], which contribute to the inflammatory process.29, 30, 31 LFs also regulate pulmonary remodeling by modulating extracellular matrix production and reabsorption that largely depend on matrix metalloproteinases (MMPs), particularly MMP2.32,33 However, the role of LFs in lung inflammation/injury linked with chronic HFHF diet–caused obesity has not previously been explored.
Dedicator of cytokinesis 2 (DOCK2), initially found to be expressed in lymphocytes and macrophages, plays critical roles in chemotaxis by neutrophils, lymphocytes, and plasmacytoid dendritic cells.34, 35, 36, 37, 38, 39 Our group found that DOCK2 regulates the smooth muscle cell phenotype during vascular remodeling.40 In addition, DOCK2 knockout (DOCK2−/−) attenuates high-fat diet–induced obesity with reduced proinflammatory cytokine expression in adipose tissue and peripheral circulation.13 The role of DOCK2 in pulmonary injury and fibrotic repair associated with chronic HFHF diet–caused obesity has likewise not, to our knowledge, been previously explored.
The current study highlights the novel finding that a chronic HFHF diet induces lung inflammation and profibrotic changes in mice. DOCK2 deficiency dramatically attenuated chronic HFHF diet–induced obesity and lung inflammatory and profibrotic injury. The in vitro data show that DOCK2 is also induced by TNF-α and IL-1β and required for their proinflammatory signaling in primary human LFs (HLFs). These findings collectively show that DOCK2 is a novel regulator of the proinflammatory phenotype of LFs and contributes to the development of lung inflammation/injury in obesity caused by a chronic HFHF diet.
Materials and Methods
Animals and Diets
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Georgia. Male and female C57BL/6 wild-type (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). DOCK2−/− mice were generated on the C57BL/6 background as reported previously.13,34,40 The WT and DOCK2−/− mice (8 to 12 weeks old) were maintained on a 12 hours’ light/12 hours’ dark cycle with free access to feed and water. Four groups of mice (n = 6 to 8 per group) were treated as follows: WT mice fed a chow diet, WT mice fed an HFHF diet, DOCK2−/− mice fed a chow diet, and DOCK2−/− mice fed an HFHF diet. The mice were examined after 20 weeks. Specifically, mice were given regular water and standard chow (25% protein, 62% carbohydrate, and 13% fat; 3.07 kcal/g; 5053, LabDiet, St. Louis, MO)13 or a high-fat diet (20% protein, 40% carbohydrate, and 40% fat; 4.5 kcal/g; D12108C, Research Diets, New Brunswick, NJ)13 plus high fructose (30% fructose, w/v; catalog #F0127, Sigma-Aldrich, St. Louis, MO)19,41 in water for the duration of the study. To assess the intermediate effects, WT and DOCK2−/− mice were also examined after 10 weeks’ treatment (n = 5) with either regular water and standard chow or the HFHF diet as described earlier. Animals were then euthanized, and lung tissues were collected and fixed in 4% paraformaldehyde for histologic examination and immunofluorescence staining analyses.
Reagents
Recombinant human TNF-α and IL-1β were purchased from R&D Systems (Minneapolis, MN). Oxidized low-density lipoprotein was purchased from Alfa Aesar (Ward Hill, MA). Actinomycin D was purchased from LC Laboratories (Woburn, MA). LY294002 and pyrrolidine dithiocarbamate were purchased from Sigma-Aldrich (St. Louis, MO). Primary antibodies against DOCK2, MMP-2, fibroblast-specific protein 1 (FSP1), α-tubulin, actin, and glyceraldehyde-3-phosphate dehydrogenase were purchased from Sigma-Aldrich. Primary antibodies against AT (Protein kinase B, or AK strain transforming), phosphor-AKT (p-AKT), NF-κB, and p-NF-κB p65 were purchased from Cell Signaling Technology (Danvers, MA). Anti-CD68 antibody was purchased from Bio-Rad (Hercules, CA). Anti–IL-6 antibody was purchased from Santa Cruz Biotechnology (Dallas, TX). Anti–MCP-1 antibody was purchased from Abcam (Cambridge, MA). Anti–4-hydroxynonenal antibody was purchased from Alpha Diagnostic International (San Antonio, TX). Anti–collagen 1A1 antibody (catalog #1310-08) was purchased from SouthernBiotech (Birmingham, AL). Secondary fluorescence-labeled antibodies, including anti-Rabbit IgG-Alexa 488, anti-Rat IgG-Alexa 488, and anti-mouse IgG-Texas Red, were purchased from Invitrogen (Carlsbad, CA).
Cell Culture
Two lines of primary HLFs isolated from histologically normal nonfibrotic lung (denoted as HLFs#1 and HLFs#2) were provided by the University of Michigan (Ann Arbor, MI) under a Material Transfer Agreement. These cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, l-glutamine, and antibiotics (penicillin/streptomycin) in a humidified hood with 5% carbon dioxide, as previously described.42 Cell starvation was performed by using Dulbecco’s modified Eagle’s medium with l-glutamine and antibiotics without fetal bovine serum.
Lung Histologic Examination
Formaldehyde-fixed lung tissues were dehydrated, embedded in paraffin, and then cut at 5 μm thickness for further staining, as previously reported.40,42 Hematoxylin and eosin and Masson’s trichrome staining were performed according to the guide of the staining kits purchased from American MasterTech Scientific (Lodi, CA).
Immunofluorescence Staining and Immunohistochemical Staining
Immunofluorescence was conducted as previously reported40,43,44 to detect CD68, MCP-1, DOCK2, and FSP1 in formaldehyde-fixed, paraffin-embedded lung tissues. Diluted primary antibodies were incubated with slides at 4°C overnight. Secondary anti-mouse, anti-rabbit, or anti-rat antibody conjugated with Texas Red or fluorescein isothiocyanate was used to stain these molecules. Fluorescent images were taken with a Ni-U microscope (Nikon, Tokyo, Japan).
For immunohistochemical staining of collagen, the formaldehyde-fixed, paraffin-embedded lung slides were processed following routine procedures, including de-waxing in xylene, rehydration, and antigen retrieval, and quenching endogenous peroxidase activity with 3% hydrogen peroxide in methanol. The slides were then blocked with 3% bovine serum albumin, followed by incubation with primary anti–collagen 1A1 antibody (catalog #1310-08, SouthernBiotech) overnight at 4°C. After washing with phosphate-buffered saline with 0.1% Tween-20 three times, the slides were then incubated with secondary streptavidin–horseradish peroxidase (016 to 030-084; Jackson ImmunoResearch, West Grove, PA) for 1 hour at room temperature. 3,3′-Diaminobenzidine (DAB) staining was subsequently performed according to the manufacturer’s guide.
Western Blotting
The procedure was described as previously reported.44, 45, 46 Whole-cell lysates were collected from serum-starved primary HLFs after treatment, as indicated in respective experiments. Radioimmunoprecipitation assay buffer with protease inhibitor cocktail and phosphatase inhibitor (Thermo Fisher Scientific, Waltham, MA) was used to lyse the cells. Denatured proteins were separated by SDS-PAGE gel and detected by using an enhanced chemiluminescence kit.
Quantitative PCR Analysis
Total RNA extraction from cultured cells was performed by using the TRIzol reagent according to the manufacturer’s guide.47,48 The purified total RNA (1 μg) was then reversely transcribed to cDNA as a template for quantitative PCR (qPCR) analysis using the iScript Kit (Bio-Rad). qPCR was performed in a Bio-Rad CFX96 real-time PCR machine using SYBR Green master mix (Bio-Rad). Each sample was amplified in triplicate. DOCK2 primers: forward 5′-TGCTGAAGTGGCGTATGAAG-3′ and reverse 5′-AATTTCCGGTCTGCAATGAG-3′. GAPDH was used as the internal control and the primers: forward 5′-TGCACCACCAACTGCTTA-3′ and reverse 5′-GGATGCAGGGATGATGTTC-3'.46
Statistical Analysis
All statistical analyses were performed by using GraphPad Prism 8 (GraphPad Software, La Jolla, CA). Data were evaluated with a two-tailed, unpaired t-test or compared by one-way analysis of variance followed by the Fisher's least significant difference test. Data are expressed as means ± SD. P < 0.05 was considered statistically significant.
Results
A Chronic HFHF Diet Causes Obesity and Lung Inflammation/Injury and Increases DOCK2 Expression in LFs of WT Mice
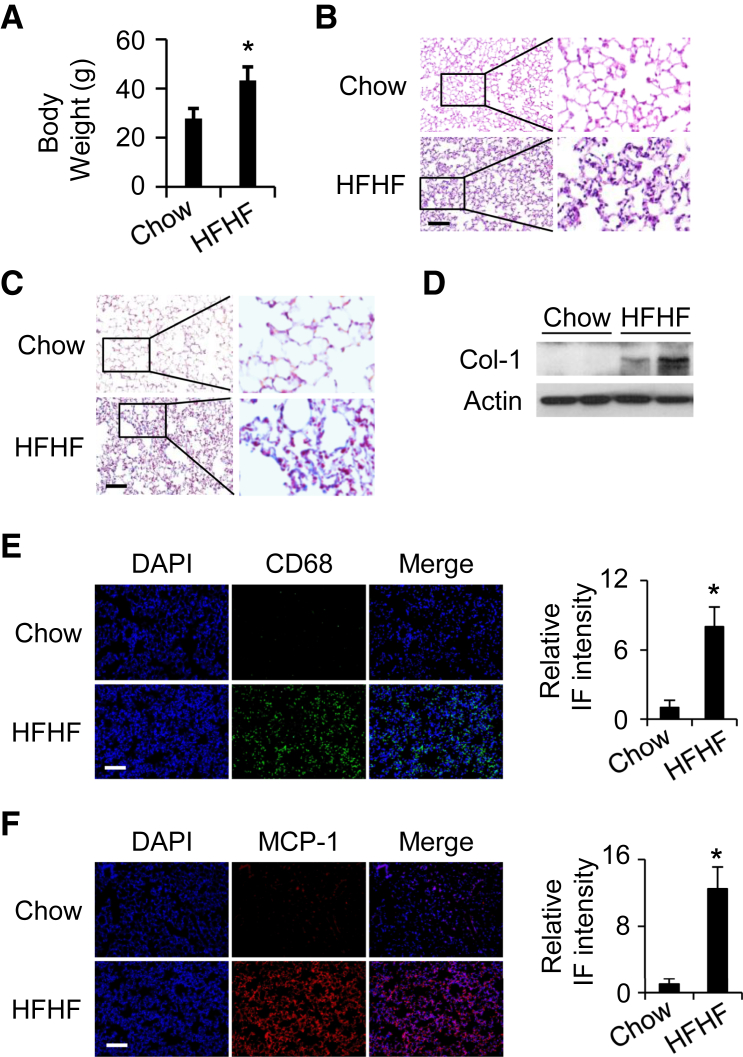
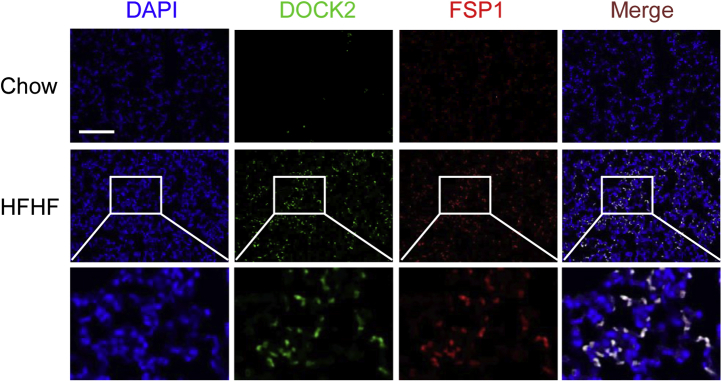
Chronic HFHF consumption has been linked with human obesity characterized by low-grade systemic inflammation.16,18 In the current study, significant gain in body weight was observed in WT mice fed an HFHF diet for 20 weeks compared with a normal chow diet (43.3 ± 5.4 g versus 27.7 ± 4.1 g; P < 0.05) (Figure 1A). Hematoxylin and eosin staining revealed that, in contrast to the chow diet, the chronic HFHF diet induced alveolar and interstitial inflammation in the lung (Figure 1B). In addition, the chronic HFHF diet caused increased collagen deposition and expression in the lung compared with the chow diet, as shown by Masson’s trichrome staining and Western blotting on lung lysates (Figure 1, C and D). An inflammatory response was also indicated as macrophage infiltration was observed in the chronic HFHF diet group and shown by increased expression of the macrophage marker CD68 (Figure 1E). MCP-1, a key chemokine in attracting macrophages to the site of inflammation,49 was likewise enhanced in the HFHF diet group (Figure 1F). Oxidative stress has been reported to be closely related to lung inflammation and fibrosis.50,51 In the current experiment, expression of an oxidative stress marker, 4-hydroxynonenal, was observed in the lung tissues of mice fed an HFHF diet. The results show that HFHF treatment for 20 weeks dramatically increased the expression of 4-hydroxynonenal (Supplemental Figure S1), suggesting that HFHF induces oxidative stress in the lung parenchyma. These data suggest that a chronic HFHF diet induces lung inflammation and profibrotic changes in WT mice. Importantly, immunofluorescence staining showed that DOCK2 was dramatically induced in the lungs of WT mice fed an HFHF diet. Furthermore, DOCK2 expression largely co-localized with that of the fibroblast marker FSP152 (Figure 2), suggesting that DOCK2 may be involved in the proinflammatory phenotype of LFs to promote lung inflammation/injury in obesity caused by a chronic HFHF diet.
Figure 1.
Chronic high-fat and high-fructose (HFHF) diet-induced lung inflammation/injury in wild-type (WT) C57BL/6 mice. A: Body weight of WT C57BL/6 mice fed with normal chow or an HFHF diet for 20 weeks. B and C: Representative hematoxylin and eosin staining (B) and Masson’s staining (C) of lung tissues from WT mice fed a chow diet or an HFHF diet for 20 weeks. D: Lung tissues from WT mice fed a chow diet or an HFHF diet for 20 weeks were collected. Two representative samples for each group were loaded to detect collagen (Col-1) protein expression by Western blotting. Actin is a loading control. E and F: Immunofluorescence (IF) shows increased CD68 (green) (E) and monocyte chemoattractant protein-1 (MCP-1) (red) (F) expression in lung tissues from WT mice fed with chow or HFHF diet for 20 weeks. DAPI stained the nuclei (blue). The black boxes indicate the areas enlarged for view. Quantification of expression levels in E and F from 5 fields per slide per mouse and 3 mice per group. n = 6 to 8 (A). ∗P < 0.05 versus WT chow diet group. Scale bar = 100 μm.
Figure 2.
Dedicator of cytokinesis 2 (DOCK2) expression. DOCK2 was induced and co-localizes with fibroblast-specific protein 1 (FSP1) in the lungs of wild-type mice fed a chronic high-fat and high-fructose (HFHF) diet. Immunofluorescence staining showed notably increased DOCK2 (green) expression and co-localization with FSP1 (red) in lung tissues of wild-type mice fed a chow diet or an HFHF diet for 20 weeks. DAPI stained the nuclei (blue). The white boxes indicate the areas enlarged for view. Scale bar = 100 μm.
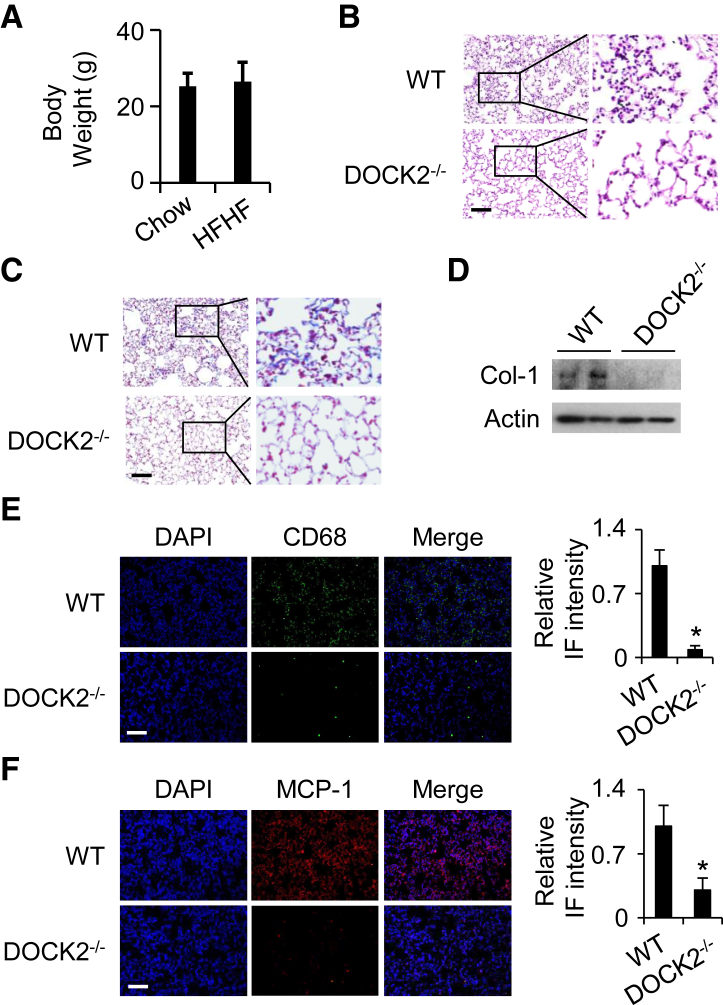
DOCK2−/− Mice Are Protected from Body Weight Gain and Lung Inflammation/Injury Induced by a Chronic HFHF Diet
DOCK2 deficiency has been reported to attenuate high-fat diet–induced obesity.13 There was no significant difference in body weight between WT and DOCK2−/− mice fed a chow diet. DOCK2 deficiency instead attenuated the gain in body weight caused by the high-fat diet.13 In this study, hematoxylin and eosin staining and Masson’s staining data show similar lung parenchyma between the WT and DOCK2−/− mice fed a chow diet (Supplemental Figure S2). Moreover, the chronic HFHF diet did not significantly induce body weight gain in DOCK2−/− mice compared with those fed a chow diet (26.5 ± 5.0 g versus 25.5 ± 3.4 g; P = 0.456) (Figure 3A). DOCK2−/− mice were also protected from chronic HFHF diet–induced lung inflammation, as indicated by hematoxylin and eosin staining (Figure 3B). DOCK2−/− mouse lungs also exhibited a reduction in HFHF diet–induced collagen production, as indicated by Masson’s staining (Figure 3C), Western blotting (Figure 3D), and immunohistochemical staining (Supplemental Figure S3). HFHF diet–induced macrophage infiltration (CD68 stain) in the lung was also significantly reduced in DOCK2−/− mice compared with that in WT control mice (Figure 3E). Accordingly, HFHF diet–induced MCP-1 expression was also significantly decreased in DOCK2−/− mice (Figure 3F). Taken together, these findings indicate that DOCK2 deficiency attenuates the lung inflammation and fibrotic repair associated with a chronic HFHF diet. To evaluate the interim effects of an HFHF diet, WT and DOCK2−/− mice were fed chow and an HFHF diet for 10 weeks. The results show that the HFHF diet for 10 weeks induced inflammatory infiltration in the lungs of WT mice but not in DOCK2−/− mice (Supplemental Figure S4). In contrast, no obvious collagen deposition was found in the WT mice or DOCK2−/− mice fed the HFHF diet for 10 weeks (Supplemental Figure S5). The data suggest that progressive inflammatory alteration and fibrosis occur in the lung due to an HFHF diet.
Figure 3.
Dedicator of cytokinesis 2 (DOCK2) deficiency protects mice from chronic high-fat and high-fructose (HFHF) diet–induced lung inflammation/injury. A: Body weight of DOCK2 knockout (DOCK2−/−) mice fed a normal chow diet or an HFHF diet for 20 weeks. B and C: Representative hematoxylin and eosin staining (B) and Masson’s staining (C) of lung tissues from wild-type (WT) or DOCK2−/− mice fed an HFHF diet for 20 weeks. D: Lung tissues from WT or DOCK2−/− mice fed an HFHF diet for 20 weeks were collected. Two representative samples for each group were loaded to detect collagen (Col-1) protein expression by Western blotting. Actin is a loading control. E and F: Immunofluorescence (IF) shows increased CD68 (green, E) and monocyte chemoattractant protein-1 (MCP-1) (red, F) expression in lung tissues from WT or DOCK2−/− mice fed an HFHF diet for 20 weeks. DAPI stained the nuclei (blue). The black boxes indicate the areas enlarged for view. Quantification of expression levels in E and F from 5 fields per slide per mouse and 3 mice per group. n = 6 to 8 (A). ∗P < 0.05 versus WT HFHF diet group. Scale bar = 100 μm.
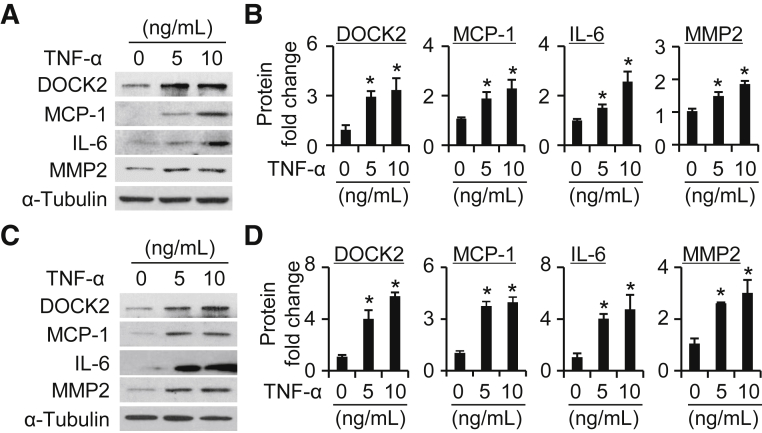
DOCK2 Is Induced in Primary HLFs by TNF-α and IL-1β
TNF-α is a well-known and potent inducer of diverse chemokines, including MCP-1, by different cell types such as vascular endothelial cells, adipocytes, and fibroblasts.49,53,54 To characterize the expression of DOCK2 in LFs, serum-starved primary HLFs were treated with TNF-α at different concentrations (0, 5, and 10 ng/mL) for 24 hours. Although the basal level of DOCK2 was low in primary HLFs, TNF-α significantly increased its expression in a dose-dependent manner (Figure 4). As expected, TNF-α also induced expression of MCP-1, IL-6, and MMP2 (Figure 4). IL-1β, a mechanistically different proinflammatory cytokine, was used to confirm the role of proinflammatory mediators in the induction of DOCK2 in primary HLFs. IL-1β increased DOCK2 expression concurrent with MCP-1, IL-6, and MMP2 (Supplemental Figure S6) in primary HLFs. Because 10 ng/mL of TNF-α maximally induced expression of DOCK2 and proinflammatory LF phenotype–related molecules, this dose was selected for time course experiments. The data show that TNF-α significantly induced DOCK2 expression by 8 hours and the highest level reached at 24 hours in primary HLFs (Figure 5). Oxidized low-density lipoprotein is known to be closely related to the effects of diet-induced injury.55, 56, 57 DOCK2 was also induced by oxidized low-density lipoprotein in HLFs along with the induction of MCP-1, IL-6, and MMP2 (Supplemental Figure S7).
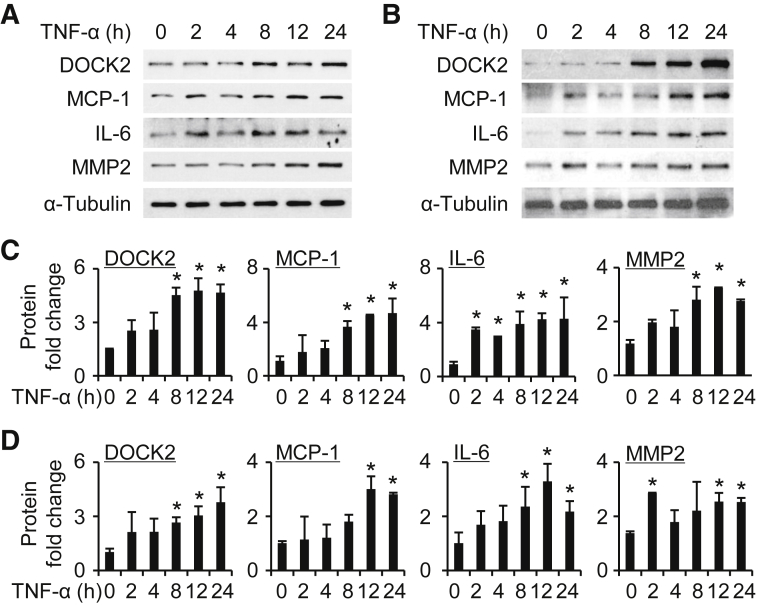
Figure 4.
Dedicator of cytokinesis 2 (DOCK2) is induced in a dose-dependent manner in human lung fibroblasts (HLFs) treated with tumor necrosis factor-α (TNF-α). A and C: Primary HLFs#1 (A) or HLFs#2 (C) were induced by TNF-α with 0, 5, and 10 ng/mL for 24 hours to detect DOCK2, monocyte chemoattractant protein-1 (MCP-1), IL-6, and matrix metalloproteinase 2 (MMP2) expression by Western blotting. α-Tubulin is a loading control. B and D: Quantification of protein expression as shown in A (B) and C (D). n = 3. ∗P < 0.05 versus vehicle group (0 ng/mL).
Figure 5.
Dedicator of cytokinesis 2 (DOCK2) is induced in a time-dependent manner in human lung fibroblasts (HLFs) treated with tumor necrosis factor-α (TNF-α). A and B: Primary HLFs#1 (A) or HLFs#2 (B) were treated with TNF-α (10 ng/mL) for various times (0, 2, 4, 8, 12, and 24 hours) and assessed for DOCK2, monocyte chemoattractant protein-1 (MCP-1), IL-6, matrix metalloproteinase 2 (MMP2), and α-tubulin expression by Western blotting. C and D: Quantification of protein expression as shown in A (C) or B (D). n = 3. ∗P < 0.05 versus vehicle group (0 hours).
DOCK2 Is Required for the Phenotypic Modulation of Proinflammatory LFs
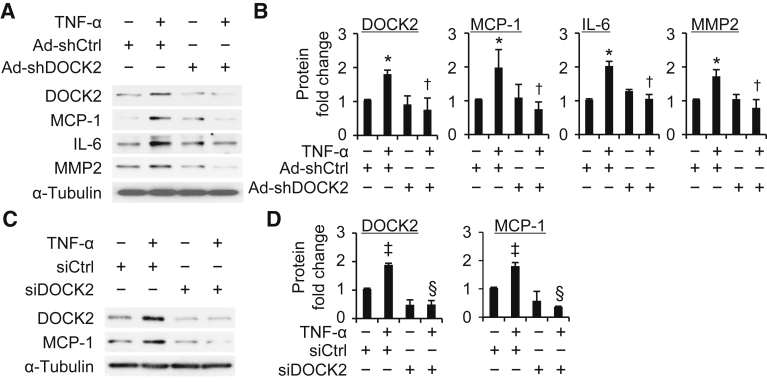
Next, the role of DOCK2 in a TNF-α–induced proinflammatory phenotypic change in LFs was explored. Primary HLFs were transduced with adenovirus control (Ad-shCtrl) or DOCK2 shRNA. Cells were then treated with TNF-α (10 ng/mL) for 24 hours to detect the expression of MCP-1, IL-6, and MMP2. TNF-α treatment increased MCP-1, IL-6, and MMP2 expression in the Ad-shCtrl groups in primary HLFs (Figure 6, A and B). Conversely, DOCK2 knockdown attenuated TNF-α–mediated induction of MCP-1, IL-6, and MMP2 (Figure 6, A and B). DOCK2 siRNA was next used to verify the role of DOCK2 in TNF-α–induced LF proinflammatory phenotypic change. HLFs were transduced with control siRNA or DOCK2 siRNA followed by TNF-α treatment. DOCK2 siRNA treatment significantly blocked TNF-α–mediated increases in DOCK2 expression in HLFs (Figure 6, C and D). Furthermore, TNF-α–induced MCP-1 expression was significantly reduced in DOCK2-downregulated HLFs compared with control siRNA cells (Figure 6, C and D). These data strongly support the inference that DOCK2 is required for LF proinflammatory phenotypic modulation.
Figure 6.
Dedicator of cytokinesis 2 (DOCK2) knockdown blocks the proinflammatory phenotype of human lung fibroblasts (HLFs) treated with tumor necrosis factor-α (TNF-α). A: Primary HLFs were infected with adenovirus scramble (Ad-shCtrl) or DOCK2 shRNA (Ad-shDOCK2) and treated with TNF-α (10 ng/mL) for 24 hours to detect DOCK2, monocyte chemoattractant protein-1 (MCP-1), IL-6, matrix metalloproteinase 2 (MMP2), and α-tubulin expression by Western blotting. B: Quantification of protein expression shown in A. C: Primary HLFs were transfected with control siRNA (siCtrl) or DOCK siRNA (siDOCK2) and treated with TNF-α (10 ng/mL) for 24 hours to detect DOCK2, MCP-1, and α-tubulin expression by Western blotting. D: Quantification of protein expression shown in C. n = 3. ∗P < 0.05 versus vehicle-treated Ad-shCtrl group; †P < 0.05 versus TNF-α–treated Ad-shCtrl group; ‡P < 0.05 versus vehicle-treated siCtrl group; §P < 0.05 versus TNF-α–treated siCtrl group.
Transcriptional Regulation of DOCK2 by TNF-α in LFs
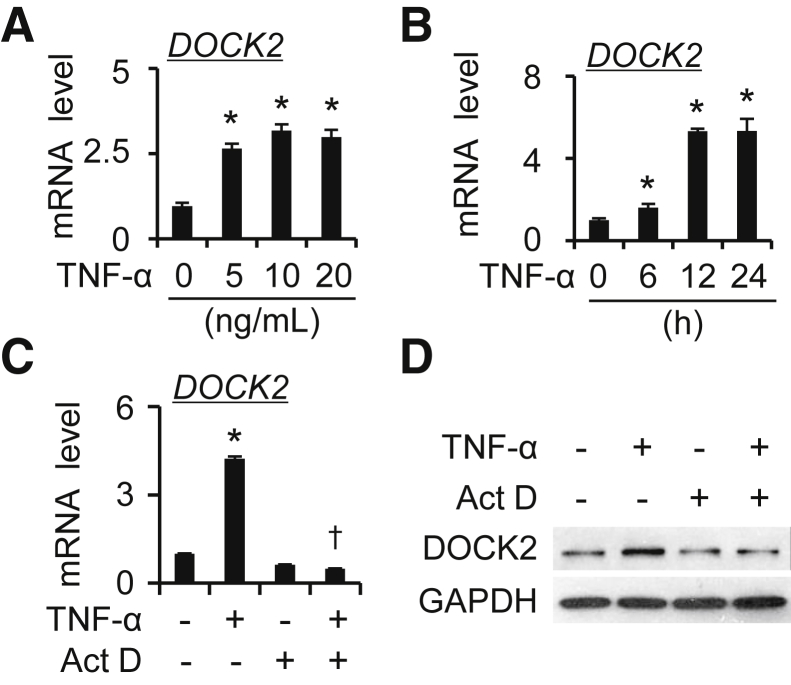
DOCK2 mRNA expression in TNF-α–treated (0, 5, and 10 ng/mL) HLFs was tested by using qPCR analyses. TNF-α treatment induced DOCK2 mRNA expression dose dependently in primary HLFs (Figure 7A and Supplemental Figure S8A). Time course analyses showed that DOCK2 mRNA was induced by TNF-α and peaked between 12 and 24 hours (Figure 7B and Supplemental Figure S8B). Moreover, pretreatment of cells with the transcription inhibitor actinomycin D (1 μg/mL) suppressed TNF-α–mediated induction of DOCK2 mRNA and protein levels in HLFs (Figure 7, C and D and Supplemental Figure S8, C and D). The results indicate that DOCK2 induction by TNF-α occurs at the transcriptional level.
Figure 7.
Dedicator of cytokinesis 2 (DOCK2) is induced by tumor necrosis factor-α (TNF-α) at the transcriptional level in human lung fibroblasts (HLFs). A: Primary HLFs#1 were treated with TNF-α (0, 5, 10, and 20 ng/mL) for 12 hours to detect DOCK2 mRNA expression by quantitative PCR (qPCR). B: Primary HLFs#1 were treated with TNF-α (10 ng/mL) for various times (0, 6, 12, and 24 hours) to detect DOCK2 mRNA expression by qPCR. C and D: Primary HLFs#1 were pretreated with actinomycin D (Act D; 1 μg/mL) for 30 minutes, followed by TNF-α (10 ng/mL) treatment for additional 12 hours. The DOCK2 mRNA and protein expression were detected by qPCR (C) and Western blotting with GAPDH as an internal control (C) or a loading control (D). n = 3. ∗P < 0.05 versus vehicle-treated group; †P < 0.05 versus TNF-α–treated group.
DOCK2 Mediates LF Proinflammatory Phenotype through PI3K/AKT and NF-κB Pathways
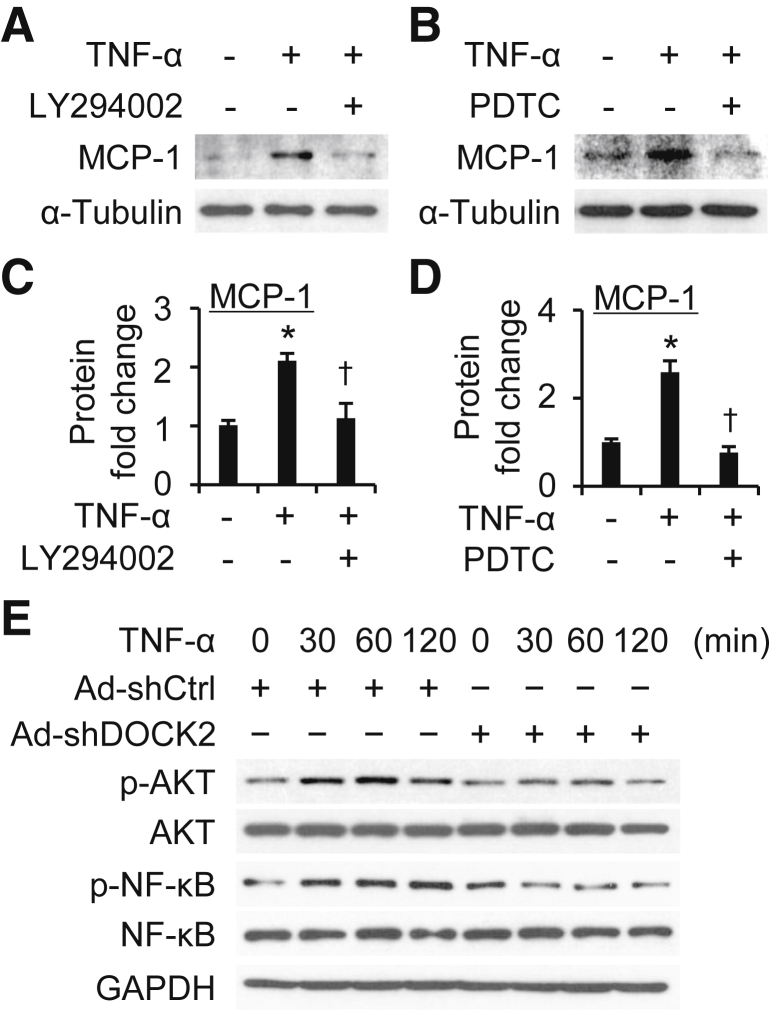
Multiple pathways have been implicated in TNF-α–induced proinflammatory responses, including phosphatidylinositol 3-kinase (PI3K)/AKT and NF-κB.54,58,59 To determine if PI3K/AKT and/or NF-κB pathways are involved in TNF-α–induced proinflammatory phenotypic modulation, HLFs were pretreated with LY294002 (PI3K/AKT inhibitor, 1 μg/mL) or pyrrolidine dithiocarbamate (NF-κB inhibitor, 1 μg/mL) for 30 minutes, followed by TNF-α treatment. MCP-1 expression was used as a reporter for these analyses. Both LY294002 and pyrrolidine dithiocarbamate successfully blocked TNF-α–induced MCP-1 expression (Figure 8, A–D), suggesting that the PI3K/AKT and NF-κB pathways are involved in TNF-α–induced LF proinflammatory phenotypic modulation.
Figure 8.
Dedicator of cytokinesis 2 (DOCK2) attenuates tumor necrosis factor-α (TNF-α)–induced lung fibroblast proinflammatory phenotypic changes through phosphatidylinositol 3-kinase/AKT and NF-κB pathways. A and B: Primary human lung fibroblasts (HLFs) were pretreated with LY294002 (1 μg/mL) (A) or pyrrolidine dithiocarbamate (PDTC) (1 μg/mL) (B) for 30 minutes, followed by TNF-α treatment to detect monocyte chemoattractant protein-1 (MCP-1) and α-tubulin expression. C and D: Quantification of protein expression shown in A (C) and B (D). E: Primary HLFs were infected with adenovirus scramble (Ad-shCtrl) or DOCK2 shRNA (Ad-shDOCK2) and treated with TNF-α for various times to detect phosphor (p-) and total AKT and NF-κB. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. n = 3. ∗P < 0.05 versus vehicle-treated group; †P < 0.05 versus TNF-α–treated group.
Next, the role of DOCK2 in TNF-α–induced activation of PI3K/AKT and/or NF-κB signaling pathways was determined. Primary HLFs were infected with Ad-shCtrl or Ad-shDOCK2 and treated with TNF-α for various times to detect phosphorylation and total PI3K/AKT and NF-κB. TNF-α rapidly induced phosphorylation of AKT and NF-κB p65 (Figure 8E) in Ad-shCtrl–transduced HLFs. Conversely, DOCK2 knockdown blocked TNF-α–induced phosphorylation of AKT and NF-κB p65 in the primary HLFs (Figure 8), indicating that DOCK2 mediates the TNF-α–induced LF proinflammatory phenotype via activation of PI3K/AKT and NF-κB signaling pathways.
Discussion
This study shows, for the first time, that DOCK2 is a novel regulator of the proinflammatory phenotype of LFs, which may contribute to the development of lung inflammation/injury in chronic HFHF diet–caused obesity. DOCK2 deficiency was found to inhibit pulmonary inflammation/injury induced by a chronic HFHF diet. DOCK2 expression was increased especially in the LFs of WT mice fed a chronic HFHF diet. DOCK2 knockdown attenuated TNF-α–induced MCP-1, IL-6, and MMP2 expression in primary HLFs, suggesting that it can regulate the severity of a chronic HFHF diet–associated lung inflammation/injury and that decreased expression of DOCK2 by LFs contributes to the response.
Obesity has been identified as a major risk factor for the development of lung diseases, including acute respiratory distress symptom, asthma, chronic obstructive pulmonary disease, and idiopathic pulmonary fibrosis.5,6 A high-fat diet has been widely used to generate obese rodent models to explore metabolic derangements.13,17 However, the fat content and fat composition of the diet, as well as the feeding period, may significantly affect the outcome. Several reports link lung injury to experimental obesity induced by long-term high fat alone or in combination with high fructose,10,22 consistent with the current findings. Herein, this study shows that pulmonary inflammation/injury occurs in WT mice with chronic HFHF diet-induced obesity. There was significant body weight gain in WT mice fed an HFHF diet for 20 weeks compared with those fed a chow diet (Figure 1A). In contrast to the chow diet group, the chronic HFHF diet caused lung inflammatory changes and increased lung collagen expression in WT mice (Figure 1, B–D), indicative of organization of the pulmonary injury. Macrophage infiltration (indicated by CD68 expression) was found to be induced by a chronic HFHF diet in WT mice (Figure 1E), consistent with a prior report.60 MCP-1 is one of the important chemokines for monocytes attraction that is inducible by TNF-α. A chronic HFHF diet indeed dramatically induced MCP-1 expression in the lung tissue of WT mice (Figure 1F). In addition, increased expression of the oxidative stress maker 4-hydroxynonenal was observed in the lungs of WT mice fed an HFHF diet (Supplemental Figure S1). These findings suggest that lung inflammation and subsequent fibrosis occur in WT mice with chronic HFHF diet-induced obesity.
Although a few prior reports have linked acute lung injury with a high-fat diet,10,22 our understanding of the underlying mechanism remains obscure. This study highlights a critical role of DOCK2 in mediating lung inflammation/injury associated with chronic HFHF diet–caused obesity. DOCK2 was dramatically induced in the lungs of WT mice fed a chronic HFHF diet, which largely co-localized with that of the fibroblast marker FSP1 (Figure 2). This finding suggests that DOCK2 can mediate a proinflammatory phenotype in LFs, contributing to lung inflammation and fibrosis associated with a chronic HFHF diet. Importantly, the data showed that DOCK2−/− inhibited the expression of CD68 and MCP-1 in the lung tissues of mice fed a chronic HFHF diet. This finding indicates that DOCK2 may regulate MCP-1 to induce macrophage infiltration in the lungs of these mice (Figure 3, B–F). In addition, DOCK2 deficiency attenuated lung inflammatory infiltration over a 10-week period of the HFHF diet (Supplemental Figure S4).
To our knowledge, this study is the first to report the critical role of DOCK2 in regulating lung inflammation induced by a chronic HFHF diet. Whether a chronic HFHF diet impairs lung function and the extent to which DOCK2 plays a role in mediating such effects require further investigation. DOCK2−/− suppresses obesity induced by a high-fat diet in WT C57BL/6 mice.13 In the current study, DOCK2−/− mice fed a high-fat diet exhibited increased mRNA and protein expression of metabolic genes related to β-oxidation, lipolysis, and thermogenesis (eg, PPAR-α, HSL, PGC1α) in adipose tissues with greater energy expenditure. Compared with WT mice fed a high-fat diet, DOCK2−/− mice fed a high-fat diet exhibited significantly increased brown adipose tissue content and brown adipose tissue marker gene expression (PGC1a, UCP1, and PRDM16), which are closely related to energy expenditure. Similarly, DOCK2−/− mice fed HFHF exhibited significantly lower body weight gain compared with WT counterparts (Figure 3A); this occurs likely due to the increased energy expenditure in adipose tissue as previously reported.13
Lung fibroblasts play a critical role in secretion of MCP-1, TNF-α, IL-1, and IL-6 in the process of lung inflammation/injury.23, 24, 25 However, little is known about the role of LFs in the pulmonary inflammation/injury associated with chronic HFHF diet–caused obesity. Increased DOCK2 expression was observed in the lungs of WT mice, particularly in the LFs of mice fed a chronic HFHF diet (Figure 2). Primary HLFs were therefore used to delineate the mechanism by which DOCK2 contributes to this response. A high-fat diet is known to induce adipose tissue inflammation, releasing TNF-α, IL-6, and other critical cytokines responsible for the systemic proinflammatory state.13,16 In addition, TNF-α has been shown to mediate lung injury associated with a high-fat diet.61,62 TNF-α was therefore used to treat primary HLFs to explore the regulation of the proinflammatory phenotype of LFs. The data showed that TNF-α induced DOCK2 expression in a dose- and time-dependent manner in primary HLFs (Figures 4 and 5). DOCK2 was also found to be induced by IL-1β dose dependently in primary HLFs (Supplemental Figure S6). Furthermore, TNF-α and IL-1β increased DOCK2 expression along with the induction of MCP-1, IL-6, and MMP2 in primary HLFs (Figures 4 and 5 and Supplemental Figure S6). Therefore, DOCK2 expression can be induced by TNF-α and IL-1β in HLFs.
Oxidized low-density lipoprotein is known to contribute to high-fat diet–induced lung injury and thus was used to treat HLFs. The data show that oxidized low-density lipoprotein also induces DOCK2 expression along with that of MCP-1, IL-6, and MMP2 in HLFs (Supplemental Figure S7). Importantly, DOCK2 knockdown by adenovirus shRNA or siRNA blocked TNF-α–induced MCP-1, IL-6, and MMP2 expression in primary HLFs (Figure 6). These data suggest that DOCK2 is required for the proinflammatory phenotypic change in LFs, which is an important process for lung injury. DOCK2 deficiency was previously reported to attenuate adipose tissue and systemic inflammation,13 which may contribute to lung injury indirectly. Therefore, DOCK may contribute to lung injury through direct effects on LF phenotypic regulation and indirectly through influencing body weight gain induced by HFHF diet.
Tissue fibrosis is a pathologic condition linked with chronic inflammatory diseases.63,64 For the first time, we found that DOCK2 is induced by an HFHF diet and DOCK2−/− significantly attenuates HFHF diet-induced lung fibrosis, as shown by collagen deposition (Figure 1, C and D, Figure 3, C and D, and Supplemental Figure S3). These findings implicate DOCK2 in both lung injury and subsequent parenchymal lung fibrosis caused by a chronic HFHF diet. The role of DOCK2 in mediating lung fibrosis is an interesting direction that is under active investigation in our group.
TNF-α induced DOCK2 expression at the mRNA level, which can be blocked by a transcriptional inhibitor, actinomycin D, in primary HLFs (Figure 7 and Supplemental Figure S8). Thus, DOCK2 was induced by TNF-α at the transcriptional level in primary HLFs. To decipher the mechanism by which DOCK2 is induced in TNF-α–treated LFs, the involvement of two of the most affected inflammatory signaling pathways downstream of TNF-α was tested (ie, the PI3K/AKT and NF-κB pathways). The results show that blockage of these two pathways with specific inhibitors abolished the effects of TNF-α–induced MCP-1 expression in primary HLFs (Figure 8, A–D). Furthermore, DOCK2 knockdown attenuated the phosphorylation of PI3K/AKT and NF-κB p65 in primary HLFs (Figure 8E), suggesting that DOCK2 mediates the TNF-α–induced proinflammatory phenotypic change in LFs with the participation of PI3K/AKT and NF-κB pathways.
A recent paper reported that DOCK2 mediates endotoxemia-induced acute lung injury through promoting macrophage activation.65 In addition to regulating a proinflammatory phenotype of LFs, DOCK2 may affect the activation and function of macrophages to contribute to lung inflammation/injury in chronic HFHF diet–caused obesity. Comprehensive examination of that possibility is beyond the scope of the current study. However, this study is the first to show that lung inflammation/injury associated with a chronic HFHF diet involves induction of DOCK2 and that DOCK2−/− mice were significantly protected from HFHF-induced organizing lung injury. A previous study reported that a high-fat diet induced progressive adipose tissue expansion into the diaphragm and impaired its contractility over a 6-month duration.10 It remains an interesting and open question whether DOCK2 is involved in HFHF-associated adverse effects on the skeletal muscles. In addition to HFHF, other methods, mainly involving genetic manipulation, can induce obesity in mice. Whether DOCK2 is implicated in lung injury associated with those methods inducing obesity is an interesting field of study that warrants further investigation.
Taken together, the findings from this study show a novel mechanistic role for DOCK2 in modulating proinflammatory phenotype of LFs, which may contribute to pulmonary inflammation/injury development in chronic HFHF diet–caused obesity. Attenuation of fibrotic repair was also found. These pivotal roles of DOCK2 raise the possibility that targeting of DOCK2 may improve lung injury associated with chronic HFHF diet–caused obesity.
Author Contributions
G.Q. and X.G. designed the study, wrote the first draft of the manuscript, and interpreted data; S.H. generated primary cells and tissues; O.A., C.S., G.Q., and X.G. collected data; and S.H., S.C., T.K., and S.I. critically reviewed the manuscript.
Footnotes
Supported by the NIH (HL141583, X.G.); University of Texas Rising Star Award (X.G.); and the University of Texas Health Science Center at Tyler Startup fund (G.Q. and X.G.).
G.Q. and O.A. contributed equally to this work.
Disclosures: S.I. declares no conflict of interest with regard to the present study but declares that he is a founder of Lung Therapeutics, Inc.; has an equity position in that company; and is paid as Chief Scientific Officer to the company. His involvement with Lung Therapeutics, Inc. is managed by a Conflict of Interest Management Plan at the University of Texas Health Science Center at Tyler. All other authors declare no conflict of interest.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2021.10.011.
Supplemental Data
Chronic high-fat and high-fructose (HFHF) diet induces 4-hydroxynonenal (4HNE) expression in the lungs of wild-type mice. Representative immunofluorescence staining of the expression of 4HNE (red) in lung tissues from wild-type C57BL/6 mice fed a chow diet or an HFHF diet for 20 weeks. DAPI stained the nuclei (blue). Scale bar = 100 μm.
No significant difference in lung parenchyma between wild-type (WT) and dedicator of cytokinesis 2 knockout (DOCK2−/−) mice fed a chow diet. Representative hematoxylin and eosin (H&E) (A) and Masson’s staining (B) of lung tissues from WT C57BL/6 mice fed a normal chow diet for 20 weeks. Scale bar = 100 μm.
Immunohistochemical staining of collagen in the lungs of wild-type (WT) and dedicator of cytokinesis 2 knockout (DOCK2−/−) mice fed a chow diet or a high-fat and high-fructose (HFHF) diet. Representative immunohistochemical staining of collagen in lung tissues from WT C57BL/6 and DOCK2−/− mice fed a chow diet or HFHF diet for 20 weeks. The black boxes indicate the areas enlarged for view. n = 6 to 8. Scale bar = 100 μm.
Lung inflammation in wild-type (WT) and dedicator of cytokinesis 2 knockout (DOCK2−/−) mice fed a 10-week chow diet or a high-fat and high-fructose (HFHF) diet. Representative hematoxylin and eosin (A) and monocyte chemoattractant protein-1 (MCP-1) (red) (B) immunofluorescence staining of lung tissues from WT C57BL/6 and DOCK2−/− mice fed a chow diet or an HFHF diet for 10 weeks. The black boxes indicate the areas enlarged for view. n = 5. Scale bar = 100 μm.
Collagen deposition in lung tissues of wild-type (WT) and dedicator of cytokinesis 2 knockout (DOCK2−/−) mice fed a 10-week chow diet or a high-fat and high-fructose (HFHF) diet. Representative Masson’s staining (A) and immunohistochemical staining (B) of collagen in lung tissues from WT C57BL/6 and DOCK2−/− mice fed a chow diet or an HFHF diet for 10 weeks. The black boxes indicate the areas enlarged for view. n = 5. Scale bar = 100 μm.
Dedicator of cytokinesis 2 (DOCK2) induced in human lung fibroblasts (HLFs) treated with IL-1β. A and C: Primary HLFs#1 (A) or HLFs#2 (C) were induced by IL-1β with 0, 5, and 10 ng/mL for 24 hours to detect DOCK2, monocyte chemoattractant protein-1 (MCP-1), IL-6, and matrix metalloproteinase 2 (MMP2) expression by Western blotting. α-Tubulin is a loading control. B and D: Quantification of protein expression as shown in A (B) and C (D). n = 3. ∗P < 0.05 versus vehicle group (0 ng/mL).
Dedicator of cytokinesis 2 (DOCK2) induced with lung fibroblast proinflammatory phenotypic change by oxidized low-density lipoprotein (ox-LDL). A: Primary human lung fibroblasts (HLFs) were induced by ox-LDL (50 μg/mL) for 24 hours to detect DOCK2, monocyte chemoattractant protein-1 (MCP-1), IL-6, and matrix metalloproteinase 2 (MMP2) expression by Western blotting. α-Tubulin is a loading control. B: Quantification of protein expression in A. ∗P < 0.05 versus vehicle group (0 ng/mL). n = 3.
Dedicator of cytokinesis 2 (DOCK2) induced by tumor necrosis factor-α (TNF-α) at the transcriptional level in human lung fibroblasts (HLFs). A: Primary HLFs were treated with TNF-α (0, 5, 10, and 20 ng/mL) for 12 hours to detect DOCK2 mRNA expression by quantitative PCR (qPCR). B: Primary HLFs were treated with TNF-α (10 ng/mL) for various times (0, 6, 12, and 24 hours) to detect DOCK2 mRNA expression by qPCR. C and D: Primary HLFs were pretreated with actinomycin D (Act D) (1 μg/mL) for 30 minutes, followed by TNF-α (10 ng/mL) treatment for additional 12 hours. The DOCK2 mRNA and protein expression were detected by qPCR (C) and Western blotting with GAPDH as an internal control (C) or as a loading control (D). n = 3. ∗P < 0.05 versus vehicle-treated group; †P < 0.05 versus TNF-α–treated group.
References
- 1.Malik V.S., Willet W.C., Hu F.B. Nearly a decade on—trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol. 2020;16:615–616. doi: 10.1038/s41574-020-00411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell N.S., Catenacci V.A., Wyatt H.R., Hill J.O. Obesity: overview of an epidemic. Psychiatr Clin North Am. 2011;34:717–732. doi: 10.1016/j.psc.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreyeva T., Sturm R., Ringel J.S. Moderate and severe obesity have large differences in health care costs. Obes Res. 2004;12:1936–1943. doi: 10.1038/oby.2004.243. [DOI] [PubMed] [Google Scholar]
- 4.Bendor C.D., Bardugo A., Pinhas-Hamiel O., Afek A., Twig G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol. 2020;19:79. doi: 10.1186/s12933-020-01052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters U., Suratt B.T., Bates J.H.T., Dixon A.E. Beyond BMI: obesity and lung disease. Chest. 2018;153:702–709. doi: 10.1016/j.chest.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong M.N., Bajwa E.K., Thompson B.T., Christiani D.C. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65:44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beuther D.A., Sutherland E.R. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyake Y., Sasaki S., Yokoyama T., Chida K., Azuma A., Suda T., Kudoh S., Sakamoto N., Okamoto K., Kobashi G., Washio M., Inaba Y., Tanaka H., Japan Idiopathic Pulmonary Fibrosis Study Group Dietary fat and meat intake and idiopathic pulmonary fibrosis: a case-control study in Japan. Int J Tuberc Lung Dis. 2006;10:333–339. [PubMed] [Google Scholar]
- 9.Scoditti E., Massaro M., Garbarino S., Toraldo D.M. Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients. 2019;11:1357. doi: 10.3390/nu11061357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buras E.D., Converso-Baran K., Davis C.S., Akama T., Hikage F., Michele D.E., Brooks S.V., Chun T.-H. Fibro-adipogenic remodeling of the diaphragm in obesity-associated respiratory dysfunction. Diabetes. 2019;68:45–56. doi: 10.2337/db18-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones R.L., Nzekwu M.-M.U. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 12.Xu P., Gärtner F., Gihring A., Liu C., Burster T., Wabitsch M., Knippschild U., Paschke S. Influence of obesity on remodeling of lung tissue and organization of extracellular matrix after blunt thorax trauma. Respir Res. 2020;21:238. doi: 10.1186/s12931-020-01502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo X., Li F., Xu Z., Yin A., Yin H., Li C., Chen S.-Y. DOCK2 deficiency mitigates HFD-induced obesity by reducing adipose tissue inflammation and increasing energy expenditure. J Lipid Res. 2017;58:1777–1784. doi: 10.1194/jlr.M073049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilg H., Moschen A.R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 15.O’Rourke R.W. Inflammation in obesity-related diseases. Surgery. 2009;145:255–259. doi: 10.1016/j.surg.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellulu M.S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buettner R., Schölmerich J., Bollheimer L.C. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 18.Kohli R., Kirby M., Xanthakos S.A., Softic S., Feldstein A.E., Saxena V., Tang P.H., Miles L., Miles M.V., Balistreri W.F., Woods S.C., Seeley R.J. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–944. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Softic S., Meyer J.G., Wang G.-X., Gupta M.K., Batista T.M., Lauritzen H.P.M.M., Fujisaka S., Serra D., Herrero L., Willoughby J., Fitzgerald K., Ilkayeva O., Newgard C.B., Gibson B.W., Schilling B., Cohen D.E., Kahn C.R. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab. 2019;30:735–753.e4. doi: 10.1016/j.cmet.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangüesa G., Baena M., Hutter N., Montañés J.C., Sánchez R.M., Roglans N., Laguna J.C., Alegret M. The addition of liquid fructose to a western-type diet in LDL-R–/– mice induces liver inflammation and fibrogenesis markers without disrupting insulin receptor signalling after an insulin challenge. Nutrients. 2017;9:278. doi: 10.3390/nu9030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragno M., Tomasinelli C.E., Vercellinatto I., Catalano M.G., Collino M., Fantozzi R., Danni O., Boccuzzi G. SREBP-1c in nonalcoholic fatty liver disease induced by Western-type high-fat diet plus fructose in rats. Free Radic Biol Med. 2009;47:1067–1074. doi: 10.1016/j.freeradbiomed.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Vedova M.C.D., Soler Garcia F.M., Muñoz M.D., Fornes M.W., Gomez Mejiba S.E., Gómez N.N., Ramirez D.C. Diet-induced pulmonary inflammation and incipient fibrosis in mice: a possible role of neutrophilic inflammation. Inflammation. 2019;42:1886–1900. doi: 10.1007/s10753-019-01051-9. [DOI] [PubMed] [Google Scholar]
- 23.Phipps R.P., Penney D.P., Keng P., Silvera M., Harkins S., Derdak S. Immune functions of subpopulations of lung fibroblasts. Immunol Res. 1990;9:275–286. doi: 10.1007/BF02935527. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman J., Graf B.A., Leung E.C., Pollock S.J., Koumas L., Reddy S.Y., Blieden T.M., Smith T.J., Phipps R.P. Fibroblasts as sentinel cells: role of the CDcd40-CDcd40 ligand system in fibroblast activation and lung inflammation and fibrosis. Chest. 2001;120 Suppl 1:53S–55S. doi: 10.1378/chest.120.1_suppl.s53. [DOI] [PubMed] [Google Scholar]
- 25.Pardo A., Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2016;13(Suppl 5):S417–S421. doi: 10.1513/AnnalsATS.201605-341AW. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z., Sun Z., Liu H., Ren Y., Shao D., Zhang W., Lin J., Wolfram J., Wang F., Nie S. Connective tissue growth factor stimulates the proliferation, migration and differentiation of lung fibroblasts during paraquat-induced pulmonary fibrosis. Mol Med Rep. 2015;12:1091–1097. doi: 10.3892/mmr.2015.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffery P. Inflammation and remodeling in the adult and child with asthma. Pediatr Pulmonol Suppl. 2001;21:3–16. [PubMed] [Google Scholar]
- 28.Profita M., Bonanno A., Siena L., Bruno A., Ferraro M., Montalbano A.M., Albano G.D., Riccobono L., Casarosa P., Pieper M.P., Gjomarkaj M. Smoke, choline acetyltransferase, muscarinic receptors, and fibroblast proliferation in chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2009;329:753–763. doi: 10.1124/jpet.108.145888. [DOI] [PubMed] [Google Scholar]
- 29.Standiford T.J., Rolfe M.R., Kunkel S.L., Lynch J.P., 3rd, Becker F.S., Orringer M.B., Phan S., Strieter R.M. Altered production and regulation of monocyte chemoattractant protein-1 from pulmonary fibroblasts isolated from patients with idiopathic pulmonary fibrosis. Chest. 1993;103 Suppl 2:121S. doi: 10.1378/chest.103.2_supplement.121s. [DOI] [PubMed] [Google Scholar]
- 30.Hogaboam C.M., Smith R.E., Kunkel S.L. Dynamic interactions between lung fibroblasts and leukocytes: implications for fibrotic lung disease. Proc Assoc Am Physicians. 1998;110:313–320. [PubMed] [Google Scholar]
- 31.Kendall R.T., Feghali-Bostwick C.A. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Chouly C.A.E., Astier A., Jacob C., Pruniaux M.-P., Bertrand C., Lagente V. Modulation of matrix metalloproteinase production from human lung fibroblasts by type 4 phosphodiesterase inhibitors. Life Sci. 2004;75:823–840. doi: 10.1016/j.lfs.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Pegorier S., Campbell G.A., Kay A.B., Lloyd C.M. Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF) Respir Res. 2010;11:85. doi: 10.1186/1465-9921-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukui Y., Hashimoto O., Sanui T., Oono T., Koga H., Abe M., Inayoshi A., Noda M., Oike M., Shirai T., Sasazuki T. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 35.Gotoh K., Tanaka Y., Nishikimi A., Inayoshi A., Enjoji M., Takayanagi R., Sasazuki T., Fukui Y. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood. 2008;111:2973–2976. doi: 10.1182/blood-2007-09-112169. [DOI] [PubMed] [Google Scholar]
- 36.García-Bernal D., Sotillo-Mallo E., Nombela-Arrieta C., Samaniego R., Fukui Y., Stein J.V., Teixidó J. DOCK2 is required for chemokine-promoted human T lymphocyte adhesion under shear stress mediated by the integrin alpha4beta1. J Immunol. 2006;177:5215–5225. doi: 10.4049/jimmunol.177.8.5215. [DOI] [PubMed] [Google Scholar]
- 37.Guo X., Chen S.-Y. Dedicator of cytokinesis 2 in cell signaling regulation and disease development. J Cell Physiol. 2017;232:1931–1940. doi: 10.1002/jcp.25512. [DOI] [PubMed] [Google Scholar]
- 38.Wang L., Nishihara H., Kimura T., Kato Y., Tanino M., Nishio M., Obara M., Endo T., Koike T., Tanaka S. DOCK2 regulates cell proliferation through Rac and ERK activation in B cell lymphoma. Biochem Biophys Res Commun. 2010;395:111–115. doi: 10.1016/j.bbrc.2010.03.148. [DOI] [PubMed] [Google Scholar]
- 39.Wu M., Hamaker M., Li L., Small D., Duffield A.S. DOCK2 interacts with FLT3 and modulates the survival of FLT3-expressing leukemia cells. Leukemia. 2017;31:688–696. doi: 10.1038/leu.2016.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo X., Shi N., Cui X.-B., Wang J.-N., Fukui Y., Chen S.-Y. Dedicator of cytokinesis 2, a novel regulator for smooth muscle phenotypic modulation and vascular remodeling. Circ Res. 2015;116:e71–e80. doi: 10.1161/CIRCRESAHA.116.305863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Sousa Rodrigues M.E., Bekhbat M., Houser M.C., Chang J., Walker D.I., Jones D.P., Oller do Nascimento C.M.P., Barnum C.J., Tansey M.G. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behav Immun. 2017;59:158–172. doi: 10.1016/j.bbi.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeffers A., Qin W., Owens S., Koenig K.B., Komatsu S., Giles F.J., Schmitt D.M., Idell S., Tucker T.A. Glycogen synthase kinase-3[beta] inhibition with 9-ING-41 attenuates the progression of pulmonary fibrosis. Sci Rep. 2019;9:18925. doi: 10.1038/s41598-019-55176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo X., Stice S.L., Boyd N.L., Chen S.-Y. A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell-derived mesenchymal cells. Am J Physiol Cell Physiol. 2013;304:C289–C298. doi: 10.1152/ajpcell.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo X., Jose P.A., Chen S.-Y. Response gene to complement 32 interacts with Smad3 to promote epithelial-mesenchymal transition of human renal tubular cells. Am J Physiol Cell Physiol. 2011;300:C1415–C1421. doi: 10.1152/ajpcell.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng L., Qian G., Zhang S., Zheng H., Fan S., Lesinski G.B., Owonikoko T.K., Ramalingam S.S., Sun S.-Y. Inhibition of mTOR complex 1/p70 S6 kinase signaling elevates PD-L1 levels in human cancer cells through enhancing protein stabilization accompanied with enhanced [beta]-TrCP degradation. Oncogene. 2019;38:6270–6282. doi: 10.1038/s41388-019-0877-4. [DOI] [PubMed] [Google Scholar]
- 46.Qian G., Wang D., Magliocca K.R., Hu Z., Nannapaneni S., Kim S., Chen Z., Sun S.-Y., Shin D.M., Saba N.F., Chen Z.G. Human papillomavirus oncoprotein E6 upregulates c-Met through p53 downregulation. Eur J Cancer. 2016;65:21–32. doi: 10.1016/j.ejca.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian G., Yao W., Zhang S., Bajpai R., Hall W.D., Shanmugam M., Lonial S., Sun S.-Y. Co-inhibition of BET and proteasome enhances ER stress and Bim-dependent apoptosis with augmented cancer therapeutic efficacy. Cancer Lett. 2018;435:44–54. doi: 10.1016/j.canlet.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Dong K., Guo X., Chen W., Hsu A.C., Shao Q., Chen J.-F., Chen S.-Y. Mesenchyme homeobox 1 mediates transforming growth factor-[beta] (TGF-[beta])-induced smooth muscle cell differentiation from mouse mesenchymal progenitors. J Biol Chem. 2018;293:8712–8719. doi: 10.1074/jbc.RA118.002350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shvedova A.A., Yanamala N., Murray A.R., Kisin E.R., Khaliullin T., Hatfield M.K., Tkach A.V., Krantz Q.T., Nash D., King C., Ian Gilmour M., Gavett S.H. Oxidative stress, inflammatory biomarkers, and toxicity in mouse lung and liver after inhalation exposure to 100% biodiesel or petroleum diesel emissions. J Toxicol Environ Health A. 2013;76:907–921. doi: 10.1080/15287394.2013.825217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hector A., Griese M., Hartl D. Oxidative stress in cystic fibrosis lung disease: an early event, but worth targeting? Eur Respir J. 2014;44:17–19. doi: 10.1183/09031936.00038114. [DOI] [PubMed] [Google Scholar]
- 52.Lawson W.E., Polosukhin V.V., Zoia O., Stathopoulos G.T., Han W., Plieth D., Loyd J.E., Neilson E.G., Blackwell T.S. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:899–907. doi: 10.1164/rccm.200311-1535OC. [DOI] [PubMed] [Google Scholar]
- 53.Yang J., Park Y., Zhang H., Gao X., Wilson E., Zimmer W., Abbott L., Zhang C. Role of MCP-1 in tumor necrosis factor-alpha-induced endothelial dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;297:H1208–H1216. doi: 10.1152/ajpheart.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu J., Yong W., Wu X., Yu Y., Lv J., Liu C., Mao X., Zhu Y., Xu K., Han X., Liu C. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun. 2008;369:471–477. doi: 10.1016/j.bbrc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 55.Mitra S., Goyal T., Mehta J.L. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25:419–429. doi: 10.1007/s10557-011-6341-5. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Q., Yu J., Guo T., Tian L., Quan J., Lin W., Niu X.E., Liu J. High glucose/ox-LDL induced hepatic sinusoidal capillarization via [alphavbeta]5/FAK/ERK signaling pathway. Biochem Biophys Res Commun. 2019;513:1055–1062. doi: 10.1016/j.bbrc.2019.04.082. [DOI] [PubMed] [Google Scholar]
- 57.Dai Y., Palade P., Wang X., Mercanti F., Ding Z., Dai D., Mehta J.L. High fat diet causes renal fibrosis in LDLr-null mice through MAPK-NF-[kappa]B pathway mediated by Ox-LDL. J Cardiovasc Pharmacol. 2014;63:158–166. doi: 10.1097/FJC.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 58.Coward W.R., Okayama Y., Sagara H., Wilson S.J., Holgate S.T., Church M.K. NF-kappa B and TNF-alpha: a positive autocrine loop in human lung mast cells? J Immunol. 2002;169:5287–5293. doi: 10.4049/jimmunol.169.9.5287. [DOI] [PubMed] [Google Scholar]
- 59.Murao K., Ohyama T., Imachi H., Ishida T., Cao W.M., Namihira H., Sato M., Wong N.C., Takahara J. TNF-alpha stimulation of MCP-1 expression is mediated by the Akt/PKB signal transduction pathway in vascular endothelial cells. Biochem Biophys Res Commun. 2000;276:791–796. doi: 10.1006/bbrc.2000.3497. [DOI] [PubMed] [Google Scholar]
- 60.Plataki M., Fan L., Sanchez E., Huang Z., Torres L.K., Imamura M., Zhu Y., Cohen D.E., Cloonan S.M., Choi A.M. Fatty acid synthase downregulation contributes to acute lung injury in murine diet-induced obesity. JCI Insight. 2019;5:e127823. doi: 10.1172/jci.insight.127823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naura A.S., Hans C.P., Zerfaoui M., Errami Y., Ju J., Kim H., Matrougui K., Kim J.G., Boulares A.H. High-fat diet induces lung remodeling in ApoE-deficient mice: an association with an increase in circulatory and lung inflammatory factors. Lab Invest. 2009;89:1243–1251. doi: 10.1038/labinvest.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J.Y., Sohn J.-H., Lee J.-H., Park J.-W. Obesity increases airway hyperresponsiveness via the TNF-[alpha] pathway and treating obesity induces recovery. PLoS One. 2015;10:e0116540. doi: 10.1371/journal.pone.0116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wuyts W.A., Agostini C., Antoniou K.M., Bouros D., Chambers R.C., Cottin V., Egan J.J., Lambrecht B.N., Lories R., Parfrey H., Prasse A., Robalo-Cordeiro C., Verbeken E., Verschakelen J.A., Wells A.U., Verleden G.M. The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J. 2013;41:1207–1218. doi: 10.1183/09031936.00073012. [DOI] [PubMed] [Google Scholar]
- 65.Xu X., Su Y., Wu K., Pan F., Wang A. DOCK2 contributes to endotoxemia-induced acute lung injury in mice by activating proinflammatory macrophages. Biochem Pharmacol. 2021;184:114399. doi: 10.1016/j.bcp.2020.114399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chronic high-fat and high-fructose (HFHF) diet induces 4-hydroxynonenal (4HNE) expression in the lungs of wild-type mice. Representative immunofluorescence staining of the expression of 4HNE (red) in lung tissues from wild-type C57BL/6 mice fed a chow diet or an HFHF diet for 20 weeks. DAPI stained the nuclei (blue). Scale bar = 100 μm.
No significant difference in lung parenchyma between wild-type (WT) and dedicator of cytokinesis 2 knockout (DOCK2−/−) mice fed a chow diet. Representative hematoxylin and eosin (H&E) (A) and Masson’s staining (B) of lung tissues from WT C57BL/6 mice fed a normal chow diet for 20 weeks. Scale bar = 100 μm.
Immunohistochemical staining of collagen in the lungs of wild-type (WT) and dedicator of cytokinesis 2 knockout (DOCK2−/−) mice fed a chow diet or a high-fat and high-fructose (HFHF) diet. Representative immunohistochemical staining of collagen in lung tissues from WT C57BL/6 and DOCK2−/− mice fed a chow diet or HFHF diet for 20 weeks. The black boxes indicate the areas enlarged for view. n = 6 to 8. Scale bar = 100 μm.
Lung inflammation in wild-type (WT) and dedicator of cytokinesis 2 knockout (DOCK2−/−) mice fed a 10-week chow diet or a high-fat and high-fructose (HFHF) diet. Representative hematoxylin and eosin (A) and monocyte chemoattractant protein-1 (MCP-1) (red) (B) immunofluorescence staining of lung tissues from WT C57BL/6 and DOCK2−/− mice fed a chow diet or an HFHF diet for 10 weeks. The black boxes indicate the areas enlarged for view. n = 5. Scale bar = 100 μm.
Collagen deposition in lung tissues of wild-type (WT) and dedicator of cytokinesis 2 knockout (DOCK2−/−) mice fed a 10-week chow diet or a high-fat and high-fructose (HFHF) diet. Representative Masson’s staining (A) and immunohistochemical staining (B) of collagen in lung tissues from WT C57BL/6 and DOCK2−/− mice fed a chow diet or an HFHF diet for 10 weeks. The black boxes indicate the areas enlarged for view. n = 5. Scale bar = 100 μm.
Dedicator of cytokinesis 2 (DOCK2) induced in human lung fibroblasts (HLFs) treated with IL-1β. A and C: Primary HLFs#1 (A) or HLFs#2 (C) were induced by IL-1β with 0, 5, and 10 ng/mL for 24 hours to detect DOCK2, monocyte chemoattractant protein-1 (MCP-1), IL-6, and matrix metalloproteinase 2 (MMP2) expression by Western blotting. α-Tubulin is a loading control. B and D: Quantification of protein expression as shown in A (B) and C (D). n = 3. ∗P < 0.05 versus vehicle group (0 ng/mL).
Dedicator of cytokinesis 2 (DOCK2) induced with lung fibroblast proinflammatory phenotypic change by oxidized low-density lipoprotein (ox-LDL). A: Primary human lung fibroblasts (HLFs) were induced by ox-LDL (50 μg/mL) for 24 hours to detect DOCK2, monocyte chemoattractant protein-1 (MCP-1), IL-6, and matrix metalloproteinase 2 (MMP2) expression by Western blotting. α-Tubulin is a loading control. B: Quantification of protein expression in A. ∗P < 0.05 versus vehicle group (0 ng/mL). n = 3.
Dedicator of cytokinesis 2 (DOCK2) induced by tumor necrosis factor-α (TNF-α) at the transcriptional level in human lung fibroblasts (HLFs). A: Primary HLFs were treated with TNF-α (0, 5, 10, and 20 ng/mL) for 12 hours to detect DOCK2 mRNA expression by quantitative PCR (qPCR). B: Primary HLFs were treated with TNF-α (10 ng/mL) for various times (0, 6, 12, and 24 hours) to detect DOCK2 mRNA expression by qPCR. C and D: Primary HLFs were pretreated with actinomycin D (Act D) (1 μg/mL) for 30 minutes, followed by TNF-α (10 ng/mL) treatment for additional 12 hours. The DOCK2 mRNA and protein expression were detected by qPCR (C) and Western blotting with GAPDH as an internal control (C) or as a loading control (D). n = 3. ∗P < 0.05 versus vehicle-treated group; †P < 0.05 versus TNF-α–treated group.