Abstract
Background
Controversy exists as to whether neoadjuvant chemotherapy improves survival in patients with invasive bladder cancer, despite randomised controlled trials (RCTs) involving over 3000 patients.
Objectives
To conduct a systematic review and meta‐analysis of individual patient data to evaluate the effect of neoadjuvant chemotherapy on survival in patients with this invasive bladder cancer.
Search methods
MEDLINE and Cancerlit searches were supplemented with information from registers and by hand searching meeting proceedings and also by discussion with relevant trialists and organisations. These have been regularly updated until June 2003.
Selection criteria
Trials that aimed to randomise patients with biopsy proven invasive (i.e. clinical stage T2 to T4a) transitional cell carcinoma of the bladder to receive local definitive treatment with or without neoadjuvant chemotherapy were eligible for inclusion.
Data collection and analysis
We collected, validated and re‐analysed updated data on all randomised patients from all available randomised trials, including 3005 patients from 11 RCTs. For all outcomes, we obtained overall pooled hazard ratios using the fixed effects model. To explore the potential impact of trial design we pre‐planned analyses that grouped trials by important aspects of their design that might influence the treatment effect. To investigate any differences in effect by pre‐defined patient subgroups we used a stratified logrank analysis on the primary endpoint of survival.
Main results
These results include data from one extra trial and so update those in the original publication ABC 2003. Platinum based combination chemotherapy showed a significant benefit on overall survival with a combined hazard ratio (HR) 0.86 (95% CI 0.77 to 0.95, P = 0.003); 14% reduction in the risk of death; 5% absolute benefit at 5 years (95% CI 1% to 7%); overall survival increased from 45% to 50%. This effect was observed irrespective of the type of local treatment and did not vary between subgroups of patients. The HR for all trials, including those that used single‐agent cisplatin, tended to favour neoadjuvant chemotherapy (HR= 0.89, 95% CI 0.81 to 0.98, P = 0.022). Although platinum based combination chemotherapy was beneficial, there was no clear evidence to support the use of single‐agent platinum, indeed there was significant difference in the effect between these groups of trials (P = 0.029).
Authors' conclusions
This improvement in survival encourages the use of platinum based combination chemotherapy for patients with invasive bladder cancer.
Plain language summary
Adding chemotherapy before surgery and/or radiotherapy in patients with invasive bladder cancer.
The standard treatment for invasive bladder cancer is surgery (to remove the bladder and surrounding tissues), and/or radiotherapy (to kill the cancer cells). This review suggests that 50 out of 100 patients will be alive at five years, when they are given chemotherapy using a platinum drug in combination with other drugs, before having surgery and/or radiotherapy. This is compared to 45 out of every 100 patients who were given surgery and/or radiotherapy without chemotherapy. This benefit of platinum‐based combination chemotherapy was seen in all types of patients and encourages its use for the treatment of invasive bladder cancer. However, chemotherapy based on a single platinum drug did not help patients live longer, and is not recommended.
Background
Bladder cancer is the second most common cancer of the genito‐urinary system; 80% of all cases are in men. Worldwide estimates suggest that the frequency of bladder cancer is about 336,000 new cases per year (Parkin 1999), of which about a third are likely to be invasive or metastatic disease.
Neoadjuvant chemotherapy, given before local treatment, may reduce primary tumour volume and could be effective in the control of metastatic disease when the volume of micrometastases is likely to be small (Soloway 1981; Fagg 1984; Raghavan 1984). This could be important since about half of the patients who present with invasive disease are likely to have occult metastases. Furthermore, patients may best tolerate chemotherapy before they have received potentially debilitating local treatment with either surgery or radiotherapy. Local treatments may also affect drug delivery by altering blood supply especially to the tissues affected by the tumour (Martinez 1998). Therefore, neoadjuvant chemotherapy has the potential to deliver the drugs more efficiently and at higher doses than in the adjuvant setting and provides an opportunity to prospectively assess the response to chemotherapy.
Several randomised trials, most of which have included platinum‐based regimens (Abol‐Enein 1997; MRC/EORTC 1999; Bassi 1999; Cortesi (unpub); Malmstrom 1996; Martinez 1995; Raghavan 1991; Sengelov 2002; Shearer 1988; Sherif 2002; Grossman 2003) have been done to investigate the use of neoadjuvant chemotherapy. These trials, which have been undertaken over almost twenty years, have mostly been of modest size and shown inconclusive results. However, combining the results of all of the relevant randomised trials in a meta‐analysis could provide sufficient evidence and increase the statistical power to reliably assess the value of neoadjuvant chemotherapy in the treatment of invasive bladder cancer. In 1991, we initiated and coordinated a systematic review and meta‐analysis of individual patient data from all of the existing trials that compared local definitive treatment with or without neoadjuvant systemic chemotherapy (ABCOC 1995). At that time, only four trials, including less than 500 patients, were available The results showed no clear evidence of either benefit or harm from the treatment and it was concluded that further large‐scale randomised evidence was necessary. Subsequent systematic reviews and meta‐analyses based on summary data extracted from trial reports have been of limited value, because of their methodological limitations and because only a subset of the trials were published at the time (Parmar 1999; Sternberg 2001). No good evidence existed to suggest that neoadjuvant chemotherapy, with either cisplatin alone, or in combination with other agents, improved survival in this group of patients.
We therefore initiated a new systematic review and meta‐analysis of individual patient data to build on the previous project and to collect, validate and re‐analyse trial data on all randomised patients from all relevant trials. Use of data from individual patients has many advantages in such a meta‐analysis (Stewart 1995). In particular, such data permit time‐to‐event analyses, which are extremely important in diseases where prolongation of survival, rather than cure, is anticipated for most patients. They also allow analyses to assess whether chemotherapy is more or less effective in different subgroups of patients. Importantly, there is evidence from the cancer field that meta‐analyses based on data extracted from published reports can give different results from those based on updated individual patient data (AOCTG 1991; Stewart 1993; Clarke 1998). Our meta‐analysis was initiated and coordinated by the Medical Research Council (UK) Clinical Trials Unit.
Objectives
This meta‐analysis forms the main part of a broader project looking at the effect of chemotherapy given in three different ways in invasive bladder cancer: (1) before local treatment (neoadjuvant); (2) during local treatment (concurrent) and (3) after local treatment (adjuvant). We followed a detailed prespecified protocol, which defines the objectives, inclusion criteria for trials, data to be obtained, and analyses to be done. A copy of the trial protocol is available on request. In the first part of the project we aimed to assess the effect of neoadjuvant chemotherapy plus standard local treatment (radical cystectomy, radical radiotherapy or preoperative radiotherapy plus cystectomy) versus the same local treatment alone. We also aimed to compare the effect of concurrent chemoradiotherapy plus local treatment with that of the same local treatment alone. Work is continuing on the separate question of adjuvant chemotherapy and results will be presented in due course.
Methods
Criteria for considering studies for this review
Types of studies
To be included in the meta‐analysis, trials had to be properly randomised and should have been closed to patient accrual at the time of the final data collation.
Types of participants
Trials should have aimed to randomise patients with biopsy proven invasive (i.e. clinical stage T2 to T4a) transitional cell carcinoma of the bladder.
Types of interventions
Patients should have been randomised to receive local definitive treatment with or without neoadjuvant chemotherapy. The comparison had to be unconfounded by additional agents or interventions. The same local treatment should have been used on each arm, i.e. control and experimental arms had to differ only by the addition of chemotherapy.
Types of outcome measures
The primary endpoint of overall survival was defined as the time from randomisation until death. Living patients were censored on the date of last follow up. Overall disease‐free survival was defined as the time from randomisation until first recurrence or progression (after randomisation) or death, whichever happened first. Loco‐regional disease‐free survival was defined as the time from randomisation to first local recurrence or progression (after randomisation) or death. Metastases‐free survival was defined as the time from randomisation to first metastases (after randomisation) or death. Patients alive without disease were censored on the date of last follow up. For all endpoints, death was defined as death by any cause.
Search methods for identification of studies
To limit publication bias, both published and unpublished trials were included. We searched MEDLINEand CancerLit databases using a version of the Cochrane Collaboration optimal search strategy (Dickersin 1994). These searches were supplemented by hand searches of the reference lists of identified trials, bibliographies of relevant books and review articles. The National Cancer Institute PDQ (Physicians Data Query) Clinical Protocols, United Kingdom Coordinating Committee for Cancer Research trials register and the Current Controlled Trials metaRegister of trials were also searched to identify unpublished and ongoing trials. All trialists who took part in the meta‐analysis were asked to help to identify additional trials. Trials that had not been published in the English language were not excluded. Initial searches were completed for the period up to and including January 1st, 2001. These were revised regularly to identify any additional new material that had appeared by our final analyses in June 2004. Two reviewers independently assessed all titles identified by search strategies for relevance. Abstracts were downloaded for all titles of potential relevance, and full papers obtained for all abstracts judged potentially relevant. In cases of uncertainty about the eligibility of a trial or particular treatment arms within a trial, the matter was discussed and resolved by consensus within the project secretariat and the international advisory group.
PT=RANDOMIZED‐CONTROLLED‐TRIAL
RANDOMIZED‐CONTROLLED‐TRIAL.DE.
RANDOM‐ALLOCATION.DE.
DOUBLE‐BLIND‐METHOD.DE.
SINGLE‐BLIND‐METHOD.DE.
1 OR 2 OR 3 OR 4 OR 5
PT=CLINICAL‐TRIAL
CLINICAL‐TRIAL#.DE.
(CLIN$ WITH TRIAL$).AB, TI.
((SINGL$ OR DOUBL$ OR TREBL$ OR TRIPL$) WITH (BLIND$ OR MASK$)).AB,TI.
PLACEBO$.DE.
PLACEBO$.AB,TI.
RANDON$.AB,TI.
RESEARCH‐DESIGN.DE
7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14
CARCINOMA#.DE.
BLADDER‐NEOPLASMS.DE.
BLADDER ADJ CARCINOMA$.AB,TI.
BLADDER ADJ CANCER$.AB,TI
BLADDER ADJ NEOPLASM$.AB,TI.
(CANCER WITH BALDDER).AB,TI.
(CARCINOMA WITH BLADDER).AB,TI.
16 AND 17
18 OR 19 OR 20 OR 21 OR 22
23 OR 24
DRUG‐THERAPY#.DE
QS NEOPLASMS# WITH DT
26 OR 27
RADIOTHERAPY#.DE
QS NEOPLASMS# WITH RT
29 OR 30
SURGERY#.DE
QS NEOPLASMS# WITH SU
32 OR 33
28 OR 31 OR 34
SUPERFICIAL
6 OR 15
37 AND 25 AND 35
38 NOT 36
Data collection and analysis
We sought up‐to‐date individual patient information on date of randomisation, survival status, local recurrence status, metastases status and date of last follow up. Details of treatment allocated, age, sex, TNM category, grade, performance status, tumour diameter, renal function and pre‐treatment haemoglobin were also obtained. To reduce potential bias, we requested information for all randomised patients including those who had been excluded from the investigators' original analyses. All data were thoroughly checked (Stewart 1995) for consistency, plausibility and integrity of randomisation and follow up. Any queries were resolved and the final database entries verified by the responsible trial investigator or statistician.
Analyses of all endpoints, subsets and subgroups were pre‐specified in the protocol and carried out on an intention‐to‐treat basis. Analyses of all of the endpoints were stratified by trial, and the log rank expected number of deaths and variance used to calculate individual trial hazard ratios and overall pooled hazard ratios (HR) using the fixed‐effect model (Yusuf 1985). [NB. Hazard Ratios calculated are labelled as Peto ORs in all of the forest plots.] Thus, the times to event (recurrence, progression or death) for individual patients were used within trials to calculate the HR, representing the overall risk of an event for those patients allocated to neoadjuvant chemotherapy compared with those allocated to no chemotherapy.
To examine the potential impact of trial design and the treatments used, we prospectively planned analyses that grouped trials by important aspects that might influence the effect of chemotherapy. Groups were defined according to the type of the chemotherapy regimen and also by the local treatment. For each of these analyses, we calculated a pooled HR for each group of trials and for all trials together. A chi‐square test for quantitative interaction was used to find out whether the effect of neoadjuvant chemotherapy differed substantially between the trial groups. These analyses focused on the primary endpoint of overall survival, however they were conducted for the other endpoints to help to support or to refute any patterns found. The effects of chemotherapy within subgroups of patients were investigated using similar stratified analyses. Analyses were performed for each pre‐specified subgroup, for example, comparing treatment and control for males and for females within each individual trial. These results were then combined to give overall HRs for males and for females.
We also calculated absolute differences at five years, using the overall HRs and event rate on the control arm (Parmar 1995). Confidence intervals for absolute differences were calculated from the baseline event rate and the HR at the 95% confidence interval boundary values. Chi2 heterogeneity tests (Stewart 1995) were used to test for statistical heterogeneity across trials. The power of this test is however acknowledged to be low and thus only gross statistical heterogeneity is likely to be detected. We used Chi‐square tests for interaction or trend to test for differences in outcome between subsets of trials or between subgroups of patients. Survival curves are presented as simple (non‐stratified) Kaplan‐Meier curves (Kaplan 1958). All P values quoted are two‐sided.
Results
Description of studies
(1) Neoadjuvant Chemotherapy trials
We identified 14 randomised trials using neoadjuvant chemotherapy that were potentially eligible for inclusion. Three of these were subsequently found to be ineligible; one because it was confounded by the use of additional treatments in both arms (Ozono 1991), a second because chemoradiotherapy was used as the local treatment (Shipley 1998) and the third compared neoadjuvant with adjuvant chemotherapy (Millikan 2001). There were therefore 11 remaining trials eligible for inclusion and data were obtained for all of these. In our original publication (ABC 2003), one of these trials, including a total of 317 patients, was not available because the investigators felt unable to supply data for inclusion in the meta‐analysis prior to publication. This trial has now been published (Grossman 2003) and the investigators have supplied data for inclusion in this update of the original meta‐analysis.
Individual patient data were supplied on 3005 patients from 11 randomised controlled trials (Abol‐Enein 1997; MRC/EORTC 1999; Cortesi (unpub); Malmstrom 1996; Martinez 1995; Raghavan 1991; Sengelov 2002; Sherif 2002; Wallace 1991; Grossman 2003) representing 98% of individuals from all known, eligible randomised trials. Data were collected for 18 of the 51 randomised patients who had been excluded from the investigators' analyses of these ten trials and were reinstated in the meta‐analysis. For the available trials, patient accrual ranged from 96 to 976. In six trials (Martinez 1995; Cortesi (unpub); Abol‐Enein 1997; Bassi 1999; Sherif 2002; Grossman 2003) the planned local treatment was radical cystectomy, two used radical radiotherapy (Wallace 1991; Raghavan 1991) and one used pre‐operative radiotherapy and cystectomy (Malmstrom 1996). Two trials used a combination of one or more of these local treatments (MRC/EORTC 1999; Sengelov 2002). All of the trials used platinum‐based chemotherapy. Ten trials used cisplatin, either as a single agent (Martinez 1995; Raghavan 1991; Wallace 1991 (3 trials)) or in combination with one or more of doxorubicin / epirubicin, methotrexate and vinblastine (MRC/EORTC 1999; Bassi 1999; Cortesi (unpub); Malmstrom 1996; Sengelov 2002; Sherif 2002; Grossman 2003 (7 trials)). Cisplatin doses ranged from 70 mg/m2 per cycle for 2 to 4 cycles to 100 mg/m2 per cycle given in 2 to 3 cycles, every 2 to 4 weeks. One further trial used carboplatin in combination with methotrexate and vinblastine (Abol‐Enein 1997). The carboplatin dose per cycle was 300 mg/m2 given in 2 cycles at 3‐week intervals.
Patient characteristics, which reflect the eligibility criteria of the included trials, are given in Table 1. Age, sex and clinical T stage data were provided for all trials. Grade and performance status were supplied for eight trials, and clinical N category for seven trials. Tumour diameter and renal function (recorded as glomerular filtration rate) were supplied in full for only one trial. Pre‐treatment haemoglobin data could not be supplied in full for any of the trials. Based on the available data, patients were mostly male with a median age across all trials of 63 years (range 30 to 90 years) with good performance status and tumours that were predominantly T2 to T3. The median follow up for surviving patients across all trials is 6.4 years. For one trial (Wallace 1991), follow up could not be updated, however survival data for this trial had been included in the 1995 IPD meta‐analysis conducted by the ABCOC 1995. These data were therefore included in our survival analysis. Two trials had only recorded survival and overall disease‐free survival and so could not be included in the analyses of locoregional disease‐free survival or metastases‐free survival (Cortesi (unpub); Grossman 2003). One trial (Abol‐Enein 1997) had only recorded overall disease‐free survival and so could only be included in the analysis of this endpoint.
1. Characteristics of included patients.
| Neo CT (n=1502) | Control (n=1503) | Total (n=3005) | ||
| Age | <55 | 265 (18%) | 287 (19%) | 552 |
| 55‐64 | 599 (40%) | 532 (35%) | 1131 | |
| ≥65 | 638 (42%) | 684 (46%) | 1322 | |
| Unknown | 0 | 0 | 0 | |
| Sex | Male | 1267 (84%) | 1273 (85%) | 2540 |
| Female | 235 (16%) | 230 (15%) | 465 | |
| Unknown | 0 | 0 | 0 | |
| T category | T0‐1 | 33 (2%) | 31 (2%) | 64 |
| T2 | 507 (34%) | 541 (36%) | 1048 | |
| T3‐T4a | 927 (62%) | 898 (60%) | 1825 | |
| T4b | 11 (1%) | 9 (1%) | 20 | |
| Unknown | 24 (2%) | 24 (2%) | 48 | |
| N category | N0 | 797 (53%) | 765 (51%) | 1562 |
| N1/N2 | 55 (4%) | 62 (4%) | 117 | |
| Nx/Unknown | 650 (43%) | 676 (45%) | 1326 | |
| Grade | G0‐1 | 155 (10%) | 136 (9%) | 291 |
| G2 | 179 (12%) | 219 (15%) | 398 | |
| G3 | 805 (54%) | 789 (52%) | 1594 | |
| G4 | 10 (1%) | 28 (2%) | 38 | |
| Unknown | 353 (24%) | 331(22%) | 684 | |
| Performance Status | 0 | 774 (52%) | 756 (50%) | 1530 |
| 1 | 292 (19%) | 312 (21%) | 604 | |
| 2 | 49 (3%) | 60 (4%) | 109 | |
| 3 | 13 (1%) | 8 (1%) | 21 | |
| Unknown | 374 (25%) | 367 (24%) | 741 | |
| Tumour diameter (cm) | <2.5 | 96 (6%) | 101 (7%) | 197 |
| 2.5‐5.0 | 247 (16%) | 284 (19%) | 531 | |
| >5.0 | 238 (16%) | 205 (14%) | 443 | |
| Unknown | 921 (61%) | 913 (61%) | 1834 | |
| Renal function (GFR) | ≦59 | 40 (3%) | 42 (3%) | 82 |
| 60‐69 | 119 (8%) | 134 (9%) | 253 | |
| >69 | 330 (22%) | 305 (20%) | 635 | |
| Unknown | 1013 (67%) | 1022 (68%) | 2035 |
(2) Concurrent Chemoradiotherapy trials
Searches identified only one moderately sized eligible trial (Coppin 1996) for this comparison. One hundred two patients were randomised and patient characteristics are similar to those for the neoadjuvant trials, most were male with a median age of 64 years (range 39 to 75 years), good performance status and with tumours that were predominantly T2 to T3. Cisplatin was given at 100 mg/m2 per cycle in 3 cycles every 2 weeks. The median follow up for living patients was just over 13 years.
(3) Neoadjuvant plus adjuvant chemotherapy trials One trial compared chemotherapy both before and after local treatment with local treatment alone (Shearer 1988) and its results are presented alongside those of the neoadjuvant chemotherapy trials. The 398 patients in this trial were mostly male with a median age of 67 years (range 41 to 87 years) and all had T3 tumours. Methotrexate was given at 100 mg/m2 per cycle for six cycles every 2 weeks. Patients in the chemotherapy arm also received a further 9 cycles of adjuvant methotrexate at 100 mg/m2 per cycle every four weeks. The median follow up for living patients was slightly under 9 years. Data for 25 patients who had been excluded from the original analysis were unavailable.
Risk of bias in included studies
All data were thoroughly checked for validity, consistency, plausibility and integrity of randomisation and follow‐up. Any queries were resolved and the final database entries verified by the responsible investigator, data manager or statistician.
Effects of interventions
Survival (1) Neoadjuvant chemotherapy trials Survival data were supplied for 10 of the 11 trials with 2809 patients included. A total of 1691 deaths have been recorded. The confidence intervals for individual trial results are wide and the results of most individual trials are inconclusive. There is no clear evidence of statistical heterogeneity (P = 0.47) or inconsistency (I2 = 0%) between trials. The overall hazard ratio (HR) of 0.89 (95% CI 0.81 to 0.98) for these trials represents an 11% relative reduction in the risk of death associated with neoadjuvant chemotherapy which is conventionally significant (P = 0.022). This is equivalent to an absolute improvement of 4% at five years (95% CI 0% to 7%) increasing overall survival from 45% to 49%.
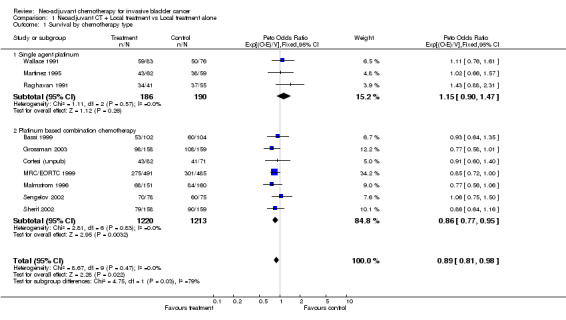
When we grouped the trials according to whether they used single agent platinum or platinum‐based combination chemotherapy as pre‐specified in the protocol (Outcome 1.01‐ Survival by chemotherapy type), we noted a difference in the effect of chemotherapy between the groups (interaction P = 0.029). The HR of 1.15 (95% CI 0.90 to 1.47) for those trials using single‐agent chemotherapy is in favour of local treatment alone. This translates to an absolute benefit of ‐5% at 5 years (95% CI ‐14% to 4%). However, few trials and patients were included in this comparison, the confidence intervals are wide and the result is not conventionally significant (P = 0.264). By contrast, the combined HR for those trials using combination chemotherapy was 0.86 (95% CI 0.77 to 0.95, P = 0.003), equivalent to a 14% relative reduction in the risk of death; an absolute benefit of 5% at 5 years (95% CI 2% to 9%) improving survival from 45% to 50%. The survival curves for the combination chemotherapy trials, which separate by six months and remain apart thereafter, illustrate this benefit (Figure 1). [NB. Hazard Ratios calculated are labelled as Peto ORs in all of the forest plots.]
1.
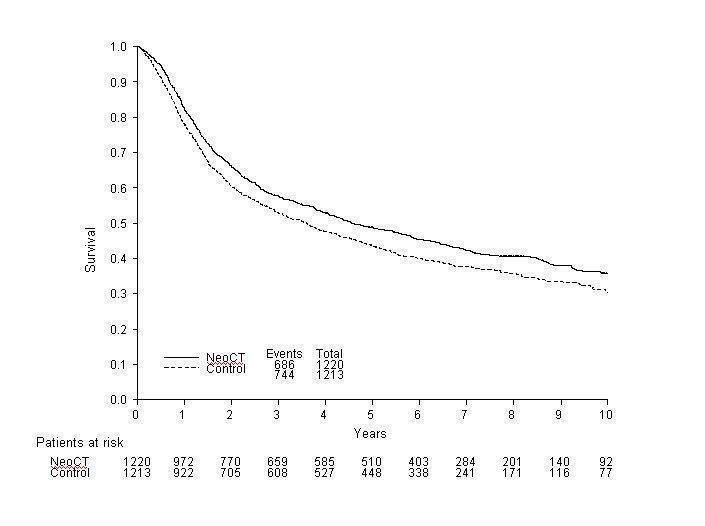
Overall survival in the cisplatin‐based combination chemotherapy trials
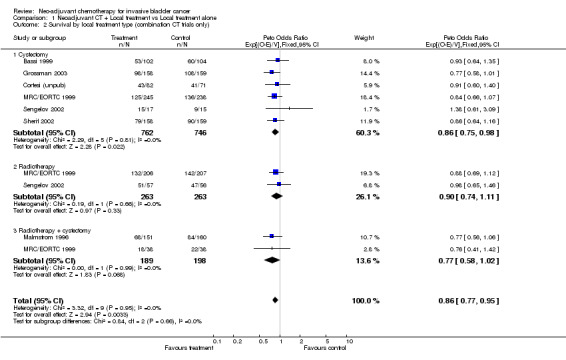
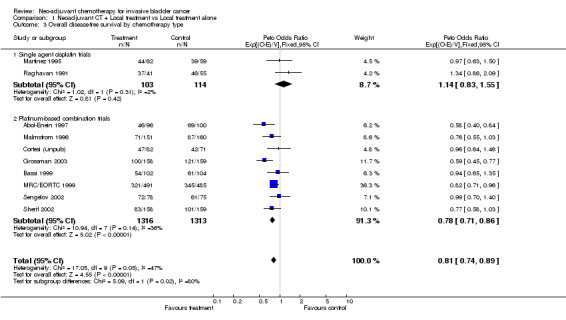
The other pre‐specified subset grouping was by planned local treatment: cystectomy alone, radical radiotherapy alone or combined radiotherapy and cystectomy. Since we had noted evidence of a difference in the effect between the single agent and combination chemotherapy group, this analysis was restricted to trials that had used combination chemotherapy (Outcome 1.02 ‐ Survival by local treatment). We noted no evidence of a difference in the effect of chemotherapy in the three local treatment groups (interaction P = 0.656). Other Endpoints Data on overall disease‐free survival were available from 10 trials that included 2846 patients and 1847 events, of which 1606 (87%) were recurrences and 241 (13%) were deaths Further information is given in Table 2. As for overall survival, in the pre‐specified analysis according to whether trials had used single agent platinum or platinum‐based combination chemotherapy, we noted a difference in the effect of chemotherapy between the group (interaction P=0.024) (Outcome 1.03 ‐ Overall disease‐free survival by chemotherapy type). For the combination chemotherapy trials, the combined HR of 0.78 (95% CI 0.71 to 03.86, P = 0.0000005) is equivalent to a 22% relative reduction in the risk of locoregional recurrence, metastases or death; an absolute disease‐free survival benefit of 9% at 5 years (95% CI 5% to 12%).
2. First events reported by treatment arm.
| First event | Neo CT (n =1502) | Control (n = 1503) |
| Alive (no recurrence) | 451 (30%) | 388 (26%) |
| Local recurrence | 208 (14%) | 213 (14%) |
| Metastasis | 150 (10%) | 196 (13%) |
| Local recurrence and metastases together | 176 (12%) | 200 (13%) |
| Death (No recurrence) | 194 (13%) | 306 (20%) |
| Unknown | 323 (22%) | 200 (13%) |
For loco‐regional disease‐free survival and metastases‐free survival, data were available for 7 trials that included 2180 patients with 1398 events (loco‐regional disease‐free survival) and 1345 events (metastases‐free survival). For both endpoints approximately half of the events were deaths. Further information is given in Table 2. Again, the results for these endpoints show a similar pattern to survival, both in terms of chemotherapy type (Table 3) and local treatment (Table 4) with a significant benefit of platinum‐based combination chemotherapy.
3. Results for all endpoints by chemotherapy type.
| Endpoint | CT Type | No patients / events | HR (95% CI) | Effect P value | Abs benefit (95% CI) | Interaction p‐value |
| Overall survival | Single agent platinum | 261/376 | 1.15 (0.90‐1.47) | 0.26 | ‐5% (‐14% to 4%) | |
| Platinum based combinations | 1430/2433 | 0.86 (0.77‐0.95) | 0.003 | 5% (2% to 9%) | 0.029 | |
| All trials | 1691/2890 | 0.89 (0.81‐0.98) | 0.022 | 4% (0% to 7%) | ||
| Disease‐free survival | Single agent platinum | 166/217 | 1.14 (0.83‐1.55) | 0.42 | ‐5% (‐16% to 7%) | |
| Platinum based combinations | 1681/2629 | 0.78 (0.71‐0.86) | 0.0000005 | 9% (5% to 12%) | 0.024 | |
| All trials | 1847/2846 | 0.81 (0.74‐0.89) | 0.000005 | 8% (4% to 11%) | ||
| Locoregional disease‐free survival | Single agent platinum | 166/217 | 1.12 (0.82‐1.52) | 0.49 | ‐4% (‐15% to 7%) | |
| Platinum based combinations | 1232/1963 | 0.87 (0.77‐0.97) | 0.012 | 5% (3% to 11%) | 0.131 | |
| All trials | 1398/2180 | 0.89 (0.80‐0.99) | 0.032 | 4% (0% to 8%) | ||
| Metastases‐free survival | Single agent platinum | 154/217 | 1.21 (0.88‐1.67) | 0.25 | ‐7% (‐18% to 5%) | |
| Platinum based combinations | 1181/1963 | 0.82 (0.73‐0.92) | 0.001 | 7% (3% to 11%) | 0.025 | |
| All trials | 1335/2180 | 0.86 (0.77‐0.95) | 0.004 | 5% (2% to 9%) |
4. Results for all endpoints by local treatment (combination CT trials only).
| Endpoint | Local treatment type | HR (95% CI) | Interaction p‐value |
| Overall survival | Cystectomy | 0.86 (0.75‐0.98) | |
| Radiotherapy | 0.90 (0.74‐1.11) | ||
| Radiotherapy + cystectomy | 0.77 (0.58‐1.02) | 0.656 | |
| Disease‐free survival | Cystectomy | 0.75 (0.66‐0.84) | |
| Radiotherapy | 0.92 (0.76‐1.11) | ||
| Radiotherapy + cystectomy | 0.71 (0.54‐0.94) | 0.158 | |
| Locoregional disease free survival | Cystectomy | 0.86 (0.69‐1.01) | |
| Radiotherapy | 0.96 (0.79‐1.16) | ||
| Radiotherapy + cystectomy | 0.73 (0.55‐0.96) | 0.286 | |
| Metastases free survival | Cystectomy | 0.82 (0.70‐0.96) | |
| Radiotherapy | 0.87 (0.71‐1.06) | ||
| Radiotherapy + cystectomy | 0.73 (0.56‐0.97) | 0.649 |
There was insufficient data available to formally investigate toxicity or quality of life in these trials. However, where it was reported in the publications, the most common chemotherapy‐related toxicities included nausea and vomiting haematological toxicities and impaired renal function.
Subsidiary Analyses of Survival in Patient Subgroups Subgroup analyses across all trials would not have been appropriate because of the differences in effect noted for single agent and combination chemotherapy. We therefore restricted these analyses to those trials that had used combination chemotherapy and had been able to provide adequate baseline data (age, sex and T category available for all trials; performance status available for six trials; N category available in full for five trials and grade available in full for four trials; tumour diameter and renal function only available for one trial). The power of these analyses was inevitably limited. However we recorded no strong evidence to suggest that combination chemotherapy was differentially effective in groups of patients defined by age, sex, t category, n category, grade, performance status or renal function. There was some suggestion of a differential effect between groups of patients defined by tumour diameter (trend P = 0.008), however this analysis was based on data that was largely from one trial (MRC/EORTC 1999) and should therefore be interpreted with caution (Table 5).
5. Results of subgroup analyses (survival ‐ combination CT trials only).
| Subgroup | Categories | Interaction | Trend |
| Age | ≺55, 55 to ≺ 65, ≧ 65 | 1.572 (2 d.f.) P=0.456 | 0.482 (1 d.f.) P=0.488 |
| Sex | Male, Female | 0.266 (1d.f) P=0.606 | ‐ |
| T category | T1‐2, T3, T4 | 0.324 (2 d.f.) P=0.850 | 0.108 (1 d.f.) P=0.742 |
| N category | N0, N1/N2 | 0.080 (1d.f.) P=0.777 | ‐ |
| Grade | G1/G2, G3/G4 | 1.154 (1d.f.) P=0.283 | ‐ |
| Tumour diameter (cm) | ≺ 2.5, 2.5 to 5.0, ≻ 5.0 | 7.733 (2 d.f.) P=0.021 | 7.079 (1d.f.) P=0.008 |
| Performance status | 0, 1, 2 to 3 | 1.511 (2 d.f.) P=0.470 | 0.004 (1 d.f.) P=0.95 |
| Renal function (GFR) | ≦ 59, 60 to 69, ≻ 69 | 3.573 (2 d.f.) P=0.168 | 3.564 (1d.f.) P=0.059 |
| d.f. = degrees of freedom |
(2) Concurrent Chemoradiotherapy Trials The HR of 0.76 (95% CI 0.49 to 1.18) suggests a 24% reduction in the relative risk of death associated with concurrent chemoradiotherapy, although this was not conventionally significant (P = 0.229) (Outcome 2.01 ‐ Survival).
(3) Neoadjuvant plus Adjuvant Chemotherapy Trials The HR of 0.85 (95% CI 0.68 to 1.05) represents a 15% relative reduction in the risk of death with neoadjuvant and adjuvant chemotherapy, compared with control, but this was not conventionally significant (P = 0.136) (Outcome 3.01 ‐ Survival).
Discussion
Our findings show a beneficial effect of neoadjuvant chemotherapy. This effect was most clear in those trials that used combination, platinum‐based chemotherapy, with a 5% improvement in survival at five years. The results for disease‐free survival, locoregional disease‐free survival and metastases‐free survival lend support to the evidence of survival benefits associated with combination chemotherapy. We had insufficient evidence to formally test whether any of the specific combinations of chemotherapy used in these trials is more or less active, however there is no evidence of statistical heterogeneity between the trials in the combination chemotherapy group. The effect of combination chemotherapy also appears independent of the local treatment used, with a similar benefit of chemotherapy seen whether cystectomy, radiotherapy or radiotherapy and cystectomy was used as local treatment. This analysis however cannot directly measure the underlying relative efficacy of the various local treatments.
The subgroup analyses are exploratory in nature and had fairly low power, these analyses should therefore be interpreted with caution. No strong evidence was seen that the effect of neoadjuvant combination chemotherapy varied across patient subgroups. However we found a suggestion of a trend in the effect of chemotherapy when patients were grouped according to tumour diameter. Although tumour diameter is difficult to measure accurately, this observation might warrant further investigation. The overall absolute benefit of 5% at 5 years therefore provides the best estimate of effect in all subgroups however the clinical interpretation of this benefit may vary due to the differing underlying prognoses of these patients. For example, at 5 years, neoadjuvant chemotherapy improves survival from 55% to 60% in patients with T1to T2 disease, from 40% to 45% in T3 and from 25% to 30% in the group of patients with T4 disease.
Despite a significant difference in the effect of neoadjuvant chemotherapy between groups of trials using single agent chemotherapy and combination chemotherapy, we did not find sufficient evidence to reliably determine the effect of single agent cisplatin chemotherapy on survival. Indeed, only three small trials including a total of 376 patients used single‐agent cisplatin and the combined HR of survival for this group is based on just 261 events. With such low power, the results are far less reliable than those for the combination chemotherapy group. Furthermore, these three trials are among the earliest trials of neoadjuvant chemotherapy and may be less relevant in today's context, where combination regimens are preferred.
Authors' conclusions
Implications for practice.
Our findings show a clear survival benefit associated with neoadjuvant combination chemotherapy for patients with invasive bladder cancer. However, few of the trials in this meta‐analysis measured toxicity or quality of life in ways that would allow data to be combined in a meta‐analysis and clearly these are major issues for patients and clinicians when considering their treatment options.
Implications for research.
In light of these results, promising new drug regimens or treatment approaches should be compared in randomised trials against neoadjuvant platinum‐based combination chemotherapy.
What's new
| Date | Event | Description |
|---|---|---|
| 17 January 2012 | Amended | Added grant info. |
History
Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 29 May 2008 | Amended | Converted to new review format. |
| 3 November 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to the British Medical Research Council for funding this work. We would like to thank all those patients who took part in these trials and contributed to this research. The meta‐analysis would not have been possible without the collaborating institutions that kindly supplied their trial data or without the help of those responsible for maintaining, updating and preparing trial data, in particular Jim Faulkner, Gareth Griffiths, David Lawrence, Rosario Madero, Jonas Nilsson, Giovanni Pappagallo, Ann‐Marie Sargeant, Nory Teriana and Barbara Uscinska. We would also like to thank Cora Sternberg for comments and assistance throughout the project and Sarah Burdett for helpful comments on the report.
Data and analyses
Comparison 1. Neoadjuvant CT + Local treatment vs Local treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival by chemotherapy type | 10 | 2809 | Peto Odds Ratio (95% CI) | 0.89 [0.81, 0.98] |
| 1.1 Single agent platinum | 3 | 376 | Peto Odds Ratio (95% CI) | 1.15 [0.90, 1.47] |
| 1.2 Platinum based combination chemotherapy | 7 | 2433 | Peto Odds Ratio (95% CI) | 0.86 [0.77, 0.95] |
| 2 Survival by local treatment type (combination CT trials only) | 7 | 2421 | Peto Odds Ratio (95% CI) | 0.86 [0.77, 0.95] |
| 2.1 Cystectomy | 6 | 1508 | Peto Odds Ratio (95% CI) | 0.86 [0.75, 0.98] |
| 2.2 Radiotherapy | 2 | 526 | Peto Odds Ratio (95% CI) | 0.90 [0.74, 1.11] |
| 2.3 Radiotherapy + cystectomy | 2 | 387 | Peto Odds Ratio (95% CI) | 0.77 [0.58, 1.02] |
| 3 Overall disease‐free survival by chemotherapy type | 10 | 2846 | Peto Odds Ratio (95% CI) | 0.81 [0.74, 0.89] |
| 3.1 Single agent cisplatin trials | 2 | 217 | Peto Odds Ratio (95% CI) | 1.14 [0.83, 1.55] |
| 3.2 Platinum‐based combination trials | 8 | 2629 | Peto Odds Ratio (95% CI) | 0.78 [0.71, 0.86] |
1.1. Analysis.
Comparison 1 Neoadjuvant CT + Local treatment vs Local treatment alone, Outcome 1 Survival by chemotherapy type.
1.2. Analysis.
Comparison 1 Neoadjuvant CT + Local treatment vs Local treatment alone, Outcome 2 Survival by local treatment type (combination CT trials only).
1.3. Analysis.
Comparison 1 Neoadjuvant CT + Local treatment vs Local treatment alone, Outcome 3 Overall disease‐free survival by chemotherapy type.
Comparison 2. Concurrent CTRT + local treatment vs local treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 1 | 102 | Peto Odds Ratio (95% CI) | 0.76 [0.49, 1.18] |
2.1. Analysis.
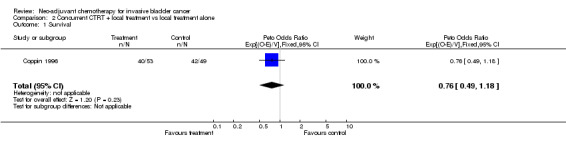
Comparison 2 Concurrent CTRT + local treatment vs local treatment alone, Outcome 1 Survival.
Comparison 3. Neoadjuvant CT + Local Treatment + Adjuvant CT vs Local Treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 1 | 398 | Peto Odds Ratio (95% CI) | 0.85 [0.68, 1.05] |
3.1. Analysis.
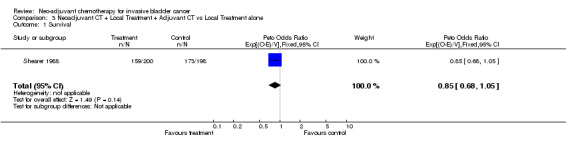
Comparison 3 Neoadjuvant CT + Local Treatment + Adjuvant CT vs Local Treatment alone, Outcome 1 Survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abol‐Enein 1997.
| Methods | RCT Accrual 1984‐1996 | |
| Participants | 196 T2‐T4a, Nx, M0 | |
| Interventions | Surgery alone vs. neoadjuvant chemotherapy + surgery Neoadjuvant CT: 2 cycles every 4 weeks of Carboplatin (300mg/m2), Methotrexate (50mg/m2) and Vinblastine (4mg/m2) |
|
| Outcomes | Disease free survival | |
| Notes | No data on overall survival | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Bassi 1999.
| Methods | RCT Accrual 1989‐01996 | |
| Participants | 206 T2‐T4, N0, M0 | |
| Interventions | Surgery alone vs. neoadjuvant chemotherapy + surgery Neoadjuvant CT: 4 cycles every 4 weeks of Cisplatin (70mg/m2), Methotrexate (30mg/m2), Vinblastine (3mg/m2) and Doxorubicin (30mg/m2) |
|
| Outcomes | Survival Disease‐free survival Locoregional disease‐free survival Metastases‐free survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Coppin 1996.
| Methods | RCT Accrual 1985‐1989 | |
| Participants | 102 T2‐T4b | |
| Interventions | Radiotherapy or radiotherapy + surgery vs. concurrent chemoradiotherapy or concurrent chemoradiotherapy + surgery CT: 3 cycles every 2 weeks of Cisplatin (100mg/m2) |
|
| Outcomes | Survival Disease‐free survival Locoregional disease‐free survival Metastases‐free survival | |
| Notes | Concurrent chemotherapy and radiotherapy used therefore not included in main comparison of neoadjuvant chemotherapy +/‐ local treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Cortesi (unpub).
| Methods | RCT Accrual 1988‐1992 | |
| Participants | 171 (18 missing) T2‐T4, N0, M0 | |
| Interventions | Surgery alone vs. neoadjuvant chemotherapy + surgery Neoadjuvant CT: 3 cycles every 4 weeks of Cisplatin (70mg/m2), Methotrexate (30mg/m2), Vinblastine (3mg/m2) and Epirubicin (40mg/m2) |
|
| Outcomes | Survival Disease‐free survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Grossman 2003.
| Methods | RCT Accrual 1987‐1998 | |
| Participants | 317 T2‐T4a, N0, M0 | |
| Interventions | Surgery alone vs. neoadjuvant chemotherapy + surgery Neoadjuvant CT: 3 cycles every 4 weeks of Cisplatin (70mg/m2), Methotrexate (30mg/m2), Vinblastine (3mg/m2) and Doxorubicin (30mg/m2) |
|
| Outcomes | Survival Overall disease free survival | |
| Notes | Not included in original analyses published in the Lancet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Malmstrom 1996.
| Methods | RCT Accrual 1985‐1989 | |
| Participants | 325 (14 missing) T1 (grade 3) ‐ T4a, Nx, M0 | |
| Interventions | Radiotherapy + surgery vs. neoadjuvant chemotherapy + radiotherapy + surgery Neoadjuvant CT: 2 cycles every 3 weeks of Cisplatin (70mg/m2) and Doxorubicin (30mg/m2) |
|
| Outcomes | Survival Disease‐free survival Locoregional disease‐free survival Metastases free‐survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Martinez 1995.
| Methods | RCT Accrual 1984‐1989 | |
| Participants | 122 (1 missing) T2‐T4a, Nx‐N2, M0 | |
| Interventions | Surgery alone vs. neoadjuvant chemotherapy + surgery Neoadjuvant CT: 3 cycles every 3 weeks of Cisplatin (100mg/m2) |
|
| Outcomes | Survival Disease free survival Locoregional disease‐free survival Metastases free‐survival | |
| Notes | Single agent cisplatin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
MRC/EORTC 1999.
| Methods | RCT Accrual 1989‐1995 | |
| Participants | 976 T2 (grade 3), T3, T4a, N0, M0 | |
| Interventions | Cystectomy (+/‐ radiotherapy) or radiotherapy alone vs. Neoadjuvant chemotherapy plus cystectomy (+/‐ radiotherapy) or radiotherapy alone Neoadjuvant CT: 3 cycles every 3 weeks of Cisplatin (100mg/m2), Methotrexate (30mg/m2) and Vinblastine (4mg/m2) |
|
| Outcomes | Survival Overall disease‐free survival Locoregional disease‐free survival Metastases‐free survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Natale 2001.
| Methods | RCT | |
| Participants | 317 | |
| Interventions | Surgery alone vs. neoadjuvant chemotherapy + surgery Neoadjuvant CT: 3 cycles every 4 weeks of Cisplatin (70mg/m2), Methotrexate (30mg/m2), Vinblastine (3mg/m2) and Doxorubicin (30mg/m2) |
|
| Outcomes | Survival | |
| Notes | Preliminary results of Grossman 2003 (presented at ASCO 2001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Raghavan 1991.
| Methods | RCT Accrual 1985‐1988 | |
| Participants | 96 T2‐T4, Nx, M0 | |
| Interventions | Radiotherapy alone vs. neoadjuvant chemotherapy + radiotherapy Neoadjuvant CT: 2 cycles every 3 weeks of Cisplatin (100mg/m2) |
|
| Outcomes | Survival Disease free survival Locoregional disease‐free survival Metastases free‐survival | |
| Notes | Single agent cisplatin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Sengelov 2002.
| Methods | RCT Accrual 1989‐1995 | |
| Participants | 153 T2‐T4b, Nx‐N0, M0 | |
| Interventions | Surgery alone or radiotherapy alone vs. neoadjuvant chemotherapy + surgery or radiotherapy Neoadjuvant CT: 3 cycles every 3 weeks of Cisplatin (100mg/m2) and Methotrexate (250mg/m2) |
|
| Outcomes | Survival Disease free survival Locoregional disease‐free survival Metastases free‐survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Shearer 1988.
| Methods | RCT Accrual 1978‐1986 | |
| Participants | 423 (25 missing) T3‐T4a, Nx, M0 | |
| Interventions | Radiotherapy alone or radiotherapy + surgery vs. Neoadjuvant chemotherapy + radiotherapy or radiotherapy and surgery + adjuvant chemotherapy Neoadjuvant CT: 6 cycles every 2 weeks of Methotrexate (100mg/m2) and Leucovorin (15mg) Adjuvant CT: 9 cycles every 4 weeks of Methotrexate (100mg/m2) a |
|
| Outcomes | Survival Disease free survival Locoregional disease‐free survival Metastases free‐survival | |
| Notes | Trial used neoadjuvant and adjuvant chemotherapy therefore not included in the main comparison of neoadjuvant chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Sherif 2002.
| Methods | RCT Accrual 1990‐1997 | |
| Participants | 317 (8 missing) T2‐T4a, Nx, M0 | |
| Interventions | Surgery alone vs. Neoadjuvant chemotherapy + surgery Neoadjuvant CT: 3 cycles every 3 weeks of Cisplatin (100mg/m2) and Methotrexate (250mg/m2) |
|
| Outcomes | Survival Disease free survival Locoregional disease‐free survival Metastases free‐survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Wallace 1991.
| Methods | RCT Accrual 1984‐1988 | |
| Participants | 159 T2‐T4, Nx, M0 | |
| Interventions | Radiotherapy alone vs. Neoadjuvant chemotherapy + radiotherapy Neoadjuvant CT: 3 cycles every3 weeks of Cisplatin (100mg/m2) |
|
| Outcomes | Survival | |
| Notes | Single‐agent cisplatin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Millikan 2001 | Trial compared neoadjuvant chemotherapy + local treatment on one arm with local treatment + adjuvant chemotherapy on the other |
| Ozono 1991 | Confounded by use of different treatments on both arms of the trial |
| Shipley 1998 | Local treatment used was chemoradiotherapy and not surgery, radiotherapy or radiotherapy + surgery |
Contributions of authors
All aspects of the meta‐analysis were carried out under the auspices of the ABC group. H Abol‐Enein, P Bassi, M Boyer, C M L Coppin, E Cortesi, H.B Grossman, R R Hall, A Horwich, P‐U Malmström, J A Martinez‐Piñeiro, L Sengeløv, A Sherif and D M A Wallace collated and supplied the individual patients' data, contributed to the discussions of the results and commented on the drafts of the report. A V Bono, P J Goebell, S Groshen, F M Torti M Stöckle and U Studer contributed to the discussions of the results and commented on the drafts of the report. The project was organised by the Advisory Group, N W Clarke, D Raghavan, J T Roberts and R Sylvester and the Secretariat, M K B Parmar, L A Stewart, J F Tierney and C L Vale, who were responsible for formulating the questions, developing the protocol and discussing the preliminary results. The secretariat, MKB Parmar, L A Stewart, J F Tierney and C L Vale, were responsible for receiving, checking and analysing data. C L Vale managed the project and drafted the report, with detailed input from J F Tierney, L A Stewart and M K B Parmar.
Sources of support
Internal sources
Medical Research Council, UK.
External sources
-
Grant no. 5R01DK63300‐4, USA.
Editing support was in part provided by the National Institutes of Health (NIH), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Declarations of interest
None given.
Edited (no change to conclusions)
References
References to studies included in this review
Abol‐Enein 1997 {published and unpublished data}
- Abol‐Enein H, El‐Mekresh M, El‐Baz M, Ghoneim MA. Neo‐adjuvant chemotherapy in the treatment of invasive transitional bladder cancer. British Journal of Urology 1997;79 (suppl 4):174. [Google Scholar]
Bassi 1999 {published and unpublished data}
- Bassi P, Pappagallo GL, Sperandio P, et al. Neoadjuvant MVAC chemotherapy of invasive bladder cancer: results of a multicentre phase III trial. Journal of Urology 1999;161:264a. [Google Scholar]
Coppin 1996 {published and unpublished data}
- Coppin CML, Gospadorowicz MK, James K, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and pre‐operative or definitive radiation. Journal of Clinical Oncology 1996;14:2901‐2907. [DOI] [PubMed] [Google Scholar]
Cortesi (unpub) {unpublished data only}
- Cortesi E. Italian Randomised Trial of Neoadjuvant MVEC in Locally Advanced Bladder Cancer.
Grossman 2003 {published and unpublished data}
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859‐66. [DOI] [PubMed] [Google Scholar]
Malmstrom 1996 {published and unpublished data}
- Malmstrom P‐U, Rintala E, Walqvist R, et al. Five year follow‐up of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial 1. Journal of Urology 1996;155:1903‐06. [PubMed] [Google Scholar]
Martinez 1995 {published and unpublished data}
- Martinez‐Pineiro JA, Gonzalez M, Arocena F, et al. Neoadjuvant cisplatin chemotherapy before radical cystectomy in invasive transitional cell carcinoma of the bladder: a prospective randomised phase III study. Journal of Urology 1995;153:964‐973. [PubMed] [Google Scholar]
MRC/EORTC 1999 {published and unpublished data}
- International Collaboration of Trialists on behalf of the Medical Research Council Advanced Bladder Cancer Working Party, EORTC Genito‐urinary Group, National Cancer Institute of Canada Clinical Trials Group, Finnbladder, Norwegian Bladder Cancer Study Group, and Club Urologico Espanol de Tratamiento Oncologico (CUETO) Group. Neoadjuvant cisplatin, methotrexate and vinblastine chemotherapy for muscle‐invasive bladder cancer: a randomised controlled trial. Lancet 1999;354:533‐40. [PubMed] [Google Scholar]
Natale 2001 {published and unpublished data}
- Natale RB, Grossman HB, Blumenstein B, et al. SWOG 8710 (INT‐0800). Randomized phase III trial of neoadjuvant MVAC + cystectomy versus cystectomy alone in patients with locally advanced bladder cancer. Proceedings of the American Society for Clinical Oncology 2001;20:3. [PubMed] [Google Scholar]
Raghavan 1991 {published and unpublished data}
- Wallace DMA, Raghavan D, Kelly KA, et al. Neo‐adjuvant (pre‐emptive) cisplatin therapy in invasive transitional cell carcinoma of the bladder. British Journal of Urology 1991;67:608‐615. [DOI] [PubMed] [Google Scholar]
Sengelov 2002 {published and unpublished data}
- Sengelov L, Maase H, Lundbech F, et al. Neoadjuvant chemotherapy with cisplatin and methotrexate in patients with muscle‐invasive bladder tumors. Acta Oncologia 2002;41:447‐456. [DOI] [PubMed] [Google Scholar]
Shearer 1988 {published and unpublished data}
- Shearer RJ, Chilvers CED, Bloom HJG, Bliss JM, Horwich A, Babiker A. Adjuvant chemotherapy in T3 carcinoma of the bladder. A prospective trial: preliminary report. British Journal of Urology 1988;62:558‐564. [DOI] [PubMed] [Google Scholar]
Sherif 2002 {published and unpublished data}
- Sherif A, Rintala E, Mestad O, et al. Neoadjuvant cisplatin‐methotrexate chemotherapy of invasive bladder cancer. Nordic Cystectomy Trial 2. Scandinavian Journal of Urology and Nephrology 2002;6:419‐425. [DOI] [PubMed] [Google Scholar]
Wallace 1991 {published and unpublished data}
- Wallace DMA, Raghavan D, Kelly KA, et al. Neo‐adjuvant (pre‐emptive) cisplatin therapy in invasive transitional cell carcinoma of the bladder. British Journal of Urology 1991;67:608‐615. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Millikan 2001 {published data only}
- Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M‐VAC versus cystectomy with both preoperative and postoperative M‐VAC.. Journal of Clinical Oncology 2001;19:4005‐4013. [DOI] [PubMed] [Google Scholar]
Ozono 1991 {published data only}
- Ozono S, kawata Y, Fukui Y, et al. Neoadjuvant therapy for locally invasive bladder cancer: results of a randomized trial in 40 patients. Urology International 1991;47(Suppl 1):116‐119. [DOI] [PubMed] [Google Scholar]
Shipley 1998 {published data only}
- Shipley W, Winter DA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of radiation therapy oncology group 89‐03. Journal of Clinical Oncology 1998;16:3576‐83. [DOI] [PubMed] [Google Scholar]
Additional references
ABCOC 1995
- Advanced Bladder Cancer Overview Collaboration. Does neo‐adjuvant cisplatin based chemotherapy improve survival of patients with locally advanced bladder cancer: a meta‐analysis of individual patient data from randomised clinical trials. British Journal of Urology 1995;75:206‐213. [DOI] [PubMed] [Google Scholar]
AOCTG 1991
- Advanced Ovarian Cancer Trialists Group. Chemotherapy in advanced ovarian cancer: An overview of randomised clinical trials. Br Med J 1991;303:884‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 1998
- Clarke M, Godwin J. Systematic reviews using individual patient data: A map for the minefields?. Annals of Oncology 1998;9:827‐33. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. British Medical Journal 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagg 1984
- Fagg SL, Dawson‐Edwards P, Hughes MA, Lateif T, Rolfe EB, Fielding JW. Cis‐diamminedichloroplatinum (DPP) as initial treatment of invasive bladder cancer. British Journal of Urology 1984;56:296‐300. [DOI] [PubMed] [Google Scholar]
Kaplan 1958
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. Journal of the American Statistical Association 1958;53:457‐81. [Google Scholar]
Martinez 1998
- Martinez‐Pineiro JA, Martinez‐Pineiro L. The role of neoadjuvant chemotherapy for invasive bladder cancer. British Journal of Urology 1998;82:33‐42. [PubMed] [Google Scholar]
Parkin 1999
- Parkin DM, Pisani P, Ferlay J. Global Cancer Statistics. CA Cancer J Clin 1999;49:33‐64. [DOI] [PubMed] [Google Scholar]
Parmar 1995
- Parmar MKB, Machin D. Survival analysis: a practical approach. John Wiley & Sons Ltd, 1995. [Google Scholar]
Parmar 1999
- Parmar MKB, Burdett S. Neoadjuvant and Adjuvant chemotherapy. In: Hall RR editor(s). Clinical management of Bladder Cancer. 1st Edition. London: Arnold, 1999:249‐63. [Google Scholar]
Raghavan 1984
- Raghavan D, Pearson B, Coorey G, et al. Intravenous cis‐platinum for invasive clinically non‐metastatic bladder cancer: safety and feasibility of a new approach. Med J Aust 1984;140:276‐278. [PubMed] [Google Scholar]
Soloway 1981
- Soloway MS, Ikard M, Ford, K. Cis‐diamminedichloro‐platinum (II) in locally advanced and metastatic urothelial cancer. Cancer 1981;47:476‐80. [DOI] [PubMed] [Google Scholar]
Sternberg 2001
- Sternberg CN, Parmar MKB. Neoadjuvant chemotherapy is not (yet) standard treatment for muscle‐invasive bladder cancer. Journal of Clinical Oncology 2001;19:21s‐26s. [PubMed] [Google Scholar]
Stewart 1993
- Stewart LA, Parmar MKB. Meta‐analysis of the literature or of individual patient data: is there a difference?. Lancet 1993;341:418‐22. [DOI] [PubMed] [Google Scholar]
Stewart 1995
- Stewart LA, Clarke MJ, on behalf of the Cochrane Working Party Group. Practical methodology of meta‐analyses (overviews) using updated individual patient data. Stat Med 1995;14:2057‐79. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta‐blockade during and after myocardial infarction: An overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
ABC 2003
- Advanced Bladder Cancer Meta‐analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta‐analysis. Lancet 2003;361:1927‐1934. [DOI] [PubMed] [Google Scholar]
ABC 2005
- Advanced Bladder Cancer Meta‐analysis Collaboration. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta‐Analysis of Individual Patient Data. European Urology 2005;48(2):202‐206. [DOI] [PubMed] [Google Scholar]
ABCOC 2004
- Advanced Bladder Cancer Overview Collaboration. Neoadjuvant cisplatin for advanced bladder cancer (Cochrane review). Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001426] [DOI] [PubMed] [Google Scholar]


