Abstract
Bi-Oxazoline (biOx) has emerged as an effective ligand framework for promoting nickel-catalyzed cross-coupling, cross-electrophile coupling, and photoredox-nickel dual catalytic reactions. This report fills the knowledge gap of the organometallic reactivity of (biOx)Ni complexes, including catalyst reduction, oxidative electrophile activation, radical capture, and reductive elimination. The biOx ligand displays no redox activity in (biOx)Ni(I) complexes, in contrast to other chelating imine and oxazoline ligands. The lack of ligand redox activity results in more negative reduction potentials of (biOx)Ni(II) complexes and accounts for the inability of zinc and manganese to reduce (biOx)Ni(II) species. On the basis of these results, we revise the formerly proposed “sequential reduction” mechanism of a (biOx)Ni-catalyzed cross-electrophile coupling reaction by excluding catalyst reduction steps.
Since their emergence in nickel-catalyzed reactions, 2,2′-linked bioxazoline (biOx) ligands have achieved extensive success in promoting Lewis acid-catalyzed Diels–Alder,1 asymmetric cross-coupling,2,3 cross-electrophile coupling,4-6 and photoredox-nickel dual catalytic reactions.7-11 The reactivity of biOx appears complementary to bis-oxazoline (box).12,13 For instance, an asymmetric diarylation of vinylarenes employs an isoleucine-derived biOx ligand, whereas box ligands gave no desired product (Scheme 1A).14 The elucidation of the organometallic steps comprising the catalytic cycle would facilitate the understanding of the overall mechanism and the integral effect of the ligand. In contrast to the recent organometallic characterization of (box)Ni complexes by Fu and coworkers,15 the fundamental reactivity of (biOx)organonickel complexes remains ill-defined.16 The only precedent is has not been related to modern cross-coupling reactions.17
Scheme 1.
Application of biOx Ligands in Nickel-Catalyzed Cross-Coupling Reactions
A seminal application of (biOx)Ni catalysts is the enantioconvergent coupling of secondary benzyl chlorides with iodoheteroarenes developed by Reisman and co-workers (Scheme 1B).4 On the basis of a recent mechanistic study on (phen)Ni (phen = 1,10-phenanthroline) catalyzed cross-electrophile coupling,18 we postulated that this reaction could follow a “sequential reduction” pathway, involving the reduction of Ni(II) intermediates to Ni(0) and Ni(I) species that subsequently activate aryl and alkyl electrophiles (Scheme 1B).19 It remains indeterminate if biOx would alter the reaction mechanisms compared to phen and other common N-ligands. In this report, we establish the fundamental organometallic reactions of (biOx)Ni complexes. The results verify the plausibility of some proposed elementary steps but contradict others. These findings form the basis for a revision of the catalytic cycle of reductive coupling reactions involving biOx ligands.
This report also addresses the redox activity of biOx in stabilizing Ni(I) intermediates, which are prevalent in Ni-catalyzed cross-coupling reactions.20-24 Ni(I) intermediates are commonly supported by imine, oxazoline, and pyridine ligands.25,26 As strong σ-donors and π-acceptors, bis-(oxazolinyl)pyridine (pybox),27 α-diimine,28-30 and pyridyl oxazoline (pyrox)31 proved redox active when stabilizing low-valent nickel radical species by delocalizing the unpaired electron into the ligand π* orbital (Figure 1).32,33 A (Ph box)Ni(Br) complex that lacks π-conjugation between the two oxazoline rings accommodates a Ni-centered radical.15 It remains unclear whether the conjugation between two oxazoline motifs in biOx would enable redox activity. In this study, characterization of a monovalent (biOx)Ni complex elucidates the redox activity of biOx, which contributes to the unusually negative redox potentials of (biOx)Ni complexes and leads to alternative mechanistic pathways in catalysis.
Figure 1.
Low-valent nickel radical complexes bearing chelating oxazoline and imine ligands and their electronic structures.
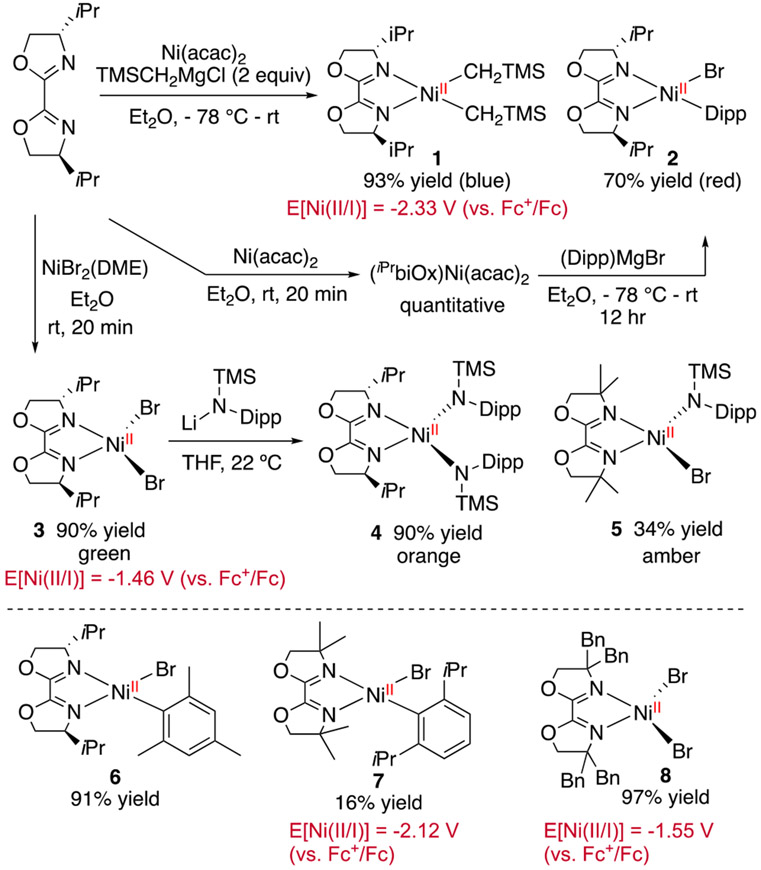
We selected iPrbiOx as the model ligand for the synthetic study of biOx-ligated organonickel complexes (Scheme 2). Addition of neosilylmagnesium chloride (TMSCH2MgCl) to a mixture of Ni(acac)2 (acac = acetylacetonate) and iPrbiOx produced (iPrbiOx)Ni(CH2TMS)2 1 in 93% yield. Following a literature protocol,17 we prepared (iPrbiOx)Ni(Dipp)Br (Dipp = 2,6-diisopropylphenyl) 2, (iPrbiOx)Ni(Mes)Br (Mes = 2,4,6-mesityl) 6, and (diMebiOx)Ni(Dipp)Br 7 by transmetalation of Grignard reagents. The diamagnetic 1H NMR spectra of 1, 2, and 6 (Figures S6-S8, and S12), in conjunction with their square planar geometry, evident from single crystal X-ray structures (Figures S36-S37), suggest that the d8 Ni(II) complexes adopt a low-spin configuration. Significant line broadening in the 1H NMR spectrum of 7 implies some geometry distortion, likely due to the steric strain (Figure S13). Mixing biOx ligands with NiBr2(DME) afforded (iPrbiOx)NiBr2 3 and (diBnbiOx)NiBr2 8. Complex 8 shows a dimeric structure of [(diBnbiOx)NiBr(μ-Br)]2 in the solid state (Figure S40). Addition of LiN(TMS)(Dipp) to 3 forms 4, whereas the bulkier diMebiOx gave 5 instead. The paramagnetic 1H NMR spectra (Figures S10-S11) and tetrahedral geometries for 4 and 5 , established by X-ray structures (Figures S38-S39), indicate high-spin d8 Ni(II) configurations caused by weak-field amido ligands.
Scheme 2.
Syntheses of (biOx)Ni(II) Complexes
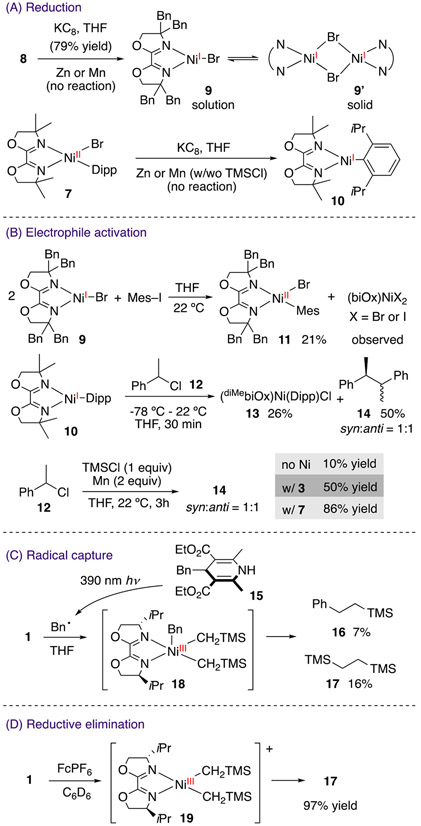
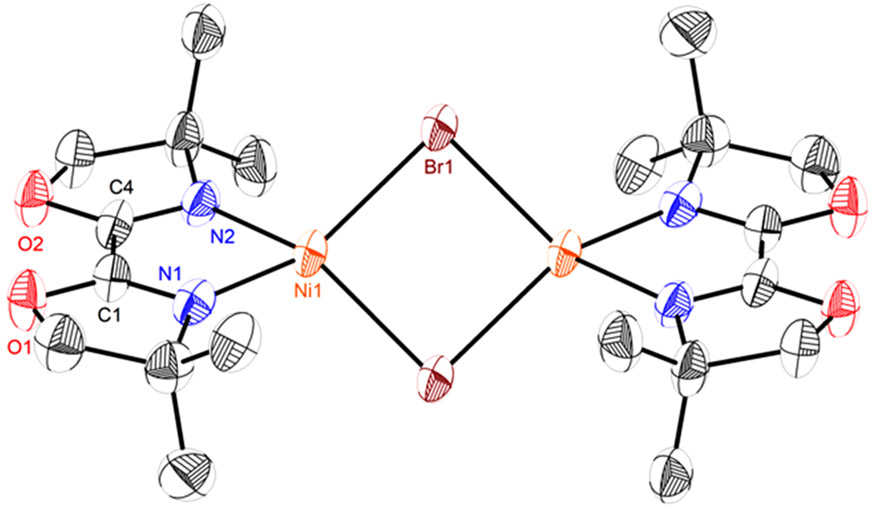
Access to complexes 1-8 provided a platform for investigating elementary reactions that possibly comprise the catalytic cycle in Scheme 1B. Reduction of 8 by KC8 in THF resulted in formation of a teal solution, displaying no signals in the 1H NMR spectrum (Scheme 3A). X-ray crystallography establishes a dimeric [(diBnbiOx)Ni(μ-Br)]2 9’ structure with an imine bond (C(1)─N(1)) length of 1.285(6) Å (Figure 2) almost identical to that of 8 (1.273(4) Å) (Figure S40). The EPR spectrum of 9 at 10 K reveals a rhombic signal with g values of [2.486, 2.175, 2.062] (Figure 3A).34,35 Data simulation ascribed the hyperfine couplings of the radical to two nitrogen atoms, assigned to the two imine nitrogen atoms of biOx. The double integration of the EPR signals against a Cu external standard established monomeric Ni(I) as the predominant species (99%) in solution. The minimal imine bond elongation in the crystal structure and the observation of a nickel-centered radical by EPR spectroscopy together suggests the lack of redox activity of biOx in 9.
Scheme 3.
Organometallic Transformations of (biOx)Ni(I), (II), and (III) Complexes
Figure 2.
X-ray structures of 9’ at 50% probability thermal ellipsoids. Hydrogen atoms are omitted and phenyl groups are truncated for clarity. Selected bond distances (Å): C(1)─N(1) = 1.285(6), C(4)─N(2) = 1.273(6), Ni─Br = 2.4560(8), Ni─Ni = 3.4620(12).
Figure 3.
X-Band EPR spectra (black) of 9 (A) and 10 (B) at10 K. The simulated spectra (red) use following parameters: 9, g = [2.486, 2.175, 2.062], AN,N = [107, 210, 0; 96, 148, 0] MHz; 10, g = [2.492, 2.179, 2.070], AN,N = [218, 31, 54; 218, 29, 50] MHz.
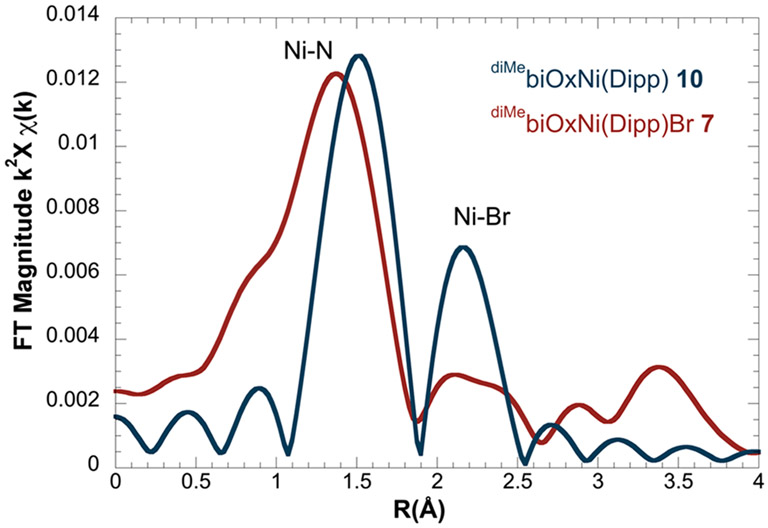
Reduction of 7 by KC8 formed a golden-brown species 10 (Scheme 3A). The EPR spectrum of 10 indicates a nickel-centered radical with g values of [2.492, 2.179, 2.070] and hyperfine features corresponding to the interaction of the radical to the two nitrogen atoms on the ligand (Figure 3B). We performed X-ray absorption spectroscopy (XAS) experiments on 10 and a series of Ni(I) and Ni(II) reference complexes. The pre-edge energies in the X-ray absorption near edge structure (XANES) span 8.3313 to 8.3346 keV across ten reference samples (Table S1) but do not show apparent correlation to the oxidation states.36 We then transformed the data to k2 weighted χ and analyzed by curve fitting of the extended X-ray absorption fine structure (EXAFS) spectra. The position of the features in the Fourier transform magnitude along the coordination shell radius (R) indicates the scattering pairs present, and the relative intensity corresponds to the coordination number. The comparison of 10 with 7 clearly indicates the dissociation of the bromide upon reduction, whereas the number of Ni─N bonds remains the same (Figure 4). Based on the above data, we assign this reduced species to (diMebiOx)NiI(Dipp) 10. DFT calculations suggest that (iPrbiOx)NiI(Dipp) is a nickel-centered radical (Figure S41).
Figure 4.
Fourier transforms of k2 χ (k) EXAFS functions of 7 (red) and 10 (blue)measured at the Ni─K edge.
Both 9 and 10 are nickel-centered radicals, evinced by EPR spectroscopy and DFT calculations. The lack of redox activity of biOx, in contrast to α-diimine,29 stems from the mesomeric effect of the oxygen atoms of oxazoline that increase the electron-density in the π orbitals. In the absence of the redox-active ligand stabilization, E[Ni(II/I)] of (biOx)Ni is noticeably more negative than complexes stabilized with redox-active bpy and phen ligands (Table 1).37 Consequently, low-valent (biOx)Ni species cannot be generated from the reduction of 7 and 8 by manganese or zinc powder, although Rieke’s manganese and zinc can reduce 7, due to an inner-sphere mechanism instead of an outer-sphere electron reduction (cf. SI).38
Table 1.
Comparison of E[Ni(II/I)]
| Complexes | Redox-activity | E1/2[Ni(II/I)] (V vs Fc+/Fc) |
|---|---|---|
| (Phen)Ni(Mes)Br39 | Yes | −1.50 |
| (bpy)Ni(Mes)Br40 | Yes | −1.79 |
| 7 | No | −2.12a |
| (6,6′-Mebpy)NiBr241 | Yes | −1.26 |
| 8 | No | −1.55a |
| 3 | No | −1.46a |
EP/2.
We then explored the activation of electrophiles by monovalent nickel complexes. (diBnbiOx)NiBr 9 proceeded to activate iodomesitylene to afford (diBnbiOx)Ni(Mes)Br 11 in 21% yield (Scheme 3B). Two possible pathways could account for this reactivity: iodomesitylene may oxidatively add to 9, followed by Mn reduction of Ni(III) or iodide radical ejection to afford 11; alternatively two molecules of 9 could engage in bimolecular oxidative addition.42 These pathways are not discerned at this point. While 9 can also activate 1-chloroethylbenzene 12, the rate is slower than that of iodomesitylene (cf. SI), consistent with a previous report that distinguishes the “radical chain” mechanism from the “sequential reduction” mechanism.18 (diMebiOx)Ni(Dipp) 10 reacted with 12 to give 13 and 14 in 26% and 50% yields, respectively. The inability of manganese to reduce 7 prompted us to postulate that alternative mechanisms were involved in the activation of 12. Control experiments reveal that 12 can readily dimerize in the presence of manganese and TMSCl,43 while the rate is increased by Ni(II) complexes, including 3 and 7 (Scheme 3B). Previous studies reveal that Lewis acids, such as nickel salts44 and ZnCl2,45 can promote the activation of allylic bromides and chlorides by manganese and TMSCl. Therefore, we attribute the formation of benzyl radical from benzyl chloride to the cooperative reactivity of manganese and TMSCl, catalyzed by Ni(II) species. This mechanism, involving manganese and TMSCl, complements the reactivity of Ni(I) complexes in C(sp3) electrophile activation46 but is restricted to benzyl halides, evident by the inactivity of chlorocyclohexane (cf. SI).
In order to probe the interaction of radicals with nickel species, we prepared 4-benzyl-1,4-dihydropyridine 15, a known radical precursor upon irradiation.47 While 1 was stable under 390 nm light irradiation, addition of 15 led to C─C bond formation to furnish 16 and 17 (Scheme 3C). The concomitant formation of 16 and 17 supports the emergence of Ni(III) 18 by radical capture, in which both neosilyl and benzyl groups are coordinated to nickel, followed by rapid reductive elimination.
Finally, we conducted the oxidation of 1 by FcPF6 (Fc = ferrocenium) (Scheme 3D). The blue solution of 1 immediately reacted to afford 17 in 97% yield (Scheme 3D). We propose that the oxidation of 1 resulted in Ni(III) 19 as an intermediate, followed by rapid reductive elimination.
The above data prompted us to reassess the initial mechanistic proposal shown in Scheme 1B (Scheme 4). Stoichiometric experiments of 9 establish that (biOx)Ni(X) can activate iodoarenes to afford (biOx)Ni(Ar)(X) intermediates (Scheme 3B). In contrast to reactions catalyzed by (phen)Ni complexes,18 (biOx)Ni(Ar)(X) is not reduced by zinc or manganese powder, due to its unusually negative reduction potential (Scheme 3A). Instead, benzyl chloride is activated by manganese and TMSCl, promoted by Ni(II) species. The resulting benzyl radical can be captured by (biOx)Ni(Ar)(X) to form a Ni(III) intermediate, followed by rapid reductive elimination to afford the cross-coupling product (Scheme 3C,D). Compared to the original proposal, the new mechanism excludes any catalyst reduction step. The radical capture step by Ni(II) species resonates with its participation in photoredox catalytic reactions by trapping photochemically generated radicals.7
Scheme 4.
Revised Mechanism of (biOx)Ni-Catalyzed Cross-Electrophile Coupling Reaction
In summary, we investigated the fundamental reactivity of a series of organometallic (biOx)Ni complexes, including reduction, electrophile activation, radical capture, and reductive elimination. BiOx lacks redox-activity in stabilizing Ni(I) complexes, in contrast to other common oxazoline, imine, and pyridine chelating ligands. This attribute results in more negative reduction potentials of Ni(II) species and prevents their reduction by zinc or manganese. As a result, we revise the mechanism of (biOx)Ni-catalyzed cross-electrophile coupling reactions, by invoking direct radical formation from the activation of alkyl chlorides by TMSCl and manganese. In this scenario, nickel catalysts serve to activate iodoarenes, capture the radical generated from alkyl chlorides, and mediate C─C bond formation, but are not reduced by manganese.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation under Award Number CHE-1654483. T.D. is a recipient of the Alfred P. Sloan Research Fellowship (FG-2018-10354) and the Camille-Dreyfus Teacher-Scholar Award (TC-19-019). T.D. acknowledges NSF (1827902) for funding to acquire the EPR spectrometer. The X-ray facility was supported partially by the National Science Foundation (CHE-0840277 and DMR-1420073).
Footnotes
Notes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c07139.
All experimental procedures, CV, NMR, XAS data (PDF)
Accession Codes
CCDC 2085350–2085355 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Contributor Information
Luchuan Ju, Department of Chemistry, New York University, New York, New York 10003, United States.
Qiao Lin, Department of Chemistry, New York University, New York, New York 10003, United States.
Nicole J. LiBretto, Department of Chemical Engineering, Purdue University, West Lafayette, Indiana 47906, United States
Clifton L. Wagner, Department of Chemistry, New York University, New York, New York 10003, United States
Chunhua Tony Hu, Department of Chemistry, New York University, New York, New York 10003, United States.
Jeffrey T. Miller, Department of Chemical Engineering, Purdue University, West Lafayette, Indiana 47906, United States.
Tianning Diao, Department of Chemistry, New York University, New York, New York 10003, United States.
REFERENCES
- (1).Kanemasa S; Adachi K; Yamamoto H; Wada E Bisoxazoline and Bioxazoline Chiral Ligands Bearing 4-Diphenylmethyl Shielding Substituents. Diels-Alder Reaction of Cyclopentadiene with 3-Acryloyl-2-oxazolidinone Catalyzed by the Aqua Nickel(II) Complex. Bull. Chem. Soc. Jpn 2000, 73, 681–687. [Google Scholar]
- (2).Do H-Q; Chandrashekar ERR; Fu GC Nickel/Bis(oxazoline)-Catalyzed Asymmetric Negishi Arylations of Racemic Secondary Benzylic Electrophiles to Generate Enantioenriched 1,1-Diarylalkanes. J. Am. Chem. Soc 2013, 135, 16288–16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Banerjee A; Yamamoto H Nickel Catalyzed Regio-, Diastereo-, and Enantioselective Cross-Coupling of 3,4-Epoxyalcohol with Aryl Iodides. Org. Lett 2017, 19, 4363–4366. [DOI] [PubMed] [Google Scholar]
- (4).Poremba KE; Kadunce NT; Suzuki N; Cherney AH; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling To Access 1,1-Diarylalkanes. J. Am. Chem. Soc 2017, 139, 5684–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Woods BP; Orlandi M; Huang C-Y; Sigman MS; Doyle AG Nickel-Catalyzed Enantioselective Reductive Cross-Coupling of Styrenyl Aziridines. J. Am. Chem. Soc 2017, 139, 5688–5691. [DOI] [PubMed] [Google Scholar]
- (6).Tu H-Y; Wang F; Huo L; Li Y; Zhu S; Zhao X; Li H; Qing F-L; Chu L Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins through Nickel-Catalyzed Cross-Electrophile Coupling. J. Am. Chem. Soc 2020, 142, 9604–9611. [DOI] [PubMed] [Google Scholar]
- (7).Tellis JC; Primer DN; Molander GA Single-Electron Transmetalation in Organoboron Cross-coupling by Photoredox/Nickel Dual Catalysis. Science 2014, 345, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ryu D; Primer DN; Tellis JC; Molander GA Single-Electron Transmetalation: Synthesis of 1,1-Diaryl-2,2,2-trifluoroethanes by Photoredox/Nickel Dual Catalytic Cross-Coupling. Chem. - Eur. J 2016, 22, 120–123. [DOI] [PubMed] [Google Scholar]
- (9).El Khatib M; Serafim RAM; Molander GA α-Arylation/Heteroarylation of Chiral α-Aminomethyltrifluoroborates by Synergistic Iridium Photoredox/Nickel Cross-Coupling Catalysis. Angew. Chem., Int. Ed 2016, 55, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ahneman DT; Doyle AG C-H Functionalization of Amines with Aryl Halides by Nickel-Photoredox Catalysis. Chem. Sci 2016, 7, 7002–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Guo L; Yuan M; Zhang Y; Wang F; Zhu S; Gutierrez O; Chu L General Method for Enantioselective Three-Component Carboarylation of Alkenes Enabled by Visible-Light Dual Photoredox/Nickel Catalysis. J. Am. Chem. Soc 2020, 142, 20390–20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lou S; Fu GC Nickel/Bis(oxazoline)-Catalyzed Asymmetric Kumada Reactions of Alkyl Electrophiles: Cross-Couplings of Racemic α-Bromoketones. J. Am. Chem. Soc 2010, 132, 1264–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hofstra JL; Cherney AH; Ordner CM; Reisman SE Synthesis of Enantioenriched Allylic Silanes via Nickel-Catalyzed Reductive Cross-Coupling. J. Am. Chem. Soc 2018, 140, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Anthony D; Lin Q; Baudet J; Diao T Nickel-Catalyzed Asymmetric Reductive Diarylation of Vinylarenes. Angew. Chem., Int. Ed 2019, 58, 3198–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yin H; Fu GC Mechanistic Investigation of Enantioconvergent Kumada Reactions of Racemic α-Bromoketones Catalyzed by a Nickel/Bis(oxazoline) Complex. J. Am. Chem. Soc 2019, 141, 15433–15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For a computational study, see: Gutierrez O; Tellis JC; Primer DN; Molander GA; Kozlowski MC Nickel-Catalyzed Cross-Coupling of Photoredox-Generated Radicals: Uncovering a General Manifold for Stereoconvergence in Nickel-Catalyzed Cross-Couplings. J. Am. Chem. Soc 2015, 137, 4896–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Walther D; Dohler T; Heubach K; Klobes O; Schweder B; Gorls H Catalytic Cyclization and Dimerization of 1.5-Hexadiene by Transition Metals: The Reactivity of NiII and PdII Complexes containing Bi- or Tridendate Ligands and of Bis(alkyne)nickel(0) Compounds. Z. Anorg. Allg. Chem 1999, 625, 923–932. [Google Scholar]
- (18).Lin Q; Diao T Mechanism of Ni-Catalyzed Reductive 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc 2019, 141, 17937–17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Diccianni JB; Katigbak J; Hu C; Diao T Mechanistic Characterization of (Xantphos)Ni(I)-Mediated Alkyl Bromide Activation: Oxidative Addition, Electron Transfer, or Halogen-Atom Abstraction. J. Am. Chem. Soc 2019, 141, 1788–1796. [DOI] [PubMed] [Google Scholar]
- (20).Hu X Nickel-catalyzed Cross Coupling of Non-Activated Alkyl Halides: A Mechanistic Perspective. Chem. Sci 2011, 2, 1867–1886. [Google Scholar]
- (21).Tasker SZ; Standley EA; Jamison TF Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Fu GC Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes. ACS Cent. Sci 2017, 3, 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Diccianni J; Diao T Mechanisms of Nickel-Catalyzed Cross Coupling Reactions. Trends Chem. 2019, 1, 830–844. [Google Scholar]
- (24).Diccianni J; Lin Q; Diao T Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res 2020, 53, 906–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lin C-Y; Power PP Complexes of Ni(I): A ″Rare″ Oxidation State of Growing Importance. Chem. Soc. Rev 2017, 46, 5347–5399. [DOI] [PubMed] [Google Scholar]
- (26).Zimmermann P; Limberg C Activation of Small Molecules at Nickel(I) Moieties. J. Am. Chem. Soc 2017, 139, 4233–4242. [DOI] [PubMed] [Google Scholar]
- (27).Schley ND; Fu GC Nickel-Catalyzed Negishi Arylations of Propargylic Bromides: A Mechanistic Investigation. J. Am. Chem. Soc 2014, 136, 16588–16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Dong Q Zhao Y; Su Y; Su J-H; Wu B; Yang X-J Synthesis and Reactivity of Nickel Hydride Complexes of an α-Diimine Ligand. Inorg. Chem 2012, 51, 13162–13170. [DOI] [PubMed] [Google Scholar]
- (29).Kuang YL; Anthony D; Katigbak J; Marrucci F; Humagain S; Diao TN Ni(I)-Catalyzed Reductive Cyclization of 1,6-Dienes: Mechanism-Controlled trans Selectivity. Chem. 2017, 3, 268–280. [Google Scholar]
- (30).Zarate C; Yang H; Bezdek MJ; Hesk D; Chirik PJ Ni(I)-X Complexes Bearing a Bulky α-Diimine Ligand: Synthesis, Structure, and Superior Catalytic Performance in the Hydrogen Isotope Exchange in Pharmaceuticals. J. Am. Chem. Soc 2019, 141, 5034–5044. [DOI] [PubMed] [Google Scholar]
- (31).Wagner CL; Herrera G; Lin Q; Hu CT; Diao T Redox Activity of Pyridine-Oxazoline Ligands in the Stabilization of Low-Valent Organonickel Radical Complexes. J. Am. Chem. Soc 2021, 143, 5295–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Anderson TJ; Jones GD; Vicic DA Evidence for a NiI Active Species in the Catalytic Cross-Coupling of Alkyl Electrophiles. J. Am. Chem. Soc 2004, 126, 8100–8101. [DOI] [PubMed] [Google Scholar]
- (33).Lu CC; Bill E; Weyhermüller T; Bothe E; Wieghardt K Neutral Bis(α-iminopyridine)metal Complexes of the First-Row Transition Ions (Cr, Mn, Fe, Co, Ni, Zn) and Their Monocationic Analogues: Mixed Valency Involving a Redox Noninnocent Ligand System. J. Am. Chem. Soc 2008, 130, 3181–3197. [DOI] [PubMed] [Google Scholar]
- (34).Zheng B; Tang F; Luo J; Schultz JW; Rath NP; Mirica LM Organometallic Nickel(III) Complexes Relevant to Cross-Coupling and Carbon-Heteroatom Bond Formation Reactions. J. Am. Chem. Soc 2014, 136, 6499–6504. [DOI] [PubMed] [Google Scholar]
- (35).Mohadjer Beromi M; Brudvig GW; Hazari N; Lant HMC; Mercado BQ Synthesis and Reactivity of Paramagnetic Nickel Polypyridyl Complexes Relevant to C(sp2)-C(sp3) Coupling Reactions. Angew. Chem., Int. Ed 2019, 58, 6094–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Sarangi R; Dey M; Ragsdale SW Geometric and Electronic Structures of the NiI and Methyl-NiIII Intermediates of Methyl-Coenzyme M Reductase. Biochemistry 2009, 48, 3146–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Lin Q; Dawson G; Diao T, Experimental Electrochemical Potentials of Nickel Complexes. Synlett. 2021, 32; DOI: 10.1055/s-0040-1719829. [DOI] [Google Scholar]
- (38).Guijarro A; Rosenberg DM; Rieke RD The Reaction of Active Zinc with Organic Bromides. J. Am. Chem. Soc 1999, 121, 4155–4167. [Google Scholar]
- (39).Yakhvarov DG; Petr A; Kataev V; Büchner B; Gómez-Ruiz S; Hey-Hawkins E; Kvashennikova SV; Ganushevich YS; Morozov VI; Sinyashin OG Synthesis, structure and electrochemical properties of the organonickel complex [NiBr(Mes)(phen)] (Mes = 2,4,6-trimethylphenyl, phen = 1,10-phenanthroline). J. Organomet. Chem 2014, 750, 59–64. [Google Scholar]
- (40).Klein A; Kaiser A; Sarkar B; Wanner M; Fiedler J The electrochemical behaviour of organonickel complexes: Mono-, di- and trivalent nickel. Eur. J. Inorg. Chem 2007, 2007, 965–976. [Google Scholar]
- (41).Sahoo B; Bellotti P; Juliá-Hernández F; Meng Q-Y; Crespi S; König B; Martin R Site-Selective, Remote sp3 C-H Carboxylation Enabled by the Merger of Photoredox and Nickel Catalysis. Chem. - Eur. J 2019, 25, 9001–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Yoo C; Lee Y A T-Shaped Nickel(I) Metalloradical Species. Angew. Chem., Int. Ed 2017, 56, 9502–9506. [DOI] [PubMed] [Google Scholar]
- (43).Takai K; Ueda T; Hayashi T; Moriwake T Activation of Manganese Metal by a Catalytic Amount of PbCl2 and Me3SiCl. Tetrahedron Lett. 1996, 37, 7049–7052. [Google Scholar]
- (44).Fürstner A; Shi N A Multicomponent Redox System Accounts for the First Nozaki-Hiyama-Kishi Reactions Catalytic in Chromium. J. Am. Chem. Soc 1996, 118, 2533–2534. [Google Scholar]
- (45).Cahiez G; Chavant P-Y Organomanganese (II) Reagents XX: Manganese Mediated Barbier and Reformatsky like Reactions an Efficient Route to Homoallylic Alcohols and β-Acetoxyesters. Tetrahedron Lett. 1989, 30, 7373–7376. [Google Scholar]
- (46).Lin Q; Fu Y; Liu P; Diao T Monovalent Nickel-Mediated Radical Formation: A Concerted Halogen-Atom Dissociation Pathway Determined by Electroanalytical Studies. J. Am. Chem. Soc 2021, 5255 DOI: 10.1021/jacs.1c05255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Buzzetti L; Prieto A; Roy SR; Melchiorre P Radical-Based C-C Bond-Forming Processes Enabled by the Photoexcitation of 4-Alkyl-1,4-dihydropyridines. Angew. Chem., Int. Ed 2017, 56, 15039–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.