The anti-CD20 monoclonal antibodies rituximab (R) and obinutuzumab (O) are used as maintenance therapy every two months for two-three years in patients with follicular lymphoma (FL) and mantle cell lymphoma (MCL). This strategy improves event-free survival (EFS) and/or overall survival (OS) after immunochemotherapy in responding patients.1-3 Severe forms of COVID-19 with prolonged carriage of the virus in patients on R maintenance have been reported due to a significant alteration of humoral immunity in this context.4,5 Therefore, during the COVID-19 epidemic, clinicians are faced with the question of whether to discontinue maintenance therapy or not. The Pfizer/BioNTech RNA vaccine BNT162b2 (Comirnaty®) has shown efficacy and safety data in all age groups and against several variants. 6,7 The specificities of mRNA vaccines also suggest that they may induce a better T lymphocyte response.8
The aim of our study was to evaluate the post-vaccination humoral and T cell response based on the serological data and enumeration of interferon gamma (IFNγ)-producing T cells in response to SARS-COV-2 specific antigens (Elispot assay) in a group of patients with a longterm anti-CD20 antibody lymphoma treatment.
Patients with lymphoma receiving or initiating their maintenance therapy by anti-CD20 antibodies (R or O) and treated in a single center - Centre Henri Becquerel, France - were selected. The study protocol was approved by the local and national ethics committees (Internal Review board N° 2106B and Comité de Protection des Personnes, Ile de France IV, registered as number NCT04918940 at clinical.trial.gov) and written informed consent was obtained. Serologies and Elispot assays were repeated between D21-D28 after the first vaccination (V1), one month after the second vaccination (V2) and 1 month after the third vaccination (V3).
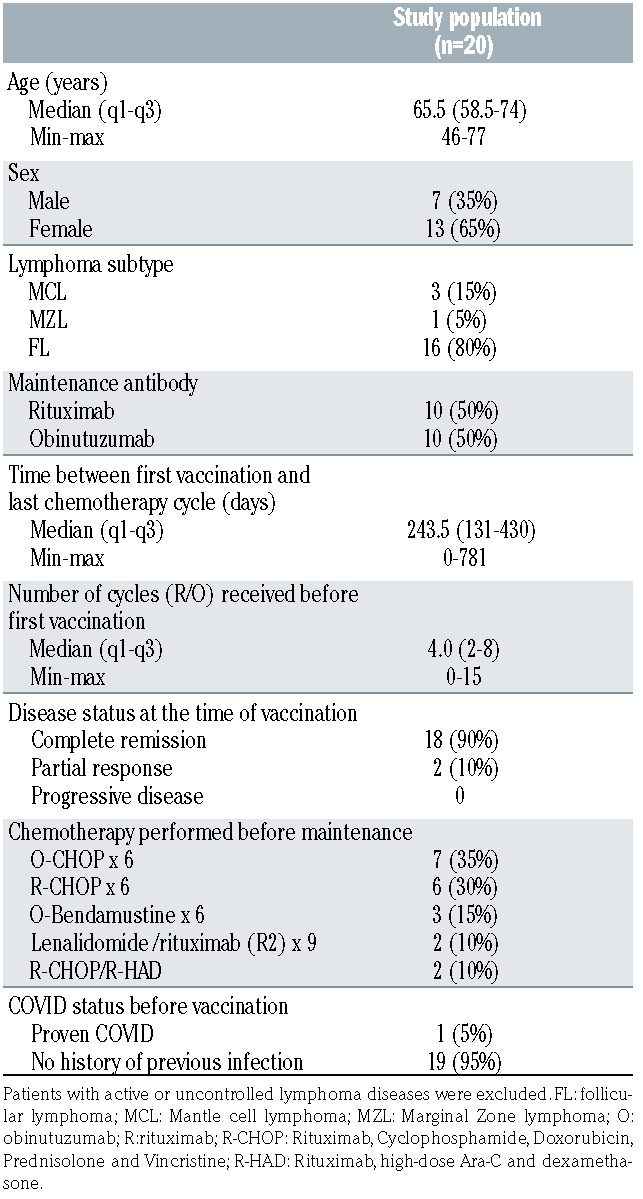
Twenty patients were enrolled in this study: 16 FL (80%); 3 MCL (15%); 1 Marginal Zone Lymphoma (5%) between 21 May 2021 and 1 July 2021. They received three doses of BNT162b2 vaccine after the initial phase of immunochemotherapy, in a steady state regarding lymphoma status (Table 1), during the maintenance phase. Ten patients were treated with R and ten with O. It should be noted that, in order to vaccinate patients as quickly as possible, the vaccine injections were carried out without any time interval rules with respect to the anti-CD20 antibody injections. The median number of R or O infusions received during the maintenance phase at the time of the first vaccination was four infusions (range 0-15, Table 1). The B-cell depletion was profound for all patients with no detectable CD19+/CD20+ cells. T-cell counts also indicated a low rate of both CD4+ and CD8+ T cells in 12/17 (70%) and 9/17 (53%) cases, respectively. Conversely, NK CD56+CD16+ cells were in the normal range in 16/17 (94%) cases (Table 2). With a median follow- up of 96 days (range 75-150), no COVID-19 infection was detected in the cohort.
The anti–SARS-CoV-2 post-vaccine antibody response against the spike protein (RBD) was centrally assessed using the ARCHITECT SARS-Cov-2 IgG II Quant (Abbott) CMIA test, with titers >50 arbitrary units (AU) per milliliter considered positive (measurement interval: 6.8–80,000 AU/ml; positive agreement, 99.4%; negative agreement, 99.6%). At baseline, antibodies against the SARS-CoV-2 virus spike protein were detected in 1/17 patients (5.9%, patient n°7, discussed hereafter). After one, two and three vaccine injections, all except this patient remained below the threshold of 50 AU/ml, indicating that the humoral response is deeply impaired in this cohort and does not seem improved by a triple-injection schedule. Therefore, the positivity rate one month after the third injection in this cohort remained unchanged at 5% (1/20 tested patients) with no additional responder. Of note, antibody titers only increase after vaccination in patient n°7 despite the fact that, as with other patients, no CD19+/CD20+ B-cells were detectable by cytometry. This male FL patient was treated initially by O-chlorambucil as a first-line treatment. He developed a COVID-19 disease after the first cycle (C1-D8) of this treatment. Other than oxygen therapy, no intensive care was required but the infection led to the postponement of immunochemotherapy for two months. The patient was thereafter treated by R-CHOP (six cycles). This led to the patient receiving one infusion of binutuzumab and six infusions of rituximab before his first vaccine injection.
Contrasting with the deep alteration of the humoral response to SARS-COV-2 vaccination, we demonstrated the induction of an anti-spike T-cell response, as assessed by IFNγ Elispot assay, irrespective of the antibody response. Elispot assays were performed, as previously reported.9
Table 1.
Population clinical features
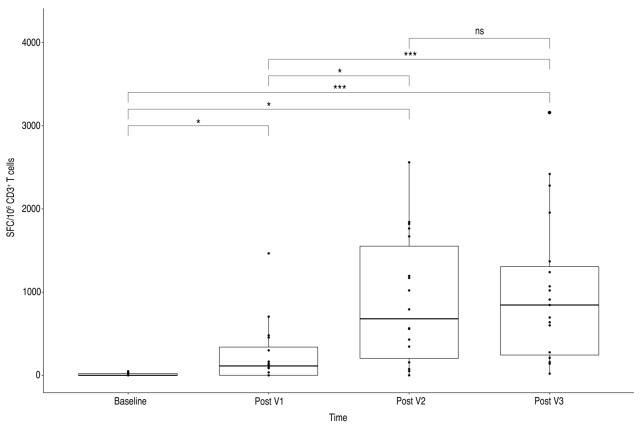
At baseline, and after one, two and three injections, the median number of SFC/106 CD3+ T cells were 0 (0-20), 112.5 (0;339), 679.0 (202;1551) and 845.0 (243; 1305), respectively. Overall, one month after V2 and V3, 17/19 patients (89%) displayed IFNγ-producing T cells reactive to S (S1+S2) peptide pools.
A significant increase in T-cell response, compared to baseline, was observed throughout the vaccination process until V2. The magnitude of T-cell response increased after the third vaccination in eight patients but appears globally unchanged for the entire cohort (Figure 1). T-cell response did not differ significantly after the complete vaccination schedule according to the type of anti-CD20 antibodies used (mean SFC/106 CD3+ T cells = 1045.0 (749; 1338) for O and 600.0 (160; 845) for R) or according to the number of anti-CD20 infusions received before vaccination (data not shown). In this cohort, no pre-existing immune memory was suggested by responses to other non-spike antigens in 19/20 patients. Conversely, as expected, patient n°7 displayed IFNγ-producing T cells reactive to N, M, and N7A SARS-CoV-2 proteins. Importantly, the level of T-cell response was at least equivalent to that observed in kidney-transplanted patients. We previously reported, using the same assay, a median rate of 212 SFCs /106 CD3+ T cells after two doses in responding patients (versus 679 SFCs /106 CD3+ T in the present cohort) and 330 SFC/106 CD3+ T cells after the third injection (versus 845 SFCs/106 CD3+T)(paper submitted, in revision).9 Similarly, the level of T-cell response was not below the that reported in healthy individuals, showing a median of 165 SFU/106 PBMCs 28 days after two doses (Angyal et al, Lancet 2021, in press). These comparisons suggest that T-cell response at least remains conserved in these highly immunocompromised patients.
However, there is no clear demonstration as to whether T-cell activity is sufficient to protect vaccinated patients from COVID-19 infection.10 Robust T-cell responses to the SARS-CoV-2 virus occur in most individuals with COVID-19.11 Furthermore, SARS-CoV-2-specific T cells have been detectable in antibody-seronegative exposed family members and convalescent individuals with a history of asymptomatic and mild COVID-19, consistent with a non-redundant role of immune protection against COVID-19.11 Importantly, after the third vaccine dose, we still observed an increased interferon-γ response to the SARS-Cov-2 spike protein. This was particularly visible for patient n°7, characterized by a previous COVID-19 disease that occurred at the beginning of his lymphoma treatment, when only one dose of obinutuzumab had been delivered.
To improve the rate of seroconversion or to maintain a humoral response in elderly individuals or immunocompromised patients, a third injection was proposed. However, the impact of such a strategy in such a case of very deep B-cell depletion is still uncertain. In the kidney transplant setting, one study has shown that a third dose of mRNA-1273 vaccine induced a serologic response in 49% of kidney transplant recipients who did not respond after two doses.12 Administration of a third dose of the BNT162b2 vaccine to solid organ transplant recipients, or to recipients of allogeneic HSCT, also significantly improved the immunogenicity of the vaccine.13,14 In our cohort characterized by complete B-cell depletion, we did not observe any improvement of the antibody response after the third injection. The vaccination schedule could also be improved by increasing time-lapse between the second and third injection or by offering heterologous prime-boost strategies that demonstrated increased levels of neutralizing antibody titers in immunocompetent patients, as compared to homologous prime-boost strategies. 15 Nevertheless, whether this optimization might result in an improved level of protection remains uncertain. REGN-COV2, a neutralizing antibody cocktail, may also be proposed as a prophylactic strategy in these immunosuppressed patients after or before SarsCov2 exposition.
The question of delaying or ceasing maintenance therapy is clearly raised for patients with MCL and FL in the current pandemic context. The benefit in terms of OS in MCL, contrasting with the benefit only for PFS in FL, is an important point to consider when considering the benefit-risk balance. Other points such as social context, the ability to follow physical protective measures and access to passive immunization with anti-S monoclonal antibody therapy, should be considered for each individual patient in order to correctly evaluate their benefit-risk balance.
Finally, given the lack of post-vaccinal humoral response observed in our cohort, vaccination may still provide a limited but significant protection by triggering a T-cell response. This should encourage patients and physicians to maintain a proactive vaccine policy.
Table 2.
Population biological parameters at baseline
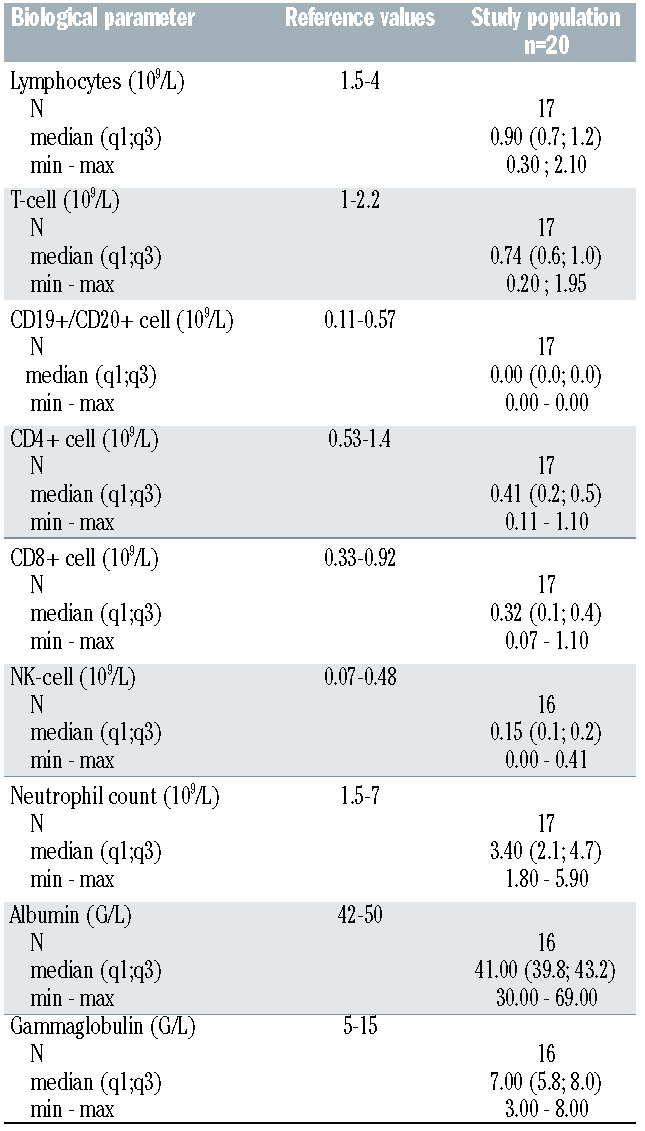
Figure 1.
SARS-CoV-2–reactive IFNγ-producing T cells after vaccination. Elispot assays were performed, as previously reported (8). Briefly, PBMCs (in concentrations adjusted to 2x105 CD3+ T cells per well) were plated in anti-IFNγ–coated Elispot 96-well plates in the presence of overlapping 15-mer peptide pools spanning the sequence of SARS-CoV-2 structural and nonstructural proteins: S (pool S1 spanning the N-terminal part of the protein including the S1-subunit, and pool S2 spanning the C-terminal part), N, M, NS3A, NS7A (JPT, Strassberg, Germany). Spots were counted with an automated ELISPOT reader (AID, Strassberg, Germany). Results were expressed as spot forming cells (SFC) per 106 CD3+ T cells. For each assay, a specific response was considered positive if the SFC number was superior to 3 standard deviations of spot numbers observed in wells without antigens (ranging between 9 and 20 SFC/106 CD3+ T cells).
Acknowledgments
The authors thank for their help and technical support: Delphine Robbe, Julie Libraire, Nathalie Breda, Laure Gaillon, Justine Loret, Laure Sulpice and Julie Lamulle.
References
- 1.Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377(13):1250-1260. [DOI] [PubMed] [Google Scholar]
- 2.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42-51. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R, Davies A, Ando K, et al. Obinutuzumab for the firstline treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331-1344. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda H, Tsukune Y, Watanabe N, et al. Persistent COVID-19 pneumonia and failure to develop anti-SARS-CoV-2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2020;20(11):774-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulery R, Lamure S, Delord M, et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non- Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021;96(8):934-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594-599. [DOI] [PubMed] [Google Scholar]
- 9.Candon S, Guerrot D, Drouot L, et al. T cell and antibody responses to SARS-CoV-2: Experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant. 2021;21(2):854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonelli MM, Mrak D, Perkmann T, Haslacher H, Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021;80(10):1355-1356. [DOI] [PubMed] [Google Scholar]
- 11.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T Cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158-168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redjoul R, Le Bouter A, Parinet V, Fourati S, Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8(10):e681-e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenbusch M, Schumacher S, Vogel E, et al. Heterologous primeboost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 2021;21(9):1212-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]