Abstract
Background and Objectives
The choroid plexus has been shown to play a crucial role in CNS inflammation. Previous studies found larger choroid plexus in multiple sclerosis (MS) compared with healthy controls. However, it is not clear whether the choroid plexus is similarly involved in MS and in neuromyelitis optica spectrum disorder (NMOSD). Thus, the aim of this study was to compare the choroid plexus volume in MS and NMOSD.
Methods
In this retrospective, cross-sectional study, patients were included by convenience sampling from 4 international MS centers. The choroid plexus of the lateral ventricles was segmented fully automatically on T1-weighted MRI sequences using a deep learning algorithm (Multi-Dimensional Gated Recurrent Units). Uni- and multivariable linear models were applied to investigate associations between the choroid plexus volume, clinically meaningful disease characteristics, and MRI parameters.
Results
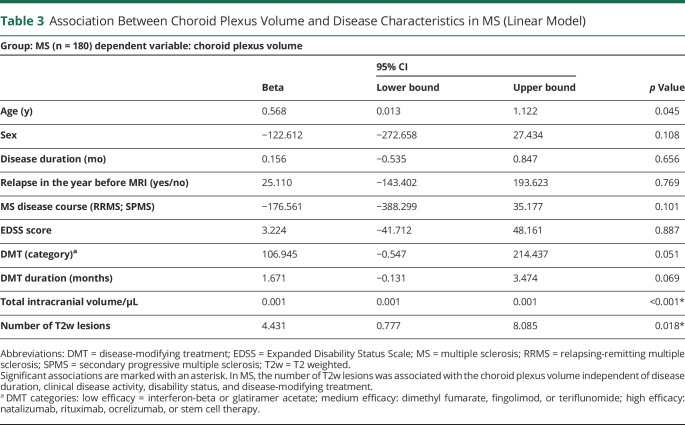
We studied 180 patients with MS and 98 patients with NMOSD. In total, 94 healthy individuals and 47 patients with migraine served as controls. The choroid plexus volume was larger in MS (median 1,690 µL, interquartile range [IQR] 648 µL) than in NMOSD (median 1,403 µL, IQR 510 µL), healthy individuals (median 1,533 µL, IQR 570 µL), and patients with migraine (median 1,404 µL, IQR 524 µL; all p < 0.001), whereas there was no difference between NMOSD, migraine, and healthy controls. This was also true when adjusted for age, sex, and the intracranial volume. In contrast to NMOSD, the choroid plexus volume in MS was associated with the number of T2-weighted lesions in a linear model adjusted for age, sex, total intracranial volume, disease duration, relapses in the year before MRI, disease course, Expanded Disability Status Scale score, disease-modifying treatment, and treatment duration (beta 4.4; 95% CI 0.78–8.1; p = 0.018).
Discussion
This study supports an involvement of the choroid plexus in MS in contrast to NMOSD and provides clues to better understand the respective pathogenesis.
The choroid plexus is constituted by cuboid epithelial cells ensheathing fenestrated blood vessels and a connective stroma.1 It is located inside the brain ventricles and produces CSF in the vertebrate brain.2 The choroid plexus is essential for the development, maintenance, and normal function of the brain1,2 and serves as a port of entry for immune cells to the CNS.2,3 It is both a target and modulator of inflammation.4
Histopathologic studies in multiple sclerosis (MS) showed inflammation and enlargement of the choroid plexus due to edema with high concentrations of T lymphocytes, dendritic cells, and activated macrophages.4,5 These studies also found increased concentrations of vascular cell adhesion protein 1 staining indicating active recruitment of peripheral inflammatory cells.2,4-8 In a postmortem study, the immune cell pattern in the choroid plexus observed in MS was similar to that seen in acute viral encephalitis with activation of immune cells and enlargement of choroid plexus stroma cells.4
Recently, a 3 tesla (3T) MRI and PET study confirmed larger choroid plexus and higher fluorine 18 fluorodeoxyglucose uptake in the choroid plexus of 97 patients with MS compared with 44 healthy controls (HCs) in vivo indicating inflammation within the choroid plexus of patients with MS.9 The same research group recently found enlarged choroid plexus in individuals with a radiologically isolated syndrome (presymptomatic stage of the disease) compared with HCs, supporting the hypothesis of enlargement of the choroid plexus due to MS-related inflammation.10
Neuromyelitis optica spectrum disorder (NMOSD) has a different pathophysiology than MS.11 In contrast to MS, NMOSD is an antibody-mediated inflammation.11 The antigen is aquaporin-4, a water channel molecule that is abundant on the astrocyte end feet in the CNS.11 The antigen-antibody reaction triggers astrocyte injury through complement-dependent cytotoxicity. So, the breakdown of the blood-brain barrier in NMOSD is less dependent on the migration and infiltration of lymphocytes into the choroid plexus as seen in MS but on antibodies against aquaporin-4.12-14 Thus, we hypothesized that the choroid plexus is larger in MS than NMOSD, and if this is true, the choroid plexus volume measured using MRI might be helpful to distinguish both conditions.
Recently, a study that compared the choroid plexus size between MS and NMOSD did not find significant differencens between the 2 diseases.15 However, that study included only a small number of patients (51 patients with MS and 32 patients with NMOSD), and used only linear measures on axial and coronal MRI slices to calculate the choroid plexus volume. Therefore, we aimed to investigate the choroid plexus volume in MS vs NMOSD in a larger study population and using high-resolution 3D MRI data in a multicenter setting.
Methods
Study Design and Participants
This study was designed as a retrospective, cross-sectional MRI study to compare the choroid plexus volume between patients with MS and NMOSD in vivo. We included healthy individuals and patients with migraine as controls. Migraine was chosen as an example of a nonautoimmune inflammatory CNS disease.
Our null hypothesis was that there is no difference in the choroid plexus volume between MS and NMOSD compared with migraine and HCs. We planned a priori to investigate the associations between the choroid plexus volume and disease characteristics in MS and NMOSD.
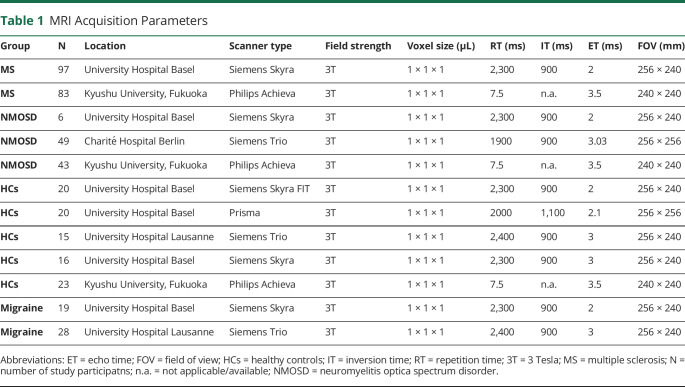
MRI and clinical data derived from 4 MS centers including the University Hospital of Basel (Switzerland; Swiss MS cohort study),16 Kyushu University Hospital in Fukuoka (Japan), Charité - Universitätsmedizin Berlin (Germany), and the Centre Hospitalier Universitaire Vaudois, Lausanne (Switzerland). The inclusion criteria for this study were the diagnosis of MS or NMOSD and a full 3T MRI data set consisting of a T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) image and a fluid-attenuated inversion recovery (FLAIR) sequence. Patients with MS had to fulfill the McDonald criteria 2010,17 patients with NMOSD the 2015 International Panel for NMO Diagnosis criteria,18 and patients with migraine the diagnostic criteria of the International Headache Society.19 HCs were included from Basel, Lausanne, and Fukuoka. The patients with migraine derived from Basel and Lausanne. We excluded individuals with any other comorbidity of the CNS (except of migraine). We also checked all MRIs for incidental findings associated with the choroid plexus (such as plexus papilloma or ependymoma), which have not been detected in any of the study participants.
Clinical Characteristics
The MS disease course (relapsing-remitting [RR] MS or secondary progressive [SP] MS) was defined according to the Lublin criteria.20 Disease duration in MS and NMOSD was defined as time between first symptoms of the disease and the MRI time point. The disability status was measured using the Expanded Disability Status Scale (EDSS).21 Relapses were defined as new or recurring neurologic deficits or symptoms associated with MS or NMOSD that lasted for at least 24 hours in the absence of fever or an infection and could not be better explained by any other comorbidity.22 Serum antibody diagnostic testing was conducted using state-of-the-art cell-based assays.23,24 To include the MS disease-modifying treatment (DMT) as covariate into the linear model, we grouped the treatments into 3 efficacy categories: 1 = first generation injectables (interferon-beta and glatiramer acetate); 2 = orals (dimethyl fumarate, teriflunomide, and fingolimod); 3 = IV (natalizumab, rituximab, ocrelizumab, mitoxantrone, and stem cell therapy).
MRI Acquisition and Analysis
MRI scanner type and acquisition parameters are provided in Table 1. T2-weighted (T2w) white matter lesions were segmented fully automatically on FLAIR images using a deep learning algorithm (Multi-Dimensional Gated Recurrent Units).25 We used the same algorithm to segment the lateral ventricle choroid plexus on 3D T1-weighted MPRAGE sequences (Figure 1). The algorithm is available on GitHub (github.com/zubata88/mdgru). The deep learning algorithm was trained with 125 manual lateral ventricle choroid plexus segmentations: 42 in patients with MS, 38 in patients with migraine, and 45 in healthy controls. These choroid plexus maps were quality checked by a board-certified neuroradiologist and served as ground truth for the machine learning algorithm. To control for the head size, we adjusted the statistical models for total intracranial volume (TIV) and in a sensitivity analysis for the lateral ventricle volume. TIV and lateral ventricle volume were measured using FreeSurfer (surfer.nmr.mgh.harvard.edu/) on white matter lesion–filled T1-weighted MPRAGE sequences. Similar to a previously published study,9 we reported the choroid plexus volume also relative to the TIV.
Table 2.
Demographics, Clinical Characteristics, and MRI Variables of the Study Participants
Figure 1. Choroid Plexus Segmentation and White Matter Parcellation.
(A) Automated segmentation (green) of the brain lateral ventricle choroid plexus in a multiple sclerosis patient using multi-dimensional gated recurrent units.25 (B) Parcellation of the white matter into periventricular bands. To investigate the association between choroid plexus volume and distance between MS and lateral ventricle wall, we parcellated the white matter into periventricular bands extending from the lateral ventricle to the cortex. As a precaution against periventricular CSF / white matter partial volume effects, we excluded data from the first periventricular band. The white matter band masks were applied on co-registered T1- and T2w lesion masks to calculate the volume of lesions in each periventricular band. Lesions are marked in dark blue and the white matter bands in red. (C) 3D-model of the choroid plexus, based on segmentation of (A). P = posterior; R = right; S = superior.
To investigate the association between choroid plexus volume and distance of T2w white matter lesions to the lateral ventricle wall, we parcellated the white matter in concentric 1-voxel thick white matter bands by repeatedly dilating the lateral ventricle mask by 1 voxel (1 × 1 × 1 mm3) using the DilM option in Functional MRI Brain Software Library 6.0.1 (FSL)26 on T1w MPRAGE (Figure 1B). To minimize partial volume effects, we eliminated the first band from the analysis. We assessed the T2w lesion number and volume in each periventricular white matter band separately. All outputs were reviewed by experienced raters and corrected manually, blinded to the clinical data using 3D slicer (slicer.org, Version 4.6.2). In total, 151/419 (36%) of automated choroid plexus segmentations needed minor corrections (only a few voxels for most of the scans).
Statistical Analysis
We used data from a recent study15 for an a priori sample size calculation (t test for independent samples). That study15 found a mean choroid plexus diameter of 2.09 +/− 0.49 mm on coronal plane in MS vs 1.89 +/− 0.48 mm in NMOSD. We calculated a minimum of 47 patients that would be needed in each group to detect a significant difference between the groups with a type I error rate of 5% and a power of 80%. Normality of the variables was evaluated visually using Q-Q plots. Normally distributed variables are displayed as mean ± 1 SD. Skewed variables are reported as median and interquartile range (IQR). We used the Mann-Whitney U test (ordinal and continuous variables) and the χ2 test (proportions) to compare baseline characteristics between the groups. Associations were analyzed using uni- and multivariable linear models adjusted for clinically meaningful covariates. Estimates (beta) are presented together with 95% CIs and p values. We performed 3 sensitivity analyses, which were planned a priori: (1) we compared the choroid plexus volume between MS and NMOSD including the infratentorial parts of the choroid plexus in a subgroup 20 patients; (2) we additionally adjusted our statistical models for different MRI scanner types and study centers; and (3) we included the lateral ventricle volumes as covariate into the multivariable linear model.
We also performed a post hoc analysis: As we found an association between T2w lesion burden and the choroid plexus volume in MS, we were interested whether the observed difference in the choroid plexus volume between MS and NMOSD and between MS and HCs is also detectable in the subgroup of patients with a low T2w lesion burden. We defined low T2w lesion burden as <13 lesions, which corresponds to the patients with the 25% lowest T2 lesion number of the MS group.
Receiver operating characteristic (ROC) curves were used to assess the sensitivity, specificity, and accuracy of the choroid plexus size to discriminate MS from NMOSD in comparison to the number of T2-weighted lesions. To obtain estimates for the association between the choroid plexus volume and the disease course (MS vs NMOSD), we performed a logistic regression model with the disease group as dependent variable and both the choroid plexus volume and clinically meaningful variables as covariates (eTable 3, links.lww.com/NXI/A701).
All statistical tests were 2 tailed. We used the conventional significance threshold of p < 0.05. Statistical analyses were performed using IBM SPSS Statistics (version 25; IBM, Armonk, NY).
Ethics Approval
This study was approved by the ethics committee North-West and Central Switzerland (reference 2020-01364).
Data Availability
Clinical and MRI data are accessible at the Department of Biomedical Engineering and the Department of Clinical Research (Clinical Trial Unit) of the University of Basel via the corresponding author.
Results
Demographics and Clinical Characteristics
We included 180 patients with MS and 98 patients with NMOSD from 3 different MS centers (Table 2). In total, 47 patients with migraine (19 from Basel and 28 from Lausanne) and 94 healthy individuals (56 from Basel, 15 from Lausanne, and 23 from Fukuoka) served as controls.
Table 1.
MRI Acquisition Parameters
The patients with NMOSD in our study were older than the patients with MS (p < 0.001), whereas there was no significant age or sex difference between MS, migraine, and healthy individuals (MS: median age 46.6 years, IQR 15.3 years, 70% female; migraine: median age 42.2 years, IQR 18.3 years, 72% female; HCs: median age 46.1 years, IQR 22.3 years, 64% female).
Typical for the difference between patients with MS and NMOSD,27 the proportion of women was higher in NMOSD than in MS (p = 0.001). Moreover, disease duration was longer in patients with MS than patients with NMOSD (p < 0.001), whereas the median EDSS score was similar between the 2 groups.
In this study, patients with NMOSD had a shorter time between last relapse and MRI than the patients with MS (median time 3.3 vs 4.5 years; p = 0.01). Moreover, only 21% and 15% of the NMOSD and MS groups, respectively, had a relapse in the year before the MRI. In total, 44/135 patients with RRMS (33%) had a relapse in the 24 months before the MRI.
Typical for NMOSD,27 the patients with NMOSD in this study had significantly fewer T2-weighted lesions than patients with MS. There was no difference between MS and NMOSD regarding the TIV.
The antibody status was available for 86/98 (88%) patients with NMOSD: 71 were positive for aquaporin-4 antibody and 11 patients for anti–myelin oligodendrocyte glycoprotein; 4 patients were negative for both antibodies. None of the patients were positive for both anti-aquaporin-4 antibody and anti-MOG antibody. In 12/98 (12%) patients with NMOSD, the antibody status was not available, and the diagnosis was made based on clinical criteria only.18 Most of the patients with MS (153/180; 85%) were treated with a DMT at time of the MRI. Details are given in the legend of Table 2.
In total, 23/47 patients with migraine (49%) had a migraine with aura. Two patients with MS also had a migraine (both without aura). Some patients with migraine had unspecific T2w white matter hyperintensities. Those were not segmented as lesions as they are believed to have no clinical significance.28
Association Between Age, Sex, Total Intracranial Volume, and the Choroid Plexus Volume in Healthy Individuals and Patients With Migraine
In HCs, there was no association between the choroid plexus volume and age or sex when adjusted for TIV. The same was true for the migraine group. Details are given in the supplementary material (eTable 1, links.lww.com/NXI/A701).
Choroid Plexus Volume in MS vs NMOSD Compared With the Control Groups
The choroid plexus was larger in MS than NMOSD (median 1,690 µL, IQR 648 µL vs 1,403 µL, IQR 510 µL; beta 121.8; 95% CI:81.8–161.8; p < 0.001) (Figure 2). Choroid plexus volume, expressed as a ratio of TIV, was on average 20.5% larger in MS than NMOSD (12.13 × 10−4± 2.75 × 10−4 vs 10.07 × 10−4 ± 2.32 × 10−4). When adjusting for age, sex, TIV, disease duration, relapses in the previous year, EDSS score, and T2w lesion number, the choroid plexus was still larger in MS than NMOSD (beta 92.8; 95% CI: 55.5–130.2; p < 0.001; eTable 2, links.lww.com/NXI/A701).
Figure 2. Choroid Plexus Volume in Multiple Sclerosis, Neuromyelitis Optica Spectrum Disorder, Migraine, and Healthy Individuals.
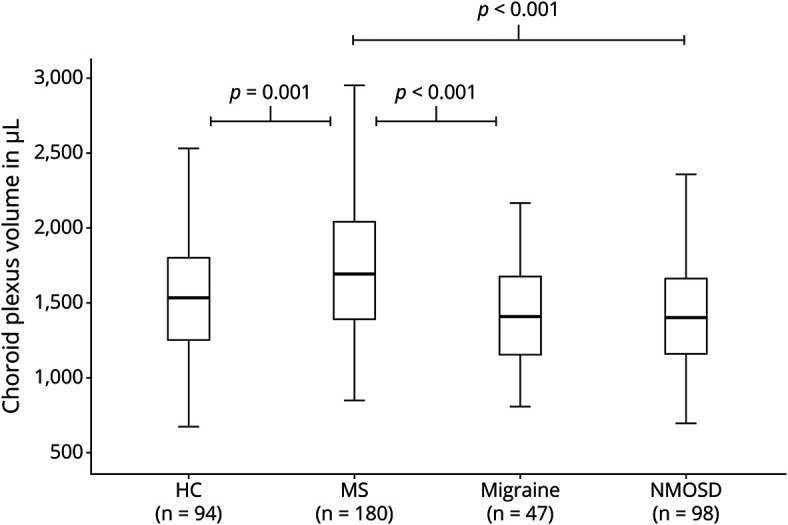
p Values given in this figure derived from linear models adjusted for age, sex and total intracranial volume. HC = healthy controls; MS = multiple sclerosis; n = number; NMOSD = neuromyelitis optica spectrum disorder.
The choroid plexus was also larger in MS than HCs (beta 292; 95% CI: 192–393; p < 0.001). Expressed as a ratio of TIV, the mean choroid plexus volume was 21.4% higher in MS than HCs (12.13 × 10−4 ± 2.75 × 10−4 vs 9.99 × 10−4 ± 2.51 × 10−4). Similarly, the choroid plexus volume was larger in patients with MS than in patients with migraine (median 1,404 µL, IQR 524 µL; beta 153; 95% CI: 83–223; p < 0.001). Relative to TIV, the mean choroid plexus volume was 23% higher in MS than migraine (12.13 × 10−4 ± 2.75 × 10−4 vs 9.86 × 10−4 ± 2.63 × 10−4). In contrast, there was no difference in the choroid plexus volume between NMOSD, migraine, and healthy individuals.
Choroid Plexus Volume and Disease Characteristics in MS
In MS, there was no association between the choroid plexus volume and disease duration, disease course, or the EDSS score at time of the MRI when adjusted for age, sex, and TIV. The choroid plexus volume was similar in patients with and without a relapse within the 12 months before the MRI. There was an association between the time to last relapse and the choroid plexus volume in the subgroup of 135 patients with RRMS (beta 1.1; 95% CI: 0.1–2.1; p = 0.03; adjusted for age, sex, and TIV): RRMS patients with longer time since last relapse had marginally higher choroid plexus volumes. However, when adjusting for T2w lesion number, the association between the time since last relapse and choroid plexus volume lost statistical significance. This indicated a strong association between the choroid plexus volume and the T2w lesion burden, and indeed, we found an association between the choroid plexus volume and the number of T2w white matter lesions in the MS group: on average, every T2w lesion in MS increased the choroid plexus volume statistically by 4.4 µL (beta 4.4; 95% CI: 0.78–8.1; p = 0.018), adjusted for age, sex, TIV, relapse in the previous year, disease course, EDSS score, DMT category, and DMT duration (Table 3). The choroid plexus volume was also higher in the subgroup of patients with RRMS with low T2w lesion number (for definition, see the Methods section) compared with HCs, adjusted for age, sex, and TIV (beta 49; 95% CI: 12–85; p = 0.009). There was no association between the volume of T2w lesions and the choroid plexus volume. We also did not find any association between the distance of the T2w lesions to the lateral ventricle and the choroid plexus volume. This was evaluated in 50 of 135 patients with RRMS.
Table 3.
Association Between Choroid Plexus Volume and Disease Characteristics in MS (Linear Model)
Choroid Plexus Volume and Disease Characteristics in NMOSD
In the NMOSD group, there was no association between the choroid plexus volume and disease duration, EDSS score, relapse in the year before the MRI, time to the last relapse, DMT category, DMT duration, T2w lesion number, or volume (data not shown).
Choroid Plexus Volume to Differentiate MS From NMOSD
The choroid plexus volume was able to differentiate MS from NMOSD with an accuracy of 0.68 (area under the ROC curve 0.68; 95% CI: 0.62–0.75; p < 0.001; Figure 3); this was marginally lower compared with the number of T2w lesions to distinguish the 2 diseases (area under the ROC curve 0.70, 95% CI: 0.63–0.77; p < 0.001; Figure 3). T2w lesion number and the choroid plexus volume were significantly higher in MS than NMOSD independent from each other.
Figure 3. Receiver Operating Characteristic Curves to Differentiate Multiple Sclerosis (n = 180) From Neuromyelitis Optica Spectrum Disorder (n = 98).
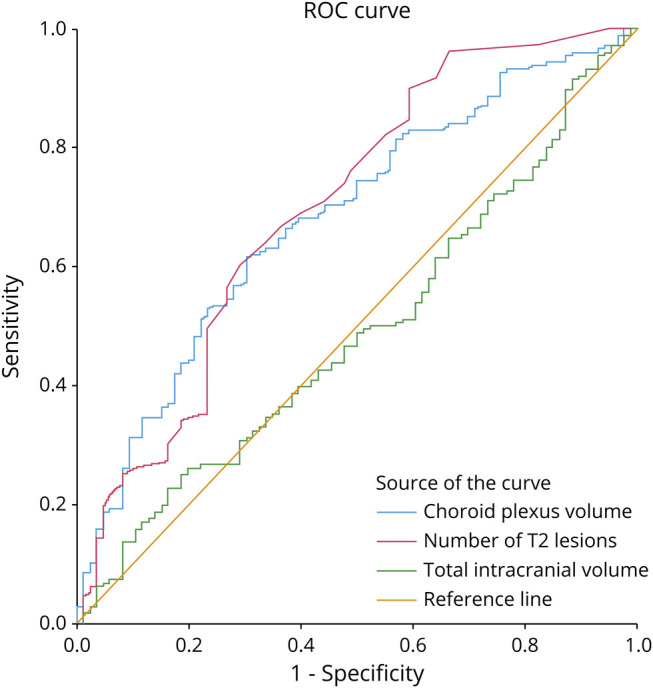
The accuracy to differentiate MS from NMOSD using the choroid plexus volume is comparable to the T2w lesion burden. Please note that the choroid plexus volume is associated with the diagnosis of MS (vs. NMOSD) independent of the number of T2w lesions (eTable 2, links.lww.com/NXI/A701).
a. Under the nonparametric assumption
b. Null hypothesis: true area = 0.5
To estimate the effect size of the choroid plexus volume to differentiate MS from NMOSD, we used a logistic regression model with disease group (MS vs NMOSD) as dependent variable and choroid plexus volume as independent parameter, adjusted for clinically meaningful parameters (eTable 3, links.lww.com/NXI/A701). The results indicated that a 100 µL larger choroid plexus increased the risk of having MS instead of NMOSD statistically by 26% (Exp(B): 1.259; 95% CI:1.130–1.404) independently of sex, disease duration, relapse in the year before the MRI, EDSS score, and TIV but also independently of the number of T2w lesions (eTable 3).
Sensitivity Analyses
In 10 patients with MS and 10 patients with NMOSD, we additionally segmented the infratentorial parts of the choroid plexus manually and blinded to the clinical data (eFigure 1, links.lww.com/NXI/A701). Including these infratentorial parts of the choroid plexus into the model did not alter the overall results. The median choroid plexus volume was larger in MS than NMOSD, adjusted for age, sex, and TIV (beta 196; 95% CI: 29–362, p = 0.024). The median choroid plexus volume was 16.7% higher in MS than NMOSD (2025 µL, IQR 503 vs 1735 µL, IQR 545 µL).
Moreover, including the scanner type, the study center, or the lateral ventricle volume as covariates to the models did not alter the overall results (all p > 0.05, estimates not shown). When analyzing the 25% of patients with MS with the lowest number of T2w lesions (n = 46), the choroid plexus volume was still larger in MS than in NMOSD, adjusted for age, sex, and total intracranial volume (beta 64; 95% CI: 20–108; p = 0.005).
Discussion
This study found larger choroid plexus in patients with MS than NMOSD, migraine, or HCs (adjusted for age, sex, and TIV), whereas there was no difference between patients with NMOSD, migraine, and HCs. These results suggest an involvement of the choroid plexus in MS-related inflammation and no or clearly less choroid plexus involvement in NMOSD.
Our results are in line with other MRI studies9 but not all.15 The enlargement of the choroid plexus in MS vs HCs was more pronounced in a previous study9 than in our study. In that study9, the choroid plexus volume adjusted for TIV was 35% larger in MS than in HCs compared with 21% in our study.9 This might be due to the inclusion of less active MS patients in our study. In our MS group, 33% of the patients with RRMS had a relapse in the 2 years before the MRI, and only 2 of 180 patients with MS had gadolinium enhancement in contrast to 80% and 32%, respectively, in the aforementioned study.9 These differences in the disease characteristics of the patients included are important as it has been shown that the choroid plexus volume is associated with both clinical and MRI disease activity in MS.9 The inclusion of more clinically and MRI stable patients with MS in our study might also explain why we have not found any difference in the choroid plexus volume between patients with and without a recent relapse. Only 15% of the patients in our study had a relapse in the 12 months before the MRI. In the entire MS group, we even found a tendency toward larger choroid plexus in patients with longer time since last relapse. However, further analysis elucidated that this association was driven by the higher T2w lesion burden in patients with longer time since last relapse because when we adjusted for the T2w lesion burden, the association between the time to last relapse and the choroid plexus volume lost statistical significance. This was also confirmed in a multivariable linear model with the choroid plexus volume as dependent variable and the number of T2w lesions and relapses in the previous year as covariates (Table 3). Relapses in the previous year were not associated with the choroid plexus volume when the T2w lesion number was included into the model. In summary, our study built on the results of a previous work9 and found larger choroid plexus in MS vs HCs even in a less active MS patient population. Similar to the aforementioned study.9 our study could not find an association between disease duration and the choroid plexus volume in MS.
In contrast, the association between the choroid plexus volume and T2w lesion burden in our study was strong and remained stable when the linear model was adjusted for clinically meaningful covariates (Table 3). Similar results were found in a previous work9 in which the choroid plexus enlargement correlated with the number of white matter lesions. As the T2w lesion burden is an objective parameter of focal inflammation, our result suggests that the larger choroid plexus in MS compared with HCs might be related directly to MS inflammation. Our study built on these data and found that this association does not seem to be driven by the lesion location, at least not near to the ventricle wall. We measured in a subgroup of patients with RRMS the distance between the T2w lesions and the lateral ventricle wall and could not find any association to the choroid plexus volume. Although the T2w lesion burden showed a strong association with the choroid plexus volume, the number of T2w lesion lesions could not entirely explain the variance in the choroid plexus size in MS. There are 2 arguments in our study supporting this hypothesis: (1) in the subgroup of patients with RRMS with a low MRI disease burden, the choroid plexus was still larger in MS than HCs (see Sensitivity Analyses), and (2) in a linear model with the choroid plexus volume as dependent variable, the disease group (MS vs NMOSD) was associated with the choroid plexus volume independent of the T2w lesion burden (eTable 2, links.lww.com/NXI/A701).
This work is not the first study that has investigated the choroid plexus size in MS vs NMOSD. A recent work15 found 9–11% larger choroid plexus in MS vs NMOSD compared with 20% in our study. In contrast to our study, the differences between MS and NMOSD in that study15 did not reach statistical significance. At first glance, this might be surprising as the aforementioned study15 included more active patients than our study. The median time between the last relapse and MRI was 11 days in NMOSD and 16 days in MS in that study15 compared with 3.3 years and 4.5 years, respectively, in our study. Moreover, the disease duration was 2–3× shorter compared with our study. On the other hand, that study15 also did not find any difference in the choroid plexus volume between MS and HCs, either. This suggests that the methods used in that study15 to measure the choroid plexus size might have been too inaccurate to find a significant difference between MS and NMOSD. A recent study that segmented the choroid plexus manually on 3D T1w sequences even found differences in the choroid plexus volume between a presymptomatic stage of MS (radiologically isolared syndrome) and HCs.10
A reason for the discrepancy between our study and the aforementioned study15 could be the relatively higher sample size in our study. Low sample sizes are prone to the type II errors, which is the risk of concluding that there is no significant difference between the groups when in fact such a difference exists. The aforementioned study15 included 51 patients with MS, 32 patients with NMOSD, and 26 HCs, whereas our study included more than 2–3× more study participants (180 patients with MS, 98 patients with NMOSD, and 94 HCs). Moreover, we segmented the choroid plexus on high-resolution 3D images (slice thickness 1 mm). They15 used linear measures on axial and coronal planes with a slice thickness of 5 mm. On the other hand, and this is certainly an advantage, they15 also measured the choroid plexus of the fourth ventricle but analyzed these results separately from the choroid plexus in the lateral ventricles. In contrast, we only included the infratentorial parts of the choroid plexus in a sensitivity analysis in a subgroup of our patients and found results compared to our main analysis. A further advantage of our study is that we included study subjects from 4 different MS centers and different regions of the world in contrast to the recent single-center work15 which might make our results more generalizable. We further have not included only healthy individuals as a control group but also patients with migraine as an example of a nonautoimmune inflammatory CNS disease, which might strengthen our results.
The larger choroid plexus volume in MS compared to NMOSD is not only important to better understand the pathophysiologic differences between these conditions. Our results might also have clinical implications in the future. The differential diagnosis between MS and NMOSD is clinically important and often challenging,18,29 in particular, if characteristic MRI findings such as longitudinal extensive transverse myelitis, Dawson fingers, or other imaging hallmarks are missing, and if the aquaporin-4 antibody status is negative. Our ROC curve analysis suggested that the choroid plexus volume might be helpful as a MRI biomarker in addition to the brain T2w lesion number to discriminate MS from NMOSD. However, further prospective studies are needed to prove this hypothesis.
Our study is not without limitations. We excluded the choroid plexus of the fourth ventricle and the subarachnoid space (Bochdalek flower baskets) as these parts of the choroid plexus were difficult to delineate. The excluded part of the choroid plexus represents about 35% of the entire choroid plexus.15 However, in a sensitivity analysis, we were able to reliably segment the infratentorial parts of the choroid plexus in 20 patients by consensus between 3 raters. Including these infratentorial parts into the analysis did not alter the overall results of the study.
Head size is an important factor affecting the choroid plexus volume. To mitigate this effect, we adjusted all statistical models similar to other studies9 for TIV and in a sensitivity analysis also for the lateral ventricle volumes, with similar results compared with the main analysis. However, residual effects might still have affected our results.
Patients were scanned on different scanners and therefore had slightly different MRI acquisition parameters, which might have influenced our results. However, we made sure that the magnetic field strength and the image resolution were constant across all scanners and scan protocols. Moreover, including the scanner type in a sensitivity analysis as a covariate into the statistical model did not alter the overall results. However, again, residual effects might have affected our results.
The choroid plexus is larger in MS than in NMOSD. The involvement of the choroid plexus in MS (in contrast to NMOSD) provides clues to better understand the respective pathogenesis. Further prospective studies are warranted to investigate whether the choroid plexus volume is useful to differentiate MS from NMOSD in clinical practice.
Acknowledgment
The authors thank Dr Nicholas Sanderson for the review of the paper.
Glossary
- DMT
disease-modifying treatment
- EDSS
Expanded Disability Status Scale
- ET
echo time
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- HCs
healthy control
- Ig
immunoglobulin
- IQR
interquartile range
- IT
inversion time
- n.a.
not applicable/available
- NMOSD
neuromyelitis optica spectrum disorder
- MOG
myelin oligodendrocyte glycoprotein
- MPRAGE
magnetization-prepared rapid gradient echo
- MS
multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- ROC
receiver operating characteristic
- RT
repetition time
- SMSC
Swiss Multiple Sclerosis Cohort
- SPMS
secondary progressive multiple sclerosis
- TIV
total intracranial volume
- T1w
T1 weighted
- T2w
T2 weighted
- 3T
3 Tesla
Appendix. Authors
Contributor Information
Jannis Müller, Email: jannis.mueller@usb.ch.
Tim Sinnecker, Email: tim.sinnecker@usb.ch.
Maria Janina Wendebourg, Email: mjwendebourg@gmail.com.
Regina Schläger, Email: regina.schlaeger@usb.ch.
Jens Kuhle, Email: jens.kuhle@usb.ch.
Sabine Schädelin, Email: sabine.schaedelin@usb.ch.
Pascal Benkert, Email: pascal.benkert@usb.ch.
Tobias Derfuss, Email: tobias.derfuss@usb.ch.
Philippe Cattin, Email: philippe.cattin@unibas.ch.
Christoph Jud, Email: christoph.jud@unibas.ch.
Florian Spiess, Email: florianmartinspiess@gmail.com.
Michael Amann, Email: michael.amann@miac.ch.
Therese Lincke, Email: therese.lincke@usb.ch.
Muhamed Barakovic, Email: muhamed.barakovic@unibas.ch.
Alessandro Cagol, Email: alessandro.cagol@unibas.ch.
Charidimos Tsagkas, Email: charidimos.tsagkas@usb.ch.
Katrin Parmar, Email: k.parmar@reha-rhf.ch.
Anne-Katrin Pröbstel, Email: anne-katrin.proebstel@usb.ch.
Sophia Reimann, Email: sophia.reimann@stud.unibas.ch.
Susanna Asseyer, Email: susanna.asseyer@charite.de.
Ankelien Duchow, Email: ankelien-solveig.duchow@charite.de.
Alexander Brandt, Email: aubrandt@hs.uci.edu.
Klemens Ruprecht, Email: klemens.ruprecht@charite.de.
Nouchine Hadjikhani, Email: nouchine@nmr.mgh.harvard.edu.
Shoko Fukumoto, Email: fshouko@neuro.med.kyushu-u.ac.jp.
Mitsuru Watanabe, Email: mwata@neuro.med.kyushu-u.ac.jp.
Katsuhisa Masaki, Email: kmasaki@neuro.med.kyushu-u.ac.jp.
Takuya Matsushita, Email: matusita@neuro.med.kyushu-u.ac.jp.
Noriko Isobe, Email: kuroki@neuro.med.kyushu-u.ac.jp.
Jun-Ichi Kira, Email: kira@neuro.med.kyushu-u.ac.jp.
Ludwig Kappos, Email: ludwig.kappos@usb.ch.
Jens Würfel, Email: jw@miac.ch.
Cristina Granziera, Email: cristina.granziera@usb.ch.
Özgür Yaldizli, Email: friedemann.paul@charite.de.
Study Funding
No targeted funding reported.
Disclosure
J. Müller received ad personam funding from the Swiss Academy of Medical Science (Schweizer Akademie der Medizinischen Wissenschaften, SAMW). T. Sinnecker is part-time employee of the Medical Image Analysis Center Basel. MJ. Wendebourg and R. Schlaeger report no competing interests. J. Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by ECTRIMS, Swiss MS Society, Swiss National Research Foundation [320030_160221], University of Basel, Bayer, Biogen, Genzyme, Merck, Novartis, Protagen AG, Roche, and Teva. Sabine Schaedelin and Pascal Benkert have nothing to disclose. T. Derfuss received speaker fees, travel support, and/or served on advisory boards or steering committees of Novartis Pharma, Merck, Biogen, GeNeuro, Mitsubishi Pharma, MedDay, Roche, Celgene, Alexion, Actelion, and Genzyme; he received research support from Biogen, Novartis, Roche, Alexion, the Swiss National Research Foundation, University of Basel, and the Swiss MS Society. P. Cattin, C. Jud, F. Spiess, M. Amann, T. Lincke, M. Barakovic, A. Cagol, Ch. Tsagkas, and K.Parmar report no competing interest. AK. Pröbstel received speaker fees, travel support, and/or served on advisory boards of Novartis Pharma and Roche; she received research support from Biogen, the Swiss National Research Foundation, the National Multiple Sclerosis Society, the European Research Council, University of Basel, the Propatient Foundation, and the Goldschmidt-Jacobson Foundation. S. Reimann reports no competing interest. S. Asseyer received travel grant from Celgene and speaker's honorary from Roche and Bayer. A. Duchow received a speakers's honorary from Roche. A. Brandt reports no competing interest. K. Ruprecht received research support from Novartis Pharma, Merck Serono, Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung), European Union (821283-2), Stiftung Charité (BIH Clinical Fellow Program), and Arthur Arnstein Foundation and received speaker honoraria from Bayer and travel grants from the Guthy-Jackson Charitable Foundation. N. Hadjikhani and S. Fukumoto report no competing interests. M. Watanabe received speaker honoraria from Novartis Pharma and a grant from JSPS KAKENHI (Grant No. 19K07995) and GlaxoSmithKline. K. Masaki reports no competing interests. T. Matsushita received speech honoraria payments from Biogen Japan, Chugai Pharmaceutical, Alexion Pharmaceuticals, Novartis Pharma, and Takeda Pharmaceutical Company. N. Isobe received grant support from Mitsubishi Tanabe Pharma, Osoegawa Neurology Clinic, Bayer Yakuhin Ltd., and Japan Blood Products Organization and speaker honoraria from Novartis Pharma, Biogen Japan, Alexion, Mitsubishi Tanabe Pharma, Chugai Pharmaceutical Co. Ltd., Teijin Pharma, and Eisai Co., Ltd. J. Kira received research funds from Dainippon Sumitomo Pharma, Daiichi Sankyo, Mitsubishi Tanabe Pharma, and Kyowa Kensetsukougyo and consultancy fees, speaking fees, and/or honoraria from Novartis Pharma, Mitsubishi Tanabe Pharma, CSL Behring, Biogen Japan, Teijin Health Care, the Takeda Pharmaceutical Company, Kyowa Kirin, Ono Pharmaceutical Co. Ltd., Alexion Pharmaceuticals Inc., Tsumura, Ricoh, EMC, and Eisai. L. Kappos' institution (University Hospital Basel) received and used exclusively for research support: steering committee, advisory board, and consultancy fees (Actelion, Bayer HealthCare, Biogen, BMS, Genzyme, Janssen, Merck, Novartis, Roche, Sanofi, Santhera, and TG Therapeutics); speaker fees (Bayer HealthCare, Biogen, Merck, Novartis, Roche, and Sanofi); support of educational activities (Allergan, Bayer HealthCare, Biogen, CSL Behring, Desitin, Genzyme, Merck, Novartis, Roche, Pfizer, Sanofi, Shire, and Teva); license fees for Neurostatus products; and grants (Bayer HealthCare, Biogen, European Union, InnoSwiss, Merck, Novartis, Roche, Swiss MS Society, and Swiss National Research Foundation). M. Amann reports no competing interests. J. Wuerfel is employee of MIAC AG; he served for advisory boards for Biogen, Idorsia, Novartis, Roche, and Sanofi; he is or was supported by the EU (Horizon2020), the German Ministeries for Economy and Science and Education. C. Granziera's institution, the University Hospital Basel (USB), has received the following fees, which were used exclusively for research support: (1) advisory board and consultancy fees from Actelion, Novartis, Genzyme-Sanofi, and F. Hoffmann-La Roche Ltd; (2) speaker fees from Biogen and Genzyme-Sanofi; and (3) collaborative research funds by F. Hoffmann-La Roche Ltd. F. Paul reports no competing interests. Ö. Yaldizli received grants from ECTRIMS/MAGNIMS, University of Basel, Pro Patient Stiftung, University Hospital Basel, Free Academic Society Basel, and the Swiss Multiple Sclerosis Society and advisory board fees from Roche, Sanofi Genzyme, Biogen, Almirall, and Novartis. Go to Neurology.org/NN for full disclosures.
Publication History
Received by Neurology: Neuroimmunology & Neuroinflammation August 3, 2021. Accepted in final form January 18, 2022. Submitted and externally peer reviewed. The handling editor was Scott S. Zamvil, MD, PhD, FAAN.
References
- 1.Johanson CE, Conn PM. The CP—CSF nexus. In: Neuroscience in Medicine. Humana Press; 2003. [Google Scholar]
- 2.Benarroch EE. CP–CSF system: recent developments and clinical correlations. Neurology. 2016;86(3):286-296. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 2014;33(1):7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vercellino M, Votta B, Condello C, et al. . Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathological study. J Neuroimmunol. 2008;199(1-2):133-141. [DOI] [PubMed] [Google Scholar]
- 5.Kaur C, Rathnasamy G, Ling EA. The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol. 2016;75(3):198-213. [DOI] [PubMed] [Google Scholar]
- 6.Lauer AN, Tenenbaum T, Schroten H, Schwerk C. The diverse cellular responses of the choroid plexus during infection of the central nervous system. Am J Physiol Cell Physiol. 2018;314(2):C152-C165. [DOI] [PubMed] [Google Scholar]
- 7.Strominger I, Elyahu Y, Berner O, et al. . The choroid plexus functions as a niche for T-cell stimulation within the central nervous system. Front Immunol. 2018;9:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol. 2010;119(1):75-88. [DOI] [PubMed] [Google Scholar]
- 9.Ricigliano VAG, Morena E, Colombi A, et al. . Choroid plexus enlargement in inflammatory multiple sclerosis: 3.0-T MRI and translocator protein PET evaluation. Radiology. 2021;301(1):166-177. [DOI] [PubMed] [Google Scholar]
- 10.Ricigliano VAG, Louapre C, Poirion E, et al. . An imaging signature in choroid plexuses in pre-symptomatic multiple sclerosis. Poster Presented at Annual Meeting of the European Charcot Foundation; Nov 14-18, 2021; Baveno, Italy.
- 11.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parratt JD, Prineas JW. Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Scler. 2010;16(10):1156-1172. [DOI] [PubMed] [Google Scholar]
- 13.Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6(1):85. [DOI] [PubMed] [Google Scholar]
- 14.Takeshita Y, Fujikawa S, Serizawa K, et al. . New BBB model reveals that IL-6 blockade suppressed the BBB disorder, preventing onset of NMOSD. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Lim YM, Kim G, et al. . Choroid plexus changes on magnetic resonance imaging in multiple sclerosis and neuromyelitis optica spectrum disorder. J Neurol Sci. 2020;415:116904. [DOI] [PubMed] [Google Scholar]
- 16.Disanto G, Benkert P, Lorscheider J, et al. . The Swiss multiple sclerosis cohort-study (SMSC): a prospective Swiss wide investigation of key phases in disease evolution and new treatment options. PLoS One. 2016;11(3):e0152347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Headache classification committee of the International Headache Society (IHS) the International Classification of Headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- 20.Lublin FD, Reingold SC, Cohen JA, et al. . Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. [DOI] [PubMed] [Google Scholar]
- 22.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430-1438. [DOI] [PubMed] [Google Scholar]
- 23.Waters P, Reindl M, Saiz A, et al. . Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2016;87(9):1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reindl M, Schanda K, Woodhall M, et al. . International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andermatt S, Pezold S, Amann M, Cattin PC. Multi-dimensional gated recurrent units for automated anatomical landmark localization. ArXiv. Preprint posted online August 9, 2017. arXiv:1708.02766. https://arxiv.org/pdf/1708.02766.pdf. [Google Scholar]
- 26.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782-790. [DOI] [PubMed] [Google Scholar]
- 27.Jarius S, Ruprecht K, Wildemann B, et al. . Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruit MC, van Buchem MA, Hofman PA, et al. . Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291(4):427-434. [DOI] [PubMed] [Google Scholar]
- 29.Geraldes R, Ciccarelli O, Barkhof F, et al. . The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol. 2018;14(4):199-213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Clinical and MRI data are accessible at the Department of Biomedical Engineering and the Department of Clinical Research (Clinical Trial Unit) of the University of Basel via the corresponding author.