Abstract
Myotonic dystrophy type 2 (DM2) is one of >40 microsatellite disorders caused by RNA repeat expansions. The DM2 repeat expansion, r(CCUG)exp (where “exp” denotes expanded repeating nucleotides), is harbored in intron 1 of the CCHC-type zinc finger nucleic acid binding protein (CNBP). The expanded RNA repeat causes disease by a gain-of-function mechanism, sequestering various RNA-binding proteins including the pre-mRNA splicing regulator MBNL1. Sequestration of MBNL1 results in its loss-of-function and concomitant deregulation of the alternative splicing of its native substrates. Notably, this r(CCUG)exp causes retention of intron 1 in the mature CNBP mRNA. Herein, we report druglike small molecules that bind the structure adopted by r(CCUG)exp and improve DM2-associated defects. These small molecules were optimized from screening hits from an RNA-focused small-molecule library to afford a compound that binds r(CCUG)exp specifically and with nanomolar affinity, facilitates endogenous degradation of the aberrantly retained intron in which it is harbored, and rescues alternative splicing defects.
INTRODUCTION
RNA repeat expansions cause >40 microsatellite disorders, including Huntington’s disease [HD, r(CAG)exp], C9orf72 amyotrophic lateral sclerosis/frontotemporal dementia [c9ALS/FTD, r(G4C2)exp], and myotonic dystrophy types 1 and 2 [DM1, r(CUG)exp and DM2, r(CCUG)exp, respectively]. (1) Repeat expansions cause disease through various mechanisms dependent upon its location within a gene. For example, an RNA gain-of-function mechanism occurs when repeat sequesters functionally inactivate proteins that regulate alternative pre-mRNA splicing. (2) Repeat expansions can also contribute to disease via an aberrant translational mechanism that generates toxic proteins, named repeat-associated non-ATG translation. (3) Recently, it was discovered that GC-rich RNA repeat expansions located in introns, such as r(CCUG)exp, r(CUG)exp, and r(G4C2)exp, cause retention of the intron in which they are harbored in mature mRNA species. (4)
The DM2 repeat expansion, r(CCUG)exp, is harbored in intron 1 of CCHC-type zinc finger nucleic acid binding protein gene (CNBP). (5) This r(CCUG)exp folds into a structure containing repeating 2 × 2 CU/UC internal loops (Figure 1A), (6) which bind and sequester muscleblind-like 1 (MBNL1), an important regulator of alternative pre-mRNA splicing, in nuclear foci. (7,8) Sequestration of MBNL1 by r(CCUG)exp results in pre-mRNA splicing defects of MBNL1-regulated genes. For example, insulin receptor (IR) exon 11 is excluded too frequently in DM2 cells (Figure 1B). (9) r(CCUG)exp can also cause dysfunction through intron retention, where CNBP itself is mis-spliced, and the r(CCUG)exp-containing intron 1 is retained in mature mRNA species (Figure 1C). (4)
Figure 1.
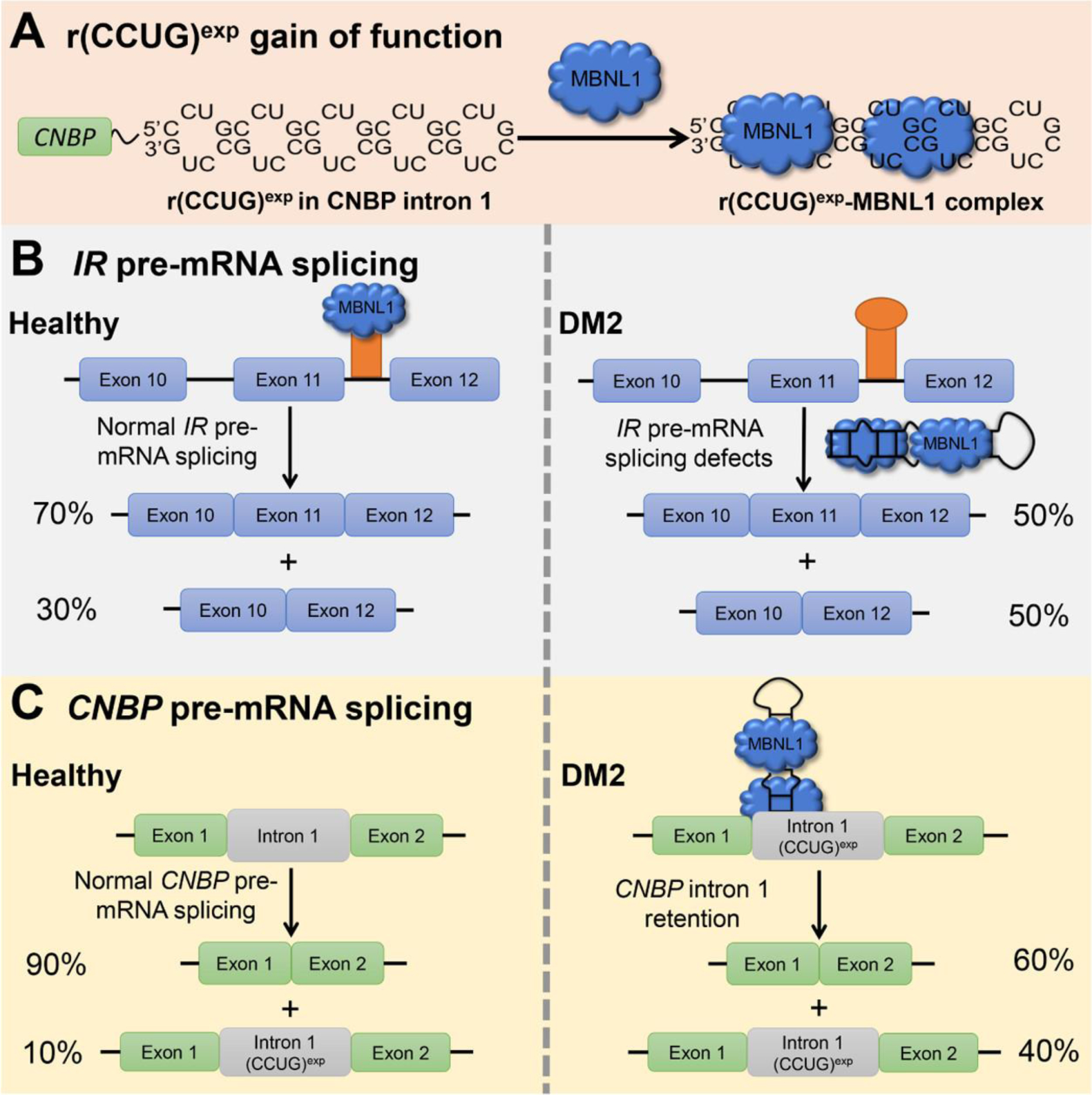
r(CCUG)exp-mediated defects in DM2. (A) DM2 is caused by r(CCUG)exp in CNBP intron 1, which folds into a structure with repeating 2 × 2 nucleotide internal loops that sequester regulatory proteins involved in pre-mRNA splicing such as MBNL1. (B) Sequestration of MBNL1 by r(CCUG)exp results in splicing defects in its native pre-mRNAs substrates. For example, IR exon 11 is included ∼70% of the time in healthy cells, but only ∼50% in DM2-affected cells. (C) r(CCUG)exp also causes intron retention where intron 1 is aberrantly retained in CNBP mature RNA.
We have previously shown that intron retention is due to MBNL1 binding; knock-down of MBNL1 reduces intron retention, whereas forced expression of MBNL1 increases intron retenion. (10) Thus, small-molecule recognition of r(CCUG)exp liberates MBNL1 and rescues deregulated splicing events and intron retention. In particular, we have shown that kanamycin A derivatives that bind r(CCUG)exp can specifically improve DM2-associated defects and reduce the levels of intron 1 containing r(CCUG)exp. (10) In another example of an intron-retained RNA repeat [r(CUG)exp in intron 3 of transcription factor 4 mRNA (TCF4) in Fuchs endothelial corneal dystrophy (FECD)], (11) small-molecule binding results in decay of the r(CUG)exp-containing intron 3 through the RNA exosome. (12) Thus, small-molecule recognition of RNA repeat expansions in retained introns can allow for targeted RNA degradation through stimulation of RNA quality control mechanisms. In addition to interfacing of disease-causing RNAs with endogenous decay pathways, other methods for targeted RNA degradation rely on chimeric compounds comprising an RNA-binding ligand attached to a cleaving module. However, this strategy increases compound molecular weight and can affect various pharmacological and physicochemical properties such as membrane permeability and solubility. (13–17)
Although our previous studies targeting r(CCUG)exp showed that small molecules can indeed interface the toxic RNA with endogenous degradation pathways, the aminoglycosides from which they are derived are not particularly druglike and provide little opportunity for optimization. (10,18) Herein, we studied a collection of RNA-focused, druglike small molecules to target r(CCUG)exp. Medicinal chemistry optimization of screening hits afforded a druglike small molecule that binds r(CCUG)exp with nanomolar affinity, specifically degrades r(CCUG)exp-containing intron 1, and improves DM2-associated defects.
RESULTS & DISCUSSION
Studying an RNA-Focused Small-Molecule Compound Collection for Targeting r(CCUG)
To identify novel small molecules that bind r(CCUG)exp, a druglike RNA-focused small-molecule library containing 3271 compounds was employed. (19) This library was previously designed to contain structurally diverse small molecules that have chemotypes that confer avidity toward RNA. (19) These chemotypes include benzimidazoles, indoles, thiazoles, and quinazolines. Furthermore, the compounds in this library are druglike as defined by satisfying Lipinksi’s Rule of 5, (20) where Log P is < 5, molecular weight is < 500, the number of hydrogen bond donors is <5, and the number of hydrogen bond acceptors is <10 (Figure 2A). Historically, druglikeness has been defined by satisfying the Rule of 5; however, an increasing number of approved orally bioavailable drugs fall outside of these guidelines, and parameters such as molecular weight are increasing. (21,22) On average, the library also contains similar physicochemical properties to known drugs in DrugBank (Figure 2A). (19) Thus, this library can be used to identify druglike small molecules that bind to RNA structures.
Figure 2.
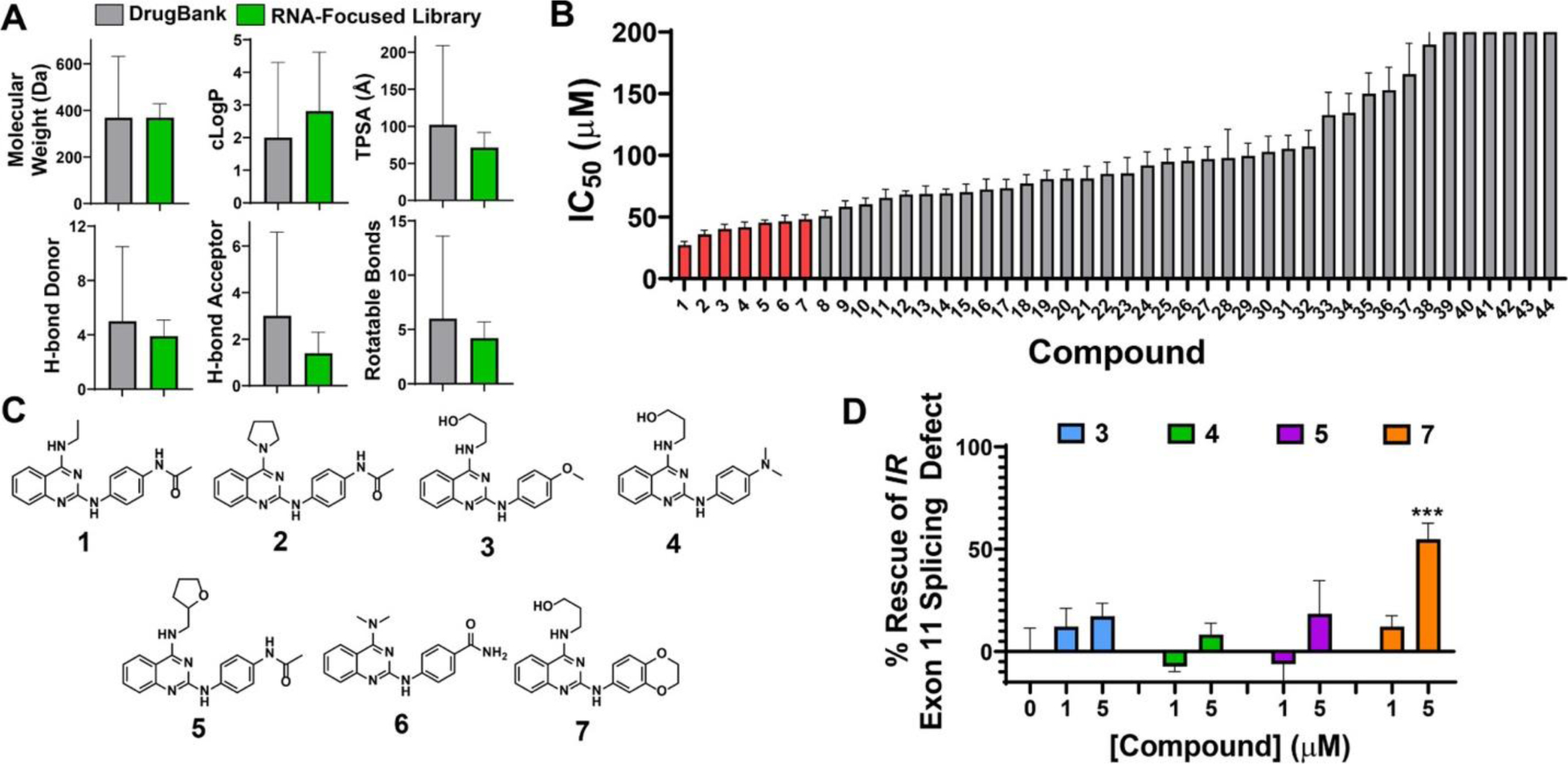
Small molecules that inhibit r(CCUG)exp–MBNL1 complex formation in vitro. (A) Characteristics of an RNA-focused small molecule library compared to characteristics of known drugs in DrugBank. (B) IC50 values of hit compounds from the RNA-focused library screen for disrupting the r(CCUG)12–MBNL1 complex (n = 2). Molecules in red have IC50s < 50 μM. (C) Chemical structures of compounds with IC50s < 50 μM. (D) Rescue of the IR exon 11 splicing defect by 3, 4, 5, and 7 in DM2 patient-derived fibroblasts (n = 2). Error bars represent standard deviation (SD) for all panels.
To identify compounds that bind r(CCUG)exp, a previously reported time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used that measures disruption of a preformed r(CCUG)12–MBNL1 complex by small molecules or other modalities. (23,24) The 3271 member library was screened at 200 μM, and 44 compounds disrupted complex formation by >60%, which corresponds to three standard deviations from the mean (or 3σ), affording a hit rate of 1.34% (Figure S1 and Table S1). Of the 44 hits, 38 contained a similar substituted N2-phenylquinazoline-2,4-diamine core, while the other hits contained benzimidazoles and indazoles (Figure S2). Importantly, these 44 compounds are druglike as they have similar physicochemical properties to the rest of the library and to known drugs in DrugBank (Figure 2A). On average, the 44 hit compounds have a molecular weight of 327 ± 35 g/mol, a c Log P of 3.2 ± 0.9, TPSA of 76 ± 15 Å, and 2.5 ± 0.9 hydrogen bond donors. The in vitro potencies (IC50s) of the 44 compounds were then measured in the TR-FRET assay, affording seven compounds (1–7) with IC50s < 50 μM (highlighted in red in Figures 2B,C and Table S1). Interestingly, all seven of these compounds contained the substituted N2-phenylquinazoline-2,4-diamine core.
Analysis of Hit Compounds
The binding of 1–7 to an RNA containing a single 2 × 2 CU/UC was further studied by NMR spectroscopy. Compounds 1, 2, and 6 showed significant aggregation in NMR experiments and were excluded from further analysis. In contrast, waterLOGSY spectra showed that 3, 4, 5, and 7 bound to the RNA, based on positive phase signals in the presence of RNA (Figures S3–S6). To confirm these interactions, one-dimensional (1D) NMR spectra of 3, 4, 5, and 7 bound to the RNA containing one 2×2 CU/UC internal loop were analyzed. Aromatic protons of the unbound RNA were assigned via a two-dimensional (2D) nuclear over-Hauser effect spectroscopy (NOESY) spectrum (Figure S7). Significant shifts in resonances corresponding to H6 and H8 protons of the RNA were observed in the presence of these four compounds, indicating binding to the RNA (Figure S8).
After confirming binding to r(CCUG) repeats by NMR spectroscopy, the compounds were evaluated for their ability to improve the deregulation of the IR exon 11 alternative splicing in DM2 patient-derived fibroblasts. All four compounds were well tolerated in the patient-derived fibroblasts, as no significant toxicity was observed up to a 20 μM dose (Figure S9). As previously mentioned, IR exon 11 is frequently excluded in DM2 patient-derived fibroblasts as compared to wild-type cells. Interestingly, the most potent compound, 7, rescued splicing by ∼50% at 5 μM (Figure 2D).
Synthesis and In Vitro Analysis of Derivatives of 7
To improve the potency of 7, a library of derivatives that replace the functional groups on the N2-phenylquinazoline-2,4-diamine core was synthesized and then evaluated for disrupting the r(CCUG)12–MBNL1 complex in vitro. As an overview of medicinal chemistry effort, analogues were synthesized to explore the quinazoline core (54–61), the aniline moiety (45–53), and combinations thereof (62–64) (Figures 3A and S10). Synthetically, compounds were prepared starting from 2,4-dichloroquinazoline or 2,4-dichloropyrimidine analogue, followed by the addition of 3-aminopropan-1-ol in a 4-position selective manner. The aniline derivatives at the N2 position were then installed in a microwave-assisted reaction (Scheme 1, Supporting Information). Of the nine compounds in which N2–2,3-dihydrobenzo[b][1,4]dioxine was replaced with various aniline analogues, three compounds containing 2-methylbenzooxazole (49), 2-methylbenzothiazole (50), and 1-methyl-1H-indazole (51) substituents had IC50s that were ∼2–8 times lower than 7 (IC50 = 48.2 ± 0.07 μM) (Figures 3A, S11, and Table S1). Eight derivatives (54–61) with various substitution patterns of quinazolines and fused pyrimidines were then evaluated, and one compound containing 6-methylquinazoline (58) was identified with an IC50 of 12.7 ± 0.2 μM, about 4 times more potent than 7 (Figures 3A, S11, and Table S1).
Figure 3.
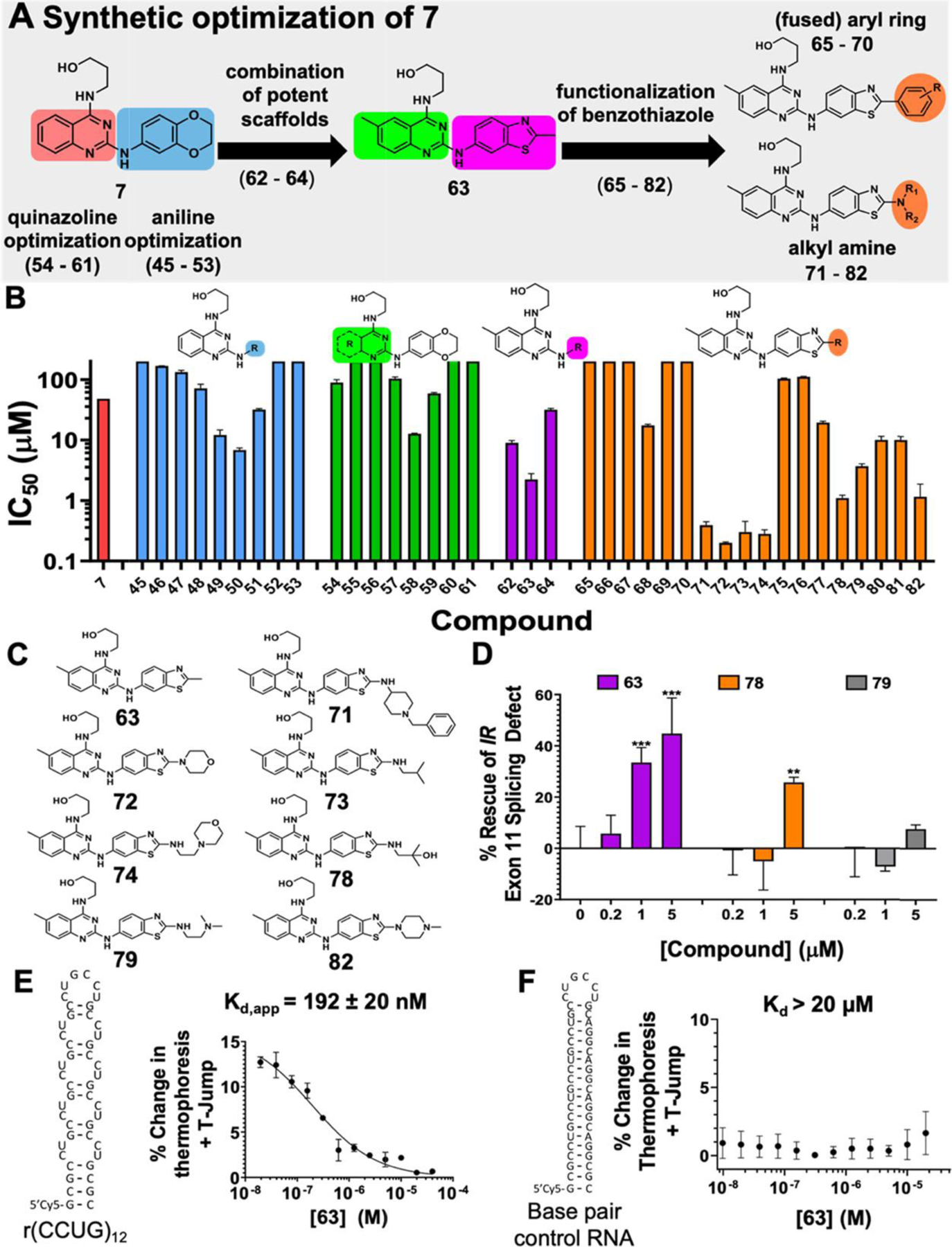
Lead optimization of 7 and activity of analogues in vitro and in DM2 patient-derived fibroblasts. (A) Synthetic optimization scheme of compound 7. (B) Structures of derivatives of 7 where “R” indicates the position(s) that were varied. IC50 values for disrupting the r(CCUG)12–MBNL1 complex for each derivative (n = 3). (C) Structures of compounds with IC50s < 5 μM. (D) Rescue of IR exon 11 mis-splicing by nontoxic compounds 63, 79, and 80 in DM2 patient-derived fibroblasts (n = 3). ** P < 0.01, *** P < 0.001, as determined by a one-way analysis of variance (ANOVA) relative to untreated (“0”). (E) Binding affinity curve of 63 and r(CCUG)12 (Kd = 192 ± 20 nM). (F) Binding affinity curve of 63 and a base pair control RNA (Kd > 20 μM). Error bars represent standard deviation (SD) for all panels.
To combine features of the derivates with improved IC50s, three additional compounds were synthesized that contained 6-methylquinazoline and 2-methylbenzooxazole (62), 2-methylbenzothiazole (63), and 1-methyl-1H-indazole (64) (Figures 3A, S11, and Table S1). Compounds 62 and 63 had slightly lower IC50s than corresponding analogues without a methyl substituent at the 6 position (49 and 50, respectively), and 63 was the most potent compound synthesized thus far with an IC50 of 2.2 ± 0.5 μM (Figures 3A, S11, and Table S1).
To explore further SAR around the benzothiazole moiety of 63, 18 analogues with extensions built onto benzothiazole were synthesized (Schemes 2 and 3 and Figures 3A and S10). Six of these analogues were synthesized via Suzuki coupling using a bromobenzothiazole intermediate of 63 (Intermediate D, Scheme 2). Notably, an aromatic ring was added to derivatives 65–70 to potentially add π-stacking interactions with r(CCUG)exp’s internal loops and thereby enhance binding interactions. The other 12 analogues were synthesized via thermal amine replacement toward the corresponding bromobenzothiazole derivative of 63 (Intermediate D, Scheme 3). This series of compounds was designed to add functional groups that could potentially interact with the RNA such as those with a positive charge (71, 74, 79, and 82), additional hydrogen bond donors and acceptors (71, 72, 74–82), or those with aromatic rings to form π–π interactions (75, 76, 77, and 81). Within these analogues, seven compounds (71, 72, 73, 74, 78, 79, and 82; Figure 3C) had IC50s < 5 μM and were chosen for further investigation, along with 63 (Figure 3B). Interestingly, four compounds (71–74) have IC50s < 500 nM, which represents a 100-fold improvement in in vitro potency compared to the starting compound, 7.
Evaluation of the Cellular Activity of Compounds
The eight compounds with in vitro IC50s < 5 μM were studied in DM2 patient-derived fibroblasts, both for their effects on cell viability and for rescue of IR exon 11 splicing. For the latter, we compared the percentage of exon 11 excluded upon compound treatment to untreated DM2 patient-derived fibroblasts and untreated WT fibroblasts, as measured by RT-PCR analysis. The percent rescue of a compound that restores the splicing pattern to that observed in WT cells is set to 100. Compounds 64, 65, 71, 72, 73, 74, and 82 displayed significant toxicity at 5 μM (Figure S11) and were not further evaluated. Of compounds with no toxicity at 5 μM (63, 78, and 79), 63 and 78 significantly improved IR splicing at 5 μM, while 63 also rescued splicing, by ∼30%, at 1 μM (Figures 3D and S12). Compound 79 did not significantly rescue splicing at any concentration studied (Figures 3D and S12). Interestingly, this derivative (79) is structurally similar to 64; however, the addition of the di-N-methylated functional group ablated its cellular activity, which could be due to reduced cellular uptake or changes to subcellar localization. At 1 μM dose, 63 rescues IR splicing more potently than 7 (33 ± 5 vs 12 ± 5; p = 0.026), and thus 63 is the most potent compound identified in these studies for improving DM2-associated splicing defects in cells.
The affinity and specificity of 63 for r(CCUG)exp were measured by microscale thermophoresis (MST). Compound 63 binds to r(CCUG)12 with a Kd,app of 192 ± 20 nM, while no binding was observed to a base-paired RNA that does not contain internal loops (Kd > 20 μM) (Figure 3E,F).
As 63 selectively bound to r(CCUG)12 over a base-paired control, its ability to improve other DM2-associated defects was assessed in DM2 patient-derived fibroblasts. Compound 63 (5 μM) reduced the number of r(CCUG)exp–MBNL1 nuclear foci per cell from 7 ± 1 in untreated patient-derived fibroblasts to 5 ± 0.5 in treated cells, or an ∼30% reduction, as determined by RNA fluorescence in situ hybridization (FISH) and MBNL1 immunofluorescence (Figure 4A,B). This decrease in the number of nuclear foci correlates with the improvement in IR receptor splicing observed at 5 μM (Figure 3D) as MBNL1 is no longer sequestered in foci and can resume its normal function. Importantly, 63 did not affect MAP4K4 exon 22a splicing, a NOVA-dependent splicing event (Figure S13). (25)
Figure 4.
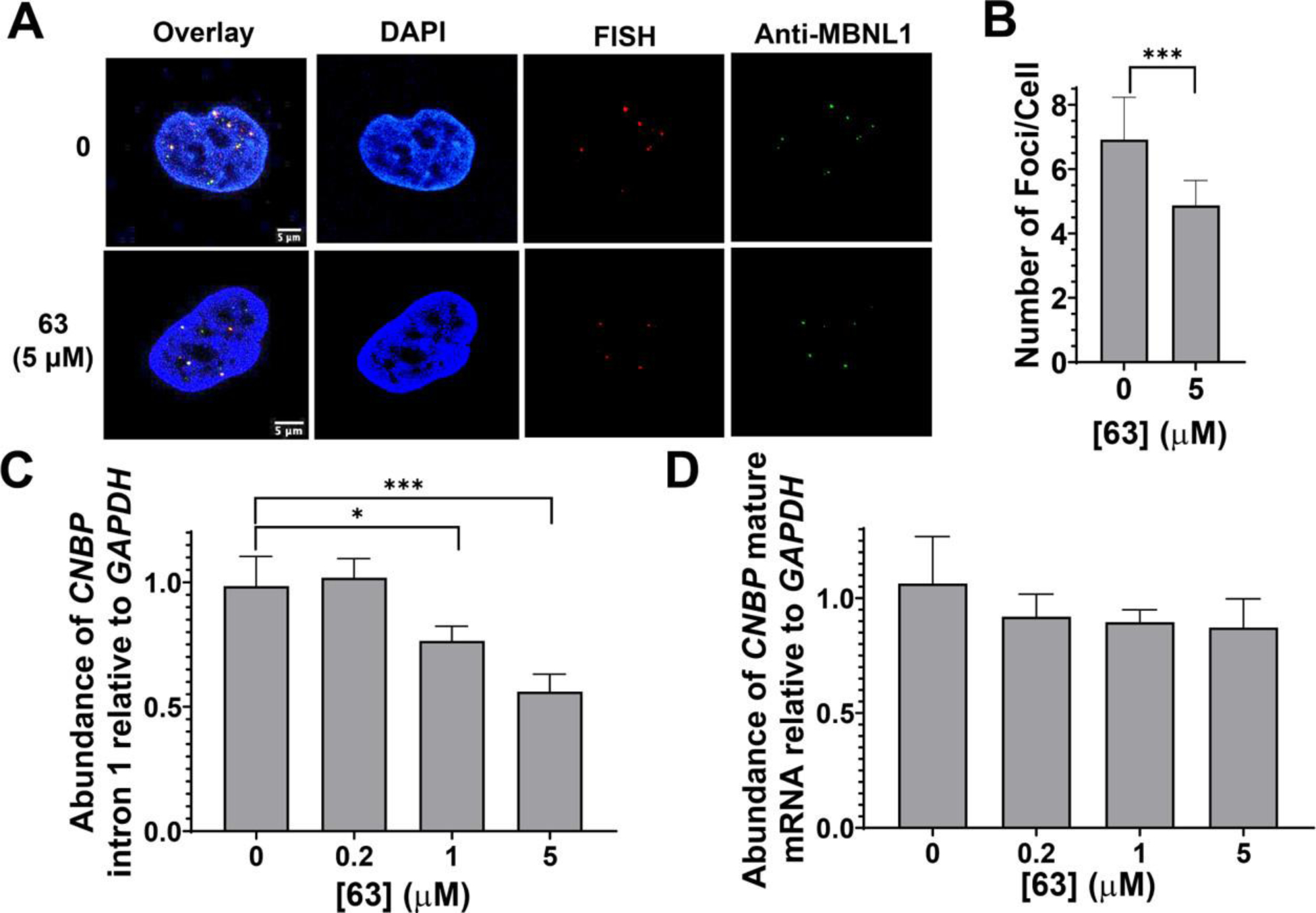
Compound 63 rescues disease-associated defects in DM2 patient-derived fibroblasts. (A) Representative RNA FISH and MBNL1 immunofluorescence images of r(CCUG)exp–MBNL1 foci. (B) Quantification of the number of nuclear foci/cell (n = 3, 40 nuclei counted/replicate). *** P < 0.001, as determined by a Student’s t-test. (C) Analysis of CNBP intron 1 levels upon treatment with 63 via RT-qPCR (n = 3).* P < 0.05, *** P < 0.001, as determined by a one-way ANOVA. (D) Analysis of CNBP mature mRNA levels upon treatment with 63 via RT-qPCR (n = 3). Error bars represent standard deviation (SD) for all panels.
As mentioned above, r(CCUG)exp causes the aberrant retention of the intron in which it is harbored in the mature mRNA. We have previously shown that small molecules that bind r(CCUG)exp can rescue defects in intron retention by facilitating removal of the retained intron, which is subsequently degraded. (10) Thus, we evaluated 63’s ability to rescue r(CCUG)exp-mediated intron retention. Indeed, 63 significantly reduced the abundance of CNBP intron 1 in DM2 patient-derived fibroblasts at doses of 5 and 1 μM (Figure 4C), as determined by RT-qPCR. Importantly, 63 did not affect the levels of CNBP mature mRNA (Figure 4D). The effects on intron 1 levels were also specific to mutant CNBP containing r(CCUG)exp, as no effects were observed in wild-type fibroblasts (Figure S14). Furthermore, 63 did not affect IR exon 11 inclusion in wild-type fibroblasts (Figure S14). Thus, 63 specifically improved r(CCUG)exp-mediated defects that are dysregulated in DM2 including nuclear foci, IR splicing, and intron retention.
Implications for Targeted Degradation of r(CCUG)exp
Importantly, all of the bioactive compounds identified herein possess druglike properties. The optimized compound, 63, has a low molecular weight (379.48 g/mol) and has a similar number of hydrogen bond donors (n = 3) to known drugs in DrugBank (n = 5). Furthermore, 63 possesses a favorable c Log P (3.75) and TPSA (83 Å) and satisfies the Rule of 5 criteria for druglikeness. Through medicinal chemistry optimization of screening hits, we identified compounds with >10-fold improvement in in vitro activity for disruption of an r(CCUG)12–MBNL1 complex. The most bioactive compound from lead optimization showed ∼5-fold improvement in its ability to improve DM2-associated splicing defects. Importantly, this lead-optimized compound (63) also rescued r(CCUG)exp-containing intron 1 retention, resulting in degradation of the expanded repeat but not the mature transcript.
Endogenous decay of r(CCUG)exp activated by a small molecule could occur in the nucleus or the cytoplasm. If the small molecule bound the mutant allele in the cytoplasm, any MBNL1 bound to the cytoplasmic repeat expansion would be liberated and would return to the nucleus. Since r(CCUG)exp–MBNL1 foci are not observed in the cytoplasm in the absence of compound in our imaging studies (Figure 4A), such a mechanism is unlikely. Alternatively, the small molecule could bind the repeat expansion in the nucleus, freeing MBNL1, increasing intron 1’s accessibility to the splicing machinery, reducing nuclear foci, and rescuing splicing defects. The liberated intron would then be shuttled through endogenous degradation pathways. In such a mechanism, intron 1 levels would be reduced while levels of the mature CNBP transcript would be unchanged. Collectively, our data (Figures 3 and 4) suggest that the latter, rather than the former, is the primary mode of action for 63.
Targeted degradation is an emerging field for affecting the biological function of disease-causing RNAs. Thus far, targeted RNA degradation can be achieved through: (i) direct cleavage of RNAs through conjugation of an RNA-binding module to bleomycin or derivatives of bleomycin; (13,26) (ii) recruitment of a ribonuclease through ribonuclease targeting chimeras (RIBOTACs), comprising an RNA-binding module and a compound that recruits RNaseL; (14,27) and (iii) small molecules that degrade repeat-containing introns, likely by increasing the accessibility of the disease-causing RNA to endogenous decay mechanisms. (10,12) As the former two approaches require chimeric compounds, the molecular weights of such modalities are larger and can negatively alter physicochemical properties. Targeted degradation through endogenous RNA decay mechanisms, however, can be accomplished with monomeric small molecules. Herein, we show that it is indeed possible to degrade r(CCUG)exp with druglike monomeric small molecules. Targeted degradation of RNA repeat expansions with monomeric compounds can have broad applications as other repeat expansions, such as r(G4C2)exp that causes c9ALS/FTD or r(CUG)exp that causes FECD, which are both found in retained introns. (4,28) Furthermore, targeted degradation of RNA through RNA quality control mechanisms may be broadly applicable with monomeric, druglike small molecules that recognize RNA structures.
Conclusions
We identified novel compounds that bind r(CCUG)exp and improve DM2-associated defects in patient-derived cells by high-throughput screening of a druglike RNA-focused small molecule library. Lead chemical optimization of small molecule binders afforded a compound with 20-fold improvement in vitro activity and 5-fold improvement in cellular activity. The lead-optimized small molecule selectively improves various DM2-associated defects including retention of r(CCUG)exp-containing intron 1, which is decayed in cells upon treatment with the compound. The physicochemical properties of the compounds identified herein indicate that druglike monomeric small molecules can indeed selectively bind RNA structures. The ability to selectively degrade introns containing RNA repeat expansions with druglike small molecules will have broad implications in drug discovery efforts toward targeting RNAs.
EXPERIMENTAL SECTION
General Synthetic Procedures
Reagents and solvents were purchased from commercial sources and used without further purification. Microwave (MW)-assisted reactions were performed by an Initiator + (Biotage).
Compounds were purified: (i) by Isolera One flash chromatography system (Biotage) using prepacked C18 columns (spherical 20–35 μm, Agela Technologies) or prepacked silica gel columns (spherical 20–35 μm, Agela Technologies) or (ii) by high-performance liquid chromatography (HPLC) (Waters 2489 and 1525) using a SunFire Prep C18 OBD 5 μm column (19 mm × 150 mm) with a 5 mL/min flow. HPLC purity analysis was performed using a SunFire C18 3.5 μm column (4.6 mm × 150 mm) with a linear gradient (0–100% methanol (MeOH) + 0.1% (v/v) trifluoracetic acid (TFA) and water + 0.1% (v/v) TFA) over 60 min at a flow rate of 1 mL/min. The purity of all derivatives was evaluated via analytical HPLC and was >95% in all cases.
NMR spectra were collected on a 400 UltraShield (Bruker) (400 MHz for 1H and 100 MHz for 13C) or Ascend 600 (Bruker) (600 MHz for 1H and 150 MHz for 13C). Chemical shifts are reported in ppm relative to tetramethylsilane (TMS) for 1H and residual solvent for 13C as internal standards. Coupling constants (J values) are expressed in hertz.
High-resolution mass spectra were recorded on an Agilent 1260 Infinity LC system coupled to an Agilent 6230 TOF (HR-ESI) with a Poroshell 120 EC-C18 column (Agilent, 50 mm × 4.6 mm, 2.7 μm). Liquid chromatography–mass spectrometry (LCMS) analysis was performed by Agilent 1260 Infinity LC system coupled to an Agilent 6130 quadrupole LC/MS (ESI) with a ZORBAX SB-C18 column (Agilent, 50 mm × 2.1 mm, 1.8 μm). Compounds 1–44 were purchased from ChemBridge and used without further purification.
General Synthetic Procedure for 7, 45–64 (General Synthetic Procedure 1)
For step 1, a mixture of dichloroquinazoline (A) (0.469 mmol, 1.0 equiv) and 3-aminopropan-1-ol (1.52 mmol, 3.2 equiv) in isopropanol (IPA) (0.40 M) was heated at 85 °C for 30 min under MW irradiation. The reaction was cooled to room temperature, and product formation was confirmed by LCMS. The reaction mixture was then concentrated in vacuo, washed with H2O (4 mL × 2), and dried to afford intermediate B. The material was used in the next reaction without further purification. Then in step 2, a mixture of B (79.5 μmol, 1.0 equiv) and a corresponding aniline (C) (159 μmol, 2.0 equiv) in ethanol (EtOH) was heated at 150 °C for 30–60 min under MW irradiation. The reaction was cooled to room temperature, and product formation was confirmed by LCMS. The reaction mixture was then concentrated in vacuo and purified by column chromatography [Agela Technologies, Silica, 20 g, 0–30% MeOH in dichloromethane (DCM)] to afford compounds 45–64.
Synthesis of 7
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (7) (12.2 mg, 44%). 1H NMR (400 MHz, MeOD) δ 8.08 (d, J = 8.2 Hz, 1H), 7.78 (d, J = 7.4 Hz, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.45 (d, J = 7.4 Hz, 1H), 7.11–6.86 (m, 3H), 4.28 (s, 4H), 3.76 (t, J = 7.1 Hz, 2H), 3.67 (t, J = 6.1 Hz, 2H), and 2.00–1.90 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 160.4, 154.7, 145.4, 143.5, 140.0, 134.2, 133.1, 123.9, 123.3, 121.7, 117.2, 114.4, 111.4, 110.1, 64.7, 64.4, 59.0, 38.9, and 32.1; HR-MS (ESI): calcd for C19H19N4O3– [M – H]−; 351.1463; found, 351.1463.
Synthesis of 45
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and benzo[d][1,3]dioxol-5-amine (21.8 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((4-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-yl)amino)quinazolin-4-yl)amino)propan-1-ol (45) (16.6 mg, 62%). 1H NMR (400 MHz, DMSO-d6) δ 10.4 (br s, 1H), 9.89 (br s, 1H), 8.48–8.37 (m, 1H), 7.83–7.73 (m, 1H), 7.55–7.47 (m, 1H), 7.45–7.29 (m, 2H), 7.05–6.97 (m, 1H), 6.97–6.91 (m, 1H), 6.06 (s, 2H), 4.75–4.57 (m, 1H), 3.65–3.59 (m, 2H), 3.53–3.47 (m, 2H), and 1.87–1.78 (m, 2H); HR-MS (ESI): calcd for C18H17N4O3– [M – H]−; 337.1306; found, 337.1321.
Synthesis of 46
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and 2,2-difluorobenzo[d][1,3]dioxol-5-amine (27.5 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)amino)quinazolin-4-yl)amino)propan-1-ol (46) (12.4 mg, 42%). 1H NMR (400 MHz, DMSO-d6) δ 10.5 (br s, 1H), 9.77 (br s, 1H), 8.40–8.33 (m, 1H), 7.91–7.75 (m, 2H), 7.60–7.56 (m, 1H), 7.51–7.41 (m, 2H), 7.37–7.31 (m, 1H), 4.79–4.43 (m, 1H), 3.69–3.58 (m, 2H), 3.57–3.46 (m, 2H), and 1.87–1.78 (m, 2H); HR-MS (ESI): calcd for C18H15F2N4O3– [M – H]−; 373.1118; found, 373.1119.
Synthesis of 47
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and 5-methyl-2,3-dihydrobenzo[b][1,4]dioxin-6-amine (26.3 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((5-methyl-2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (47) (29.0 mg, 100%). 1H NMR (400 MHz, DMSO-d6) δ 9.86–8.69 (m, 2H), 8.24 (d, J = 7.8 Hz, 1H), 7.72–7.65 (m, 1H), 7.46 (d, J = 7.6 Hz, 1H), 7.35–7.27 (m, 1H), 6.96 (d, J = 8.6 Hz, 1H), 6.75 (d, J = 8.6 Hz, 1H), 4.58–4.54 (m, 1H), 4.33–4.28 (m, 2H), 4.27–4.22 (m, 2H), 3.59–3.49 (m, 2H), 3.49–3.43 (m, 2H), 2.06 (s, 3H), and 1.80–1.73 (m, 2H); HR-MS (ESI): calcd for C20H21N4O3– [M – H]−; 365.1619; found, 365.1622.
Synthesis of 48
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and 4-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-amine (26.1 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((4-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-yl)amino)quinazolin-4-yl)amino)propan-1-ol (48) (6.3 mg, 22%). 1H NMR (400 MHz, MeOD) δ 7.86–7.82 (m, 1H), 7.86–7.82 (m, 1H), 7.57–7.52 (m, 1H), 7.39–7.35 (m, 1H), 7.20 (d, J = 2.4 Hz, 1H), 7.16–7.11 (m, 1H), 7.01 (dd, J = 8.6, 2.5 Hz, 1H), 6.70 (d, J = 8.7 Hz, 1H), 4.31–4.27 (m, 1H), 3.72–3.65 (m, 4H), 3.21–3.17 (m, 2H), 2.84 (s, 3H), and 1.97–1.88 (m, 2H); HR-MS (ESI): calcd for C20H22N5O2– [M – H]−; 364.1779; found, 364.1786.
Synthesis of 49
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (23.5 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2-methylbenzo[d]oxazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (49) (8.2 mg, 30%). 1H NMR (400 MHz, MeOD) δ 8.29 (d, J = 1.8 Hz, 1H), 7.94 (dd, J = 8.2, 1.0 Hz, 1H), 7.68–7.62 (m, 1H), 7.54–7.45 (m, 2H), 7.43 (dd, J = 8.6, 2.0 Hz, 1H), 7.29–7.23 (m, 1H), 3.75 (t, J = 7.0 Hz, 2H), 3.69 (t, J = 6.2 Hz, 2H), 2.63 (s, 3H), and 2.00–1.92 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.1, 160.6, 151.2, 138.6, 135.8, 133.6, 128.5, 126.0, 124.3, 123.5, 122.7, 118.8, 117.0, 112.0, 101.6, 59.1, 38.7, 32.3, and 14.6; HR-MS (ESI): calcd for C19H18N5O2– [M – H]−; 348.1466; found, 348.1470.
Synthesis of 50
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and 2-methylbenzo[d]thiazol-6-amine (26.1 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2-methylbenzo[d]thiazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (50) (7.7 mg, 27%). 1H NMR (400 MHz, MeOD) δ 8.56 (d, J = 2.0 Hz, 1H), 7.93–7.88 (m, 1H), 7.70 (d, J = 8.8 Hz, 1H), 7.66–7.63 (m, 1H), 7.63–7.57 (m, 1H), 7.49–7.45 (m, 1H), 7.24–7.17 (m, 1H), 3.74 (t, J = 7.0 Hz, 2H), 3.70 (t, J = 6.2 Hz, 2H), 2.79 (s, 3H), and 2.00–1.92 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.8, 160.6, 157.4, 151.4, 147.6, 139.5, 136.3, 133.0, 125.8, 123.2, 122.0, 121.9, 118.7, 112.2, 110.1, 59.2, 38.5, 32.4, and 20.1; HR-MS (ESI): calcd for C19H18N5OS– [M – H]−; 364.1238; found, 364.1245.
Synthesis of 51
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and 1-methyl-1H-indazol-6-amine (23.4 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((1-methyl-1H-indazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (51) (25.4 mg, 92%). 1H NMR (400 MHz, MeOD) δ 8.31 (br s, 1H), 7.97–7.93 (m, 1H), 7.89 (d, J = 0.9 Hz, 1H), 7.67–7.61 (m, 2H), 7.52–7.48 (m, 1H), 7.28–7.21 (m, 2H), 3.10 (s, 3H), 3.80 (t, J = 6.9 Hz, 2H), 3.69 (t, J = 6.1 Hz, 2H), and 2.02–1.94 (m, 2H); HR-MS (ESI): calcd for C19H19N6O– [M – H]−; 347.1626; found, 347.1630.
Synthesis of 52
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and 1-methyl-1H-indazol-3-amine (23.4 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((1-methyl-1H-indazol-3-yl)amino)quinazolin-4-yl)amino)propan-1-ol (52) (5.2 mg, 19%). 1H NMR (400 MHz, DMSO-d6) δ 12.8 (br s, 0.5H), 11.5 (br s, 0.5H), 9.87 (br s, 1H), 8.51 (d, J = 8.1 Hz, 1H), 8.02 (d, J = 8.1 Hz, 1H), 7.90–7.82 (m, 1H), 7.79–7.72 (m, 1H), 7.68 (d, J = 8.6 Hz, 1H), 7.55–7.45 (m, 2H), 7.23–7.15 (m, 1H), 4.72–4.56 (m, 1H), 4.10 (3H, s), 3.75–3.56 (m, 2H), 3.52–3.39 (m, 2H), and 1.88–1.70 (m, 2H); HR-MS (ESI): calcd for C19H19N6O– [M – H]−; 347.1626; found, 347.1635.
Synthesis of 53
Following general synthetic procedure 1 using 2,4-dichloroquinazoline (93.3 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 105.0 mg, 94%) and N-methyl-2,3-dihydrobenzo[b][1,4]dioxin-6-amine (26.3 mg, 159 μmol) in step 2 (18.9 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)(methyl)amino)quinazolin-4-yl)amino)propan-1-ol (53) (7.5 mg, 26%). 1H NMR (400 MHz, DMSO-d6) δ 9.63 (br s, 1H), 8.36 (d, J = 8.2 Hz, 1H), 7.75–7.69 (m, 2H), 7.40–7.34 (m, 1H), 7.04 (d, J = 2.2 Hz, 1H), 7.00–6.91 (m, 2H), 4.65–4.58 (m, 1H), 4.32–4.28 (m, 4H), 3.58–3.49 (m, 2H), 3.52 (s, 3H), 3.48–3.41 (m, 2H), and 1.81–1.70 (m, 2H); HR-MS (ESI): calcd for C20H21N4O3– [M – H]−; 365.1619; found, 365.1636.
Synthesis of 54
Following general synthetic procedure 1 using 2,4-dichloro-5,6,7,8-tetrahydroquinazoline (95.2 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 86.3 mg, 76%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (19.2 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)-5,6,7,8-tetrahydroquinazolin-4-yl)amino)propan-1-ol (54) (15.4 mg, 54%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (br s, 1H), 7.60 (br s, 1H), 7.30 (d, J = 2.2 Hz, 1H), 7.01 (dd, J = 8.8, 2.2 Hz, 1H), 6.77 (d, J = 8.8 Hz, 1H), 4.54 (t, J = 4.7 Hz, 1H), 4.26–4.17 (m, 4H), 3.51–3.42 (m, 4H), 2.54–2.46 (m, 2H), 2.28–2.21 (m, 2H), and 1.79–1.66 (m, 6H); HR-MS (ESI): calcd for C19H23N4O3– [M – H]−; 355.1778; found, 355.1773.
Synthesis of 55
Following general synthetic procedure 1 using 2,4-dichloro-5,7-dihydrofuro[3,4-d]pyrimidine (89.6 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 88.8 mg, 82%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (18.2 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)-5,7-dihydrofuro[3,4-d]pyrimidin-4-yl)amino)propan-1-ol (55) (7.8 mg, 29%). 1H NMR (400 MHz, DMSO-d6) δ 8.86 (br s, 1H), 7.45 (d, J = 2.5 Hz, 1H), 7.12 (dd, J = 8.8, 2.5 Hz, 1H), 7.02 (t, J = 5.2 Hz, 1H), 6.69 (d, J = 8.8 Hz, 1H), 4.80 (br s, 2H), 4.68–4.63 (m, 2H), 4.45 (t, J = 4.6 Hz, 1H), 4.23–4.13 (m, 4H), 3.53–3.38 (m, 4H), and 1.77–1.68 (m, 2H); HR-MS (ESI): calcd for C17H19N4O4– [M – H]−; 343.1412; found, 343.1418.
Synthesis of 56
Following general synthetic procedure 1 using 4,6-dichloro-3-methyl-1H-pyrazolo[3,4-d]pyrimidine (95.2 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 44.0 mg, 39%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (19.2 mg, 79.5 μmol of monochloro intermediate) afforded 3-((6-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)-3-methyl-1H-pyrazolo[3,4-d]pyrimidin-4-yl)amino)propan-1-ol (56) (12.5 mg, 44%). 1H NMR (400 MHz, DMSO-d6) δ 12.4 (br s, 1H), 8.79 (br s, 1H), 7.62–7.41 (m, 1H), 7.20–7.05 (m, 1H), 6.91 (br s, 1H), 6.79–6.65 (m, 1H), 4.84–4.49 (m, 1H), 4.28–4.13 (m, 4H), 3.64–3.50 (m, 4H), 2.45 (br s, 3H), and 1.85–1.74 (m, 2H); HR-MS (ESI): calcd for C17H19N6O3– [M – H]−; 355.1524; found, 355.1537.
Synthesis of 57
Following general synthetic procedure 1 using 2,4-dichloro-8-methylquinazoline (100.0 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 104.6 mg, 89%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (20.0 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)-8-methylquinazolin-4-yl)amino)propan-1-ol (57) (4.2 mg, 14%). 1H NMR (400 MHz, MeOD) δ 7.95 (d, J = 7.9 Hz, 1H), 7.34 (d, J = 7.3 Hz, 1H), 7.39–7.35 (m, 1H), 7.29–7.24 (m, 1H), 6.99 (d, J = 8.7, 2.6 Hz, 1H), 6.89 (d, J = 8.7 Hz, 1H), 4.31–4.25 (m, 4H), 3.78–3.74 (m, 2H), 3.67 (t, J = 6.2 Hz, 2H), 2.51 (s, 3H), and 1.99–1.91 (m, 2H); HR-MS (ESI): calcd for C20H21N4O3– [M – H]−; 365.1619; found, 365.1628.
Synthesis of 58
Following general synthetic procedure 1 using 2,4-dichloro-6-methylquinazoline (100.0 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 103.8 mg, 88%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (20.0 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (58) (9.0 mg, 31%). 1H NMR (400 MHz, MeOD) δ 7.90 (br s, 1H), 7.65–7.60 (m, 1H), 7.41 (d, J = 8.5 Hz, 1H), 7.06 (br s, 1H), 6.95–6.88 (m, 2H), 4.27 (s, 4H), 3.75 (t, J = 7.1 Hz, 2H), 3.67 (t, J = 6.2 Hz, 2H), 2.47 (s, 3H), 1.99–1.89 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 160.2, 152.4, 143.7, 140.9, 138.7, 136.4, 134.1, 131.3, 123.8, 118.6, 117.5, 115.6, 111.3, 110.7, 64.7, 64.5, 58.8, 39.3, 31.9, and 21.2; HR-MS (ESI): calcd for C20H21N4O3– [M – H]−; 365.1619; found, 365.1622.
Synthesis of 59
Following general synthetic procedure 1 using 2,4-dichloro-6-fluoroquinazoline (101.8 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 93.8 mg, 78%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (20.3 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)-6-fluoroquinazolin-4-yl)amino)propan-1-ol (59) (8.0 mg, 27%). 1H NMR (400 MHz, DMSO-d6) δ 9.59 (br s, 1H), 8.92 (br s, 1H), 8.19–8.08 (m, 1H), 7.65–7.56 (m, 1H), 7.56–7.48 (m, 1H), 7.42–7.28 (m, 1H), 7.16–7.05 (m, 1H), 6.82 (d, J = 8.7 Hz, 1H), 4.64–4.50 (m, 1H), 4.29–4.18 (m, 4H), 3.65–3.56 (m, 2H), 3.56–3.49 (m, 2H), and 1.87–1.78 (m, 2H); HR-MS (ESI): calcd for C19H18FN4O3– [M – H]−; 369.1368; found, 369.1375.
Synthesis of 60
Following general synthetic procedure 1 using 2,4-dichloro-7-fluoroquinazoline (101.8 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 46.1 mg, 38%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (20.3 mg, 79.5 μmol of monochloro intermediate) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)-7-fluoroquinazolin-4-yl)amino)propan-1-ol (60) (3.0 mg, 10%). 1H NMR (400 MHz, MeOD) δ 8.21–8.12 (m, 1H), 7.31–7.19 (m, 2H), 7.11–7.00 (m, 1H), 6.98–6.87 (m, 2H), 4.28 (s, 4H), 3.75 (t, J = 7.1 Hz, 2H), 3.67 (t, J = 6.1 Hz, 2H), and 1.99–1.90 (m, 2H); HR-MS (ESI): calcd for C19H18FN4O3– [M – H]−; 369.1368; found, 369.1383.
Synthesis of 61
Following general synthetic procedure 1 using 2,4-dichloro-8-fluoroquinazoline (101.8 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 76.5 mg, 64%) and 2,3-dihydrobenzo[b][1,4]dioxin-6-amine (24.0 mg, 159 μmol) in step 2 (20.3 mg, 79.5 μmol of monochloro intermediate was used) afforded 3-((2-((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)amino)-8-fluoroquinazolin-4-yl)amino)propan-1-ol (61) (11.5 mg, 39%). 1H NMR (400 MHz, DMSO-d6) δ 9.21 (br s, 1H), 8.38 (br s, 1H), 7.91 (d, J = 8.2 Hz, 1H), 7.73–7.56 (m, 1H), 7.51–7.40 (m, 1H), 7.30–7.21 (m, 1H), 7.17–7.07 (m, 1H), 6.75 (d, J = 8.7 Hz, 1H), 4.64–4.47 (m, 1H), 4.30–4.15 (m, 4H), 3.66–3.48 (m, 4H), and 1.90–1.78 (m, 2H); HR-MS (ESI): calcd for C19H18FN4O3– [M – H]−; 369.1368; found, 369.1383.
Synthesis of 62
Following general synthetic procedure 1 using 2,4-dichloro-6-methylquinazoline (100.0 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 103.8 mg, 88%) and 2-methylbenzo[d]oxazol-6-amine (23.5 mg, 159 μmol) in step 2 (20.0 mg, 79.5 μmol of monochloro intermediate) afforded 3-((6-methyl-2-((2-methylbenzo[d]oxazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (62) (6.2 mg, 21%). 1H NMR (400 MHz, MeOD) δ 8.11 (d, J = 1.8 Hz, 1H), 7.84 (br s, 1H), 7.60–7.55 (m, 2H), 7.44–7.38 (m, 2H), 3.75 (t, J = 7.0 Hz, 2H), 3.67 (t, J = 6.1 Hz, 2H), 2.64 (s, 3H), 2.46 (s, 3H), and 2.00–1.92 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.8, 160.3, 154.1, 151.1, 136.9, 135.8, 133.2, 128.6, 126.0, 123.3, 121.5, 119.1, 117.9, 111.3, 102.9, 58.9, 39.0, 32.0, 21.3, and 14.6; HR-MS (ESI): calcd for C20H20N5O2– [M – H]−; 362.1623; found, 362.1624.
Synthesis of 63
Following general synthetic procedure 1 using 2,4-dichloro-6-methylquinazoline (100.0 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 103.8 mg 88%) and 2-methylbenzo[d]thiazol-6-amine (26.1 mg, 159 μmol) in step 2 (20.0 mg, 79.5 μmol of monochloro intermediate) afforded 3-((6-methyl-2-((2-methylbenzo[d]thiazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (63) (15.4 mg, 56%). 1H NMR (400 MHz, MeOD) δ 8.31 (d, J = 2.0 Hz, 1H), 7.91–7.85 (m, 2H), 7.62–7.58 (m, 2H), 7.41 (d, J = 8.5 Hz, 1H), 3.76 (t, J = 7.1 Hz, 2H), 3.66 (t, J = 6.1 Hz, 2H), 2.83 (s, 3H), 2.47 (s, 3H), and 1.98–1.90 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 164.7, 160.3, 155.4, 148.5, 145.9, 138.0, 136.2, 135.2, 132.2, 123.5, 122.8, 122.0, 119.6, 111.6, 59.1, 38.8, 32.3, 21.3, and 20.1; HR-MS (ESI): calcd for C20H22N5OS– [M – H]−; 378.1394; found, 378.1403.
Synthesis of 64
Following general synthetic procedure 1 using 2,4-dichloro-6-methylquinazoline (100.0 mg, 0.469 mmol) in step 1 (obtained monochloro intermediate; 103.8 mg, 88%) and 1-methyl-1H-indazol-6-amine (23.4 mg, 159 μmol) in step 2 (20.0 mg, 79.5 μmol of monochloro intermediate) afforded 3-((6-methyl-2-((1-methyl-1H-indazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (64) (7.9 mg, 27%). 1H NMR (400 MHz, MeOD) δ 8.27 (br s, 1H), 7.89 (d, J = 0.9 Hz, 1H), 7.78 (br s, 1H), 7.67–7.63 (m, 1H), 7.53–7.48 (m, 1H), 7.41 (d, J = 8.5 Hz, 1H), 7.24–7.20 (m, 1H), 4.04 (s, 3H), 3.79 (t, J = 6.9 Hz, 2H), 3.69 (t, J = 6.1 Hz, 2H), 2.45 (s, 3H), and 2.02–1.93 (m, 2H); HR-MS (ESI): calcd for C20H21N6O– [M – H]−; 361.1782; found, 361.1778.
Synthesis of intermediate D
Following general synthetic procedure 1 using 2,4-dichloro-6-methylquinazoline (1.50 g, 7.04 mmol) in step 1 (obtained monochloro intermediate; 1.56 g, 88%) and 2-bromobenzo[d]thiazol-6-amine (500 mg, 2.18 mmol) in step 2 (1.10 g, 4.37 mmol of monochloro intermediate) afforded 3-((2-((2-bromobenzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (D) (1.00 g, quant). 1H NMR (400 MHz, MeOD) δ 8.32–8.25 (m, 1H), 8.02–7.94 (m, 2H), 7.69 (dd, J = 8.5, 1.5 Hz, 1H), 7.64 (dd, J = 8.8, 2.2 Hz, 1H), 7.44 (d, J = 8.5 Hz, 1H), 3.80–3.74 (m, 2H), 3.65 (t, J = 6.1 Hz, 2H), 2.49 (s, 3H), and 1.99–1.89 (m, 2H); HR-MS (ESI): calcd for C19H18BrN5OS+ [M + H]+; 444.0488; found, 444.04777.
General Synthetic Procedure for 65–70 (General Synthetic Procedure 2)
A mixture of 3-((2-((2-bromobenzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (D) (20.0 mg, 45.0 μmol, 1.0 equiv), Ar-boronic acid (67.5 μmol, 1.5 equiv), Pd(PPh3)4 (5.2 mg, 4.5 μmol, 0.1 equiv), and K3PO4 (19.1 mg, 90.0 μmol, 2.0 equiv) in dioxane/H2O (2/1, 0.50 mL) was heated at 120 °C overnight. The reaction was cooled to room temperature, and product formation was confirmed by LCMS. The reaction mixture was then concentrated in vacuo and purified by column chromatography as described above (Agela Technologies, Silica, 20 g, 0–20% MeOH in DCM) and HPLC to afford compounds 65–70.
Synthesis of 65
Following general synthetic procedure 2 using D (20.0 mg, 45.0 μmol) and (4-fluorophenyl)boronic acid (9.4 mg, 67.5 μmol) afforded 3-((2-((2-(4-fluorophenyl)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (65) (5.0 mg, 24%). 1H NMR (400 MHz, DMSO-d6) δ 12.6 (br s, 1H), 10.6 (br s, 1H), 9.55 (br s, 1H), 8.51 (br s, 1H), 8.19–8.06 (m, 4H), 7.73–7.65 (m, 2H), 7.54–7.40 (m, 3H), 4.79–4.49 (m, 1H), 3.72–3.63 (m, 2H), 3.57–3.49 (m, 2H), 2.43 (s, 3H), and 1.90–1.80 (m, 2H); HR-MS (ESI): calcd for C25H23FN5OS+ [M + H]+; 460.1602; found, 460.1613.
Synthesis of 66
Following general synthetic procedure 2 using D (20.0 mg, 45.0 μmol) and (3-fluorophenyl)boronic acid (9.4 mg, 67.5 μmol) afforded 3-((2-((2-(3-fluorophenyl)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (66) (5.0 mg, 24%). 1H NMR (400 MHz, DMSO-d6) δ 12.7 (br s, 1H), 10.6 (br s, 1H), 9.58 (br s, 1H), 8.57 (br s, 1H), 8.15–8.07 (m, 2H), 7.96–7.87 (m, 2H), 7.75–7.61 (m, 3H), 7.53–7.42 (m, 2H), 4.77–4.56 (m, 1H), 3.72–3.63 (m, 2H), 3.57–3.49 (m, 2H), 2.43 (s, 3H), and 1.90–1.80 (m, 2H); HR-MS (ESI): calcd for C25H23FN5OS+ [M + H]+; 460.1602; found, 460.1621.
Synthesis of 67
Following general synthetic procedure 2 using D (20.0 mg, 45.0 μmol) and o-tolylboronic acid (9.2 mg, 67.5 μmol) afforded 3-((6-methyl-2-((2-(o-tolyl)benzo[d]thiazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (67) (9.4 mg, 46%). 1H NMR (400 MHz, DMSO-d6) δ 12.6 (br s, 1H), 10.6 (br s, 1H), 9.60 (br s, 1H), 8.53 (br s, 1H), 8.16–8.07 (m, 2H), 7.83 (d, J = 7.3 Hz, 1H), 7.75–7.63 (m, 2H), 7.53–7.38 (m, 4H), 4.75–4.52 (m, 1H), 3.73–3.63 (m, 2H), 3.58–3.50 (m, 2H), 2.65 (s, 3H), 2.43 (s, 3H), and 1.91–1.80 (m, 2H); HR-MS (ESI): calcd for C26H26N5OS+ [M + H]+; 456.1853; found, 456.1872.
Synthesis of 68
Following general synthetic procedure 2 using D (20.0 mg, 45.0 μmol) and (1H-pyrazol-3-yl)boronic acid (7.6 mg, 67.5 μmol) afforded 3-((2-((2-(1H-pyrazol-3-yl)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (68) (18.5 mg, 95%). 1H NMR (400 MHz, DMSO-d6) δ 13.4 (s, 1H), 12.5 (br s, 1H), 10.6 (br s, 1H), 9.66 (br s, 1H), 8.42 (br s, 1H), 8.19–7.93 (m, 3H), 7.71–7.60 (m, 2H), 7.54–7.46 (m, 1H), 6.93 (s, 1H), 4.73–4.57 (m, 1H), 3.72–3.62 (m, 2H), 3.57–3.49 (m, 2H), 2.43 (s, 3H), and 1.89–1.82 (m, 2H); HR-MS (ESI): calcd for C22H22N7OS+ [M + H]+; 432.1601; found, 432.1613.
Synthesis of 69
Following general synthetic procedure 2 using D (20.0 mg, 45.0 μmol) and (4-carbamoylphenyl)boronic acid (11.1 mg, 67.5 μmol) afforded 4-(6-((4-((3-hydroxypropyl)amino)-6-methylquinazolin-2-yl)amino)benzo[d]thiazol-2-yl)benzamide (69) (12.5 mg, 57%). 1H NMR (400 MHz, DMSO-d6) δ 12.9 (br s, 1H), 10.7 (br s, 1H), 9.56 (br s, 1H), 8.59 (br s, 1H), 8.22–8.02 (m, 6H), 7.76–7.64 (m, 2H), 7.57 (s, 1H), 7.49 (d, J = 8.4 Hz, 1H), 6.93 (s, 1H), 4.78–4.52 (m, 1H), 3.76–3.62 (m, 2H), 3.60–3.51 (m, 2H), 2.43 (s, 3H), and 1.91–1.81 (m, 2H); HR-MS (ESI): calcd for C26H25N6O2S+ [M + H]+; 485.1754; found, 485.1778.
Synthesis of 70
Following general synthetic procedure 2 using D (20.0 mg, 45.0 μmol) and (1H-indol-5-yl)boronic acid (10.9 mg, 67.5 μmol) afforded 3-((2-((2-(1H-indol-5-yl)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (70) (19.6 mg, 91%). 1H NMR (400 MHz, DMSO-d6) δ 12.6 (br s, 1H), 11.5 (s, 1H), 10.6 (br s, 1H), 9.60 (br s, 1H), 8.43 (br s, 1H), 8.32 (d, J = 1.7 Hz, 1H), 8.13 (s, 1H), 8.03 (d, J = 8.7 Hz, 1H), 7.87 (dd, J = 8.5, 1.8 Hz, 1H), 7.71–7.61 (m, 2H), 7.59–7.48 (m, 3H), 6.64–6.61 (m, 1H), 4.83–4.44 (m, 1H), 3.72–3.63 (m, 2H), 3.57–3.51 (m, 2H), 2.43 (s, 3H), and 1.90–1.81 (m, 2H); HR-MS (ESI): calcd for C27H28N6OS+ [M + H]+; 481.1805; found, 481.1829.
General Synthetic Procedure for 71–82 (General Synthetic Procedure 3)
A mixture of 3-((6-bromo-2-((2-methylbenzo[d]thiazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (D) (15.0 mg, 33.8 μmol, 1.0 equiv), N,N-diisopropylethylamine (DIPEA; 35.4 μL, 203 μmol, 6.0 equiv) and amine (203 μmol, 6.0 equiv) in 3-pentanol (0.10 M) was heated in a microwave vial at 120 °C overnight. The reaction was cooled to room temperature, and product formation was confirmed by LCMS. The reaction mixture was then concentrated in vacuo and purified by column chromatography (Biotage SNAP cartridge, KP-NH, 11g, 2%–30% MeOH in DCM) to afford compounds 71–82.
Synthesis of 71
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 1-benzylpiperidin-4-amine (38.6 mg, 203 μmol) afforded 3-((2-((2-((1-benzylpiperidin-4-yl)amino)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (71) (13.8 mg, 74%). 1H NMR (400 MHz, MeOD) δ 8.11 (d, J = 1.9 Hz, 1H), 7.71–7.67 (m, 1H), 7.46–7.25 (m, 9H), 3.81–3.64 (m, 5H), 3.56 (s, 2H), 2.97–2.86 (m, 2H), 2.42 (s, 3H), 2.28–2.19 (m, 2H), 2.13–2.04 (m, 2H), 1.96–1.89 (m, 2H), and 1.69–1.55 (m, 2H); HR-MS (ESI): calcd for C31H36N7OS+ [M + H]+; 554.2697; found, 554.2722.
Synthesis of 72
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and morpholine (17.7 mg, 203 μmol) afforded 3-((6-methyl-2-((2-morpholinobenzo[d]thiazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (72) (7.3 mg, 48%).1H NMR (400 MHz, MeOD) δ 8.26 (d, J = 1.6 Hz, 1H), 7.71–7.67 (m, 1H), 7.48–7.40 (m, 3H), 7.33 (d, J = 8.5 Hz, 1H), 3.84–3.79 (m, 4H), 3.74–3.64 (m, 4H), 3.60–3.54 (m, 4H), 2.42 (s, 3H), and 2.00–1.89 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 167.3, 160.2, 157.0, 149.7, 146.5, 136.9, 134.5, 131.0, 130.7, 125.6, 122.4, 118.9, 118.3, 111.9, 110.6, 66.0 (2C), 59.2, 48.7 (2C), 38.4, 32.4, and 21.3; HR-MS (ESI): calcd for C23H27N6O2S+ [M + H]+; 451.1911; found, 451.1929.
Synthesis of 73
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 2-methylpropan-1-amine (14.8 mg, 203 μmol) afforded 3-((2-((2-(isobutylamino)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (73) (11.6 mg, 79%). 1H NMR (400 MHz, MeOD) δ 8.11 (d, J = 2.0 Hz, 1H), 7.70–7.67 (m, 1H), 7.45–7.38 (m, 2H), 7.35–7.31 (m, 2H), 3.74–3.65 (m, 4H), 3.23 (d, J = 7.0 Hz, 2H), 2.42 (s, 3H), 2.03–1.89 (m, 3H), 1.02 (s, 3H), and 1.00 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 165.0, 160.2, 157.1, 149.8, 147.1, 136.2, 134.4, 130.7, 130.6, 125.6, 122.4, 117.9, 117.7, 111.8, 110.8, 59.3, 52.1, 38.4, 32.5, 28.2, 21.3, and 20.6 (2C); HR-MS (ESI): calcd for C23H27N6OS– [M – H]−; 435.1973; found, 435.1989.
Synthesis of 74
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 2-morpholinoethan-1-amine (26.4 mg, 203 μmol) afforded 3-((6-methyl-2-((2-((2-morpholinoethyl)amino)benzo[d]thiazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (74) (12.6 mg, 75%).1H NMR (400 MHz, MeOD) δ 8.14–8.09 (m, 1H), 7.68 (br s, 1H), 7.45–7.29 (m, 4H), 3.77–3.63 (m, 8H), 3.57 (d, J = 6.4 Hz, 2H), 2.66 (d, J = 6.4 Hz, 2H), 2.60–2.49 (m, 4H), 2.41(s, 3H), and 1.98–1.88 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δ 164.8, 160.2, 157.1, 149.8, 147.0, 136.3, 134.4, 130.8, 130.6, 125.6, 122.4, 118.0, 117.7, 111.9, 110.8, 66.6(2C), 59.3, 57.6, 53.8(2C), 41.5, 38.4, 32.5, 21.3; HR-MS (ESI): calcd for C25H32N7O2S+ [M + H]+; 494.2333; found, 494.2357.
Synthesis of 75
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and benzo[d][1,3]dioxol-5-ylmethanamine (29.1 mg, 203 μmol) afforded 3-((2-((2-((benzo[d][1,3]dioxol-5-ylmethyl)amino)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (75) (8.9 mg, 51%). 1H NMR (400 MHz, MeOD) δ 7.89 (s, 1H), 7.83 (d, J = 2.0 Hz, 1H), 7.60 (dd, J = 8.5, 1.4 Hz, 1H), 7.48 (d, J = 8.5 Hz, 1H), 7.40 (d, J = 8.5 Hz, 1H), 7.37–7.32 (m, 1H), 6.91–6.86 (m, 2H), 6.79 (d, J = 11.8 Hz, 1H), 5.93 (s, 2H), 4.54 (s, 2H), 3.74 (t, J = 7.1 Hz, 2H), 3.65 (t, J = 6.1 Hz, 2H), 2.46 (s, 3H), 1.98–1.88 (m, 2H); HR-MS (ESI): calcd for C27H27N6O3S+ [M + H]+; 515.1860; found, 515.1883.
Synthesis of 76
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 2-phenoxyethan-1-amine (27.8 mg, 203 μmol) afforded 3-((6-methyl-2-((2-((2-phenoxyethyl)amino)benzo[d]thiazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (76) (6.3 mg, 37%).1H NMR (400 MHz, MeOD) δ 7.95–7.89 (m, 2H), 7.68–7.63 (m, 1H), 7.56–7.51 (m, 1H), 7.48–7.41 (m, 2H), 7.31–7.24 (m, 2H), 6.99–6.90 (m, 3H), 4.25 (t, J = 5.2 Hz, 2H), 3.90 (t, J = 5.2 Hz, 2H), 3.76 (t, J = 7.1 Hz, 2H), 3.65 (t, J = 6.1 Hz, 2H), 2.47 (s, 3H), and 1.98–1.88 (m, 2H); HR-MS (ESI): calcd for C27H27N6O3S+ [M + H]+; 515.1860; found, 515.1883.
Synthesis of 77
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 2-(benzyloxy)ethan-1-amine (30.7 mg, 203 μmol) afforded 3-((2-((2-((2-(benzyloxy)ethyl)amino)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (77) (7.7 mg, 44%). 1H NMR (400 MHz, MeOD) δ 7.98–7.91 (m, 2H), 7.68–7.63 (m, 1H), 7.52–7.46 (m, 2H), 7.44 (d, J = 8.5 Hz, 1H), 7.36–7.21 (m, 5H), 4.59 (s, 2H), 3.79–3.70 (m, 6H), 3.65 (t, J = 6.1 Hz, 2H), 2.48 (s, 3H), and 1.98–1.88 (m, 2H); HR-MS (ESI): calcd for C28H31N6O2S+ [M + H]+; 515.2224; found, 515.2246.
Synthesis of 78
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 1-amino-2-methylpropan-2-ol (18.1 mg, 203 μmol) afforded 3-((2-((2-((2-hydroxy-2-methylpropyl)amino)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (78) (14.0 mg, 91%). 1H NMR (400 MHz, MeOD) δ 8.12 (d, J = 2.1 Hz, 1H), 7.68 (br s, 1H), 7.44–7.37 (m, 2H), 7.35–7.30 (m, 2H), 3.73–3.64 (m, 4H), 3.44 (s, 2H), 2.41 (s, 3H), 1.98–1.87 (m, 2H), and 1.27 (s, 6H); HR-MS (ESI): calcd for C23H29N6O2S+ [M + H]+; 453.2067; found, 453.2069.
Synthesis of 79
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and N1,N1-dimethylethane-1,2-diamine (17.9 mg, 203 μmol) afforded 3-((2-((2-((2-(dimethylamino)ethyl)amino)benzo[d]thiazol-6-yl)amino)-6-methylquinazolin-4-yl)amino)propan-1-ol (79) (12.9 mg, 84%). 1H NMR (400 MHz, MeOD) δ 8.14 (d, J = 2.0 Hz, 1H), 7.68 (br s, 1H), 7.44–7.38 (m, 2H), 7.38–7.30 (m, 2H), 3.73–3.65 (m, 4H), 3.56 (t, J = 6.7 Hz, 2H), 2.64 (t, J = 6.7 Hz, 2H), 2.42 (s, 3H), 2.32 (s, 6H), and 1.98–1.89 (m, 2H); HR-MS (ESI): calcd for C23H30N7OS+ [M + H]+; 452.2227; found, 452.2242.
Synthesis of 80
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 2,2′-azanediylbis(ethan-1-ol) (21.3 mg, 203 μmol) afforded 2,2′-((6-((4-((3-hydroxypropyl)amino)-6-methylquinazolin-2-yl)amino)benzo[d]thiazol-2-yl)azanediyl)bis(ethan-1-ol) (80) (5.2 mg, 33%). 1H NMR (400 MHz, MeOD) δ 8.10–8.02 (m, 1H), 7.95–7.90 (m, 1H), 7.65 (dd, J = 8.5, 1.3 Hz, 1H), 7.56–7.50 (m, 2H), 7.44 (d, J = 8.5 Hz, 1H), 3.95–3.88 (m, 4H), 3.88–3.82 (m, 4H), 3.76 (t, J = 7.2 Hz, 2H), 3.66 (t, J = 6.1 Hz, 2H), 2.47 (s, 3H), and 1.98–1.88 (m, 2H); HR-MS (ESI): calcd for C23H29N6O3S+ [M + H]+; 469.2016; found, 469.2031.
Synthesis of 81
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 4-(2-aminoethyl)benzenesulfonamide (40.7 mg, 203 μmol) afforded 4-(2-((6-((4-((3-hydroxypropyl)amino)-6-methylquinazolin-2-yl)amino)benzo[d]thiazol-2-yl)amino)ethyl)benzenesulfonamide (81) (7.5 mg, 39%). 1H NMR (400 MHz, MeOD) δ 7.94–7.91 (m, 1H), 7.91–7.82 (m, 3H), 7.67 (dd, J = 8.5, 1.4 Hz, 1H), 7.53–7.40 (m, 5H), 3.82–3.71 (m, 4H), 3.65 (t, J = 6.1 Hz, 2H), 3.11 (t, J = 7.0 Hz, 2H), 2.48 (s, 3H), and 1.99–1.89 (m, 2H); HR-MS (ESI): calcd for C27H30N7O3S2+ [M + H]+; 564.1846; found, 564.1856.
Synthesis of 82
Following general synthetic procedure 3 using D (15.0 mg, 33.8 μmol) and 1-methylpiperazine (20.3 mg, 203 μmol) afforded 3-((6-methyl-2-((2-(4-methylpiperazin-1-yl)benzo[d]thiazol-6-yl)amino)quinazolin-4-yl)amino)propan-1-ol (82) (2.9 mg, 18%). 1H NMR (400 MHz, MeOD) δ 8.25 (d, J = 1.7 Hz, 1H), 7.69 (br s, 1H), 7.47–7.39 (m, 3H), 7.37–7.30 (m, 1H), 3.75–3.58 (m, 8H), 2.65 (t, J = 10.2 Hz, 4H), 2.42 (s, 3H), 2.36 (s, 3H), and 1.98–1.88 (m, 2H); HR-MS (ESI): calcd for C24H30N7OS+ [M + H]+; 464.2227; found, 464.2235.
High-Throughput Screen of the RNA-Focused Small-Molecule Library
Measurements for disruption of the r(CCUG)12–MBNL1 complex were completed using a previously reported TR-FRET assay with minor modifications. (23,24) Briefly, 5′-biotinylated r(CCUG)12 was folded in 1 × Folding Buffer (20 mM HEPES, pH 7.5, 110 mM KCl, and 10 mM NaCl) at 60 °C for 5 min then cooled to room temperature. The buffer was adjusted to 1 × Assay Buffer (20 mM HEPES, pH 7.5, 110 mM KCl, 10 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, 5 mM DTT, 0.1% BSA, and 0.5% Tween-20). Then, MBNL1-His6 was added, and the samples were incubated at room temperature for 15 min and added to a white 384-well plate using an Aurora FRD-IB reagent dispenser. Compounds from the RNA-focused small molecule library were then delivered to each well using a Beckman Biomek NXP liquid handler with a pin tool, and the samples were incubated for another 15 min at room temperature. The final concentration of r(CCUG)12 was 80 nM, while the final concentration of MBNL1 was 60 nM. A solution of streptavidin-XL665 and anti-His6-Tb antibody was then added to a total volume of 10 μL using an Aurora FRD-IB reagent dispenser, with final concentrations of 40 nM and 0.44 ng/μL, respectively. The sample plate was incubated for 30 min at room temperature and TR-FRET was measured on a Molecular Devices SpectraMax M5 plate reader using an excitation wavelength of 345 and a 420 nm cutoff. The ratios of fluorescence intensity at 545 and 665 nm were calculated, and ratios in the absence of a compound and in the absence of RNA were used to calculate percent disruption.
In Vitro IC50 Measurements
The IC50s for disruption of r(CCUG)12–MBNL1 by hit compounds from the RNA-focused small-molecule library were completed as described above except compounds were added manually to wells at varying concentrations to afford a dose response. The resulting curves were fit to eq 1 to determine IC50 values
| (1) |
where y is the ratio of fluorescence intensities at 545 and 665 nm (F545/F665), x is the concentration of a compound, B is F545/F665 value at max FRET effect (solution has RNA and protein but no compound added); A is F545/F665 value at min FRET effect (solution has antibodies but no RNA, protein, or compound); and IC50 is the concentration of a compound where half of the protein is displaced by a compound.
NMR Sample Preparation
A self-complementary RNA construct, r(5′-GACAGCCUGCUGUC-3′), was purchased from GE Healthcare Dharmacon, Inc., deprotected according to the manufacturer’s recommended protocol, and desalted with PD-10 columns (GE Healthcare, cat: 17–0851-01) also per the manufacturer’s protocol. RNA samples were dissolved in NMR Buffer [10 mM KH2PO4/K2HPO4 and 0.05 mM ethylenediamine tetraacetic acid (EDTA) (pH 6.0)] and folded by heating to 95 °C for 3 min and slowly cooling to room temperature.
NMR Spectroscopy
NMR spectra were acquired at 25 °C on Bruker Avance III 600 and 700 MHz spectrometers equipped with cryoprobes. One-dimensional NMR spectra were acquired on samples containing 100 μM of RNA alone in 100% D2O. Compounds were each titrated into separate samples at 0.5, 1.0, 1.5, and 2.0 compound: RNA molar ratios. WaterLOGSY (water-ligand observed via gradient spectroscopy) spectra (29) were acquired on samples containing 300 μM of each compound alone or in the presence of 15 μM of RNA at a 20:1 compound: RNA ratio in H2O, to which D2O was added to 5% by volume. WaterLOGSY spectra were phased to give negative NOEs for nonbinders. Two-dimensional NOESY and DQF-COSY spectra were acquired on samples containing 400 μM of RNA alone in 100% D2O. Two-dimensional NMR spectra were processed with nmrPipe (30) and assigned with SPARKY. (31)
Cell Lines and Cell Culture
Compounds were tested in two cell lines: (i) DM2 patient-derived fibroblasts (generous gift from University of Florida, Center for NeuroGenetics) and (ii) fibroblasts from a healthy donor (wild-type; WT GM07492; Coriell Institute). Cells were maintained at 37 °C with 5% CO2. DM2 fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/high glucose (HyClone) supplemented with 20% (v/v) fetal bovine serum (FBS) (Sigma) and 1% (v/v) antibiotic–antimycotic solution (Corning). Wild-type fibroblasts were cultured in MEM (Corning) supplemented with 10% (v/v) FBS, 1% (v/v) Glutagro (Corning), and 1% (v/v) antibiotic–antimycotic solution.
Evaluation of Cell Viability
Compound toxicity in DM2 fibroblasts was evaluated using a CellTiter-Glo Kit (Promega) as described in the manufacturer’s protocol. Briefly, after 48 h treatment in 96-well plates, 100 μL of the CellTiter-Glo reagent was added to each well. The plate was then incubated at room temperature for 10 min, and luminescence was measured using BioTek FLX-800 luminescence plate reader (n = 6 replicates; 1 independent experiment).
Evaluation of Pre-mRNA Splicing via RT-PCR
Pre-mRNA splicing was analyzed as previously described. (10) Briefly, cells were grown in 6-well plates and treated with the desired compound in growth medium at ∼40% confluency. After 48 h, the cells were lysed, and total RNA was harvested using a Zymo Quick RNA Miniprep Kit. Approximately 1 μg of total RNA was reverse transcribed using a qScript cDNA synthesis kit (20 μL of total reaction volume, Quanta BioSciences); 2 μL of the RT reaction was used for PCR using GoTaq DNA polymerase (Promega). RT-PCR products were observed after 35 cycles of 95 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 5 min. Products were separated on a 2% agarose gel (110 V for 1 h in 1 × TBE buffer), visualized by staining with ethidium bromide, and imaged using a Typhoon 9410 variable mode imager. Gels were quantified using ImageJ. Percent rescue was calculated by dividing the difference between treated and untreated DM2 samples by the difference between untreated DM2 and WT samples (eq 2).
| (2) |
Evaluation of CNBP Abundance via RT-qPCR
CNBP abundance was evaluated as previously described. (10) Briefly, cells were grown in 6-well plates and treated with the desired compound in growth medium at 40% confluency. After 48 h, the cells were lysed, and total RNA was harvested using a Zymo Quick RNA Miniprep Kit per the manufacturer’s protocol. Approximately 1 μg of total RNA was reverse transcribed using a qScript cDNA synthesis kit (20 μL of total reaction volume, Quanta BioSciences); 2 μL of the reverse transcription (RT) reaction was used for each primer pair (Table S2) for quantitative (q)PCR with SYBR Green Master Mix, performed on a QuantStudio 5, 384-well Block Real-Time PCR System (Applied Biosciences). Relative abundance was determined by normalizing to GAPDH (n = 6 replicates per concentration; 2 independent experiments).
Evaluation of Nuclear Foci via RNA Fluorescence In Situ Hybridization (FISH)
RNA FISH was used to determine the small molecules’ effects on the number of nuclear foci as previously described. (10) The number of foci was counted in 40 nuclei/replicate (120 total nuclei counted); n = 3 replicates; 1 independent experiment.
Affinity Measurements
Binding affinity measurements were performed via microscale thermophoresis (MST) on a Monolith NT.115 system (NanoTemper Technologies) with Cy5-labeled (CCUG)12 (5′-Cy5-GCG(CCUG)12CGC; Dharmacon) and Cy5-labeled base pair control (BP; 5′-Cy5-GCG(CCUG)7(GCAG)5CGC; Dharmacon), which were deprotected according to the manufacturer’s protocol and then desalted using a PD-10 column (GE LifeSciences) per the manufacturer’s recommended procedure. RNA (5 nM) was prepared in 8 mM Na2HPO4, pH 7.0, 185 mM NaCl, and 1 mM EDTA and folded by heating at 60 °C for 5 min and slowly cooling to room temperature. Compound dilutions (1:1) were prepared using 1 × MST Buffer (8 mM Na2HPO4, pH 7.0, 185 mM NaCl, 1 mM EDTA) and 0.1% (v/v) Tween-20 was added. After cooling, the RNA solution was added to the compound solution (0.05% (v/v) Tween-20 final concentration). Samples were incubated for 30 min at room temperature and then loaded into standard capillaries (NanoTemper Technologies). The following parameters were used to acquire thermophoretic data: 5–20% LED, 80% MST power, laser-on time = 30 s, laser-off time = 5 s. Fluorescence was detected using excitation wavelengths of 605–645 nm and emission wavelengths of 680–685 nm. The resulting data were analyzed by thermophoresis analysis and fitted using a quadratic binding equation in the MST analysis software (NanoTemper Technologies). Dissociation constants were then determined using eq 3. The reported Kd values are an average of two independent sets of experiments.
| (3) |
where c is the ligand concentration, cT is the concentration of the RNA, and Kd is the dissociation constant.
SMILES and Physicochemical Properties
Marvin 20.8.0 (ChemAxon; https://www.chemaxon.com) was used to generate SMILES and to calculate physicochemical properties.
Supplementary Material
Acknowledgments
The authors thank the University of Florida, Center for NeuroGenetics for the generous gift of the DM2 fibroblast cell line used in this paper. The authors also thank the agencies that funded this work including the National Institutes of Health (R35 NS116846 to M.D.D. and F31-NS110269 to A.J.A., and S10-OD021550 to The Scripps Research Institute), the Muscular Dystrophy Association (grant 380467 to M.D.D.), the Myotonic US Fellowship Research grant (to R.I.B.), and to the National Ataxia Foundation Fellowship Research grant (to R.I.B.).
ABBREVIATIONS
- CNBP
CCHC-type zinc finger nucleic acid binding protein
- DCM
dichloromethane
- DIPEA
N,N-diisopropylethylamine
- DMF
dimethylformamide
- DMSO
dimethyl sulfoxide
- DM2
myotonic dystrophy type 2
- HCl
hydrochloric acid
- ESI
electrospray ionization
- EtOH
ethanol
- FISH
fluorescence in situ hybridization
- HPLC
high-performance liquid chromatography
- IPA
isopropanol
- IR
insulin receptor
- LCMS
liquid chromatography mass spectrometry
- MBNL1
muscleblind-like 1
- MeOH
methanol
- MW
microwave
- NMR
nuclear magnetic resonance
- TFA
trifluoroacetic acid
- TR-FRET
time-resolved fluorescence resonance energy transfer
Footnotes
The authors declare the following competing financial interest(s): M.D.D. is a founder of Expansion Therapeutics, and K.V. and A.J.A are current employees of Expansion Therapeutics.
SUPPORTING INFORMATION
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c00414.
• Approximate IC50s for the disruption of the r(CCUG)exp–MBNL1 complex (Table S1); Sequences of primers (Table S2); Results of a high-throughput screen (Figure S1); Structures of compounds 1–44 (Figure S2); WaterLOGSY NMR analysis of 3 (Figure S3); WaterLOGSY NMR analysis of 4 (Figure S4); WaterLOGSY NMR analysis of 5 (Figure S5); WaterLOGSY NMR analysis of 7 (Figure S6); Two-dimensional NOESY NMR analysis (Figure S7); One-dimensional NMR analysis of compounds (Figure S8); Cell viability of DM2 fibroblasts treated with 3, 4, 5, and 7 (Figure S9); Structures of compounds 45–82 (Figure S10); Cell viability of DM2 fibroblasts treated with lead-optimized compounds (Figure S11); Cellular activity of compounds 63, 78, and 79 (Figure S12); Compound 63 does not affect MAP4K4 alternative splicing (Figure S13); Compound 63 has no effect on CNBP levels or IR exon 11 splicing (Figure S14); synthetic schemes, compound characterization; HPLC traces; and NMR spectra (PDF)
• All DM2 hit compounds (SMILES + DATA) (CSV)
Contributor Information
Sarah Wagner-Griffin, Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida 33458, United States.
Masahito Abe, Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida 33458, United States.
Raphael I. Benhamou, Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida 33458, United States
Alicia J. Angelbello, Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida 33458, United States
Kamalakannan Vishnu, Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida 33458, United States.
Jonathan L. Chen, Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida 33458, United States.
Jessica L. Childs-Disney, Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida 33458, United States
Matthew D. Disney, Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida 33458, United States.
REFERENCES
- 1.Paulson H Repeat expansion diseases. Handbook of Clinical Neurology; Elsevier, 2018; Vol. 147, pp 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hale MA; Johnson NE; Berglund JA Repeat-associated RNA structure and aberrant splicing. Biochim. Biophys. Acta, Gene Regul. Mech 2019, 1862, 194405, DOI: 10.1016/j.bbagrm.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleary JD; Pattamatta A; Ranum LPW Repeat-associated non-ATG (RAN) translation. J. Biol. Chem. 2018, 293, 16127–16141, DOI: 10.1074/jbc.R118.003237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sznajder ŁJ; Thomas JD; Carrell EM; Reid T; McFarland KN; Cleary JD; Oliveira R; Nutter CA; Bhatt K; Sobczak K Intron retention induced by microsatellite expansions as a disease biomarker. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 4234, DOI: 10.1073/pnas.1716617115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liquori CL; Ricker K; Moseley ML; Jacobsen JF; Kress W; Naylor SL; Day JW; Ranum LPW Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 2001, 293, 864, DOI: 10.1126/science.1062125 [DOI] [PubMed] [Google Scholar]
- 6.Childs-Disney JL; Yildirim I; Park H; Lohman JR; Guan L; Tran T; Sarkar P; Schatz GC; Disney MD Structure of the myotonic dystrophy type 2 RNA and designed small molecules that reduce toxicity. ACS Chem. Biol. 2014, 9, 538–550, DOI: 10.1021/cb4007387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fardaei M; Rogers MT; Thorpe HM; Larkin K; Hamshere MG; Harper PS; Brook JD Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum. Mol. Genet. 2002, 11, 805–814, DOI: 10.1093/hmg/11.7.805 [DOI] [PubMed] [Google Scholar]
- 8.Mankodi A; Urbinati CR; Yuan QP; Moxley RT; Sansone V; Krym M; Henderson D; Schalling M; Swanson MS; Thornton CA Muscleblind localizes to nuclear foci of aberrant RNA in myotonic dystrophy types 1 and 2. Hum. Mol. Genet. 2001, 10, 2165–2170, DOI: 10.1093/hmg/10.19.2165 [DOI] [PubMed] [Google Scholar]
- 9.Savkur RS; Philips AV; Cooper TA Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat. Genet. 2001, 29, 40–47, DOI: 10.1038/ng704 [DOI] [PubMed] [Google Scholar]
- 10.Benhamou RI; Angelbello AJ; Wang ET; Disney MD A toxic RNA catalyzes the cellular synthesis of its own inhibitor, shunting It to endogenous decay pathways. Cell Chem. Biol. 2020, 27, 223–231, DOI: 10.1016/j.chembiol.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieben ED; Aleff RA; Tosakulwong N; Butz ML; Highsmith WE; Edwards AO; Baratz KH A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2–2) gene predicts Fuchs corneal dystrophy. PLoS One 2012, 7, e49083–e49086, DOI: 10.1371/journal.pone.0049083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelbello AJ; Benhamou RI; Rzuczek SG; Choudhary S; Tang Z; Chen JL; Roy M; Wang KW; Yildirim I; Jun AS A small molecule that binds an RNA repeat expansion stimulates its decay via the exosome complex. Cell Chem. Biol. 2021, 28, 34–45, DOI: 10.1016/j.chembiol.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelbello AJ; Rzuczek SG; Mckee KK; Chen JL; Olafson H; Cameron MD; Moss WN; Wang ET; Disney MD Precise small-molecule cleavage of an r(CUG) repeat expansion in a myotonic dystrophy mouse model. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 7799–7804, DOI: 10.1073/pnas.1901484116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costales MG; Aikawa H; Li Y; Childs-Disney JL; Abegg D; Hoch DG; Pradeep Velagapudi S; Nakai Y; Khan T; Wang KW Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 2406–2411, DOI: 10.1073/pnas.1914286117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disney MD Targeting RNA with small molecules to capture opportunities at the intersection of chemistry, biology, and medicine. J. Am. Chem. Soc. 2019, 141, 6776–6790, DOI: 10.1021/jacs.8b13419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benhamou RI; Angelbello AJ; Andrews RJ; Wang ET; Moss WN; Disney MD Structure-specific cleavage of an RNA repeat expansion with a dimeric small molecule is advantageous over sequence-specific recognition by an oligonucleotide. ACS Chem. Biol. 2020, 15, 485–493, DOI: 10.1021/acschembio.9b00958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benhamou RI; Abe M; Choudhary S; Meyer SM; Angelbello AJ; Disney MD Optimization of the linker domain in a dimeric compound that degrades an r(CUG) repeat expansion in cells. J. Med. Chem. 2020, 63, 7827–7839, DOI: 10.1021/acs.jmedchem.0c00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong C-H; Fu Y; Ramisetty SR; Baranger AM; Zimmerman SC Selective inhibition of MBNL1–CCUG interaction by small molecules toward potential therapeutic agents for myotonic dystrophy type 2 (DM2). Nucleic Acids Res. 2011, 39, 8881–8890, DOI: 10.1093/nar/gkr415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haniff HS; Graves A; Disney MD Selective small molecule recognition of RNA base [DOI] [PMC free article] [PubMed]
- 20.Lipinski CA; Lombardo F; Dominy BW; Feeney PJ Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23, 3–25, DOI: 10.1016/S0169-409X(96)00423-1 [DOI] [PubMed] [Google Scholar]
- 21.Shultz MD Two decades under the influence of the Rule of Five and the changing properties of approved oral drugs. J. Med. Chem. 2019, 62, 1701–1714, DOI: 10.1021/acs.jmedchem.8b00686 [DOI] [PubMed] [Google Scholar]
- 22.DeGoey DA; Chen H-J; Cox PB; Wendt MD Beyond the Rule of 5: lessons learned from AbbVie’s drugs and compound collection. J. Med. Chem. 2018, 61, 2636–2651, DOI: 10.1021/acs.jmedchem.7b00717 [DOI] [PubMed] [Google Scholar]
- 23.Costales MG; Rzuczek SG; Disney MD Comparison of small molecules and oligonucleotides that target a toxic, non-coding RNA. Bioorg. Med. Chem. Lett. 2016, 26, 2605–2609, DOI: 10.1016/j.bmcl.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 24.Chen CZ; Sobczak K; Hoskins J; Southall N; Marugan JJ; Zheng W; Thornton CA; Austin CP Two high-throughput screening assays for aberrant RNA-protein interactions in myotonic dystrophy type 1. Anal. Bioanal. Chem. 2012, 402, 1889–1898, DOI: 10.1007/s00216-011-5604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ule J; Ule A; Spencer J; Williams A; Hu JS; Cline M; Wang H; Clark T; Fraser C; Ruggiu M Nova regulates brain-specific splicing to shape the synapse. Nat. Genet. 2005, 37, 844–852, DOI: 10.1038/ng1610 [DOI] [PubMed] [Google Scholar]
- 26.Angelbello AJ; DeFeo ME; Glinkerman CM; Boger DL; Disney MD Precise targeted cleavage of a r(CUG) repeat expansion in cells by using a small-molecule–deglycobleomycin conjugate. ACS Chem. Biol. 2020, 15, 849–855, DOI: 10.1021/acschembio.0c00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haniff HS; Tong Y; Liu X; Chen JL; Suresh BM; Andrews RJ; Peterson JM; O’Leary CA; Benhamou RI; Moss WN Targeting the SARS-CoV-2 RNA genome with small molecule binders and ribonuclease targeting chimera (RIBOTAC) degraders. ACS Cent. Sci 2020, 6, 1713–1721, DOI: 10.1021/acscentsci.0c00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeJesus-Hernandez M; Mackenzie IR; Boeve BF; Boxer AL; Baker M; Rutherford NJ; Nicholson AM; Finch NA; Flynn H; Adamson J Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256, DOI: 10.1016/j.neuron.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalvit C; Pevarello P; Tato M; Veronesi M; Vulpetti A; Sundstrom M Identification of compounds with binding affinity to proteins via magnetization transfer from bulk water. J. Biomol. NMR 2000, 18, 65–68, DOI: 10.1023/A:1008354229396 [DOI] [PubMed] [Google Scholar]
- 30.Delaglio F; Grzesiek S; Vuister GW; Zhu G; Pfeifer J; Bax A NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293, DOI: 10.1007/BF00197809 [DOI] [PubMed] [Google Scholar]
- 31.Goddard TD; Kneller DG SPARKY, NMR Assignment and Integration Software, 3; University of California: San Francisco, 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


